Abstract
Background
Surface modification of medical polymers can improve biocompatibility. Pure polystyrene is hydrophobic and cannot provide a suitable environment for cell cultures. The conventional method for surface modification of polystyrene is treatment with plasma. In this study, conventional polystyrene was exposed to microwave plasma treatment with oxygen and argon gases for 30, 60, and 180 seconds.
Methods and results
Attenuated total reflection Fourier transform infrared spectra investigations of irradiated samples indicated clearly the presence of functional groups. Atomic force microscopic images of samples irradiated with inert and active gases indicated nanometric surface topography. Samples irradiated with oxygen plasma showed more roughness (31 nm) compared with those irradiated with inert plasma (16 nm) at 180 seconds. Surface roughness increased with increasing duration of exposure, which could be due to reduction of the contact angle of samples irradiated with oxygen plasma. Contact angle analysis showed reduction in samples irradiated with inert plasma. Samples irradiated with oxygen plasma showed a lower contact angle compared with those irradiated by argon plasma.
Conclusion
Cellular investigations with unrestricted somatic stem cells showed better adhesion, cell growth, and proliferation for samples radiated by oxygen plasma with increasing duration of exposure than those of normal samples.
Introduction
Control of surface properties is very important for good adhesion. Biomaterial wettability is an important factor in the surface modification of materials. Surface modification of hydrophobic polymer surfaces can be achieved by wet (acid, alkali), dry (plasma), and radiation treatments (ultraviolet radiation and laser).Citation1–Citation3 Nonthermal and low-pressure plasma has been used in a series of surface treatment applications. The majority of plasma-assisted technologies are based on low-pressure processes.Citation4 Treatment of polymeric materials with plasma is frequently used to achieve surface modification, control chemical composition, and modify surface topography. Moreover, microwave discharges are routinely employed in the processing of materials to deposit films and coatings.Citation5–Citation7 Physical surface modification and its effects on wettability are an interesting field for surface engineers. It should be noted that there has been a lot of scientific work done on molecularly smooth or modeled ‘simply rough’ surfaces,Citation8 but little work has been done on wettability and the spreading phenomena of real engineering surfaces.Citation9 From a practical point of view, a simple methodology is needed to account for the influence of a heterogeneous rough surface on wetting and contact angle measurements. The first attempt at this was made by Wenzel, and his simple model was based on the assumption that rough surfaces extend the solid-liquid interface area in comparison with projected smooth surfaces. Plasma treatment is generally used for surface modification of polymers because the processes involved are solvent-free and dry, the consumption of chemicals is extremely low, and the need for sterilization of the products is eliminated.Citation10 Moreover, these processes are precisely controllable. The surface can be treated homogeneously and the surface chemistry can be tailored for the required end use.Citation11 The plasma method uses energetic neutral atoms, ions, and electrons, which act on a surface to change its physicochemical properties. The nature of the gas species used and the energy of the ions are important parameters affecting the properties of a polymer surface immersed in plasma.
Polystyrene is a polymer which is commonly used in biomedical applications and is generally hydrophobic in nature. Certain applications, such as cell culture discs, require a more hydrophilic surface but without alteration of the bulk properties of the polymer. Hence, surface modification of such polymers becomes important.Citation11 In this study, we used unrestricted somatic stem cells (USSCs), first isolated from umbilical cord blood in 2003 by Jager et al who evaluated their differentiation capacity for transplantation.Citation12 In 2004, Koghler et al also evaluated these cells for their ability to produce cytokines. USSCs are pluripotent and are considered to be rare cell populations in umbilical cord blood. USSCs have a high potential to proliferate and differentiate, and so are considered to be valuable in cell therapy.Citation13 USSCs are CD45-negative, adherent, and HLA Class II-negative stem cells with a long telomerase. Moreover, these cells possess a unique cytokine profile and are associated with self-renewing factors. Unlike other cord blood-derived mesenchymal cells, which differentiate only into osteoblasts, chondrocytes, adipocytes,Citation14 and neurons,Citation13 USSCs have the potential to differentiate into osteoblasts, chondrocytes, blood cells, neurons, hepatocytes, and heart tissue in ex vivo conditions.Citation13 These cells express a wide range of factors, including adherent cells, growth factors, and various cytokines, including stem cell factor, vascular endothelial growth factor, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, transforming growth factor-1β, interleukin (IL)-6, granulocyte colony-stimulating factor, leukemia inhibitory factor, Flt3 ligand, thrombopoetin, hepatocyte growth factor, stromal cell-derived-1α, IL-15, IL-12, IL-8, and IL-1β.Citation14 In this work, we demonstrated the effect of plasma on the surface properties of polystyrene, using argon (inert) and oxygen (reactive) gases, and the influence of surface roughness on its wetting properties.
Materials and methods
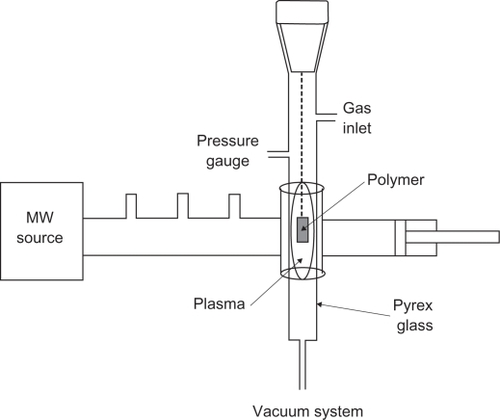
We used a microwave plasma source to modify the surfaces of the polystyrene samples. Microwave sources can be operated at low pressures of 10−3 to 10−1 millibars, which reduce the risk of gas phase contamination during processing. Moreover, plasma properties can be controlled conveniently by adjusting the microwave power. We demonstrate the effect of microwave plasma on the surface property of polystyrene with argon (inert), and oxygen (active) at different times. Culture dishes (orange) with a diameter of 60 mm, size 1 × 1 cm well, were placed in ethanol solution for 24 hours to eliminate pollution. Samples were then placed in a plasma chamber and exposed to oxygen and argon gases for 30, 60, and 180 seconds. Samples irradiated for these times were investigated by structural analysis and microscopy. Plasma surface treatment was achieved in microwave-induced plasma, with surface waves at a power level of 100 W. The experimental apparatus is shown in .
Attenuated total reflection Fourier transform infrared analysis
The samples were studied before and after surface modification using an infrared spectrometer device (Thermo Nicolet, Nexus 870, Madison, WI). Samples were cleaned beforehand. The test was performed based on the frequency absorption of covalent bands.
Atomic force microscopic analysis
An atomic force microscope (Easyscan, Nanosurf Inc, Boston, MA) was used to study surface roughness resulting from treatment with plasma.
Contact angle analysis
The static contact angles of the sample surfaces were investigated by a contact angle measuring device (G10, Krüss, Matthews, NC) using the sessile drop method. The contact angle formed was defined as the angle between the solid/liquid and liquid/vapor join surface.
Cellular analysis
Culture and isolation of USSC from umbilical cord blood
After receiving consent from the mothers (mean age 28 years), the infants’ umbilical cords were obtained. Only 40% of the cord blood samples contained USSCs. After collecting the samples, the red blood cells were lysed using ammonium, and the isolation procedure was done using Ficoll. The samples were then rinsed twice with sterile phosphate-buffered saline (pH 7.4). After centrifugation, the cells were placed in Dulbecco’s modified Eagle’s medium (DMEM) with low glucose and enriched with 100 nm dexamethasone, 10% fetal bovine serum, and penicillin and streptomycin antibiotics. The first medium exchange process was done at 24 hours, and every four days thereafter. When 80% of the flask surface area was covered with cells, the cells were passaged using 0.25% trypsin and ethylenediamine tetra-acetic acid (ETDA). USSCs were regularly expanded on the culture medium, and 37°C temperature and 5% CO2 were required for growth. The USSCs were first trypsinized and counted. The tubes containing 105–106 cells were incubated on a rocker rotator for 6–10 hours, centrifuged at 1000 rpm for six minutes, and 3% human serum was then added for cell deposition. The mixture obtained was kept at room temperature for 30 minutes. The cells were again centrifuged, and phosphate-buffered saline was added to the cell deposit. The cell mixture was passed through a nylon mesh, and 100 μL of cells was added to each tube with the following antibodies: anti-CD90, anti-CD105, anti-CD166, anti-CD45, anti-CD73, and anti-CD34. Thereafter, they were kept at 4°C out of light for 45 minutes. After washing, the cells were fixed in 100 μL of 1% paraformaldehyde. Finally, flow cytometric analysis was carried out. Before and after coculture of mouse embryonic stem cells with USSC, karyotype analysis was performed on both cell types. The first and last passages were chosen for karyotype analysis. The cells were first placed in an incubator with 0.1 μg/mL colcemid for 3–4 hours. Next, they were trypsinized, and 0.075 M KCl solution was added. The cells were incubated with 5% CO2 at 37°C for 20 minutes. In the next stage, methanol and acetic acid were added in a 1:3 ratio to fix the samples. Finally, the cells were mounted on slides and the chromosomes were subjected to karyotype analysis.
Polymer surface cell cultures
The samples and control were cleaned and autoclaved. Individual samples were placed in Petri dishes using a sterilized pincer. The USSC suspension was transferred to a flask (25 mL) containing 5 mL DMEM, 2 mM glutamine, penicillin 100 μL/mL, streptavidin 100 μL/mL, and fetal bovine serum 10%. The suspension was then placed in an incubator (5% CO2 at 37°C). The fibroblast cells were proliferated in the flask and were then washed using fetal bovine serum and EDTA. Trypsin and EDTA was added into the flask at 4°C, and the flask was incubated for two minutes. Culture medium (fetal bovine serum and DMEM) was added to the flask and the cells were carefully pipetted. The cell suspension was transferred to a 15 mL Falcon tube and centrifuged at 1410 rpm for five minutes. The solution was removed and the precipitate was transferred to a new flask (75 mL) for reculturing. Pieces of cell culture (1 × 1 cm) from the Petri dish (control) and the main sample were placed individually in one of the Petri dish wells using a sterilized pincer. Thereafter, 100,000 cells/well were seeded into a 24-well culture plate, removed by pipetting, and poured onto the control and main samples. All samples were then placed in a Memmert® incubator (Schwabach, Germany) at 37°C for 48 hours and studied using a Ceti microscope (Wolf Laboratories, Pocklington, York, UK). Cellular viability was determined by MTT assay. The MTT tetrazolium compound is reduced by living cells into a colored formazan product that is soluble in tissue culture medium. The quantity of formazan product is directly proportional to the number of viable cells in the culture. The assays were performed by adding 1 mL of MTT solution (Sigma, St Louis, MO) and 9 mL of fresh medium to each well after aspirating the spent medium and incubation at 37°C for four hours in the dark. Colorimetric measurement of the formazan dye was performed at a wavelength of 606 nm using a Rayto microplate reader.
Results
Attenuated total reflection Fourier transform infrared analysis
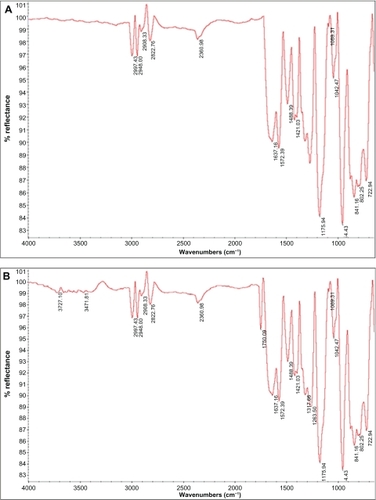
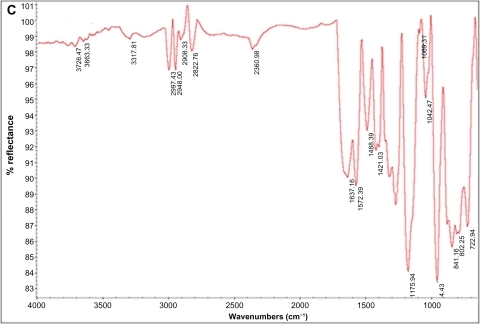
Attenuated total reflection Fourier transform infrared analysis (ATR-FTIR) spectra results for normal and modified polystyrene samples treated with plasma irradiation (oxygen and argon gases) at different times are shown in . The ATR-FTIR spectra of polystyrene irradiated with argon plasma is shown in . There was no significant difference between normal samples and those irradiated with argon plasma. However, a significant difference was observed at 3400–3700 cm−1 that could be related to an OH group and demonstrated the effect of surface treatment in comparison with the normal sample. The ATR-FTIR spectra of polystyrene irradiated with oxygen plasma is shown in . The spectrum observed at 1750 cm−1 could be related to the C═O group, and showed successful surface modification with oxygen plasma. The tension peak at 1000–1300 cm−1 could be related to the C═O group and a peak at 3000 cm−1 could be related to the –CH3 group. Moreover, the tension peak observed at 3400–3700 cm−1 could be related to an OH group that demonstrated the effect of surface treatment on the samples.
Atomic force microscopic analysis
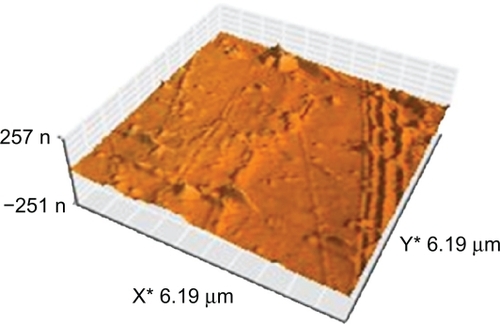
The surface topology of the normal and polystyrene samples irradiated for different times and exposures (active and inert gases) were investigated using atomic force microscopy. The three-dimensional topography of the normal samples is shown in . In this figure, a smooth surface with little roughness can be observed. This roughness could be due to scratches resulting from cleaning of the samples.
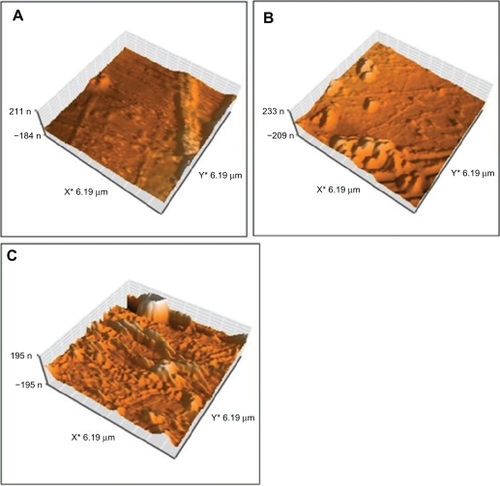
shows the topography of the polystyrene modified with argon plasma at 30, 60, and 180 seconds. A difference was observed between the topography of samples irradiated with argon plasma for different times compared with the normal samples. Surface roughness increased with increasing exposure, although little difference was observed in surface topography.
Figure 4 Topography of irradiated polystyrene by argon plasma at A) 30 seconds, B) 60 seconds, and C) 180 seconds.
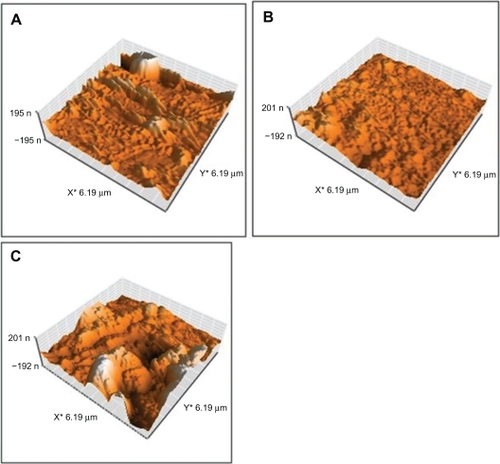
shows the topography of polystyrene modified by oxygen plasma at 30, 60, and 180 seconds. The topography of samples irradiated with oxygen plasma at different times differed from that of normal samples modified with argon plasma. Surface roughness increased with increasing duration of exposure. A lot of difference was observed in surface topology with increasing duration of exposure.
Figure 5 Topography of irradiated polystyrene by oxygen plasma at A) 30 seconds, B) 60 seconds, and C) 180 seconds.
The surface roughness of the normal samples and samples irradiated with oxygen and argon plasma are shown in . The results show that the roughness of the samples modified with oxygen plasma was higher in comparison with samples modified with argon plasma. Samples irradiated with oxygen plasma should double the surface roughness (31 nm) seen for inert plasma (16 nm) at 180 seconds.
Table 1 Surface roughness of normal and irradiated samples
Contact angle analysis
shows the angles obtained for normal samples and those modified with oxygen and argon plasma and at different durations of exposure. The results showed that samples modified using either gas reduced the contact angle. Samples modified with oxygen plasma showed a higher level of contact angle reduction in comparison with the sample modified with argon plasma, indicating that the sample modified with oxygen plasma was more hydrophilic than the normal sample.
Table 2 Contact angles of normal and irradiated samples
Cell results
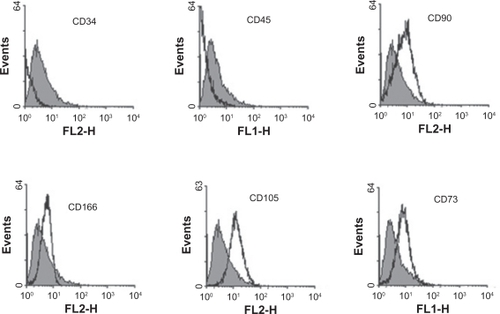
USSCs isolated from cord blood were cultured during 50 passages. USSCs have a high proliferation capacity because they need to repeat passages. The proliferation and morphology of these cells were very similar to each other before and after freezing. No indication of viral or mycoplasma infection was observed during the different stages of work on USSCs. USSCs are morphologically adherent spindle-shaped cells with a size of 20–25 μm (). After three passages, flow cytometric analysis was performed on the USSCs in order to express stem cell markers. The markers were as follows: CD34, CD45, CD73, CD105, CD90, and CD166 (). For USSCs, the expression of surface markers, such as CD90, CD105, CD166, and CD73 were positive, but CD34 and CD45 were negative.
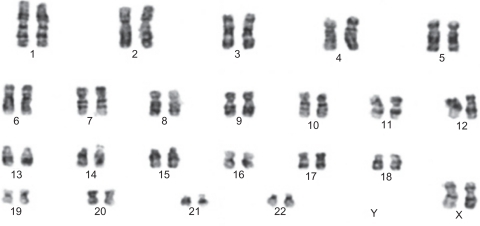
Figure 7 Markers of flow cytometric analysis performed on unrestricted somatic stem cells. Before beginning the experiments, karyotype analysis was performed on unrestricted somatic stem cells of passage 2, revealing a normal 44, XX karyotype. After 48 passages, these cells were subjected to karyotype analysis once again, and they were shown to have a normal chromosome karyotype of 44, XX (see ).
Cell study
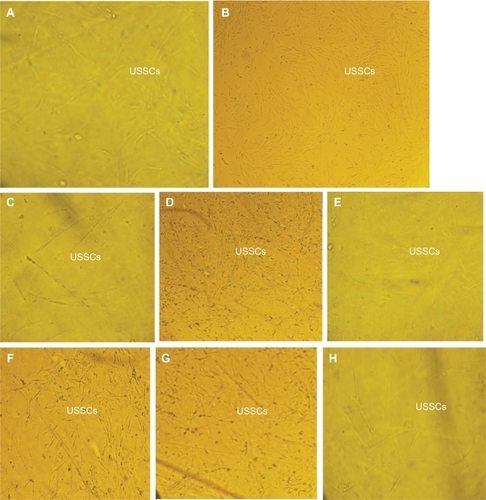
shows the MTT assays for control, normal, and polystyrene films irradiated with plasma (oxygen and argon gases) at 30, 60, and 180 seconds. The results show high viability of samples irradiated with plasma at different times, ie, 61% with argon plasma for 30 seconds, 65% with argon plasma for 60 seconds, 121% with argon plasma for 180 seconds, 81% with oxygen plasma for 30 seconds, 91% with oxygen plasma for 60 seconds, and 31% with oxygen plasma for 180 seconds. In addition, the films irradiated with plasma for longer durations showed better viability. Samples irradiated with plasma for 180 seconds also showed more proliferation of cells. shows cell culture images on the normal and irradiated films, as well as those for control samples. The cellular images showed good growth on irradiated films, especially those irradiated with active gases for longer durations.
Table 3 MTT analysis of the samples
Figure 9 Unrestricted somatic stem cell culture on A) normal (untreated polystyrene), B) control surfaces, C) polystyrene treated with argon plasma for 30 seconds, D) polystyrene treated with argon plasma for 60 seconds, E) polystyrene treated with argon plasma for 180 seconds, F) polystyrene treated with oxygen plasma for 30 seconds, G) polystyrene treated with oxygen plasma for 60 seconds, and H) polystyrene treated with oxygen plasma for 180 seconds.
Discussion
In this study, the surface modification and topography of normal polystyrene and polystyrene irradiated with oxygen plasma and argon plasma for three periods of time were investigated. Microwave plasma has a significant advantage over other techniques, ie, radiofrequency glow discharge, and is frequently used in polymer surface modifications.Citation15 ATR-FTIR analysis of the samples modified with these gases demonstrated the existence of functional groups, including C═O, CO, and OH. Atomic force microscopic images showed much surface roughness on the samples modified by oxygen plasma in comparison with normal samples and those modified with argon plasma. Contact angle analysis showed further contact angle reduction for samples modified with oxygen plasma in comparison with samples modified using argon plasma. These differences could be related to physical modification or doubled roughness of samples modified by oxygen plasma in comparison with samples modified using argon plasma. Cellular investigations using USSCs showed better adhesion, growth, and viability of films radiated with either plasma, especially oxygen plasma, than did normal films. These irradiated films could be used as substrates for cellular culture.
Disclosure
The authors report no conflicts of interest in this work.
References
- LuHWLuQHChenWTCell culturing on nanogrooved polystyrene Petri dish induced by ultraviolet laser irradiationMater Lett2004582932
- RoucoulesGMathiaTLanteriPHydrophobic mechanochemical treatment of metallic surfaces. Wettability measurements as means of assessing homogeneityAdv Colloid Interface Sci200297177201
- HayKMDragilaMILiburdyJTheoretical model for the wetting of a rough surfaceJ Colloid Interface Sci200832547247718586259
- KogelschatzUDielectric-barrier discharges: Their history, discharge physics and industrial applicationsPlasma Chem Plasma Proc200323146
- KuzmichevAIIon plasma sources based on a microwave ovenInstrum Exp Tech199437648653
- AsmussenVElectron cyclotron resonance microwave discharge for etching and thin film depositionJ Vac Sci Technol19897883889
- TakahashiCJinYNishimuraKMatsuoSAnisotropic etching of Si and WSiN using ECR plasma of SF6-CF4 gas mixtureJpn J Appl Phys20003936723676
- PrabhuKNFernandesPKumarGEffect of surface roughness on wetting characteristics of vegetable oilsMater Design200930297305
- KubiakKJMathiaTGWilsonMCTMethodology for metrology of wettability versus roughness of engineering surfacesProceedings of Fourteenth International Congress of MetrologyParis, FranceJune 22–25, 2009
- WenzelRNResistance of solid surfaces to wetting by waterInd Eng Chem193628988994
- ChanCMKoTMHiraokaHPolymer surface modification by plasmas and photonsSurf Sci Rep1996241
- JagerMSagerMKnipperAIn vivo and in vitro bone regeneration from cord blood derived mesenchymal stem cellsDer Orthopäde20043313611372 German.
- KoglerGSenskenSAireyJAA new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potentialJ Exp Med200420012313515263023
- KoglerGRadkeTLefortACytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cellsExp Hematol20053357358315850835
- NowakSGröningPKüttelOMCollaudMDietlerGElectron cyclotron resonance plasma experiment for in situ surface modification, deposition, and analysisJ Vac Sci Technol19921034193425