Abstract
Background
Titanium dioxide (TiO2) nanotubes are often used as carriers for loading materials such as drugs, proteins, and growth factors.
Materials and methods
In this study, we loaded tetracycline onto TiO2 nanotubes to demonstrate its antibacterial properties and biocompatibility. The two-layered anodic TiO2 nanotubes with a honeycomb-like porous structure were fabricated by using a two-step anodization, and they were loaded with tetracycline by using a simplified lyophilization method and vacuum drying. Their physical properties, such as chemical compositions, wettability, and surface morphologies of the different samples, were observed and measured by X-ray photoelectron spectroscopy (XPS), contact angle measurement, and scanning electron microscopy (SEM). The in vitro growth behaviors of mouse bone marrow stromal cells (BMSCs) on these substrates were investigated.
Results
The TiO2 nanotube (NT) substrates and the tetracycline-loaded TiO2 nanotube (NT-T) substrates revealed a crucial potential for promoting the adhesion, proliferation, and differentiation of BMSCs. Similarly, the NT-T substrates displayed a sudden release of tetracycline in the first 15 minutes of their administration, and the release tended to be stable 90 minutes later. The antibacterial performances of the prepared substrates were assessed with Porphyromonas gingivalis. The result showed that NT and NT-T substrates had antibacterial capacities.
Conclusion
Overall, this research provides a promising method with potential for clinical translation by allowing local slow release of antimicrobial compounds by loading them onto constructed nanotubes.
Introduction
Pure titanium (PT) and its alloys are widely used in implant materials in the dental and orthopedic fields on account of their good biocompatibility and their high resistance to corrosion.Citation1,Citation2 Dental implants have been applied for more than 30 years to reconstruct the masticatory function or for esthetic concerns associated with missing teeth.Citation3 Meanwhile, titanium-based surfaces can provide favorable conditions for bacterial adhesion and biofilm formation.Citation4 Bacterial colonization and biofilm formation may lead to increased occurrences of infection,Citation5,Citation6 such as peri-implantitis, which is a common occurrence. Peri-implantitis is an inflammatory disease that originates from infection and ultimately results in the loss of the alveolar bone that contacts implants.Citation7–Citation10 It is caused by biofilms formed around the implant. During biofilm formation, Porphyromonas gingivalis is regarded as the primary pathogen that comprises these biofilms, which is involved in the initiation and progression of severe forms of peri-implantitis.Citation11,Citation12 Therefore, effective control of the adhesion and growth of pathogenic P. gingivalis is crucial for inhibiting or treating peri-implantitis and improving the success rate of dental implants.
Systemic antibiotic treatment, such as perioperative antibiotic prophylaxis, is a routine procedure used to prevent postsurgical infection.Citation13 However, it may also give rise to many relevant complications ranging from diarrhea to life-threatening allergic reactions. Moreover, the emergence of antibiotic-resistant bacteria is another major concern relating to the widespread use of antibiotics.Citation14–Citation16 Hence, it is necessary and important to prevent implant-related infection by improving surface antibacterial properties and to develop methods to deliver antibiotics topically around dental implants that could resolve the problem and maintain adequate concentrations of antibiotics around the implant.
Titania nanotubes can be fabricated regularly with nanotopographical features by electrochemical anodization. Titania nanotubes can provide abundant space to improve osteogenic activity and can act as carriers for drug delivery.Citation17–Citation19 A previous study showed that nanotubes can exhibit moderate antibacterial ability.Citation17 Tetracycline (TET), a broad-spectrum antibiotic, can inhibit gram-negative and some gram-positive pathogens.Citation20 These pathogens can cause implant-related infections such as peri-implantitis. Nanotubes loaded with TET can deliver high levels of antibiotics locally to control bacterial colonization around the implant without causing systemic toxicity; simultaneously, these nanotubes would not change the osseointegrative properties of the surface.
In this work, a titanium surface coated with double layers of nanotubes was fabricated using the two-step electrochemical anodic oxidation method. The biocompatibility of double- layered titania nanotube arrays has already been verified in previous studies. The surfaces of double-layered titania nanotubes promoted adhesion, proliferation, and differentiation of mouse bone marrow stromal cells (BMSCs).
In this article, contact angle, XPS, and SEM measurements were used to analyze the contact angle of the surface of the TET-loaded titania nanotubes to the chemical elements and the microstructure. The ability to inhibit antibiotic release from the nanotubes was analyzed. Osteogenic activity and antibacterial ability (against P. gingivalis) of TET-loaded and nonloaded double-layered titania nanotubes were investigated and compared.
Materials and methods
Fabrication of two-layered honeycomb- like porous anodic TiO2 nanotubes
Two-layered anodic titanium dioxide (TiO2) nanotubes with a honeycomb-like porous structure were fabricated by two-step anodization of PT foil (99.99%). Prior to the electrochemical anodization treatment, PT foils (12 mm in diameter and 0.25 mm in thickness) were polished step-by-step using metallographic sandpaper (from 800 # to 7,000 #) to achieve a mirror effect and then were sonicated with acetone, ethyl alcohol, and deionized water. The electrolyte concentration was 88 mmol/L ammonium fluoride in ethylene glycol. In the first step, PT foils were anodized under a voltage of 60 V for 2.5 hours at room temperature to form large-sized TiO2 nanotube arrays. Then, the titanium foils were processed in deionized water using ultrasonic processing to remove the as- formed TiO2 nanotube arrays. Regularly arranged TiO2 nanocell arrays with shallow bowl shapes that were remaining on the titanium foil were obtained. In the second step, the remaining titanium foils were anodized again under a voltage of 12 V for 40 minutes to form small nanopores below each shallow, bowl- shaped honeycomb cell. We found that the diameter of the top shallow, bowl-shaped cells increased so that the bowl-shaped cells changed into a honeycomb shape. These prepared titanium foils were sonicated with deionized water and then dried in the ambient atmosphere, and the foils were denoted as “NT.” More details on the above fabrication of similar two-layered anodic TiO2 nanotubes are further described elsewhere.Citation21
TET loading
TET was loaded into the TiO2 nanotubes by a simplified lyophilization method followed by vacuum drying according to previous reports.Citation22,Citation23 Briefly, the NT surfaces were cleaned with deionized water before loading. A TET solution (Sangon Biotech) of 50 mg/mL was prepared in PBS. A volume of 20 μL of the TET solution was pipetted onto the nanotube surfaces and gently spread to ensure even coverage. The surfaces were dried using a vacuum for 20 minutes at room temperature. After drying, the abovementioned loading step was repeated until the nanotubes were loaded with 60 μg of TET. After the last drying step, the sample surfaces were quickly washed by pipetting 500 μL of PBS to remove any excess drug ingredients. The washed solutions were collected and stored for further analysis. The titanium foils were then dried in an ambient atmosphere, and these foils were denoted as “NT-T.”
Surface characterization
The surface morphology was viewed by SEM (Sirion-200, FEI, Hillsboro, OR, USA). The chemical states and chemical compositions of the specimen surfaces were investigated by XPS (ESCALAB 250, Thermo Scientific, Waltham, MA, USA).
Contact angle measurement
After 1 μL of deionized water was gently dropped onto the surface of these specimens, the water contact angle was measured with a pendant drop method using a contact angle meter (SL200B, Solon Technology Co., Shanghai, China) at room temperature.
Drug release evaluation
To measure how TET was released from the two-layered nanotubes, the sample surfaces were immersed into 500 mL of PBS in a 24-well plate while it was subjected to orbital shaking at 70 rpm at room temperature. A 200 μL sample of the solution was taken from the 24-well plate at 15-minute intervals to ascertain the release kinetics of TET. In the meantime, 200 mL of fresh PBS was added into the plate to keep the total amount solution unchanged at 500 mL. Samples of this solution were collected regularly for up to 120 minutes. The samples of this solution were analyzed for drug content using a HPLC system (LC-20AD, SHIMADZU Co., Kyoto, Japan). A standard curve with known concentrations of TET was used to ascertain the unknown concentrations.
Bacterial culture
P. gingivalis (ATCC 33277) was used in this study. These strains were stored at −80°C in glycerol stock and were propagated overnight in brain-heart infusion (BHI) sheep blood solid medium. All cultures were incubated under anaerobic conditions at 37°C. A sterile 10 μL loop was used to withdraw bacteria colonies from the BHI solid medium, which were then inoculated into 10 mL of BHI broth and cultured overnight. Samples of NT, PT, and PT-T were sterilized for 2 hours at 55°C with ethylene oxide.
Bacterial adhesion experiment using the spread plate method
Antibacterial activity of PT and PT-T samples was tested in P. gingivalis using a bacterial adhesion test that was quantified by counting colony-forming units (CFUs). The inoculum of each strain was prepared by regulating the concentration of an overnight bacterial culture to a turbidity equal to a 0.5 McFarland standard in BHI broth.
Confined in a laminar flow hood, samples were put into sterile petri dishes. A 20 μL volume of the P. gingivalis suspension was pipetted onto the sample surface with the cover glass coated to spread the liquid, where it evenly covered the surface of the specimen and was incubated under a lid at 37°C for 3 hours under anaerobic conditions. Petri dishes were filled with wet cotton balls to encourage an appropriate level of humidity to ensure that suspension liquid evaporation was kept to a minimum. Then, the samples were removed with sterile forceps and carefully washed with 5 mL sterile PBS three times to remove loosely adherent bacteria. The samples were then placed into fresh centrifuge tubes that contained 5 mL of fresh PBS, and the bacteria that were adherent onto the disc were dislodged by using a whirlpool mixer (XH-B, Medical Apparatus Co., Ltd, Jiangsu, China) at its maximum power for 5 minutes. This method has been shown to allow the removal of biomaterial-adherent bacteria.Citation24,Citation25 A 100 μL volume of the vortexed solutions was coated evenly in triplicate onto BHI sheep blood solid medium and then incubated for 48 hours at 37°C. The number of CFUs on the BHI medium was counted.
The antibacterial ratio was counted by using the following formula: (A−B)/A×100%, where A is the average number of bacteria in the PT specimen (CFU/specimen), B is the average number of bacteria in the NT and NT-T specimens (CFU/specimen).
Bacterial adhesion assessed using SEM
The bacterial culture was as described above. Bacteria were incubated under a lid at 37°C for 3 hours in anaerobic conditions. After this incubation, the surfaces were fixed with 2.5% glutaraldehyde for 2 hours and were washed with sterile PBS. The samples were subjected to a gradient dehydration in ethanol: 25%, 50%, 75%, 95%, and 100% ethanol concentrations for 10 minutes each. The surfaces were then air-dried for 30 minutes. The surfaces of the specimens were then treated with gold spray and observed using SEM.
Bacterial adhesion assessed using fluorescence microscopy
The inoculum for each of the strains was prepared by adjusting the concentration of an overnight bacterial culture to a turbidity equal to a 2 McFarland standard in BHI broth. Confined in a laminar flow hood, samples were put into sterile petri dishes. A 20 μL volume of the P. gingivalis suspension was pipetted onto the samples’ surfaces with the cover glass coated to spread the bacteria liquid to evenly cover the surface of the specimen and were incubated under a lid at 37°C for 3 hours under anaerobic conditions. Petri dishes were filled with a wet cotton ball to control the level of humidity and ensure that suspension liquid evaporation was kept to a minimum. Afterward, the samples were removed with sterile forceps and were carefully washed three times with 5 mL of sterile PBS to remove loosely adherent bacteria. The samples were placed into fresh centrifuge tubes with 5 mL of fresh PBS, and the adherent bacteria on the disc were dislodged by using a whirlpool mixer at maximum power for 5 minutes. DAPI (Beyotime Biotech, Jiangsu, China) staining solution was added to the vortexed solutions on glass slide for 5 minutes. After washing with PBS three times with a 3-minute interval, slides were covered with 50% glycerol in water. Fluorescence images were captured using a fluorescence microscope (BX53, OLYMPUS Corporation, Tokyo, Japan).
Cell adhesion
The mouse BMSCs were purchased from Cyagen Biotech Company. The cells were cultured in stem cell complete culture medium and were used within five passages in this study.
PT, NT, and NT-T were randomly chosen and were placed in 24-well plates. The cells were seeded at a density of 5×104/well. The discs were first incubated for 120 minutes at 37°C in an incubator and were then carefully washed three times with PBS followed by 2.5% glutaraldehyde fixation for 24 hours. Their surfaces were then air-dried for 30 minutes. The specimens were then sputter coated with gold and inspected utilizing SEM.
Cell proliferation
PT, NT, and NT-T were randomly chosen and were placed in 24-well plates. Then, BMSCs were seeded at a density of 1×104/well. After culturing for 1, 3, and 5 days, cell proliferation in the samples was assessed using the MTT assay. Cells were carefully washed with PBS three times at each time point, and then 200 μL of the MTT solution (5 mg/mL) and 800 μL of DMEM without serum and phenol red were added into each sample. The samples were incubated for 4 hours at 37°C, the supernatant was removed, and 1 mL DMSO was put into each well. The absorbance of the solution was analyzed using a spectrophotometer at 490 nm.
Cell differentiation
PT, NT, and NT-T were randomly chosen and were put in 24-well plates. Then, BMSCs were seeded at a density of 1×105/well. After culturing for 3, 5, and 7 days, 0.2% Triton X-100 solution was used to lyse cells for 30 minutes. Then, the lysis solution was treated with an ALP staining kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
The results are expressed as the means ± SD with all groups conducted in triplicate. The one-way ANOVA and least significant difference post hoc tests were used for statistical analysis, where P<0.05 was considered significant and P<0.01 was considered highly significant.
Results and discussion
Surface appearances
SEM, XPS, and a contact angle meter were used to investigate the surface physicochemical properties of PT, NT, and NT-T samples. PT sheets were polished by a physical treatment that showed a relatively rough surface morphology with visible scratches ().Citation26 Nanoporous titanium was prepared onto PT substrates by using an anodization approach.Citation27 As previous studies have reported, anodic oxidation is a controlled and cost-effective technology to form TiO2 nanoscale structures with tunable morphology that is achieved by adjusting voltage and electrolyte concentrations relating to the procedure.Citation28,Citation29 shows the SEM images of two layers of the cellular TiO2 nanotube array. From the superior aspect, the sample was divided into a large amount of homogeneous hexagonal nanoscale cells that each had a diameter of approximately 160 nm, and there were approximately 50 nanopores with a diameter approximately 15 nm in each hexagonal cell. The specific description can be observed in our previous work.Citation21 After TET was loaded onto the NT surface, the intersmall nanotube diameter was narrowed and outer large nanotubes were partially blocked (). This suggests that TET-deposited TiO2 nanotubes arrays were partially covered.
Figure 1 SEM images of the surface morphologies of different substrates: (A) PT, (B) NT, and (C) NT-T.
Note: Inserted images display the observed morphologies at high magnification.
Abbreviations: NT, nanotubes; NT-T, tetracycline-loaded nanotubes; PT, pure titanium; SEM, scanning electron microscopy.

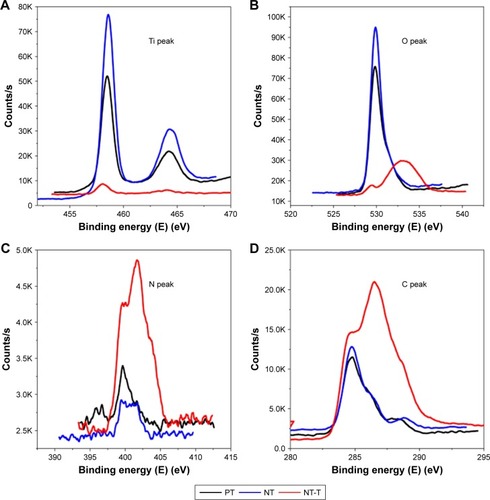
Each chemical composition analysis of PT, NT, and NT-T samples was characterized by XPS ( and ). Approximately 15.12% of Ti, 52.79% of O, 30.36% of C, and 1.72% of N (atomic concentration) were detected in a PT sample. As previously reported,Citation30–Citation32 elemental carbon on the surface of PT may be due to the physical adhesion of hydrocarbons from the surrounding environment, which is unavoidable (). Approximately 19.41% of Ti, 50.54% of O, 19.84% of C, and 0.84% of N were observed in an NT sample. The occurrence of nitrogen can be ascribed to having been derived from the ammonia fluoride in the electrolyte used to anodize the samples (). Approximately 65.81% of C (59.40% of C in the TET) and 5.55% of N were observed in an NT-T sample. As shown in , the PT and NT substrates show only one peak approximately 529 eV, while the NT-T substrate shows a new peak approximately 533 eV with significant suppression of the peak approximately 529 eV. In the PT and NT substrates, the oxygen valence state should be −2, while the oxygen in the TET is from a C–O covalent bond. This explains the origin of the different peak positions for oxygen in the XPS results. A sharp increase in carbon () and nitrogen () content was derived with successful TET loading. These results suggested that TET was deposited into TiO2 nanotubes successfully.
Table 1 The chemical compositions of the PT, NT, and NT-T substrates by XPS
Figure 2 The XPS patterns of the PT, NT, NT-T substrates: (A) Ti, (B) O, (C) N, and (D) C.
Abbreviations: NT, nanotubes; NT-T, tetracycline-loaded nanotubes; PT, pure titanium; XPS, X-ray photoelectron spectroscopy.
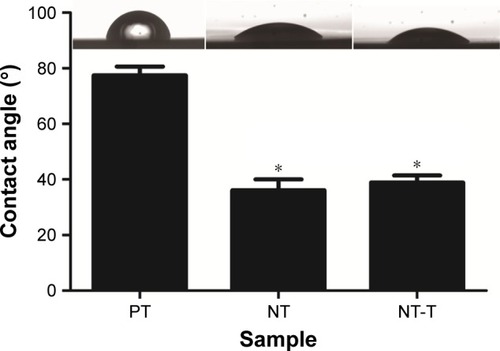
To further confirm the successful loading of NT-T samples, the hydrophilic capability of different samples was characterized with water contact angle measurements (). The PT substrates showed contact angles of approximately 77.45°±9.45° (n=3), which were similar to what has been observed in previous studies.Citation31,Citation33 Consistent with previous studies,Citation34,Citation35 the NT substrates displayed hydrophilic properties with contact angles of approximately 36.15°±11.67° (n=3). After TET had been deposited, the water contact angles of NT-T samples increased to approximately 38.85°±7.76° (n=3). Furthermore, when compared with PT, the reduction of contact angles in NT and NT-T was significant. The result suggests that NT-T was successfully fabricated.
Figure 3 The surface contact angles and water drop profiles of the PT, NT, and NT-T substrates.
Notes: The contact angle is expressed as a measure of hydrophilicity of different substrates. The error bars represent means ± SD (n=3). *A significant difference compared with PT (P<0.05).
Abbreviations: NT, nanotubes; NT-T, tetracycline-loaded nanotubes; PT, pure titanium.
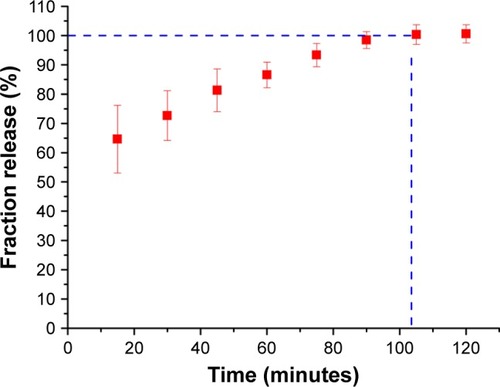
TET release
The drug loading and release characteristics of the nanotubes to prevent bacterial adhesion were investigated to explore the feasibility of using NT-T substrates as a drug carrier for local therapeutic effects in a surrounding environment. shows the fraction release profiles of TET from NT-T samples. Approximately 64.24% of TET was released from NT-T after incubation for an initial 15 minutes; the possible reasons for the sudden release of TET is that the binding of TET present in the outermost layer of TiO2 nanotubes was relatively loose, or some TET molecules did not bind but existed inside the molecular voids. After 90 minutes, the TET release curve tended to be stable. The result indicates that TET was partially deposited into the wells of TiO2 nanotubes arrays (NT-T). The result is of great significance for further study of functional titanium-based implants with antibacterial abilities.
Bacterial adhesion
The antibacterial properties of the substrates were characterized using P. gingivalis. P. gingivalis is a nonglycolytic gram-negative anaerobe that is one of the most potent and well-documented pathogens associated with periodontal disease and is widely used for antibacterial assays. Bacterial adhesion on the surfaces of the PT, NT, and NT-T were surveyed using SEM and fluorescence microscopy for a period of 3 hours. In this research, we evaluated P. gingivalis by SEM and fluorescence microscopy.
As shown in the SEM images in , there was only a small amount of single bacterial colonies scattered on the surfaces of NT-T for 3 hours, and disintegrating bacteria splints can be seen (). There were more bacterial colonies on the NT surfaces than on the NT-T surface after 3 hours (). Compared with the NT and NT-T surfaces, multiple bacterial colonies formed colony masses on the PT surfaces during the 3 hours used to survey the materials (). Additionally, as seen in , there were significantly fewer bacterial colonies in the NT and NT-T groups compared with the PT group; moreover, when compared with the NT group, there were significantly fewer bacterial colonies in the NT-T groups during the 3 hours used to survey each sample.
Figure 5 Evaluation of antimicrobial activity of different substrates for 3 hours.
Notes: (A) SEM of bacterial adhesion on the surfaces of the PT, NT, and NT-T for 3 hours (a–c); Fluorescence microscope images of bacterial colonies adhered on the PT, NT, and NT-T (d–f). (B) Bacterial CFU from different substrates for 3 hours. The error bars represent means ± SD (n=3). *A significant difference compared with PT (P<0.05). #A significant difference compared with NT (P<0.05).
Abbreviations: CFU, colony-forming units; NT, nanotubes; NT-T, tetracycline-loaded nanotubes; PT, pure titanium; SEM, scanning electronic microscopy.
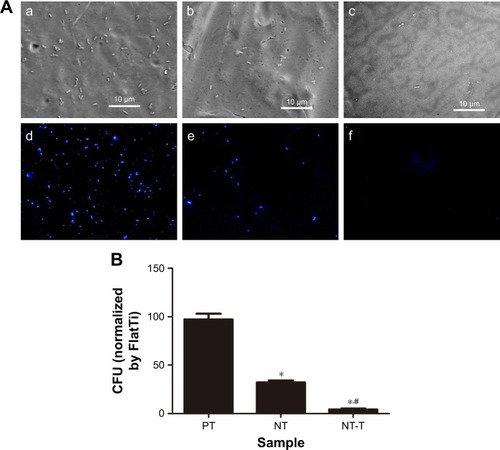
In the fluorescence microscopy images in , the PT surface has a strong fluorescence intensity. The NT surface’s fluorescence intensity was weaker compared with the PT surfaces. Formation of bacterial colonies on the NT-T surface was sparse. This result is consistent with the results obtained from SEM imaging. The above results indicated that the deposition of TET mainly contributed to the antibacterial effects of the material.
Cell adhesion, proliferation, and differentiation
shows SEM images of morphologies of different samples cocultured with BMSCs for 120 minutes. The BMSCs grown onto NT substrates () displayed more cell spreading than those adhered to the PT samples (). BMSCs cocultured with NT-T samples () exhibited similar cell spreading compared with those cells that were grown onto NT samples (). More BMSCs were observed on NT and NT-T samples than those in PT samples, whereas the number of BMSCs in NT and NT-T substrates was similar. These results suggest that deposited TET has no adverse effect on BMSCs’ adhesion.
Figure 6 BMSCs on the substrates.
Notes: (A–C) The comparison of bacterial colonies adhered on the PT, NT, and NT-T at the 48-hour point. (D) MTT assay. Formazan absorbance expressed as a measure of cell proliferation from BMSCs cultured on different substrates for 1, 3, and 5 days. (E) Alkaline phosphatase activity of BMSCs cultured on different substrates for 3, 5, and 7 days. The error bars represent means ± SD (n=3). *A significant difference compared with PT (P<0.05).
Abbreviations: BMSCs, bone marrow stromal cells; NT, nanotubes; NT-T, tetracycline-loaded nanotubes; PT, pure titanium.
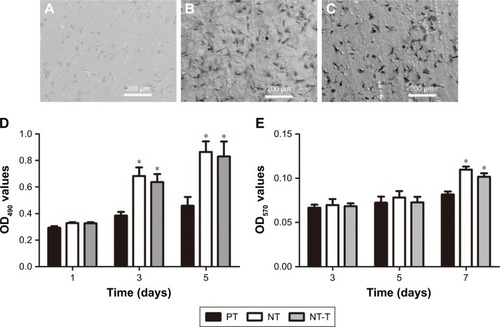
An MTT assay was performed for evaluating the cytocompatibility to assess the behavior of substrates that were cocultured with BMSCs. The production of formazan is a by-product of deoxidation formed by viable cells, thus indirectly indicating cellular proliferation. shows the absorbance of formazan, measured using a spectrophotometer, that is produced by BMSCs that have adhered to different samples. After BMSCs were cultured for 1 day, no prominent difference was observed in cell proliferation between all these groups. After culturing for 3 days, BMSCs grown onto NT and NT-T samples exhibited prominently higher cell proliferation than those in PT samples (P<0.05). However, no significant difference in cell proliferation was observed between NT and NT-T samples. The results of cell proliferation adherence on different substrates at 5 days of growth were similar to those results observed at 3 days of growth.
The ALP activity was measured after culture for 3, 5, and 7 days () to investigate the behavior of different samples cocultured with BMSCs on differentiation. After BMSCs were cultured for 3 and 5 days, all groups showed no significant differences. The absorbance representing ALP activity in the NT and NT-T substrates was significantly higher (P<0.05) than the absorbance from the PT samples after 7 days of culture. However, no significant difference in ALP activity was observed between NT and NT-T substrates. The expression of ALP activity is an early marker for osteogenesis, which is related to the bone formation and bone matrix mineralization in skeletal tissues. These results suggest that TET loading in TiO2 nanotubes has no adverse effect on the adhesion, proliferation, and differentiation of BMSCs, and NT-T substrates were beneficial for these properties in BMSCs.
Conclusions
In this study, we prepared NT and NT-T specimens successfully. Antibacterial experiments in vitro revealed that the NT surface had antibacterial properties; meanwhile, the NT-T surface had increased antibacterial properties. The results from TET loading showed that TET could be deposited onto TiO2 nanotubes. The TET release curve was also explored. We also studied the cocultivation of BMSCs with specimens. The results showed that NT and NT-T specimens’ surface could also promote the biological activities of BMSCs. In summary, TET-loaded TiO2 nanotubes have the potential to be applied to develop titanium implants with antibacterial properties.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Anhui Province (1508085MH156).
Disclosure
The authors report no conflicts of interest in this work.
References
- HanawaTMaterials for metallic stentsJ Artif Organs2009122737919536623
- AnanthHKundapurVMohammedHSAnandMAmarnathGSMankarSA review on biomaterials in dental implantologyInt J Biomed Sci201511311312026508905
- WassmannTKreisSBehrMBuergersRThe influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implantsInt J Implant Dent2017313228714053
- de AvilaEDVerganiCEMollo JuniorFAJuniorMJShiWLuxREffect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva communityJ Prosthet Dent2017118448148728343672
- ZaatrehSHaffnerDStraussMThin magnesium layer confirmed as an antibacterial and biocompatible implant coating in a co-culture modelMol Med Rep20171541624163028260022
- KolenbranderPEPalmerRJPeriasamySJakubovicsNSOral multispecies biofilm development and the key role of cell-cell distanceNat Rev Microbiol20108747148020514044
- LindheJMeyleJGroup D of European Workshop on PeriodontologyPeri-implant diseases: Consensus Report of the Sixth European Workshop on PeriodontologyJ Clin Periodontol2008358 Suppl28228518724855
- LangNPBosshardtDDLulicMDo mucositis lesions around implants differ from gingivitis lesions around teeth?J Clin Periodontol201138Suppl 1118218721323714
- Heitz-MayfieldLJPeri-implant diseases: diagnosis and risk indicatorsJ Clin Periodontol2008358 Suppl29230418724857
- ZitzmannNUBerglundhTDefinition and prevalence of peri-implant diseasesJ Clin Periodontol2008358 Suppl28629118724856
- PyeADLockhartDEDawsonMPMurrayCASmithAJA review of dental implants and infectionJ Hosp Infect200972210411019329223
- HeydenrijkKMeijerHJvan der ReijdenWARaghoebarGMVissinkAStegengaBMicrobiota around root-form endosseous implants: a review of the literatureInt J Oral Maxillofac Implants200217682983812507243
- MoslemiNShahnazABahadorATorabiSJabbariSOskoueiZAEffect of postoperative amoxicillin on early bacterial colonization of peri-implant sulcus: a randomized controlled clinical trialJ Dent2016135309317
- ParkJTennantMWalshLJKrugerEIs there a consensus on antibiotic usage for dental implant placement in healthy patients?Aust Dent J2018631253328543332
- MainjotAD’HooreWVanheusdenAvan NieuwenhuysenJPAntibiotic prescribing in dental practice in BelgiumInt Endod J200942121112111719912383
- LewisMAWhy we must reduce dental prescription of antibiotics: European Union Antibiotic Awareness DayBr Dent J20082051053753819023306
- LinWTTanHLDuanZLInhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diametersInt J Nanomedicine201491215123024634583
- YeniyolSHeZYükselBAntibacterial activity of as-annealed TiO2 nanotubes doped with Ag nanoparticles against periodontal pathogensBioinorg Chem Appl2014201482949682949825202230
- PopatKCEltgrothMLatempaTJGrimesCADesaiTADecreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubesBiomaterials200728324880488817697708
- NguyenFStarostaALArenzSSohmenDDönhöferAWilsonDNTetracycline antibiotics and resistance mechanismsBiol Chem2014395555957524497223
- HuXMengGHuangQLarge-scale homogeneously distributed Ag-NPs with sub-10 nm gaps assembled on a two-layered honey- comb-like TiO2 film as sensitive and reproducible SERS substratesNanotechnology2012233838570522948006
- ArianiMDMatsuuraAHirataIKuboTKatoKAkagawaYNew development of carbonate apatite-chitosan scaffold based on lyophilization technique for bone tissue engineeringDent Mater J201332231732523538769
- ChowSFWanKYChengKKDevelopment of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilizationEur J Pharm Biopharm20159443644926143368
- SherertzRJRaadIIBelaniAThree-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratoryJ Clin Microbiol199028176822405016
- BjerkanGWitsøEBerghKSonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitroActa Orthop200980224525019404811
- XuDYangWHuYSurface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responsesColloids Surf B Biointerfaces201311022523523732798
- SophaHSamorilTPaleschEIdeally hexagonally ordered TiO2 nanotube arraysChemistryOpen20176448048328794939
- AguirreREcheverry-RendónMQuinteroDFormation of nanotubular TiO2 structures with varied surface characteristics for biomaterial applicationsJ Biomed Mater Res A201810651341135429316200
- KhudhairDAmani HamedaniHGaburroJEnhancement of electro-chemical properties of TiO2 nanotubes for biological interfacingMater Sci Eng C Mater Biol Appl20177711112028531985
- LiuPZhaoYYuanZConstruction of Zn-incorporated multilayer films to promote osteoblasts growth and reduce bacterial adhesionMater Sci Eng C Mater Biol Appl201775998100528415556
- XuDYangWHuYSurface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responsesColloids Surf B Biointerfaces201311022523523732798
- CaiKLaiMYangWSurface engineering of titanium with potassium hydroxide and its effects on the growth behavior of mesenchymal stem cellsActa Biomater2010662314232119963080
- HuYCaiKLuoZJandtKDLayer-by-layer assembly of β-estradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasisAdv Mater201022374146415020717987
- PengZNiJZhengKDual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesionInt J Nanomedicine201383093310523983463
- YangLZhangMShiSEffect of annealing temperature on wettability of TiO2 nanotube array filmsNanoscale Res Lett20149162125426006