Abstract
Background
Spinal cord injury (SCI) is a traumatic disease of the central nervous system, accompanied with high incidence and high disability rate. Tissue engineering scaffold can be used as therapeutic systems to provide effective repair for SCI.
Purpose
In this study, a novel tissue engineering scaffold has been synthesized in order to explore the effect of nerve repair on SCI.
Patients and methods
Polycaprolactone (PCL) scaffolds loaded with actived Schwann cells (ASCs) and induced pluripotent stem cells -derived neural stem cells (iPSC-NSCs), a combined cell transplantation strategy, were prepared and characterized. The cell-loaded PCL scaffolds were further utilized for the treatment of SCI in vivo. Histological observation, behavioral evaluation, Western-blot and qRT-PCR were used to investigate the nerve repair of Wistar rats after scaffold transplantation.
Results
The iPSCs displayed similar characteristics to embryonic stem cells and were efficiently differentiated into neural stem cells in vitro. The obtained PCL scaffolds werê0.5 mm in thickness with biocompatibility and biodegradability. SEM results indicated that the ASCs and (or) iPS-NSCs grew well on PCL scaffolds. Moreover, transplantation reduced the volume of lesion cavity and improved locomotor recovery of rats. In addition, the degree of spinal cord recovery and remodeling maybe closely related to nerve growth factor and glial cell-derived neurotrophic factor. In summary, our results demonstrated that tissue engineering scaffold treatment could increase tissue remodeling and could promote motor function recovery in a transection SCI model.
Conclusion
This study provides preliminary evidence for using tissue engineering scaffold as a clinically viable treatment for SCI in the future.
Introduction
Spinal cord injury (SCI) is a serious medical condition that causes significant problem in the medical community.Citation1–Citation3 Due to the disability and multiple complications caused by the SCI, tens of thousands of people are afflicted each year, and this has a devastating impact on the well-being and financial stabilities of patients and their families.Citation4 However, there is still no definitive repair methods for SCI, especially, complete SCI. The main problems faced by nerve repair are lack of self-repair and neurotrophic factors, primary and secondary neuronal apoptosis, and factors that inhibit the regeneration of axons locally.Citation5,Citation6 Effective repair can not only greatly improve the quality of life for patients with SCI but also relieve their psychological and economic burden. Therefore, improving the quality of life has become the focus and primary goal of researchers. In recent years, drugs and pharmacology, surgical repair of nerve defects, neurotrophic factors, biological activity material, tissue engineering, and genetic engineering studies became mainstream research methods in the field of SCI.Citation7
The microenvironment of the spinal cord changes immediately after a mechanical crushing or stretching of the spinal cord.Citation8–Citation10 Axonal damage and cell membrane disruption cause glia loss in the primary injury of the spinal cord.Citation11 Then, a cascade of molecular and signaling pathways initiate a series of secondary injuries to the spinal cord. Inflammatory cells such as microglia, macrophages, and neutrophils infiltrate the injury site as a result of disruption of the blood–spinal cord barrier during the secondary injuries.Citation12 Secondary injury cascade and oxidative stress cause more neuronal and glial deaths.Citation13 Therefore, much attention has been directed at new therapeutic strategies for SCI. Bioactive materials and cell therapy have been recognized as major treatment methods for SCI in recent years.Citation14,Citation15
Induced pluripotent stem cells (iPSCs) have become the focus in the field of stem cell research and have wide application prospects in many fields such as autologous cell transplantation, tissue engineering research, and drug development.Citation16–Citation18 IPSCs provide a cell source that has characteristics of embryonic stem cells but are associated with fewer moral and ethical issues. In 2007, Takahashi et al successfully reprogrammed human cells into iPSCs for the first time, which made these cells an ideal autologous transplantation cell resource.Citation19 Recently, in the Rikagaku Kenkyūjo Center of Japan, iPSCs have been used in clinical research. The Takahashi group utilized retinal pigment epithelium cells derived from iPSCs to treat age related macular degeneration. To this day, no complications have been reported from this surgery.Citation20 Due to the gradual improvement of the safety and efficiency in iPSCs reprogramming, it has a broader application in regenerative medicine and cell transplantation.
Schwann cells (SCs) are glial cells of the peripheral nerves.Citation21,Citation22 It is one of the most important seed cells in the repair of SCI due to the ability to secrete a variety of nutritional factors and extracellular matrix that can improve the local microenvironment. Waller’s degeneration occurs immediately after peripheral nerve injury. Activated SCs (ASCs) proliferate and migrate to the lesion site, co-phagocytose damaged axonal myelin with macrophages and guide the regeneration, elongation, and myelination of axons.Citation23 Previous studies have shown that, after transplantation of SCs, the host’s endogenous P75+ cells are activated and infiltrated into the lesion site, indicating that endogenous SCs may play an important role in the repair after cell transplantation.Citation24 In addition, the neurotrophic factors and cell adhesion molecules secreted by ASCs can also induce axon myelination and neuronal survival.Citation25 While several studies have shown that single SC repair of SCI is still limited, researchers try to transplant it with other stem cells in order to achieve better effects.Citation26–Citation28 Coculture of SCs with neural stem cells (NSCs) can remarkably increase the proportion of neuron differentiation and effectively improve the recovery of nerve endings.Citation29
Polycaprolactone (PCL) is an aliphatic, biodegradable, nontoxic polyester with good mechanical properties. Due to its deficient biological activity and hydrophobicity it is widely used in absorbable suture materials, surgical dressing, and internal fixations.Citation30–Citation32 It is also approved by the United States Food and Drug Administration (FDA). The popularity of PCL in these applications can be attributed to its biocompatibility, biodegradability, and high processability.Citation33 Its longer degradation cycle allows it to be used as a carrier for cell transplantation and integrate the seed cells into host tissues. Our previous study investigated the biocompatibility of electrospinning PCL scaffolds and coculture system, which consisted of NSCs and ASCs.Citation34 ASCs and iPSCs-derived NSCs (iPSCs-NSCs) can grow well on these electrospinning PCL scaffolds, and ASCs could distribute along the fibers. However, the extraction of human NSCs is ethically unacceptable and, therefore, finding a viable and safe tissue engineering strategy is the common research goal in this field.
In this study, we used the electrospinning method to fabricate nanofibrous PCL scaffolds. We reprogrammed human umbilical cord blood (hUCB) cells into iPSCs and then induced it to differentiate into NSCs. Finally, we transplanted the engineered PCL scaffolds containing ASCs and iPSCs-NSCs to the site of injury of the rats.
Materials and methods
Ethics
All animal handling procedures and experimental protocols were approved by the Animal Care and Use Committee of Tianjin Medical University. All procedures involving animals were consistent with the ethical standards set by the above-mentioned institutions. Pregnant Institute-of-Cancer-Research (ICR) mice (100±10 g) and Wistar rats (230–250 g) were obtained from Laboratory Animal Center of Chinese People’s Liberation Army General Hospital (Beijing, China, Approval Number: SCXK2012-0004). HUCB was obtained from Tianjin First Central Hospital. The Ethical Committee of Tianjin Medical University General Hospital approved this study.
Preparation and characterization of cells
Mouse embryonic fibroblast (MEF)
Isolation, culture, and inactivation of ICR MEF were performed as described previously.Citation35
IPSCs
Fresh hUCB samples were obtained from the Tianjin First Central Hospital after full term delivery with written informed consent of the mother. The umbilical cord blood mononuclear cells were separated from the hUCB using Ficoll-Paque PLUS (Solarbio, Beijing, China). Cells (~5×104) were seeded in a 6-well plate with a culture medium 1640 (Thermo Fisher Scientific, Waltham, MA USA) containing 1% penicillin– streptomycin (Thermo Fisher Scientific) and 10% FBS (Thermo Fisher Scientific). After ~24 hours, the mononuclear cells were treated with the lentivirus (EMD Millipore, Billerica, MA, USA) with addition of polybrene (1 µg/µL) (EMD Millipore) and subjected to further incubation for 24–48 hours. Next day, after the cells were washed thrice with 1× PBS (Solarbio), 2 mL of fresh medium was added. Four days after viral transduction, inactivated ICR MEF feeder layers were prepared to support the cells, followed by further incubation with daily change of the human iPSCs medium (Thermo Fisher Scientific). IPSCs-like colonies were selected as a clone at ~15–18 days. Passage of iPSCs colonies with stem cell passaging tool (Thermo Fisher Scientific).
IPSCs-derived NSCs
When colonies reached ~80% confluency, medium in the well was aspirated and neural induction medium was added according to previous protocol.Citation36 Briefly, after treatment with Tryple, iPSCs were detached from 6-well plates, and the cells were plated at 5×105 cells per well to generate embryoid bodies (EBs) cultured with EBs medium (1% GlutaMAX, 1% N2, 2% B27, 1% penicillin–streptomycin, 1.25 µM dorsomorphin, and neurobasal medium). After treatment with Accutase®, the EBs were digested into single cells and then plated at a density of 2×106 cells per T75 with NSCs medium. This medium was comprised of DMEM/F12, 2% B27 supplement, 1% penicillin–streptomycin, epidermal growth factor (20 ng/mL) (all from Thermo Fisher Scientific), and fibroblast growth factor (20 ng/mL).
ASCs
ASCs were isolated from the sciatic nerves of adult Wistar rats as described previously.Citation37 Briefly, following the removal of the connective tissues, epineurium, and blood vessels, sciatic nerve explants were cut into ~1 mm3 and incubated for 40 minutes in 5 mL 0.05% collagenase (Solarbio). Then, the sciatic nerve explants were washed thrice in PBS and placed into T75 containing DMEM/F12, 10% FBS, and 1% penicillin–streptomycin.
Immunostaining
For the antibody staining, cells fixed with 4% paraformaldehyde were washed thrice with PBS and then were permeabilized with 0.5% Triton X-100. After being blocked with 5% normal goat serum for 40 minutes at room temperature, cells were incubated with primary antibody at 4°C for 24 hours. IPSCs were evaluated by immunostaining of Oct4, Sox2, Nanog, SSEA4, and TRA-1–60. ASCs were investigated by immunostaining of S100, and NSCs were examined by Tuj1, O4, and GFAP. The nucleus was stained with DAPI (all from Abcam, Cambridge, MA, USA). A summary of antibodies mentioned above is provided in . Cells were observed under an inverted fluorescence microscope (Leica, Wetzlar, Germany).
Table 1 Primary antibodies
Preparation of PCL electrospun fiber membrane
A total of 15 wt% poly(e-caprolactone) was dissolved in the solution (dimethylformamide and acetone, 7:3) at a constant temperature of 80°C. This solution was used to generate a degradable PCL electrospun fiber membrane with a pore diameter of <10 µm and ~0.5 mm in thickness. After being dried in a vacuum dryer at 30°C for 72 hours, the membrane was cut into 4×4 mm pieces, which were put up with cobalt 60 irradiation for sterilization. Before cell seeding, the PCL electrospun fiber membrane was sterilized with 70% alcohol, and then washed thrice with PBS. A 5-µL aliquot of medium containing ASCs and/or NSCs was then slowly injected ints each scaffold (a total of 5×105 cells/scaffold).
Spinal cord transection and transplantation
Based on the different treatments, 60 Wistar rats were divided into four experimental groups, in order to minimize the sacrifice and suffering of animals, we refer to previous experiments and cancel the sham operation group.Citation28 The control group is Group 1.
Group 1: PCL scaffolds group (n=15)
Group 2: PCL scaffolds+ASCs group (n=15)
Group 3: PCL scaffolds+NSCs group (n=15)
Group 4: PCL scaffolds+ASCs+NSCs group (n=15).
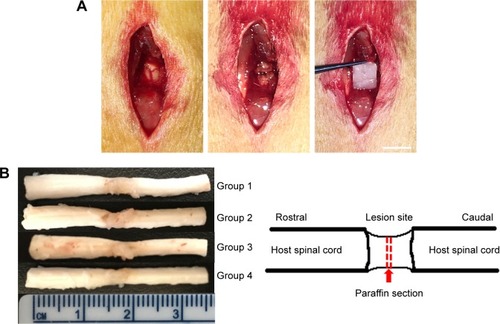
Adult female Wistar rats (230–250 g) were used for spinal cord transection. Animals were acclimatized for at least one weeks before surgery. Before surgery, all rats subjected to SCI were deeply anesthetized with isoflurane. Following laminectomy at the T10 vertebral level, a 2-mm segment of spinal cord was completely removed. Then the lesion gap was covered with tissue engineered scaffolds as previously described.Citation38 After the surgical incision closure, the rats received intensive postoperative care, including manual emiction twice daily till their automatic micturition function reestablished. Penicillin (40,000 U/kg/d) was intramuscular injected for 5 days to prevent any possible infections.
Immunohistochemistry and histological assessment
Eight weeks after transplantation, all animals were anesthetized with isoflurane, after perfused intracardially with 250 mL of saline followed by 250 mL of 4% paraformaldehyde in PBS. Six spinal cord tissue samples in each group were prepared as paraffin sagittal sections at 10 µm thickness, the rest were stored at −80°C. Paraffin sections were stained with hematoxylin–eosin (H&E) (Solarbio), as described previously.Citation39 Finally, the stained sections were observed under the microscope (Leica).
Assessment of locomotor recovery
After surgery, hindlimb function of the rats was evaluated with the Basso, Beattie, and Bresnahan (BBB) open field locomotor test every week.Citation40 Score ranges from 0 (no hindlimb movement) to 21 (normal hindlimb movement). Two independent researchers blinded to these different experimental treatments determined the BBB scores.
Western blot analysis
After weighed and minced with eye scissors on ice, the samples were homogenized in lysis buffer, centrifuged at 12,000 rpm, 4°C for 10 minutes. Then, the samples were subjected to SDS-PAGE. Next, the protein was electrophoretically transferred to a polyvinylidene fluoride filter membrane. The membrane was blocked with 4% nonfat dry milk and incubated with primary antibodies against neurotrophin 3 (NT3), glial cell-derived neurotrophic factor (GDNF), and nerve growth factor (NGF) at 4°C overnight. Next day, the membranes were washed thrice with tris buffered saline tween and were incubated for more than 2 hours at room temperature with second antibodies. The proteins were visualized with an enhanced chemiluminescence system (ECL; R&D system, Minneapolis, MN, USA). The scanned images were then semi-quantitated using the Quantity One software and normalized with β-actin.
Quantitative real-time PCR
Total RNA was extracted from spinal cord tissues using a TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. One microgram of total RNA per sample was reverse transcribed using a Reverse Transcription Kit (TaKaRa, Shiga Perfecture, Japan). Quantitative real-time RT-PCR was performed on a LightCycler® 480 Real-Time PCR System (Hoffman-La Roche Ltd., Basel, Switzerland) using SYBR Green (TaKaRa). β-actin acted as internal control. All samples were analyzed in duplicate, and then the average value of the duplicates was used for quantification. The primers are listed in .
Table 2 Information of primer sequences
Statistical analysis
The Prism statistical software (v6.01; GraphPad Software, Inc., La Jolla, CA, USA) was employed for data analysis. Statistical differences between two groups were analyzed using Student’s t-test. One-way ANOVA analysis was used to compare the mean BBB scores in different groups. All data were presented as mean±standard error of mean. P<0.05 was considered a statistically significant difference.
Results
Isolation of mononuclear cells for iPSCs generation from cord blood
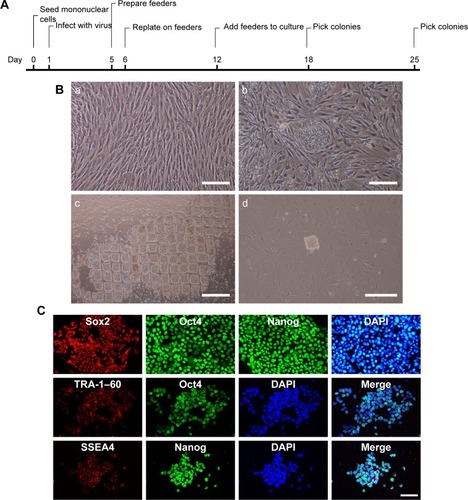
After adding Ficoll-Paque PLUS, cord blood was separated into four layers, namely plasma, lymphocyte, mononuclear cells, and red cells layers. The entire reprogramming process needs about 25 days (). Before adding the lentivirus, the prepared MEF should be frozen in advance (). Eighteen days after the isolation of mononuclear cells and virus infection, small iPSCs colonies may start to appear (). Passage of iPSCs colonies with stem cell passaging tool was used to adapt them to multicelled growth conditions (). Immunostaining demonstrated that the iPSCs robustly expressed undifferentiated cell markers Sox2, Oct4, Nanog, SSEA4, and TRA-1–60 (). As previously reported, we used four transcription factors (Oct4, Sox2, Klf4, and c-Myc) to induce iPSCs, and the human iPSCs colonies formed in our study displayed characteristics similar to human embryonic stem cells.
Figure 1 Preparation and characterization of iPSCs.
Notes: (A) A time-course schema of reprogramming hUCB mononuclear cells. (B) Preparation of MEF (a), and passage of iPSCs colonies (b) with stem cell passaging tool (c) to adapt them to multicelled growth conditions (d). (C) Immunofluorescent staining images of Sox2, SSEA4, TRA-1–60 (red), Oct4, Nanog (green), and DAPI (blue) of iPSCs. Scale bar =100 µm.
Abbreviations: DAPI, 4′,6-Diamidino-2-Phenylindole; hUCB, human umbilical cord blood; iPSCs, induced pluripotent stem cells; MEF, mice embryonic fibroblast; Nanog, homeobox protein NANOG; Oct4, octamer-binding transcription factor 4; SSEA4, stage specific embryonic antigen 4; Sox2, SRY (sex determining region Y)-box 2; TRA, terato-related antigen.
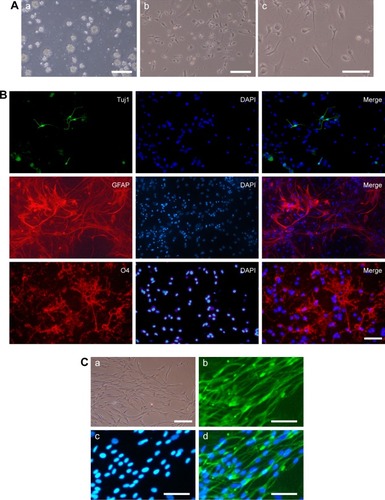
Generation and characterization of ASCs and iPSCs-NSCs
One week after the addition of EBs medium, the homogeneous EBs can be seen in the 6-well plate (). The EBs were cultured for another week to allow the formation of self-organized neuroepithelium that will give rise to NSCs (). The NSCs displayed positive Tuj1, GFAP, and O4 staining (). The above immunostaining results indicate that the induced NSCs have the ability to differentiate into oligodendrocytes, neurons, and astrocytes. In addition, ASCs were extracted from Wistar rats. At 9 days post-isolation of the cells, they proliferated and covered the entire T75 bottom (). Based on immunocytochemical staining, the ASCs express the peripheroneural markers S100 (). Our previous studies have demonstrated that ASCs have better adhesion and proliferation than normal SCs.Citation41 Therefore, in this study, ASCs were used instead of normal SCs in tissue engineering.
Figure 2 Preparation and characterization of iPSCs-NSCs and ASCs.
Notes: (A) The EBs (a) were cultured to allow the formation of self-organized neuroepithelium (b) that will give rise to iPSCs-NSCs (c). (B) Immunofluorescent staining images of Tuj1 (green), GFAP (red), O4 (red), and DAPI (blue) of iPSCs-NSCs. (C) ASCs (a) express the peripheroneural markers S100 (green) (b) and DAPI (blue) (c and d). Scale bar =100 µm.
Abbreviations: ASCs, activated Schwann cells; DAPI, 4′,6-Diamidino-2-Phenylindole; EBs, embryoid bodies; GFAP, glial fibrillary acidic protein; iPSCs-NSCs, induced pluripotent stem cells-derived neural stem cells; O4, oligodendrocyte marker O4; Tuj1, Class III β-tubulin.
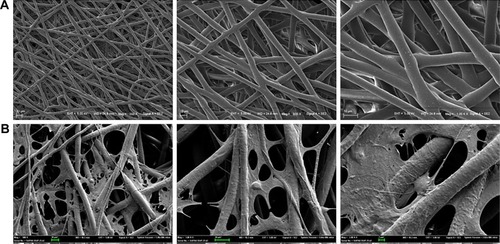
Features of the cocultured cells in PCL scaffold
PCL scaffolds used in this study were prepared by electro-spinning technique, and the membranes were designed by a punch process: 0.8 cm2. Fibers were arranged randomly. Electron microscopy indicated that the pore diameter was <10 µm (). The morphometric scanning electron microscope results indicated that the ASCs and/or iPSCs-NSCs grew well on PCL electrospun fiber membrane (). These results indicated the great biocompatibility of PCL scaffold.
Tissue remodeling in injured spinal cord
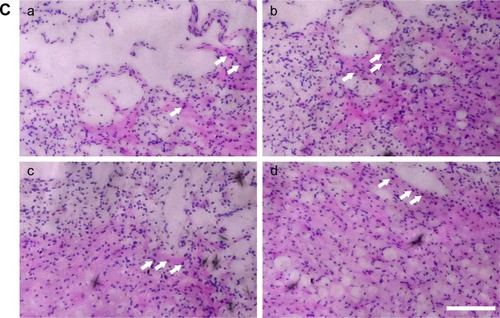
The histopathological manifestations in injured spinal tissues at 8 weeks post-operation were analyzed. Grafts integrated better with the host tissues of the spinal cord by covering rostral and caudal stumps in the cell-containing PCL scaffold groups than those compared with the PCL scaffold group (). In Group 1, atrophic neuronal cells, larger cavities, and cell infiltration were observed in the spinal cord lesion. H&E staining 8 weeks after SCI showed that cell transplantation reduced the cavity and some cell infiltration in the spinal cord. Eight weeks after SCI, in Group 2 and Group 3, some cell infiltration was observed in the injured spinal cord. In Group 4, there are more infiltrating cells and fewer cavities than Group 2 and Group 3 (). This indicates that transplantation of cells and PCL scaffolds can improve the remodeling of injured spinal cord tissue.
Figure 4 Axonal regeneration and remyelination of transplanted cells in injured spinal cord.
Notes: (A) Spinal cord transection and transplantation. Scale bar =0.5 cm. (B) Gross morphology and cavity formation of spinal cord tissue. (C) H&E staining of Group 1 (a), Group 2 (b), Group 3 (c), and Group 4 (d). Arrows: infiltrated cells in spinal cord. Scale bar =100 µm.
Abbreviation: H&E, hematoxylin–eosin.
Behavioral outcome at 8 weeks post-injury
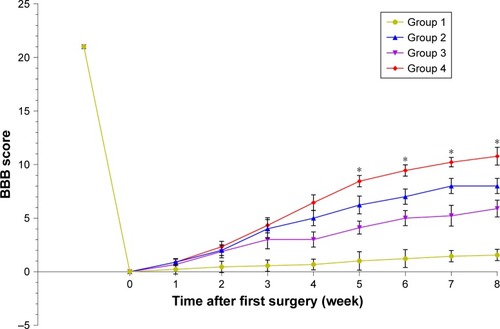
Before and after tissue engineering scaffold transplantation, behavioral analysis was performed to rate open-field locomotion by BBB scale. The hindlimbs of all of groups of Wistar rats were completely paralyzed after the T10 spinal cord tissue was transected. One week after surgery, all surgical treatments of the animals showed some slight recovery. Thereafter, animals treated with PCL scaffolds alone showed smaller improvements while rats treated with PCL scaffolds+ASCs+NSCs showed faster and better, but nonsignificant, recovery. At 8 weeks post-surgery, the BBB score of PCL scaffolds+ASCs+NSCs group reached a level of 12, indicating tissue engineering scaffolds that contain ASCs and NSCs may have a positive influence on motor recovery ().
Analysis of NGF and neurotrophic factors
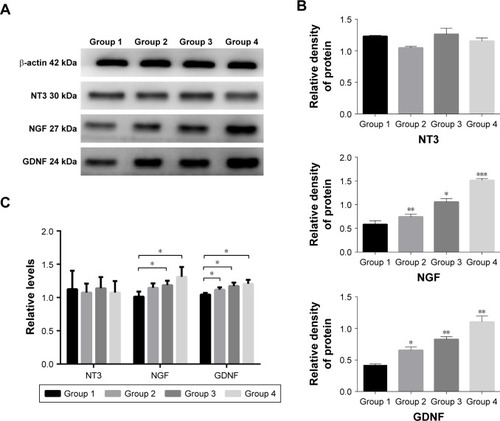
To probe the possible function of NT3, NGF, and GDNF in SCI, we used Western blotting analysis and real-time PCR to investigate the protein and gene expressions of NT3/NGF/GDNF in the spinal cord tissue. Eight weeks after transplantation, in spinal cord tissue, the protein expression of NGF in Groups 2, 3, and 4 was higher than that of Group 1 (P<0.05). Among them, the protein expression of NGF was the highest in Group 4. Compared with the control group (Group 1), we found that the protein expression of GDNF was increased in Groups 2, 3, and 4 (P<0.05). While, NT3 levels in the spinal cord were not different among each group (). In addition, the results of PCR showed that the gene expression levels of NGF and GDNF were consistent with the protein expression levels (P<0.05). Similarly, the relative gene expression levels of NT3 in the spinal cord were still not different among each group (). These results show that after SCI, cell transplantation affected the protein and gene expressions of GDNF and NGF in spinal cord tissues.
Figure 6 Analysis of NGF and neurotrophic factors.
Notes: The protein expression of NGF and GDNF (A) was increased in Group 2, Group 3, and Group 4 (B). The gene expression levels of NGF and GDNF were consistent with the protein expression levels (C). *P<0.05, **P<0.01, ***P<0.005.
Abbreviations: GDNF, glial cell-derived neurotrophic factor; NGF, nerve growth factor; NT3, neurotrophin 3.
Discussion
SCI is a common central nervous system disorder characterized by destruction or dysfunction of spinal cord caused by trauma or other causes.Citation42–Citation44 SCI can cause a variety of complications that lead to irreversible loss of sensory and motor functions. Recently, the incidence of SCI showed a significant upward trend especially in developing countries, and this is a heavy burden for the patients’ family and society.Citation45 While many strategies, such as surgery and drugs, failed to achieve a good therapeutic effect, the advent of stem cell therapy brings hope to the patients with SCI. In this study, hUCB-iPSCs were induced into NSCs as “seed cells” and was cocultured with ASCs on the PCL bioscaffold. After establishing the SCI model, the tissue engineering scaffolds were transplanted into the animals with SCI. Western blot, real-time PCR, immunohistochemical staining, and behavioral analyses were used to explore the effectiveness of repairing SCI, in order to provide new ideas and theoretical foundation for its clinical repair.
A number of studies have confirmed the efficacy of NSCs combined with SCs in the treatment of SCI.Citation46,Citation47 At present, it is illegal and unethical to extract human NSCs. Therefore, the purpose of this study was to explore a safer and more convenient way to obtain NSCs. IPSCs have unlimited self-renewal and pluripotent potential of embryonic stem cells, which allow them to differentiate into multiple types of mature cells in vitro.Citation48 Moreover, it avoids many problems similar to embryonic stem cells, such as lack of cell origin, ethical, and allogeneic immune rejection.Citation49 Takahashi and Yamanaka first used imitative transcription factors to successfully reprogram iPSCs from mouse fibroblasts in 2006.Citation50 IPSCs are gradually applied to disease models, drug screening, regenerative medicine, and other fields.Citation51,Citation52 Führmann et al have reported a peptide-modified, minimally invasive, injectable hydrogel containing hyaluronan and methylcellulose.Citation53 They used the biomaterial to enhance the survival of human iPSCs-derived oligodendrocyte progenitor cells and cell differentiation. In their study, hydrogel attenuated teratoma formation by promoting cell differentiation. Similarly, there are many studies that suggest the use of γ-secretase inhibitor could promote neuronal differentiation and maturation in vitro in order to reduce teratoma formation after cell transplantation.Citation54 However, unlike previous studies, our research use PCL electrospun fiber membrane for the preparation of tissue engineering scaffolds. In addition, many cells derived from iPSCs, such as cardiomyocytes, hepatocytes, and osteoblasts, should be used for further preclinical studies.
Biological materials are divided into two types: natural formation and artificial synthesis. They should have the following characteristics: degradable and biocompatible with a certain 3D structure.Citation55 Among the many types of biodegradable materials, polylactic acid, chitosan, and gelatin are widely used.Citation56 Most of the previous reports linked the PCL scaffolds with many diseases such as cardiovascular diseases and bone defects. Tardajos et al introduced chitosan as an antibacterial agent in a 3D printed PCL scaffold and used L929 fibroblasts to demonstrate the cell-adhesion and cell-viability capacity of the modified scaffolds.Citation57 It is an innovative approach, and the antibacterial technology has been applied in this study in order to increase the safety and proliferation of cell cultures in scaffold. PCL, due its benign drug permeability, is often administrated in combination with other drugs to treat SCI.Citation58 Previous studies have reported the use of dexamethasone acetate (DA)-loaded polymeric micelles to improve DA’s water solubility, biocompatibility, and in reducing its side effects.Citation59 In summary, the purpose of combining drugs or cells with biomaterials is to improve hindlimb functional recovery, decrease neuron loss and promote axon regeneration. This research strongly supports previous viewpoints and proposes that the use of PCL electrospun fiber membrane for SCI should be further explored.
In addition to seed cells and scaffolds, selected suitable growth factor could maximize the repair effect using tissue engineering method in SCI. Many NGFs and neurotrophic factors could not be ignored, including brain-derived neurotrophic factor, GDNF, NGF, NT3, and others, among which the most important are NGF, NT3, and GDNF. NGF was first discovered in 1950 and plays an important role in the survival, development, and maturation of neurons in the peripheral nervous system.Citation60 In the spinal cord, the expression of NGF may induce hyperalgesia and robust spouting of nociceptive axons.Citation61 GDNF is one of the important neurotrophic factors. GDNF and its receptors are widely expressed in the central nervous system in adults.Citation62 Previous studies have demonstrated that in addition to the role of GDNF in axon regeneration,Citation63,Citation64 it can also promote the remodeling of spinal cord tissue by affecting the behavior of glial cells. The effect of the above factors on the repair of SCI is consistent with the results of our experiment, which found that the degree of spinal cord recovery and remodeling was closely related to NGF and GDNF. NT3 is a multifunctional gene and has an effect on maintaining the neuron and dopaminergic neuron differentiation.Citation65 NT3 mRNA is highly expressed in the spinal cord in motor neurons.Citation66 Several studies have shown that NT3 not only plays an important role in the development and differentiation of neurons, but also promotes the hindlimb functional recovery of motor function in rats with SCI.Citation62,Citation67,Citation68 In our study, the NT3 levels in the spinal cord were unchanged after tissue engineering scaffolds transplantation, and these results may explain the slow recovery of hindlimb motor function in rats with SCI.
In this study, hUCB-iPSCs-derived NSCs combined with PCL electrospun fiber membrane were used for the first time to make tissue engineering scaffolds. The innovation of this study is to resolve ethical problems and utilize the good biocompatibility and degradability of the scaffold. Nevertheless, there are still some limitations in this study. First, the study did not continue to explore the cell’s activity and proliferation ability on the PCL. Second, we did not observe whether there was tumor formation at the spinal cord after cell transplantation. Finally, the specific epigenetic mechanisms of cell transplantation for SCI should be further elucidated. Therefore, our next step will focus on epigenetic changes before and after cell induction and transplantation. In addition, the therapeutic efficacy of other cells or scaffolds on SCI remains to be further explored.
In recent years, the repair of SCI by cell transplantation has become hotspots in the field of cell therapy, but there are significant differences in the effect and role of the repair of different cell combinations. Cell transplantation therapy can promote regeneration and remyelination of axons, replace apoptotic cells, thereby promoting the repair of spinal cord injuries, and creating favorable conditions for the recovery of sensory and motor functions. Therefore, the application of tissue engineering scaffold to promote nerve regeneration after SCI is the focus of our future research.
Conclusion
In this work, a novel tissue engineering scaffold was successfully synthesized. PCL electrospun fiber membrane loaded with iPSCs-NSCs and ASCs were prepared and evaluated for the treatment of SCI in vitro and in vivo. Cell-containing PCL scaffolds in this study have good biodegradability and biocompatibility. It plays a role in promoting tissue remodeling and secretion of neurotrophic factors. In addition, this tissue engineered scaffold could promote motor function recovery in a SCI model. Therefore, cell-containing PCL scaffolds maybe a clinically viable therapeutic strategy for SCI in the future.
Acknowledgments
This work was financially supported by the State Key Program of National Natural Science Foundation of China (81330042), State General Program National Natural Science Foundation of China (81371957), and International Cooperation Program of National Natural Science Foundation of China (81620108018).
Disclosure
The authors report no conflicts of interest in this work.
References
- NiclisJCTurnerCDurnallJLong-distance axonal growth and protracted functional maturation of neurons derived from human induced pluripotent stem cells after intracerebral transplantationStem Cells Transl Med2017661547155628198124
- WangQHeYZhaoYA thermosensitive heparin-poloxamer hydrogel bridges aFGF to treat spinal cord injuryACS Appl Mater Interfaces2017986725674528181797
- LiGShenFFanZDynasore improves motor function recovery via inhibition of neuronal apoptosis and astrocytic proliferation after spinal cord injury in ratsMol Neurobiol20175497471748227822712
- LuPWoodruffGWangYLong-distance axonal growth from human induced pluripotent stem cells after spinal cord injuryNeuron201483478979625123310
- WangHLiuCMeiXBerberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injuryOncotarget2017858983129832129228691
- ZhouZLiuCChenSActivation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injuryOncotarget2017832520785209328881715
- HeQQXiongLLLiuFMicroRNA-127 targeting of mitoNEET inhibits neurite outgrowth, induces cell apoptosis and contributes to physiological dysfunction after spinal cord transectionSci Rep201663520527748416
- MaoLGaoWChenSEpothilone B impairs functional recovery after spinal cord injury by increasing secretion of macrophage colony-stimulating factorCell Death Dis2017811e316229095439
- NagashimaKMiwaTSoumiyaHPriming with FGF2 stimulates human dental pulp cells to promote axonal regeneration and locomotor function recovery after spinal cord injurySci Rep2017711350029044129
- PilttiKMFunesGMAvakianSNIncreasing Human Neural Stem Cell Transplantation Dose Alters Oligodendroglial and Neuronal Differentiation after Spinal Cord InjuryStem Cell Reports2017861534154828479305
- ChenJWangZZhengZNeuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injuryCell Death Dis2017810e309028981091
- LeeJYKangSRYuneTYFluoxetine prevents oligodendrocyte cell death by inhibiting microglia activation after spinal cord injuryJ Neurotrauma201532963364425366938
- SpringerJEVisavadiyaNPSullivanPGHallEDPost-Injury Treatment with NIM811 Promotes Recovery of Function in Adult Female Rats after Spinal Cord Contusion: A Dose-Response StudyJ Neurotrauma201835349249928967329
- MalikmammadovETanirTEKiziltayAHasirciVHasirciNPCL and PCL-based materials in biomedical applicationsJ Biomater Sci Polym Ed2018297–986389329053081
- NicaiseAMBandaEGuzzoRMiPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury responseExp Neurol201728811412127865736
- SampaziotisFde BritoMCMadrigalPCholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validationNat Biotechnol201533884585226167629
- KawamataSKanemuraHSakaiNTakahashiMGoMJMjGDesign of a Tumorigenicity Test for Induced Pluripotent Stem Cell (iPSC)-Derived Cell ProductsJ Clin Med20154115917126237025
- OvchinnikovDASunJWolvetangEJGeneration of Footprint-Free Induced Pluripotent Stem Cells from Human Fibroblasts Using Epi-somal Plasmid VectorsMethods Mol Biol20151330374526621587
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell2007131586187218035408
- CyranoskiDJapanese woman is first recipient of next-generation stem cellsNature2014 Epub2014912
- JessenKRMirskyRLloydACSchwann cells: development and role in nerve repairCold Spring Harb Perspect Biol201577a02048725957303
- FeltriMLPoitelonYPrevitaliSCHow Schwann cells sort axons: new conceptsNeuroscientist201622325226525686621
- ZhouXHNingGZFengSQTransplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-upCell Transplant201221Suppl 1394721929867
- HillCEMoonLDWoodPMBungeMBLabeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survivalGlia200653333834316267833
- MarcolWŚlusarczykWLarysz-BryszMGrafted activated Schwann cells support survival of injured rat spinal cord white matterWorld Neurosurg201584251151925910924
- SaadaiPWangANoutYSHuman induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningoceleJ Pediatr Surg201348115816323331809
- RomanyukNAmemoriTTurnovcovaKBeneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repairCell Transplant20152491781179725259685
- UezonoNZhuYFujimotoYPrior treatment with anti-high mobility group box-1 antibody boosts human neural stem cell transplantation-mediated functional recovery after spinal cord injuryStem Cells201836573775029517828
- NovikovaLNKolarMKKinghamPJTrimethylene carbonate-caprolactone conduit with poly-p-dioxanone microfilaments to promote regeneration after spinal cord injuryActa Biomater20186617719129174588
- RumboCTamayo-RamosJACasoMFColonization of electrospun polycaprolactone fibers by relevant pathogenic bacterial strainsACS Appl Mater Interfaces20181014114671147329558795
- KularatneRNWashingtonKEBulumullaCHistone deacetyinhibitor (HDACi) conjugated polycaprolactone for combination cancer therapyBiomacromolecules20181931082108929485283
- SharifiFPatelBBDzuilkoAKMontazamiRSakaguchiDSHashemiNPolycaprolactone microfibrous scaffolds to navigate neural stem cellsBiomacromolecules201617103287329727598294
- JoshiMKTiwariAPPantHRIn situ generation of cellulose nanocrystals in polycaprolactone nanofibers: effects on crystallinity, mechanical strength, biocompatibility, and biomimetic mineralizationACS Appl Mater Interfaces2015735196721968326295953
- FanBZhouXWangLIn vitro study of neural stem cells and activated Schwann cells cocultured on electrospinning polycaprolactone scaffoldsJournal of Neurorestoratology20175155165
- Coelho de OliveiraVCSilva dos SantosDVairoLHair follicle-derived mesenchymal cells support undifferentiated growth of embryonic stem cellsExp Ther Med20171351779178828565767
- HanXYuLRenJEfficient and fast differentiation of human neural stem cells from human embryonic stem cells for cell therapyStem Cells Int20172017940520429075299
- WoodhooAAlonsoMBDroggitiANotch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticityNat Neurosci200912783984719525946
- LiuCHuangYPangMTissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (lactide-co-glycolide)/polyethylene glycol scaffoldsPLoS One2015103e011770925803031
- FanHChenKDuanLWangYZJuGBeneficial effects of early hemostasis on spinal cord injury in the ratSpinal Cord2016541192493227137123
- BassoDMBeattieMSBresnahanJCA sensitive and reliable locomotor rating scale for open field testing in ratsJ Neurotrauma19951211217783230
- ZhouXHLinWRenYMComparison of DNA Methylation in Schwann Cells before and after Peripheral Nerve Injury in RatsBiomed Res Int20172017539326828459064
- HanDYuZLiuWPlasma Hemopexin ameliorates murine spinal cord injury by switching microglia from the M1 state to the M2 stateCell Death Dis20189218129415995
- GwakS-JMacksCBaeSCecilNLeeJSPhysicochemical stability and transfection efficiency of cationic amphiphilic copolymer/pDNA polyplexes for spinal cord injury repairSci Rep2017711124728900263
- MotheAJTassewNGShabanzadehAPRGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injurySci Rep2017711052928874746
- JazayeriSBBeygiSShokranehFHagenEMRahimi-MovagharVIncidence of traumatic spinal cord injury worldwide: a systematic reviewEur Spine J201524590591824952008
- WangDLiangJZhangJLiuSSunWMild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injuryNeural Regen Res20149242189219625657741
- LaiBQWangJMLingEAWuJLZengYSGraft of a tissue-engineered neural scaffold serves as a promising strategy to restore myelination after rat spinal cord transectionStem Cells Dev201423891092124325427
- HoshinaAKawamotoTSuetaS-IDevelopment of new method to enrich human iPSC-derived renal progenitors using cell surface markersSci Rep201881637529686294
- YoshidaYYamanakaSInduced pluripotent stem cells 10 years later: for cardiac applicationsCirc Res2017120121958196828596174
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell2006126466367616904174
- BhartiyaDShaikhAAnandSEndogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look aheadHum Reprod Update2016231417627614362
- KarakikesITermglinchanVCepedaDAA comprehensive TALEN-based knockout library for generating human-induced pluripotent stem cell-based models for cardiovascular diseasesCirc Res2017120101561157128246128
- FührmannTTamRYBallarinBInjectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury modelBiomaterials201683233626773663
- OkuboTIwanamiAKohyamaJPretreatment with a γ-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injuryStem Cell Reports20167464966327666789
- TeuschlAHeimelPNürnbergerSvan GriensvenMRedlHNauTNovel silk fiber-based scaffold for regeneration of the anterior cruciate ligament: histological results from a study in sheepAm J Sports Med20164461547155726957219
- GaiMFruehJKudryavtsevaVLYashchenokAMSukhorukovGBPolylactic acid sealed polyelectrolyte multilayer microchambers for entrapment of salts and small hydrophilic molecules precipitatesACS Appl Mater Interfaces2017919165361654528452456
- TardajosMGCamaGDashMChitosan functionalized poly-ε-caprolactone electrospun fibers and 3D printed scaffolds as antibacterial materials for tissue engineering applicationsCarbohydr Polym201819112713529661300
- WangJWangJLuPLocal delivery of FTY720 in PCL membrane improves SCI functional recovery by reducing reactive astrogliosisBiomaterials201562768726036174
- WangYWuMGuLEffective improvement of the neuroprotective activity after spinal cord injury by synergistic effect of glucocorticoid with biodegradable amphipathic nanomicellesDrug Deliv201724139140128165815
- Levi-MontalciniRHamburgerVSelective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryoJ Exp Zool1951116232136114824426
- GwakYSNamTSPaikKSHulseboschCELeemJWAttenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the ratNeurosci Lett2003336211712012499054
- AwadBICarmodyMASteinmetzMPPotential role of growth factors in the management of spinal cord injuryWorld Neurosurg201583112013123334003
- IannottiCLiHYanPLuXWirthlinLXuXMXmXGlial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injuryExp Neurol2003183237939314552879
- MillsCDAllchorneAJGriffinRSWoolfCJCostiganMGDNF selectively promotes regeneration of injury-primed sensory neurons in the lesioned spinal cordMol Cell Neurosci200736218519417702601
- JiWCZhangXWQiuYSSelected suitable seed cell, scaffold and growth factor could maximize the repair effect using tissue engineering method in spinal cord injuryWorld J Exp Med201663586227622154
- KeefeKMSheikhISSmithGMTargeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord InjuryInt J Mol Sci2017183548
- DaviaudNGarbayoESchillerPCPerez-PinzonMMontero-MeneiCNOrganotypic cultures as tools for optimizing central nervous system cell therapiesExp Neurol201324842944023899655
- ParkSSByeonYERyuHHComparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3Cell Transplant20112011–121867188021375803