?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Calcium sulphate cement (CSC) is widely used as an osteoconductive biomaterial in bone repair and regeneration.
Purpose
In this study, porous TiO2 microspheres were added to CSC to achieve a controlled and sustained drug (gentamicin) release.
Methods
Scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray powder diffraction (XRD), and Brunauer-Emmett-Teller (BET) surface area analysis were conducted to analyse the morphology, phase composition, and surface area of the TiO2 micro-spheres and composite cements. In addition, the injection time, compressive strength, degradation behaviour, and antibacterial ability of the composite cements were examined during in vitro degradation. Gentamicin release profile was recorded using an ultraviolet spectrophotometer.
Results
The results revealed the excellent drug loading ability of the TiO2 microspheres. The addition of TiO2 microspheres improved the injectability and compressive strength of the composite cements, the maximum value of which was achieved at a TiO2 loading of 5 wt.%. When immersed in simulated body fluid (SBF), the composite cements doped with TiO2 microspheres were observed to release gentamicin in a stable and sustained manner, especially in the latter stages of in vitro degradation. During degradation, CSC doped with TiO2 microspheres exhibited a typical apatite-like behaviour. Further, antibacterial analysis showed that CSC doped with TiO2 microspheres exhibited long-term antibiotic activity.
Conclusion
Thus, as an effective sustained-release formulation material, TiO2 microspheres show a great potential for application in bone cements.
Introduction
Developments in the field of bone-filling materials have improved the chances of success for millions of patients facing surgery. In clinical applications, bone cements are one of the most widely used bone filling materials.Citation1 However, post-operative infections and complications are still potent threats that delay healing after bone cement injection.Citation2 Although the rate of infection in orthopedic surgery is fairly low (reported to be 1%–3%),Citation3–Citation6 post-operative infections and complications can be very troublesome and often cause severe pain and loss of bone tissue; moreover, they may lead to the removal of implants, and thus, a second surgical operation.Citation7 Current antibacterial therapies rely on the systematic administration of antibiotics.Citation8 Gentamicin is a common broad-spectrum, thermostable antibiotic and is one of the most widely used compounds in clinical applications.Citation9,Citation10 In order to avoid infection at the site of implantation, antibiotic-loaded bone cements have been developed.Citation11 However, an instantaneous burst release of the antibiotic, most of which is loaded in the cement matrix, leads to a low antibacterial activity because the amount of antibiotic able to reach the surgical site would be too low.Citation10,Citation12 Therefore, developing a solution to curb the burst release of antibiotics is a topic of significant interest for researchers working on bone filling materials.
TiO2 is widely used in implantology due to its nontoxicity, hydrophilicity, low cost, and good stability.Citation13–Citation19 TiO2 microparticles are capable of delivering antibiotic molecules and the process of drug release has been reported and their kinetics analyzed.Citation20 Several researchers have reported that TiO2 can be used to deliver antibiotics. Pawlik et al prepared anodic TiO2 nanotube layers for the co-delivery of ibuprofen and gentamicin.Citation21 Flak et al synthesized ZnPc@ TiO2 nanotubes to deliver doxorubicin.Citation22 In both these studies, coatings were deposited on the surfaces of titanium alloys, which hindered drug release from the composite cement system. Meanwhile, it has been postulated that antibiotic carriers should preferably have a porous microcosmic structure, which would help in drug loading and release via diffusion, thus effectively eliminating instant release and prolonging the drug release process.Citation23–Citation25 Therefore, porous TiO2 microspheres can be a promising sustained antibiotic media in bone cement composites.
Herein, novel porous TiO2 microspheres were designed to act as antibiotic carriers. Further, the relevance of compressive strength and injectable time of composite cements with TiO2 microspheres doping concentration was discussed; the degradation stability, drug release profile, surface morphology, phase transformation, and antibacterial properties of composite cements doped with TiO2 microspheres were investigated.
Materials and methods
Preparation of TiO2 microspheres
Amorphous TiO2 microspheres were prepared by the hydrolysis of Ti(SO4)2 according to a previously reported method.Citation26 A de-ionized solution of Ti(SO4)2 (75 mL, 4 mmol) was mixed in 75 mL of 1-propanol along with 2.0 g of poly-vinylpyrrolidone. The mixture was stirred in a beaker for 3 hours at 70°C and washed and aged in a 1 mol/L alkaline solution. Subsequently, the precipitates were solvothermally treated in a stainless steel autoclave.
Characterization of TiO2 microspheres
TiO2 microspheres were mixed with gentamicin (Aladdin Co., Ltd, Shanghai, China) in de-ionized water by stirring and the wet mixture was dried at room temperature for 24 hours. Surfaces of the TiO2 microspheres were visualized by a scanning electron microscope (HITACHI S-4800, Tokyo, Japan) operating at an accelerating voltage of 5 kV in the secondary electron imaging mode and by transmission electron microscopy (Philips Tecnai G2F20, Amsterdam, Netherlands) at an accelerating voltage of 200 kV. Phase analysis of the TiO2 microspheres was conducted using an X-ray powder diffraction (XRD) instrument (RIGAKU/DMAX 40 kV, 200 mA, Cu Kα radiation, λ=1.5418 Å). The Brunauer-Emmett-Teller (BET) surface areas of original TiO2 microspheres and gentamicin-loaded TiO2 microspheres were measured on a Micromeritics instrument (3Flex 577) using N2 adsorption/desorption isotherms. A dynamic light scattering (Malvern Zetasizer NANO-ZS90, Malvern, UK) was used to determine the zeta potential of original TiO2 microspheres and gentamicin-loaded TiO2 microspheres.
Preparation of gentamicin/TiO2-calcium sulfate cement (G/TiO2-CSC)
Calcium sulfate (CS) has a long history of being used as a bone filling material owing to its advantageous features of a suitable setting time, good mechanical properties, and biodegradability.Citation9,Citation27 In this study, we chose CS as the bone cement matrix to be doped with TiO2 microspheres. The cement powder consisted of CS hemihydrate (analytical reagent [AR], 97.0%; Aladdin). A total of 0.1875 g gentamicin and different weightsCitation28,Citation29 of TiO2 (prepared by solvothermal synthesis) were dispersed by ultrasonication in 2.5 mL of de-ionized water as the liquid monomer. The doping concentration of TiO2 was determined by injection and compressive tests. The cement powder was mixed with the liquid monomer at a liquid:solid ratio of 0.5 mL/g in a beaker to form a paste. Subsequently, the cement paste was injected through a 5 mL syringe into a polytetrafluoroethylene mold to fabricate cylindrical specimens for compressive testing and gentamicin antibiotic-elution assay. The liquid monomer without gentamicin was set as the control group.
Characterization of G/TiO2-CSC
Antibiotic release kinetics during in vitro degradation
Drug release studies were conducted by separately soaking each cylindrical specimen (6 mm in diameter and 12 mm in height for compressive testing, 12 mm in diameter and 2 mm in height for antibacterial assay) in 5 mL of simulated body fluid (SBF);Citation30 these specimens were then incubated at 37°C for 2 weeks in a shaking incubator. The SBF solution was drawn out at pre-defined time intervals (1, 3, 5, 7, and 14 days) to examine the quantity of gentamicin released. The measurement was carried out using an ultraviolet spectrophotometer (UV-2700220V CH) at a wavelength of 253 nm.Citation31 To determine the cumulative release of gentamicin from different samples, a calibration curve was initially plotted using different concentrations of pristine gentamicin solution; the amount of drug released from the tested sample could then be calculated from the measured absorbance using the generated calibration equation.
Mechanical properties and stability during in vitro degradation
After the antibiotic release studies, the specimens were removed from the SBF solution and dried at 60°C in an oven for 24 hours. A universal testing machine was used to evaluate the compressive strength of the composite cement specimens; the weight loss in the specimens was measured using the following equation in which W0 refers to the initial weight and Wt refers to the weight at time t.
The pH value of the SBF solution from which the specimens were removed was measured using a pH meter.
Morphology and phase analysis during in vitro degradation
The surface morphology of the G/TiO2-CSC specimens after in vitro degradation was observed using a scanning electron microscope and their composition was analyzed by energy dispersive X-ray analysis. The phase composition was determined by XRD.
Antibacterial activity test
The antibacterial activity of the G/TiO2-CSC specimens was evaluated by the agar disc diffusion method. Escherichia coli and Staphylococcus aureus were individually inoculated in Luria-Bertani (LB) broth (liquid medium) (5 mL) and agitated overnight in a shaking incubator (37°C, 220 rpm). The concentration of the bacterial cells was measured by optical spectrometry at 600 nm; it was calculated to be 105 cells/mL. Subsequently, 50 µL of the bacterial solution was inoculated on a plate of LB solid medium. Then specimens of G/TiO2-CSC and G-CSC obtained from different degradation times (3, 7, 14 days) in “Antibiotic release kinetics during in vitro degradation” section were placed carefully on the bacteria inoculated agar surface. These agar plates were then incubated at 37°C for 24 hours. Subsequently, agar plates were taken out to observe the inhibition zones. The diameters of the inhibition zones were measured from the photographs of the agar plate, which were captured using a digital camera.
Statistical analysis
Quantitative data were processed with Origin 8.6 (Origin Lab Corporation, Northampton, MA, USA). All the experiments were repeated at least five times. A Student’s t-test was used to compare the means of different specimens. Statistical significance was set at a CI of >95% (P<0.05).
Result and discussion
Characterization of the TiO2 microspheres
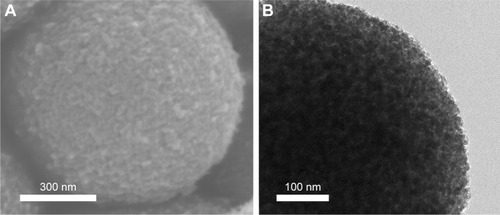
The scanning electron microscopy (SEM) images of TiO2 microspheres are shown in . The top right inset presents a magnified image of the homologous main image. TiO2 microcrystals were observed to be homogeneous inerratic and well dispersed. The surfaces of the TiO2 micro-spheres were lychee-like and their diameter was in the range of 700–1,200 nm, which is the aggregation state of TiO2 crystalline grain. shows the morphology of a single TiO2 microsphere, which indicates its highly porous nature. A large surface area plays an important role in achieving a high specific capacity, which can in turn provide high drug loading capacity.Citation32 depicts the XRD pattern of TiO2 microspheres. The characteristic peaks are consistent with those of anatase TiO2 (JCPDS no. 21-1272).
Figure 1 (A) SEM morphologies of TiO2 microspheres; (B) TEM morphology of a TiO2 microsphere; (C) XRD pattern of TiO2 microspheres.
Abbreviations: SEM, scanning electron microscopy; TEM, transmission electron microscopy; XRD, X-ray powder diffraction.

The morphology of TiO2 microspheres loaded with gentamicin is shown in . After immersion in gentamicin solution, the surface morphology of the TiO2 microspheres became much smoother, which indicates that gentamicin was loaded onto the surface micropores of TiO2 microspheres. The decrease in the surface microstructure proves the ability of TiO2 microspheres to load drugs.
Figure 2 (A) SEM morphology and (B) TEM morphology of a TiO2 microsphere loaded gentamicin.
Abbreviations: SEM, scanning electron microscopy; TEM, transmission electron microscopy.
shows the N2 adsorption-desorption isotherms of the original TiO2 microspheres and TiO2 loaded with gentamicin; the curves depict type IV isotherms with a hysteresis loop at relative pressures (P/P0) of 0.4–0.8 and 0.5–0.9, respectively, which suggest capillary condensation in the micropores. shows the pore diameter distribution of the original TiO2 microspheres and the TiO2 microspheres loaded with gentamicin. No peaks corresponding to micropores could be observed. The BET surface areas of TiO2 before and after loading with gentamicin were 241.4678 m2/g and 181.0672 m2/g, respectively. The microspheres had a larger pore volume and a relatively larger BET surface area. Because the gentamycin molecules were approximately 5 Å in width and 10 Å in length,Citation33 the pores on the surface of TiO2 microspheres were large enough to accommodate them. Therefore, the reduced surface area of the microspheres could be attribute to the loading with gentamycin.
Figure 3 (A) N2 adsorption-desorption isotherms; (B) pore size distribution; and (C) zeta potentials of TiO2 before and after being loaded with gentamicin.
Abbreviations: G/TiO2, gentamicin/TiO2; STP, standard temperature and pressure.
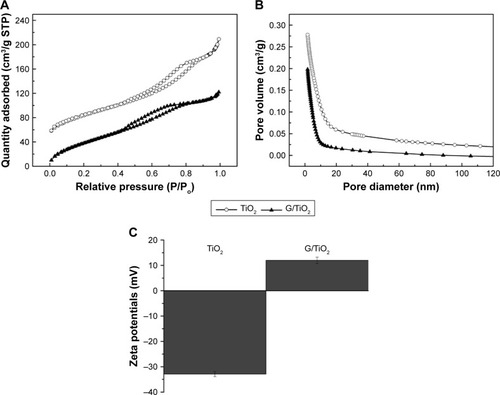
The drug release performance of a drug delivery system depends strongly on its morphology.Citation21 TiO2 microspheres with a nanoporous structure have a significant influence on the drug release profile. The BET results showed that TiO2 microspheres are porous in nature; these pores can be used to load antibiotics. The adsorption process of gentamicin was further characterized by determining the change in zeta potential values of gentamycin loaded TiO2 microspheres. As shown in , the average zeta potential of TiO2 microspheres was −32.9 mV, while it turned to positive values (+12 mV) when the gentamycin was loaded. The observations indicated the electrostatic interaction between the positively charged gentamycin and the negative surface of TiO2 microspheres. The large surface area and pore diameter of TiO2-CSC provide more suitable conditions for hosting and controlled release gentamicin compared to the original CSC. Gentamicin molecules were embedded in that cement matrix, thus, they could easily be eluted away which resulted in a burst release when the cement was placed in SBF.
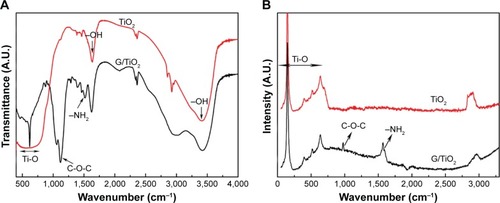
The Fourier-transform infrared spectra of samples were characteristic of TiO2 and gentamicin TiO2 compound, as shown in . In the graph, compounds with gentamicin exhibited absorption bands between 1,550 and 1,645 cm−1, which can be attributed to the bending of N-H bond of the primary and secondary amines of gentamicin. The bands between 1,100 and 1,200 cm−1 were assigned to the stretching vibration of C-O-C of gentamicin. The broad absorption band around 600 cm−1 is due to the stretching vibrations of Ti-O-Ti and Ti-O bonds that are characteristic of the formation of a Ti-O-Ti network. The results corresponded with Raman spectra, as shown in . The bands of Ti-O-Ti were observed apparently between 143 and 608 cm−1 and compounds also showed the characteristic peaks of gentamicin, which could prove the successful combination of TiO2 microspheres and gentamicin.
Characterization of G/TiO2-CSC
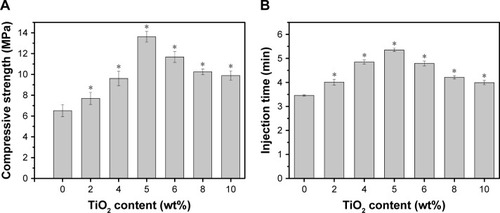
shows the mechanical properties of TiO2-CSC specimens with different TiO2 concentrations, while shows the variation in their injectability at different TiO2 concentrations. The compressive strength and injection time both followed the same trend. They initially increased with an increase in the TiO2 content but gradually decreased at TiO2 concentrations greater than 5%. When the TiO2 content reached 5%, TiO2-CSC exhibited the optimum compressive strength (13–14 MPa), which is almost twice as large as that of the control group without TiO2. Additionally, TiO2-CSC with 5% TiO2 concentration exhibited the maximum injection time for different samples (5–6 minutes), which is suitable in clinical applications for lengthening the operation time. The TiO2 replacement of cement can accelerate the hydration rate of CSC which can increase the compressive strength. It is known that sulfate ions could dissolve into the mixing water and affect the hydration of cement.Citation34 The incorporation of TiO2 accelerates the hydration rate of CSC, which can increase the compressive strength. However, when it was 5%, the compressive strength reached the maximum. More TiO2 would induce the generation of defects and weak points due to the aggregation of TiO2 microspheres.Citation35 What is more, the TiO2 inclusion in the cement causes a dilution effect, which could contribute to the delayed injection time. But for the cases over 5%, the decrease of injection time may be attributed to a mass of TiO2 microspheres interacting together to form a 3D-network.Citation36 Thus, it can be used as a good bioactive ingredient and reinforcing agent in bone composite cements. On the basis of these results, a TiO2 concentration of 5% was chosen to conduct the subsequent drug release experiments.
In vitro drug release studies
displays the cumulative gentamicin release profiles of the experimental group (with TiO2) and the control group (without TiO2). It was found that the cumulative gentamicin release curve of G-CSC had a biphasic profile, with a fast initial release followed by a continuous and slow release. In contrast to the control group, the cumulative gentamicin release curve of G/TiO2-CSC was relatively sustainable and efficient. In the first stage of degradation (<1 week), the cumulative release from the experimental group was lower than that from the control group. They reached an approximate standard after approximately 7–10-day degradation. In the long run, the cumulative release from the experimental group (approximately 70%) was higher than that of the control group (approximately 65%). The absorption of gentamicin on TiO2 contributed to the slow release in the initial stage. In the following stage, the relatively faster release of gentamicin was mainly dependent on the incorporation of TiO2 in the cement matrix, which may form effective diffusion network pathways to encourage SBF penetration and dissolve gentamicin deep within the composite cement network, as reported previously.Citation37 The initial burst release of gentamicin can be a consequence of the presence of gentamicin on the surface of the composites. When it comes to G/TiO2-CSC, the adsorption of TiO2 microspheres reduces the instantaneous burst release of gentamicin. The subsequent gentamicin release of G/TiO2-CSC comes from bulk diffusion and TiO2 microspheres. According to , G-CSC and G/TiO2-CSC had similar trends of weight loss. Because there was non-degradable TiO2 microspheres in G/TiO2-CSC, the net weight loss of calcium sulfate matrix in G/TiO2-CSC was higher than that in G-CSC. It may be attributed to the weak points formed by TiO2 aggregation, which accelerates the degradation of calcium sulfate. Thus, there was more gentamicin release in G/TiO2-CSC and the subsequent efficient release was reported to improve the rate of healing in the extended period.Citation38 The antibacterial activity of G/TiO2-CSC was weaker than that of G-CSC during the post-operative relief period, which can be attributed to the porous structure of TiO2 microspheres.
Figure 6 Cumulative gentamicin release profile (A), weight loss (B), and pH change (C) of the different samples soaking in the SBF for different times.
Abbreviations: GTMC, gentamicin; G/TiO2-CSC, gentamicin/TiO2-calcium sulfate cement; SBF, simulated body fluid.
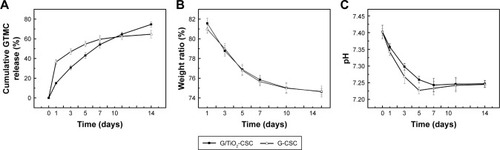
Weight loss of the composite cements with/without TiO2 during degradation is shown in . The weight loss increased with an increase in the extent of composite cement degradation; both composite cements (with/without TiO2) had the same tendency of a slightly slow rate of degradation. When the samples were soaked in SBF for 2 weeks, the weight loss was approximately 25%. This result was in agreement with other previous studies about degradation rate of bone cement.Citation39 There was no significant difference in the degradation profile of G/TiO2-CSC and G-CSC, which indicated that the difference of gentamicin release was attributed to TiO2 microspheres. shows the changes in the pH of the composite cements (with/without TiO2) soaked in SBF for different time periods. The pH decreased during the first five days of immersion, after which it exhibited a small increase. G-CSC has a relatively lower pH value because of the amount of gentamicin released in an initial burst. As the degradation process continued, pH values of both reached an equilibrium state in the range of 7.2–7.4. Cement pH is an important factor controlling calcium and phosphorus concentrations in the cement solution. In this range, it won’t change body fluid environment (7–7.4) or induce aseptic inflammation, which is caused by a low pH value of the body fluid environment, can be avoided.Citation40 It is also relevant to bone metabolic pro cess; a relatively low pH is ill-suited to bone metabolism and mineralization.Citation41
Morphology of composite cements during in vitro drug release
shows the surface morphology and composition of composite cements. reveals the variation in the morphology of G/TiO2-CSC and G-CSC when subjected to degradation in SBF. Calcium sulfate dihydrate has a block-shaped crystal structure;Citation42 such structures are evident in the matrix and the TiO2 microspheres are depicted by red dotted circles. These microspheres, which were approximately 700–800 nm in diameter, were dispersed homogeneously in the cement matrix (). In contrast, there were only block-shaped structures in . The surface morphology of the composite cements exhibited a gradual change with an increase in the extent of degradation. At higher degradation times, the composite cements with TiO2 exhibited more obvious changes. The amount of bone-like apatite remarkably increased and after a week of soaking in SBF, an apatite layer was formed on the surface. In the latter stages of degradation, the loose apatite surface provided a more bioactive environment for tissue cell attachment and growth. shows that the degradation rate of TiO2 microspheres was slower than that of calcium sulfate dihydrate. Thus, TiO2 microspheres can persistently control the release of gentamicin, especially in the latter stages after the implantation of composite cements.
Figure 7 SEM representative images of G-CSC during different degradation times (A: 1 day, B: 3 days, C: 7 days, D: 14 days) and G/TiO2-CSC during different degradation times (E: 1 day, F: 3 days, G: 7 days, H: 14 days).
Note: TiO2 microspheres were depicted by red dotted circles.
Abbreviations: SEM, scanning electron microscopy; G-CSC, gentamicin-calcium sulfate cement; G/TiO2, gentamicin/TiO2.

Phase of composite cements during in vitro drug release
depicts the XRD patterns of G-CSC and G/TiO2-CSC after soaking in SBF for different degradation times (1 day, 3 days, 7 days, and 14 days). The peaks at 25.4°, 37.8°, and 48.0°, which correspond to the structure of anatase TiO2, can be seen in . The peaks at 11.6°, 20.7°, 29.1°, and 31.1°, which were found both in G-CSC and G/TiO2-CSC before soaking in SBF, correspond to the structure of calcium sulfate dehydrate (gypsum) according to JCPDS no 33-0311. As can be seen in the figure, the half-peak width of the characteristic peaks (11.6°, 20.7°, and 29.1°) increased, which indicated a decrease in the degree of crystallinity of the samples. With an increase in the degradation time, the structure and phase obviously changed. After the samples were soaked in SBF for 2 weeks, the characteristic peaks (11.6°, 23.4°, and 29.2°) indicated phosphate (brushite) (JCPDS no. 09-0077). The addition of TiO2 microspheres had little influence on the peak position but enhanced the peak intensity.
Figure 8 XRD of G-CSC (A) and G/TiO2-CSC (B) during different degradation times (1 day, 3 days, 7 days, 14 days).
Abbreviations: A.U., absorbance unit; CSD, calcium sulfate dihydrate; XRD, X-ray powder diffraction; G-CSC, gentamicin-calcium sulfate cement.
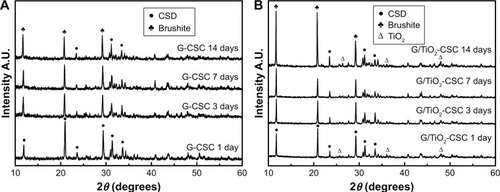
The XRD patterns () were analyzed along with the SEM images (). According to the structure and phase of the specimens, they were found to be phosphate (brushite) in the latter stages of degradation; through SEM, the specimens were found to have a loose apatite morphology. During the degradation of calcium sulfate dehydrate, Ca2+ combined with the phosphate groups in SBF to form a loose phosphate precipitate (brushite).Citation43 Brushite displays a high resorption rate when used as a bone composite cement and shows excellent biocompatibility and integration with the surrounding tissue.Citation44–Citation47 Thus, the addition of TiO2 microspheres promoted degradation and enhanced the osteoconductive and osteoinductive properties of composite cements.
Antibiotic activity
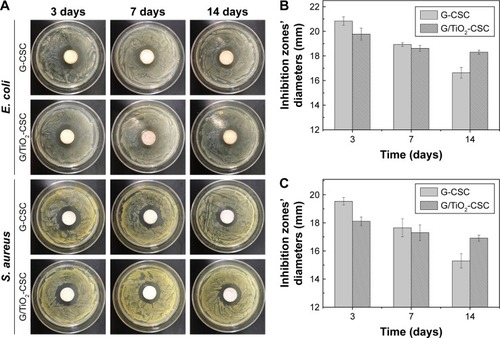
shows the results of antibiotic assay of all the samples after different degradation times (3 days, 7 days, and 14 days). The diameter of the samples was approximately 12 mm. shows the diameters of the inhibition zones of G-CSC and G/TiO2-CSC cultivated in two different bacterial strains. From the figure, we can see that the diameters of the inhibition zones against S. aureus were generally larger than those against E. coli. This is because gentamicin is more effective against gram-positive bacterial strains, such as S. aureus.Citation48 The results indicate that with an increase in SBF soaking time, the quantity of released gentamicin increased and the inhibition zones became smaller. During the first week of degradation, the inhibition zones of G/TiO2-CSC were smaller than those of G-CSC. In the next week, the inhibition zones of G/TiO2-CSC gradually became larger than those of G-CSC. According to the gentamicin release profile, in the beginning of drug diffusion (1 week), specimens of G-CSC had an initial burst rather than G/TiO2-CSC. Thus, G-CSC specimens released more gentamicin than G/TiO2-CSC specimens and caused the bigger inhibition zones. Conversely, the inhibition zones of G/TiO2-CSC were bigger than those of G-CSC at day 7, which may be due to the subsequent efficient gentamicin diffusion of G/TiO2-CSC from bulk diffusion and TiO2 microspheres contained. The rate of gentamicin release of G/TiO2-CSC was lower in the beginning and then the enhanced antibacterial ability may have been due to the sustained release in the next stage. Also, TiO2 is known to have antibacterial ability, so it may have a synergistic antibacterial effect together with gentamicin.
Conclusion
Porous TiO2 microspheres were synthesized using a solvothermal method and added to CSCs to achieve a sustained and controlled drug (gentamicin) release performance. The TiO2 microspheres exhibited a porous surface morphology and a high drug loading ability. The addition of TiO2 microspheres improved the injectability and compressive strength of the composite cements (maximum at 5 wt% TiO2). When immersed in SBF, TiO2 microsphere-doped composite cements were observed to exhibit a sustained and stable gentamicin release performance, especially in the latter stages of in vitro degradation. The TiO2 microsphere-doped composite cements exhibited a typical apatite behavior and maintained a relatively stable SBF environment during degradation in SBF. Meanwhile, the TiO2 microsphere-doped composite cements exhibited long-term antibiotic properties, as evidenced by the results of disc diffusion analysis. These results indicate that TiO2 microspheres can be used as an effective sustained-release formulation material in bone cements.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (grant no. 31570970).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuF-HSynthesis of bioceramic scaffolds for bone tissue engineering by rapid prototyping techniqueJ Solgel Sci Technol2012643704710
- Ter BooGJGrijpmaDWMoriartyTFRichardsRGEglinDAntimicrobial delivery systems for local infection prophylaxis in orthopedic-and trauma surgeryBiomaterials20155211312525818418
- HarrisWHSledgeCBTotal hip and total knee replacement (2)N Engl J Med1990323128018072136367
- ShiZNeohKGKangETWangWAntibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticlesBiomaterials200627112440244916338001
- KurtzSMLauEWatsonHSchmierJKParviziJEconomic burden of periprosthetic joint infection in the United StatesJ Arthroplasty2012278 Suppl616522554729
- SetyawatiMIKuttyRVTayCYYuanXXieJLeongDTNovel theranostic DNA nanoscaffolds for the simultaneous detection and killing of Escherichia coli and Staphylococcus aureusACS Appl Mater Interfaces2014624218222183124941440
- CampocciaDMontanaroLArciolaCRThe significance of infection related to orthopedic devices and issues of antibiotic resistanceBiomaterials200627112331233916364434
- MonteiroNMartinsMMartinsAAntibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicinActa Biomater20151819620525749293
- ChenZKangLMengQYDegradability of injectable calcium sulfate/mineralized collagen-based bone repair material and its effect on bone tissue regenerationMater Sci Eng C Mater Biol Appl2014459410225491806
- NaraharisettiPKLewMDFuYCLeeDJWangCHGentamicin-loaded discs and microspheres and their modifications: characterization and in vitro releaseJ Control Release2005102234535915653156
- MoskowitzJSBlaisseMRSamuelREThe effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone modelBiomaterials201031236019603020488534
- SilvermanLDLukashovaLHermanOTLaneJMBoskeyALRelease of gentamicin from a tricalcium phosphate bone implantJ Orthop Res2007251232917034052
- AwMSSimovicSAddai-MensahJLosicDPolymeric micelles in porous and nanotubular implants as a new system for extended delivery of poorly soluble drugsJ Mater Chem201121207082
- AwMSGulatiKLosicDControlling Drug Release from Titania Nanotube Arrays Using Polymer Nanocarriers and Biopolymer CoatingJ Biomater Nanobiotechnol201125477484
- AwMSLosicDUltrasound enhanced release of therapeutics from drug-releasing implants based on titania nanotube arraysInt J Pharm20134431–215416223313837
- KumeriaTMonHAwMSAdvanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial propertiesColloids Surf B Biointerfaces201513025526325944564
- ChaoCSLiuKHTungWLChenSYLiuDMChangYPBioactive TiO2 ultrathin film with worm-like mesoporosity for controlled drug deliveryMicroporous Mesoporous Mater20121525863
- GulatiKAwMSFindlayDLosicDLocal drug delivery to the bone by drug-releasing implants: perspectives of nano-engineered titania nanotube arraysTher Deliv20123785787322900467
- LeeDWYunYPParkKKimSEGentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegrationBone201250497498222289658
- DashSMurthyPNNathLChowdhuryPKinetic modeling on drug release from controlled drug delivery systemsActa Pol Pharm201067321720524422
- PawlikAJaroszMSyrekKSulkaGDCo-delivery of ibuprofen and gentamicin from nanoporous anodic titanium dioxide layersColloids Surf B Biointerfaces20171529510228088017
- FlakDYateLNowaczykGJurgaSHybrid ZnPc@TiO2 nanostructures for targeted photodynamic therapy, bioimaging and doxorubicin deliveryMater Sci Eng C Mater Biol Appl2017781072108528575942
- GuptaPVermaniKGargSHydrogels: from controlled release to pH-responsive drug deliveryDrug Discov Today200271056957912047857
- HorcajadaPSerreCMaurinGFlexible porous metal-organic frameworks for a controlled drug deliveryJ Am Chem Soc2008130216774678018454528
- SlowingIIVivero-EscotoJLWuCWLinVSMesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriersAdv Drug Deliv Rev200860111278128818514969
- ZhaoWWangHFengXZhangYZhangSControl over the morphology of TiO2 hierarchically structured microspheres in solvothermal synthesisMater Lett2015158174177
- HughesEYanniTJamshidiPGroverLMInorganic cements for biomedical application: calcium phosphate, calcium sulphate and calcium silicateAdv Appl Ceramic201511426576
- van de BeltHNeutDSchenkWGentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formationActa Orthop Scand200071662562911145392
- NeutDDijkstraRJThompsonJIvan der MeiHCBusscherHJAntibacterial efficacy of a new gentamicin-coating for cementless prostheses compared to gentamicin-loaded bone cementJ Orthop Res201129111654166121491478
- ChoSBNakanishiKKokuboTDependence of apatite formation on silica gel on its structure: effect of heat treatmentJ Am Ceramic Soc199578717691774
- SukhorukovaIVSheveykoANManakhovASynergistic and long-lasting antibacterial effect of antibiotic-loaded TiCaPCON-Ag films against pathogenic bacteria and fungiMater Sci Eng C Mater Biol Appl20189028929929853094
- PangQNazarLFLong-Life and High-Areal-Capacity Li-S Batteries Enabled by a Light-Weight Polar Host with Intrinsic Polysulfide AdsorptionACS Nano20161044111411826841116
- SlaneJVivancoJMeyerJPloegHLSquireMModification of acrylic bone cement with mesoporous silica nanoparticles: effects on mechanical, fatigue and absorption propertiesJ Mech Behav Biomed Mater20142945146124211354
- LeeBYKurtisKEInfluence of TiO2 Nanoparticles on Early C3S HydrationJ Am Ceram Soc2010931033993405
- MohammadiMHesarakiSHafezi-ArdakaniMInvestigation of bio-compatible nanosized materials for development of strong calcium phosphate bone cement: Comparison of nano-titania, nano-silicon carbide and amorphous nano-silicaCeram Int201440683778387
- JohariNMadaah HosseiniHRSamadikuchaksaraeiAOptimized composition of nanocomposite scaffolds formed from silk fibroin and nano-TiO2 for bone tissue engineeringMater Sci Eng C Mater Biol Appl20177978379228629081
- ShenSCNgWKShiZChiaLNeohKGTanRBMesoporous silica nanoparticle-functionalized poly(methyl methacrylate)-based bone cement for effective antibiotics deliveryJ Mater Sci Mater Med201122102283229221786132
- FrutosGPastorJYMartínezNVirtoMRTorradoSInfluence of lactose addition to gentamicin-loaded acrylic bone cement on the kinetics of release of the antibiotic and the cement propertiesActa Biomater20106380481119703595
- PeiPWeiDZhuMThe effect of calcium sulfate incorporation on physiochemical and biological properties of 3D-printed mesoporous calcium silicate cement scaffoldsMicroporous Mesoporous Mater20172411120
- WangSLiuFZengZYangHJiangHThe Protective Effect of Bafilomycin A1 Against Cobalt Nanoparticle-Induced Cytotoxicity and Aseptic Inflammation in Macrophages In vitroBiol Trace Elem Res201616919410526054709
- TakahashiTUedaSTakahashiKScowROpH-dependent multilamellar structures in fetal mouse bone: possible involvement of fatty acids in bone mineralizationAm J Physiol19942663 Pt 1C590C6008166222
- HuanZChangJSelf-setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cementsActa Biomater20073695296017588507
- GenerosiASmirnovVVRauJVAlbertiniVRFerroDBarinovSMPhase development in the hardening process of two calcium phosphate bone cements: an energy dispersive X-ray diffraction studyMater Res Bullet200843561571
- EngstrandJAbergJEngqvistHInfluence of water content on hardening and handling of a premixed calcium phosphate cementMater Sci Eng C Mater Biol Appl201333152753125428105
- AbergJEngstrandJEngqvistHInfluence of particle size on hardening and handling of a premixed calcium phosphate cementJ Mater Sci Mater Med201324482983523392965
- HabrakenWHabibovicPEppleMBohnerMCalcium phosphates in biomedical applications: materials for the future?Mater Today20161926987
- ApeltDTheissFEl-WarrakAOIn vivo behavior of three different injectable hydraulic calcium phosphate cementsBiomaterials2004257–81439145114643619
- ErskineRJWalkerRDBolinCABartlettPCWhiteDGTrends in antibacterial susceptibility of mastitis pathogens during a seven-year periodJ Dairy Sci20028551111111812086045