Abstract
Alzheimer’s disease (AD), an age-related neurodegenerative disease, the most common causes of dementia is a multifactorial pathology categorized by a complex etiology. Numerous nutraceuticals have been clinically evaluated, but some of the trials failed. However, natural compounds have some limitations due to their poor bioavailability, ineffective capability to cross the blood–brain barrier, or less therapeutic effects on AD. To overcome these disadvantages, nanoparticle-conjugated natural products could promote the bioavailability and enhance the therapeutic efficacy of AD when compared with a naked drug. This application generates and implements new prospect for drug discovery in neurodegenerative diseases. In this article, we confer AD pathology, review natural products in clinical trials, and ascertain the importance of nanomedicine coupled with natural compounds for AD.
Introduction
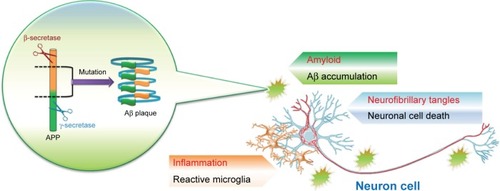
Alzheimer’s disease (AD), a progression and age-related neurodegeneration disease, is a multifactorial pathology categorized by a complex etiology. AD is increasingly documented as one of the most vital medical problems affecting the elderly and is the most common type of dementia. The symptoms of AD include progressive memory loss, cognitive impairment such as difficulty solving problems, and disorientation in time and space, among others in an aging population that causes a severe damage of cholinergic neurons in a particular area of the brain, that is, hippocampus. Remarkably, AD is a prominent cause of death in the USA since 2013; mortality of advanced stage AD increase by 11% per year. 2016 statistics of Medicaid for the AD predicted to be 19 times more for recipients >65 years, who do not have the following symptom.Citation1 Apart from the disease itself, it is the burden of care cost that seriously jeopardizes the health and financial security of the patient’s family. Of note, three hallmarks of AD pathology are beta-amyloid (Aβ) accumulations, tau phosphorylation, and inflammation that have been postulated.Citation2 The molecular pathology of AD illustrates the abnormal shearing of β- and γ-secretase resulting in Aβ accumulation (). 1) The accumulation of Aβ: Aβ accumulation is caused by inaccurate cleavage of the amyloid precursor protein (APP) with β- and γ-secretase;Citation2 and subsequently, the massive accumulation of Aβ mainly formed the plaques. Recently, the hallmarks of molecular features in senile plaque are the unfolding/misfolding of specific proteins/peptides. Aβ peptide consequently becomes susceptible to aggregate into toxic assemblies and deposits that are a crucial histopathological feature, neurofibrillary (tau) tangles, synapse loss, ROS production, and extensive oxidative stress.Citation3 This Aβ-induced oxidative stress is demonstrated via several clues such as protein oxidation, lipid peroxidation, free radical formation, DNA oxidation, and neuronal cell death.Citation4 Novel metabolic processing events such as δ-secretase and β-secretase have revealed that they generate previously uncharacterized APP metabolic fragments with the impending to be involved in AD pathogenesis.Citation5 The accumulation of amyloid plaque in the brain due to the accumulation caused by Aβ1-40 and Aβ1-42 peptides, Aβ 1-40 and Aβ 1-42 inhibit endocytosis, and Aβ 1-40 can inhibit lysosomes. The uptake led to increased accumulations of Aβ 1-42, Aβ 1-40, and Aβ 1-42, which also perturbs neuronal trafficking and vesicular dynamics.Citation6 2) Hyperphosphorylation of tau protein: tau protein is a microtubule-associated protein expressed in the axons and soma of nerve cells.Citation7 Phosphorylation of tau protein is due to phosphorylation of serine and threonine sites for the tau protein.Citation8 The tau protein hyperphosphorylation led to neurofibrillary tangles because cognitive impairment may be a more direct effect than Aβ accumulations.Citation9 And 3) microglia communication driving for AD-related functional impairments. In AD, microglia aging is owing to cytokines that complement extracellular vesicles. The consequence of these changes includes augmented inflammation, reduced phagocytosis, and declined motility.Citation10 Furthermore, aged microglia would augment the levels of IL-1β, tumor necrosis factor-α, and IL-6.Citation11 Microglia enhances inflammatory responses and inhibits phagocytosis that presumably might be increased by age and led to decreased synaptic plasticity.Citation12
Figure 1 Alzheimer’s disease pathology includes the abnormal shearing of β- and γ-secretase resulting in Aβ accumulation.
Notes: Phosphorylation of tau protein causes nerve entanglement. Inflammation and phagocytosis are induced by microglia.
Abbreviations: Aβ, beta amyloid; APP, amyloid precursor protein.
Clinically, the early stages of AD have been treated by using acetylcholinesterase inhibitors.Citation13 As we know, there are five Food and Drug Administration-approved treatment medicines for the management of AD, which all offer symptomatic benefits. Tacrine, donepezil, galantamine, and rivastigmine are acetylcholinesterase inhibitors and are N-methyl-d-aspartate receptor antagonists.Citation14 Unfortunately, none of these drugs could cure or delay the commencement of AD due to various causes of dementia and neuropathology in many patients.Citation15 This failure could be owing to the unclear underlying pathways of AD. For example, the accumulation of neurotoxic Aβ peptides in the brain exemplifies a pathogenic hallmark of AD, which is the most general form of dementia in an aging population.Citation16 It was found that the decreased clearance rather than the production of Aβ is the primary formation of the deleterious Aβ plaques in the brain.Citation17 The lessened removal of Aβ from the brain into the blood can be moderately attributed to the dysfunction of P-gp function, leading to the progression of AD.Citation18–Citation20 Furthermore, it has been shown that Aβ can downregulate the P-gp expression along with other transporters, which consequently results in further accelerated neurodegeneration.Citation21 Hence, it has been suggested that the increased Aβ clearance from the brain by restoring blood–brain barrier (BBB) P-gp function to diminish Aβ brain accumulation is a new strategy for medical treatment in the early stage of AD.Citation22,Citation23 Unexpectedly, as of 2017, Alzheimer’s treatment drugs, verubecestat and solanezumab, have been discontinued in phase III clinical trials of Aβ protein, but they have also led to the worldwide pathogenesis of AD, and new candidates require more attention to accelerate drug discovery for clinical use.Citation24–Citation26 Therefore, the devotion of discovering agents against amyloidosis has turned to the search from natural compounds for meeting this demand.
Natural compounds act as nutraceuticals
The alternative approach instead of standard medical treatments in clinic for treating the patients is broadly recognized by using chemical derivatives,Citation27–Citation30 natural sources including herb, and traditional medicine even natural compounds. In this article, we mainly focus on the benefits of natural compounds as an alternative use for neurodegenerative disease to confer this theme. Natural compounds are found abundantly in nature, particularly in daily foods, such as edible vegetable and fruit juices, green tea, wine, turmeric, and even cigarettes.Citation31 All of which contain antioxidative substances, especially polyphenols that act as both ROS scavengers and transition metal chelators.Citation32 Their antioxidant effects are naturally linked to anti-AD potential. In addition, their evolutional structure has made them become beneficial enzyme activators, channel openers, and receptor agonists.Citation33 Owing to the antioxidant activity in higher plants, consideration has improved about the defending activity of its natural antioxidants against chronic disorders caused by the oxidative process.Citation34 Antioxidants and nutrition have long been deliberated as an approach to alleviate AD progression. Numerous investigations have exposed that folic acid, vitamin B12, choline, vitamin C, vitamin K3, vitamin D3, vitamin E, zinc, selenium, s-ethyl cysteine, s-propyl cysteine, citicoline, rivastigmine, memantine, tea polyphenol, curcumin, caffeine, α-lipoic acid, N-acetylcysteine, and dietary polyphenols are able to interact with gene expressions and epigenetic mechanisms.Citation35–Citation39 A growing evidence also suggests that epigenetic alterations are elicited by dietary nutrients that possess an imperative role in health and the preventative occurrence of some diseases, particularly neurodegenerative disorders. Assured natural dietary polyphenolic phytochemicals have paid extensively recent attention as alternative candidates for AD therapy. In particular, curcumin, resveratrol, piperine and spices, extra virgin olive oil, red wine, red berries, and green tea catechins contained antiamyloidogenic, antioxidative, and anti-inflammatory properties that have been postulated to have the preventative potential for AD.Citation40,Citation41 Therefore, the attention of discovering agents against amyloidosis has turned to the search of natural compounds. Prominently, natural compounds have often been demonstrated to have better pharmacological properties than synthetic small molecules, especially with regard to less toxicity and good absorption.Citation33 Emergent studies revealed that some natural compounds isolated from Chinese herbs could be administered for disease or cancer therapy.Citation42,Citation43 Based on these successes, we have listed the natural substances of the most studied on neuroprotective and reviewed the following natural compounds: 1) scyllo-inositol, 2) curcumin, 3) Ginkgo biloba extract, 4) resveratrol, and 5) epigallocatechin gal-late along with their current clinical trials on AD treatment (). And the chemical structures of four discussed compounds are also exhibited underneath.
Table 1 Summary of natural products in clinical trial
Scyllo-inositol
Scyllo-inositol, an inositol stereoisomer, is abundant in the coconut palm. Numerous studies have shown that scyllo-inositol can bind and inhibit Aβ aggregation and the formation of Aβ fibrils in vitro.Citation44 In the TgCRND8 mice model, the amyloid pathology has delayed in a dose-dependent fashion.Citation45 The phenomenon of neuronal autophagy in TgCRND8 model has also been explored.Citation46 The clinical trial assessing the safety and efficacy of multiple oral dosages of scyllo-inositol has applied to the treatment of AD, which started in 2007. However, serious adverse events occurred, including the death of nine patients, and have forced the company to stop the highest two doses and retain 250 mg scyllo-inositol twice a day until 2017. The differences between the 250 mg scyllo-inositol and placebo groups were not significant for the coprimary or secondary endpoints.
Curcumin
Curcumin (diferuloylmethane), one component of turmeric, is isolated from the rhizome of Curcuma longa and abounds in ginger family (Zingiberaceae). Numerous studies have focused on the various facets of curcumin due to its antioxidant and anti-inflammatory properties; curcumin also plays a significant advantageous and pleiotropic regulatory role in various pathological conditions which including hyperglycemia, oxidative stress, and cancer,Citation47 cardiovascular disease, AD, anti-inflammation,Citation48 neurological disorders,Citation49 and various malignant diseases.Citation50 Apart from these well-known suppressing activities, this natural polyphenolic compound also exerts its profitable effects by mediating different signaling molecules such as transcription factors, chemokines, cytokines, tumor suppressor genes, adhesion molecules, and microRNAs.Citation51 Notably, oxidative stress (free radicals) and inflammation are responsible for many human health problems including aging, arthritis, cancer, cardiovascular disease, diabetes, neurological disorders, AD, Parkinson’s disease, mild cognitive impairment, alcohol-induced liver disease, ulcerative colitis, and atherosclerosis.Citation52 The neuroprotective effects of curcumin and curcuma oil exert its significant action in the decline of NO-induced peroxynitrite formations and cell apoptosis in the transient middle cerebral artery occlusion (MCAo) model and focal embolic stroke model rat.Citation53,Citation54 Furthermore, curcuminoids have been used to perform as latent therapeutic implications for various neurodegenerative diseases.Citation55 The mechanism of curcumin for protecting the rat hippocampus combating with the neurotoxicity of homocysteine oxidative stress could be possible by increasing the endogenous defenses against oxi-dative stress via inhibiting the ROS generation of brain.Citation56 In cellular studies, curcumin inhibits cyclooxygenase-2, tumor necrosis factor-α, and IL-1 expression.Citation57 It downregulates IL-6 signaling via suppressing phosphorylation of STAT3Citation58 and inhibits Aβ-induced microglial inflammation through modulating ERK1/2 and p38 signaling pathways.Citation59 Cur-cumin can serve as a neuroprotective reagent through various signal pathways: acting against endothelin-1-mediated cell death (decreasing proapoptotic signaling) via blocking an increase in c-Jun levels in primary hippocampal neuronsCitation60 and inhibiting Aβ generation through induction of autophagy by downregulating PI3K/Akt/mTOR signaling pathway.Citation61 Furthermore, chronic application of curcumin might ameliorate AD-related cognitive deficits and upregulate brain-derived neurotrophic factor-ERK signaling in the hippocampus.Citation62 There was a marked reduction in the production of Aβ when human neuroblastoma cells were treated with curcumin.Citation63 The aggregation of Aβ in a rat model of AD was also reduced after oral curcumin administration, as well as with the improvement of cognitive impairment in a spatial learning and memory test, suggesting that curcumin could be a candidate for treating AD. Currently, there are three clinical trials investigating the effect of anti-Aβ formation, but all of them failed to exhibit clinical or biochemical evidence of efficacy for AD. Even the underlying mechanisms of curcumin will be hampering the formation and promoting the disaggregation of Aβ plaques, mitigating the hyperphosphorylation of tau and augmenting its clearance, binding copper, lowering cholesterol, amending microglial activity, inhibiting acetylcholinesterase, regulating the insulin signaling pathway, and is an antioxidant as well.Citation64
EGb-761
EGb-761 is made of ginkgo leaves, one dry extract that is adjusted to contain ginkgo flavonoids and terpene lactones.Citation65 In the cellular study, EGb-761 decreases free cholesterol levels and neuronal Aβ production, and the level of Alzheimer’s amyloid precursor in the brain is also decreased.Citation66 It has been found in rescuing impaired mitochondrial function and improving neuronal energy supply.Citation67 The protective and rescuing abilities of EGb-761 are attributable to the antioxidant properties and the ability to inhibit NO-stimulated protein kinase C activity.Citation68 The increased production of toxic mediators such as hydrogen peroxide and platelet-activating factor in the brain may be critical in the pathological mechanism of neurodegenerative diseases particularly AD and is blocked by EGb-761.Citation69 In the current study, the dopamine level increased in the rat prefrontal cortex after EGb-761 administration, which implied a benefit for memory function.Citation70 Although the active ingredients of EGb-761 have not been explored, it still has the potential to treat or prevent AD. Nevertheless, the clinical trial for evaluating G. biloba’s ability to abrogate memory loss or delay dementia still leads to negative outcomes.
Resveratrol
Resveratrol (Res), a phytochemical, has been found in many plant species such as herbs, berries, grapes, and peanuts. Res exhibits diverse biochemical properties, such as anti-plateletCitation71 and anti-inflammatory properties.Citation72 It has been shown to reduce oxidative stress and stabilize mitochondria through regulating Sirt1 pathway. Res is reported as a potential nutraceutical agent for AD; evidence indicates that Res alleviated Tau hyperphosphorylation at Ser396 site and oxidative damage in rat hippocampal slices exposed to vanadate via ERK1/2 and glycogen synthase kinase-3β signaling cascades.Citation73
Res retreating Aβ-induced learning and memory disorder may involve the regulation of neuronal inflammation and apoptosis via phosphodiesterase-4-related Cyclic adenosine monophosphate (cAMP)-CREB-brain-derived neurotrophic factor signalingCitation74 and inhibit Aβ-induced neuronal apoptosis through reversion of silent information regulator 1 activity and subsequently the downregulation of Rho-associated kinase 1 signaling pathway.Citation75 Recently, one study showed that resveratrol changed three Aβ conformers into nontoxic alternations, suggesting that Res could mediate Aβ toxicity.Citation76 It also lowered Aβ levels in Tg2576 mice by stimulating nonamyloidogenic processing of APP.Citation77 According to its amyloidogenic-delaying and antioxidant effects, Res could be helpful in fighting AD. In recent years, four clinical trials assessing the effects on AD were established. However, none of them succeeded in different phases due to insufficient evidence and nonsignificant outcomes. Among these natural compounds with high neuroprotective effects that have broadly studied, have extensively used as an antioxidant for free radical scavenger. Nevertheless, the antioxidant and anti-Aβ formation activity of these natural compounds in vitro/in vivo failed to be well translated into therapeutic effects for patients with AD in clinical trial.Citation78–Citation82 In fact, the therapeutic success of many pharmaceutical remain moderate because of their low penetration across BBB, which limits their targeting. Therefore, carrying sufficient drugs through BBB becomes a long-term issue. Despite such phenomenal advances in medicinal applications, the clinical implication of native curcumin is hindered due to poor aqueous solubility, physicochemical instability, low compatibility, rapid metabolism, and poor pharmacokinetics.Citation83 Therefore, low compatibility and low bioavailability may hinder its usefulness as a therapeutic agent. Once the defy of low bioavailability is overcome, curcumin-based medications for AD might be in the prospect.
In view of nanoparticles posture that is extensively researched in many fields, that provides this new approach for facilitating the efficiency of disease therapy particular in neurodegenerative diseases including AD. According to the abovementioned five natural compounds for AD treatment in clinical trial, however, no extensive studies on the potential pharmaceutical applications of combinations of Epigallocatechin gallate, EGb-761, and Scyllo-inositol with nanoparticles and their synergistic effects have been performed. Furthermore, conventional strategies failed to treat AD in clinical trials, partly due to the poor solubility, low bioavailability, and ineffectiveness of the tested natural compounds to cross BBB. We will plausibly deliberate the beneficial effect or potency of nanocarriers conjugated with two natural compounds (resveratrol and curcumin) and feasibly postulate a potential natural candidate quercetin to complement a new expectation for AD treatment.
Nanoparticles conjugated natural products for AD
The nanocarrier formulations, which are featured in its particle size, adjustable component, and surface charges, have demonstrated to encapsulate commercial drugs or molecules.Citation84,Citation85 The technique may provide an alternative way to augment drug transport through the BBB in neurodegenerative disorders.Citation86 In addition, by conjugating specific antibodies, nanoparticle may target specific regions.Citation87 Therefore, to address natural compounds for the brain via nanocarriers becomes a popular topic. Next, we describe nanoparticle as a successful delivery carrier in the development of AD therapeutics and diagnostics.
Nanoparticle-conjugated curcumin
Gold
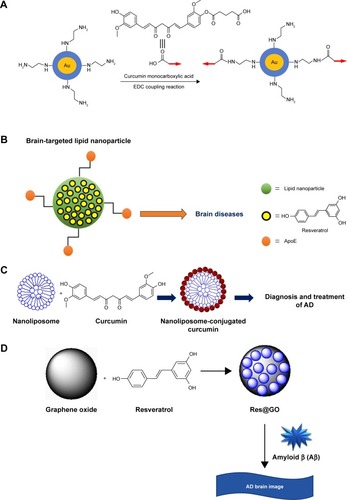
Gold nanoparticles (AuNPs) are one of the major components of bionanotechnology applications.Citation88 Special properties of AuNPs are low toxicity, highly biocompatible,Citation89 well functionalization, and plasmon-based strong optical characters that are used for detection/imaging.Citation90–Citation92 In addition, curcumin is hydrophobic in nature that shows less solubility limited to therapeutic applications;Citation93 however, some results show that curcumin is conjugated with hyaluronic acid and polymers on the surface of AuNPs to improve bioavailability.Citation94,Citation95 In one previous study, curcumin was modified by monocarboxylic acid conjugated with primary amine-terminated silica-coated AuNPs that was applied to evaluate water solubility of conjugated complex (). In nanoparticle conjugated curcumin becomes more water-soluble and can efficiently interact with amyloid protein/peptide, offering enhanced performance in inhibiting amyloid fibrillation and dissolving amyloid fibrils. Curcumin monocarboxylic acid derivative was measured using hen egg white lysozymes compared with naked curcumin and Au-curcumin modification in which Au-curcumin derivatives show high inhibits of Aβ fibrillation and dissolve/disintegrate Aβ fibrils.Citation96 After this conjugation, the data confirmed to improve the water solubility of curcumin for a promising therapeutic approach. However, unmodified curcumin has poor sensitivity, specifically it is difficult to penetrate the blood–brain barrier, researchers choosing alternatively, the polyhydroxyl substituted squaraine dyes 1–3 under investigation act as effective protein-labeling and destabilizing agents of the protein amyloidogenesis as well.Citation97 Undeniably, AuNPs were more expensive than silver (Ag). Recently, fluorescence quenching by lipid encased Ag nanoparticle is performed to discover that membrane-inserted Aβ oligomers have a preferred molecular orientation.Citation98 These new approaches might be useful for accelerating the investigation of molecular mechanism in AD treatment to discover new drug for therapy.
Figure 2 The applications of natural compounds conjugated with nanoparticles.
Notes: (A) Synthesis of curcumin-functionalized gold nanoparticles by using EDC coupling. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. reproduced from Palmal S, Maity AR, Singh BK, Basu S, Jana NR. Inhibition of amyloid fibril growth and dissolution of amyloid fibrils by curcumin-gold nanoparticles. Chemistry. 2014;20(20):6184–6191.Citation96 (B) Resveratrol-conjugated solid lipid nanoparticles. Copyright ©2016 Neves et al. Reproduced from Neves AR, Queiroz JF, Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J Nanobiotechnology. 2016;14(1):27.Citation131 (C) Nanoliposomes conjugated with a curcumin derivative. (D) Simple construction of Res@GO for the rapid fluorogenic probing of Aβ. Adapted with permission from He XP, Deng Q, Cai L, et al. Fluorogenic resveratrol-confined graphene oxide for economic and rapid detection of Alzheimer’s disease. ACS Appl Mater Interfaces. 2014;6(8):5379–5382. Copyright 2014 American Chemical Society.Citation132
Abbreviations: Aβ, beta amyloid; AD, Alzheimer’s disease; APP, amyloid precursor protein; APoE, apolipoprotein E; EDC, N-(3-dimethyl amino propyl)-N-ethyl carbodiimide; GO, graphene oxide.
Nanogels
Hydrogels are hydrophilic three-dimensional polymersCitation99 that are manipulated into nano dimensions called nanogels.Citation100 The diameter of nanogels varies from 10 to 300 nm and depends on component ratio.Citation101,Citation102 This nanogel has special features such as high loading capacity and stability and variable environment factors (ionic strength, pH, temperature) that provide a novel platform for drug delivery. Additionally, it has a microheterogeneous structure, small size, and high surface to volume ratio.Citation103,Citation104 Furthermore, nanogels create hydrophobic interactions that can increase oral and brain delivery of low-molecular-weight drugs and biomacromolecules.Citation104 Nanogel-conjugated poor water-soluble drugs such as curcumin could improve their bioavailability.Citation105,Citation106 Another drawback of curcumin was unstable and it is easy to be degraded in alkaline aqueous solutions.Citation107,Citation108 To overcome this limitation, multifunctional nanogels conjugated with curcumin giving the improvement of stability and the protection of curcumin degradation as well, making curcumin more potentially bioavailable for therapeutic applications in AD that could more efficiently inhibit Aβ fibrillogenesis and amyloid cytotoxicity than those of nonconjugation curcumin.Citation106
Polymers
Polymer nanoparticles are characterized as solid and colloidal whose particles size ranges from 10 to 1,000 nm.Citation109,Citation110 They show excellent properties such as biocompatibility and biodegradability for therapeutic applications.Citation111,Citation112 Furthermore, hydrophobic drugs such as curcumin conjugated or encapsulated with polymers could enhance the curcumin bioavailability.Citation113–Citation115 In addition, curcumin encapsulation with poly(butyl)cyanoacrylate nanoparticles was conjugated on outer surface with apolipoprotein E3 to promote therapeutic efficacy against Aβ-induced cytotoxicity in SH-SY5Y neuroblastoma cells; interestingly, poly(butyl)cyanoacrylate-loaded curcumin shows high therapeutic efficacy compared with nonencapsulation curcumin.Citation116 Water-soluble poly(lactide-co-glycolide) (PLGA) nanoparticle loaded curcumin covalently conjugated with the Tet-1 peptide is able to destroy amyloid aggregation and enhance antioxidative and noncytotoxic properties of this complex are examined by in vitro studies.Citation117 Another interesting fact is that PLGA nanoparticles encapsulated with curcumin in the ratio of 50:50 (w/w) are able to suppress or cease phosphorylation of protein kinase B (Akt) and tau proteins in human neuroblastoma SK-N-SH cells. The formulations have immense applications in pharmacology and have also been applied to treat neurodegenerative diseases such as AD.Citation118 The encapsulation of curcumin with PLGA nanoparticles in a diameter that varies from 80 to 120 nm is nontoxic to the SK-N-SH cells and protects neurons from oxidative damage in AD.Citation119 In recent studies, the functionalization of PLGA nanoparticles with glutathione (GSH) was loaded with curcumin using click reaction innovative strategy for neuronal cell delivery.Citation120
Nanoliposomes
Liposome is originally derived from two Greek words: “Lipos” meaning fat and “Soma” meaning body. Liposomes were first described in 1960s by Bangham et alCitation121 and implicated as a potential drug delivery system in early 1970s.Citation122,Citation123 Liposomes are made up of phospholipids and are self-enclosed to form spheres of lipid bilayers and with an aqueous core in its bilayers. Owing to the side of the hydrophobic and hydrophilic character (in addition to biocompatibility), liposomes are accomplished systems for drug delivery.Citation124
Curcumin shows high affinity for Aβ peptide with the fluorescence character, however, extremely low aqueous solubility limits its clinical use. Thereby, curcumin-conjugated nanoliposomes were designed for monitoring the amyloid peptide deposits as they were more stable and appeared as monodisperse. They had nontoxic in vitro properties, down-regulated the secretion of Aβ peptide, and partially prevented Aβ-induced toxicity. Additionally, this conjugation firmly marked Aβ deposits in postmortem brain tissues of AD patients and APP/PS1 mice. Moreover, curcumin-conjugated nanoliposomes were injected into the hippocampus and the neocortex of APP/PS1 mice; the data showed that curcumin nanoliposomes were specifically stained in the Aβ deposits in vivo. Therefore, curcumin-conjugated nanoliposomes could discover the application of diagnosis and targeted drug delivery in AD patients ().Citation125
Nanoliposomes conjugated with a curcumin derivative formed a planar structure for interaction with Aβ that could be detected by using surface plasmon resonance experiments. The second type of liposomes was conjugated with phospholipid in which the planar structure of curcumin does not show the planner structure. Both types of curcumin-decorated vesicles with diameters between (131–207 nm) and marginally negative ζ-potential values based on their lipid composition. Also, they were highly stable lasting up to 20 days. They likewise showed high integrity during incubation in the presence of plasma protein delivery in AD. In surface plasmon resonance experiments, the measurements of the binding of flowing liposomes to immobilized Aβ 1-42 revealed that the liposomes exposing to the curcumin derivative (maintaining the planarity) have an extremely high affinity for Aβ 1-42 fibrils (1–5 nM), owing to the occurrences of multivalent interactions; whereas nonplanar curcumin did not bind to Aβ 1-42. However, curcumin derivative demonstrated that planar with a high affinity for Aβ 1-42 fibrils is taken into considerations as vectors in the targeted delivery of new diagnostic and therapeutic molecules for AD.Citation126
Nanoparticle-conjugated resveratrol
Resveratrol (Res), a polyphenol compound, has shown great significance in therapeutic effects, including anticancer, antioxidation, and anti-inflammation. However, there are some limitations in pharmacokinetic characters such as low aqueous solubility and poor bioavailability. Recently, nanoformulations are viewed as a novel technique for enhancing the pharmacokinetic features as well as enhancing target ability and bioavailability of Res.Citation127 Also, Res is able to inhibit the formation and aggregation of Aβ peptides, which are liable for neuronal dysfunction and death associated with AD due to its ROS-generating action.Citation128 When rats received a single intracerebroventricular injection of Aβ 1-42 (2 nmol), and 1 day after Aβ infusion, they were intraperitoneally administered either free Res or (Res)-loaded lipid-core nanocapsules (5 mg/kg, each 12 hours) for 14 days. Aβ 1-42-infused animals were proved to be a significant impairment of learning memory capacity, which was associated with a significant decrease in hippocampal synaptophysin levels. Furthermore, astrocytes and microglial cells of animals are activated as well as the disturbance in c-Jun N-terminal kinase and glycogen synthase kinase-3β (GSK-3β) activated, beyond destabilization of β-catenin levels. These results remarkably reveal that by utilizing lipid-core nanocapsules, Res could protect the deleterious effects of Aβ 1-42 while treatments with Res alone present only partial beneficial effects. The increase of Res concentrations might explain these findings in the brain tissues achieved by lipid-core nanocapsules.Citation129 It is noteworthy that AD treated with grape skin and grape seed extracts increases the inhibitory effect on Aβ aggregation. However, after intravenous injection, Res is rapidly metabolized into both glucuronic acid and sulfate conjugated with the phenolic groups in the liver and intestinal epithelial cells (within <2 hours), which are then eliminated. A recent report has demonstrated that solid lipid nanoparticles (SLNs) functionalized with the antitransferrin receptor monoclonal antibody (OX26 mAb) can work as a possible carrier for transporting this extract to target the brain. In human brain-like endothelial cells, experiments illustrate that the cellular uptake of the OX26 SLNs is considerably more efficient than that of normal SLNs and SLNs functionalized with an nonspecific antibody. Consequently, the transcytosis ability of these different SLNs is higher when functionalized with OX26.Citation130 On the contrary, Res encapsulation SLNs were functionalized with apolipoprotein E, which can be perceived by the overexpressed BBB of low-density lipoprotein receptors (). In vitro cytotoxicity of hCMEC/D3 cell line assessed by MTT and LDH assays, the results revealed no toxicity up to 50 µM over 4 hours of incubation. The permeability through hCMEC/D3 monolayers showed a significant increase (1.8-fold higher) in Res encapsulation of SLNs functionalized with apolipoprotein E compared with nonfunctionalized ones.Citation131
Additionally, Res is performed to detect AD for increasing an advantageous potential of the real-time probing concerning Aβ that is closely implicated in AD or ought to assist higher understanding or monitoring the disease. Res combined with graphene oxide (GO) for the rapid, fluorogenic recognition of Aβ. This Res@GO composite could capture both Aβ monomers and fibers in a physiological buffer solution within 3 minutes that have been proved in this study. And Res@GO composite can be used to detect the fluorescent image of Aβ deposits in a mouse brain within 30 mins. This instant protocol is much cheaper and faster than conventional immunofluores-cence staining technique clinically employed and provides an economic approach for detection of AD ().Citation132
The potential candidates for AD treatment
Nanoparticle-conjugated quercetin
Quercetin, a flavonoid existing in various foodstuffs, has antioxidative properties and increases GSH levels and antioxidant enzyme function. Extensive consideration has focused on increasing the intracellular GSH levels in many diseases, including AD. Aβ 1-42 peptide when elevated in the brain of AD is associated with oxidative stress and neurotoxicity.Citation133 In this study, nanoparticle-conjugated quercetin plays an important role in AD as the results demonstrated that neuronal cell death is attributable to metal-induced oxidative stress. Prominent among redox active metals initiating oxidative stress is Cu(II). Bioactive hybrid nanoparticles are developed for overcoming oxidative stress, and they are capable of working as host carriers for potent antioxidants such as the quercetin is detected in the release profiles of the loaded nanoparticle under oxidative stress in neuronal cultures. The bioactivity profile of quercetin nanoparticles in a neurodegenerative environment brought on by Cu(II) denotes the improved specificity of antioxidant reactivity counteracting oxidative stress and sets the stage for the development of molecular protection and preventive medical nanotechnology of relevance to neurodegenerative AD.Citation134
PLGA nanoparticles conjugated quercetin
In vitro cytotoxicity studies of PLGA-conjugated quercetin (PLGA@QT)NPs inhibited and disassembled Aβ42 fibrils neu-roblastoma in SH-SY5Y cells; PLGA@QTNPs led to a concentration behavior with low cytotoxicity, illustrating that PLGA@ QTNPs can inhibit the neurotoxicity of Zn2+-Aβ42 system and improve the viability of neuron cells. Additionally, PLGA@ QTNPs are injected into APP/PS1 mice that led to partially abrogate memory impairments and also ameliorate cognition. Most interestingly, in vivo systemic toxicity of PLGA@QTNPs did not show any deterioration according to the histological analysis of significant organs in mice. Thereby, quercetin-based nanocarrier can augment therapeutic effects and subsequently reduce side effects, suggesting that the PLGA@QTNPs may be a potential candidate for AD treatment.Citation135
Conclusion
Searching natural sources to be exploited as a complementary and alternative medicine such as nutraceuticals to meet urgent demand for the treatment of neurodegenerative disease has become a critical issue. Evidence base shows some natural compounds that contained the potential for AD treatment. However, the characters of natural compounds hindered the divergence including low water solubility, physicochemical instability, low aqueous stability, low bioavailability, low biocompatibility, rapid metabolism, and poor pharmacokinetics. This discrepancy leads to low efficacy of compounds in medical use. Nanoparticles possessing the unique properties become a new approach to improve this weakness. The nanocarrier formulations are featured in its particle size, adjustable component, and surface charges, which have been demonstrated to encapsulate commercial drugs or molecules. Hereby, nanoparticles conjugated with natural products could enhance the bioavailability and promote the efficacy of AD therapy when compared with naked drugs. Achievably, new delivery strategy creates and implements a new hope for drug discovery and is more effective for the application in the treatment of neurodegenerative diseases including AD. We warrant further studies in human subjects using nanocarriers conjugated with these potential natural compounds.
Author contributions
KRK, S-HY, and L-WW contributed to this review article writing. Both C-HL and C-FW executed as a supervisor to discuss and modify this manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrookmeyerREvansDAHebertLNational estimates of the prevalence of Alzheimer’s disease in the United StatesAlzheimers Dement201171617321255744
- IizukaTShojiMKawarabayashiTIntracellular generation of amyloid beta-protein from amyloid beta-protein precursor fragment by direct cleavage with beta- and gamma-secretaseBiochem Biophys Res Commun199621812382428573139
- NesiGSestitoSDigiacomoMRapposelliSOxidative stress, mitochondrial abnormalities and proteins deposition: multitarget approaches in Alzheimer’s diseaseCurr Top Med Chem201717273062307928595557
- PocernichCBLangeMLSultanaRButterfieldDANutritional approaches to modulate oxidative stress in Alzheimer’s diseaseCurr Alzheimer Res20118545246921605052
- NorstromEMetabolic processing of the amyloid precursor protein – new pieces of the Alzheimer’s puzzleDiscov Med20172312726927628595039
- OmtriRSThompsonKJTangXDifferential effects of Alzheimer’s disease Aβ40 and 42 on Endocytosis and intraneuronal traffickingNeuroscience201837315916829337241
- YoshiyamaYLeeVMTrojanowskiJQTherapeutic strategies for tau mediated neurodegenerationJ Neurol Neurosurg Psychiatry201384778479523085937
- AlonsoADDi ClericoJLiBPhosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegenerationJ Biol Chem201028540308513086020663882
- NelsonPTAlafuzoffIBigioEHCorrelation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literatureJ Neuropathol Exp Neurol201271536238122487856
- UdeochuJCSheaJMVilledaSAMicroglia communication: parallels between aging and Alzheimer’s diseaseClin Exp Neuroimmunol20167211412527840659
- YeSMJohnsonRWIncreased interleukin-6 expression by microglia from brain of aged miceJ Neuroimmunol1999931–213914810378877
- RitzelRMPatelARPanSAge- and location-related changes in microglial functionNeurobiol Aging20153662153216325816747
- LinHQHoMTLauLSWongKKShawPCWanDCAnti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer’s diseaseChem Biol Interact20081751–335235418573242
- HaasCStrategies, development, and pitfalls of therapeutic options for Alzheimer’s diseaseJ Alzheimers Dis201228224128121987594
- BeckerREGreigNHAlzheimer’s disease drug development: old problems require new prioritiesCNS Neurol Disord Drug Targets20087649951119128207
- GosseletFSaint-PolJCandelaPFenartLAmyloid-β peptides, Alzheimer’s disease and the blood-brain barrierCurr Alzheimer Res201310101015103324156262
- MawuenyegaKGSigurdsonWOvodVDecreased clearance of CNS beta-amyloid in Alzheimer’s diseaseScience20103306012177421148344
- van AssemaDMLubberinkMBauerMBlood-brain barrier P-glycoprotein function in Alzheimer’s diseaseBrain2012135Pt 118118922120145
- JedlitschkyGVogelgesangSKroemerHKMDR1-P-glycoprotein (ABCB1)-mediated disposition of amyloid-β peptides: implications for the pathogenesis and therapy of Alzheimer’s diseaseClin Pharmacol Ther201088444144320856238
- CascorbiIFlühCRemmlerCAssociation of ATP-binding cassette transporter variants with the risk of Alzheimer’s diseasePharmacogenomics201314548549423556446
- BrennAGrubeMPetersMBeta-amyloid downregulates MDR1-P-Glycoprotein (Abcb1) expression at the blood-brain barrier in miceInt J Alzheimers Dis2011201169012169012621660212
- NeuweltEABauerBFahlkeCEngaging neuroscience to advance translational research in brain barrier biologyNat Rev Neurosci201112316918221331083
- WolfABauerBHartzAMABC Transporters and the Alzheimer’s disease enigmaFront Psychiatry20123545422675311
- De StrooperBKarranEThe cellular phase of Alzheimer’s diseaseCell2016164460361526871627
- PratiFBottegoniGBolognesiMLCavalliABACE-1 inhibitors: from recent single-target molecules to multitarget compounds for Alzheimer’s diseaseJ Med Chem201861361963728749667
- GoldMPhase II clinical trials of anti-amyloid β antibodies: when is enough, enough?Alzheimers Dement201733402409
- LengJQinHLZhuKEvaluation of multifunctional synthetic tetralone derivatives for treatment of Alzheimer’s diseaseChem Biol Drug Des201688688989827434226
- XieSChenJLiXSynthesis and evaluation of selegiline derivatives as monoamine oxidase inhibitor, antioxidant and metal chelator against Alzheimer’s diseaseBioorg Med Chem201523133722372925934229
- XiaNLiuLNingXLinLMetallothioneins and synthetic metal chela-tors as potential therapeutic agents for removal of aberrant metal ions from metal-Aβ speciesMini Rev Med Chem201414327128124456271
- BahramikiaSYazdanparastRInhibition of human islet amyloid polypeptide or amylin aggregation by two manganese-salen derivativesEur J Pharmacol20137071–3172523528352
- DeyABhattacharyaRMukherjeeAPandeyDKNatural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventionsBiotechnol Adv201735217821628043897
- Sandoval-AcuñaCFerreiraJSpeiskyHPolyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actionsArch Biochem Biophys2014559759024875147
- BeghynTDeprez-PoulainRWillandNFolleasBDeprezBNatural compounds: leads or ideas? Bioinspired molecules for drug discoveryChem Biol Drug Des200872131518554253
- GarciaEJOldoniTLAlencarSMReisALoguercioADGrandeRHAntioxidant activity by DPPH assay of potential solutions to be applied on bleached teethBraz Dent J2012231222722460310
- FarinaNLlewellynDIsaacMTabetNVitamin E for Alzheimer’s dementia and mild cognitive impairmentCochrane Database Syst Rev201744CD00285428418065
- SezginZDincerYAlzheimer’s disease and epigenetic dietNeurochem Int20147810511625290336
- ZhangLNSunYJPanSNa+-K+-ATPase, a potent neuroprotective modulator against Alzheimer diseaseFundam Clin Pharmacol20132719610323033963
- AlamPChaturvediSKSiddiqiMKVitamin k3 inhibits protein aggregation: implication in the treatment of amyloid diseasesSci Rep201662675927230476
- MizwickiMTMenegazDZhangJGenomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophagesJ Alzheimers Dis2012291516222207005
- RigacciSStefaniMNutraceuticals and amyloid neurodegenerative diseases: a focus on natural phenolsExpert Rev Neurother2015151415225418871
- SawikrYYarlaNSPelusoIKamalMAAlievGBishayeeAChapter two – neuroinflammation in Alzheimer’s disease: the preventive and therapeutic potential of polyphenolic nutraceuticalsDonevRAdvances in Protein Chemistry and Structural BiologyMassachusettsAcademic Press20173357
- LodiASahaALuXCombinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolismNPJ Precis Oncol201711pii18
- ZasadilLMAndersenKAYeumDCytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindlesSci Transl Med20146229229ra43
- MclaurinJGolombRJurewiczAAntelJPFraserPEInositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta-induced toxicityJ Biol Chem200027524184951850210764800
- MclaurinJKiersteadMEBrownMECyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse modelNat Med200612780180816767098
- LaiAYMcLaurinJInhibition of amyloid-beta peptide aggregation rescues the autophagic deficits in the TgCRND8 mouse model of Alzheimer diseaseBiochim Biophys Acta20121822101629163722800931
- ShanmugamMKRaneGKanchiMMThe multifaceted role of curcumin in cancer prevention and treatmentMolecules20152022728276925665066
- CianciulliACalvelloRPorroCTrottaTSalvatoreRPanaroMAPI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microgliaInt Immunopharmacol20163628229027208432
- EsmailyHSahebkarAIranshahiMAn investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trialChin J Integr Med201521533233825776839
- MattsonMPSonTGCamandolaSViewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicalsDose Response20075317418618648607
- GhoshSBanerjeeSSilPCThe beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent updateFood Chem Toxicol20158311112426066364
- AlamMNBristiNJRafiquzzamanMReview on in vivo and in vitro methods evaluation of antioxidant activitySaudi Pharm J201321214315224936134
- DoharePVarmaSRayMCurcuma oil modulates the nitric oxide system response to cerebral ischemia/reperfusion injuryNitric Oxide200819111118485279
- DoharePGargPJainVNathCRayMDose dependence and therapeutic window for the neuroprotective effects of curcumin in throm-boembolic model of ratBehav Brain Res2008193228929718611416
- ZhangLJWuCFMengXLComparison of inhibitory potency of three different curcuminoid pigments on nitric oxide and tumor necrosis factor production of rat primary microglia induced by lipopolysaccharideNeurosci Lett20084471485318838107
- AtaieASabetkasaeiMHaghparastAMoghaddamAHKazeminejadBNeuroprotective effects of the polyphenolic antioxidant agent, curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the ratPharmacol Biochem Behav201096437838520619287
- AbeYHashimotoSHorieTCurcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophagesPharmacol Res1999391414710051376
- BhartiACDonatoNAggarwalBBCurcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cellsJ Immunol200317173863387114500688
- ShiXZhengZLiJCurcumin inhibits Aβ-induced microglial inflammatory responses in vitro: involvement of ERK1/2 and p38 signaling pathwaysNeurosci Lett201559410511025818332
- StankowskaDLKrishnamoorthyVREllisDZKrishnamoorthyRRNeuroprotective effects of curcumin on endothelin-1 mediated cell death in hippocampal neuronsNutr Neurosci201720527328326651837
- WangCZhangXTengZZhangTLiYDownregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic miceEur J Pharmacol201474031232025041840
- ZhangLFangYXuYCurcumin improves amyloid β-peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathwayPLoS One2015106e013152526114940
- XiongZHongmeiZLuSYuLCurcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s diseasePharmacol Rep20116351101110822180352
- TangMTaghibiglouCThe mechanisms of action of curcumin in Alzheimer’s diseaseJ Alzheimers Dis20175841003101628527218
- UdeCSchubert-ZsilaveczMWurglicsMGinkgo biloba extracts: a review of the pharmacokinetics of the active ingredientsClin Pharmacokinet201352972774923703577
- AugustinSRimbachGAugustinKSchliebsRWolfframSCermakREffect of a short- and long-term treatment with Ginkgo biloba extract on amyloid precursor protein levels in a transgenic mouse model relevant to Alzheimer’s diseaseArch Biochem Biophys2009481217718218996078
- Abdel-KaderRHauptmannSKeilUStabilization of mitochondrial function by Ginkgo biloba extract (EGb 761)Pharmacol Res200756649350217977008
- BastianettoSZhengWHQuirionRThe Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicityJ Neurochem20007462268227710820186
- ShiCWuFXuJH2O2 and PAF mediate Abeta1-42-induced Ca2+ dyshomeostasis that is blocked by EGb761Neurochem Int201056889390520362023
- YoshitakeTYoshitakeSKehrJThe Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortexBr J Pharmacol2010159365966820105177
- JangJYMinJHWangSBResveratrol inhibits collagen-induced platelet stimulation through suppressing NADPH oxidase and oxidative inactivation of SH2 domain-containing protein tyrosine phosphatase-2Free Radic Biol Med20158984285126482867
- CianciulliADragoneTCalvelloRIL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cellsInt Immunopharmacol201524236937625576658
- JhangKAParkJSKimHSChongYHResveratrol ameliorates tau hyperphosphorylation at Ser396 site and oxidative damage in rat hippocampal slices exposed to vanadate: implication of ERK1/2 and GSK-3β signaling cascadesJ Agric Food Chem201765449626963429022339
- WangGChenLPanXThe effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signalingOncotarget2016714173801739226980711
- FengXLiangNZhuDResveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathwayPLoS One201383e5988823555824
- LadiwalaARLinJCBaleSSResveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformersJ Biol Chem201028531242282423720511235
- WangJHoLZhaoZModerate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s diseaseFASEB J200620132313232017077308
- RingmanJMFrautschySATengEOral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled studyAlzheimers Res Ther2012454323107780
- QuinnJFRamanRThomasRGDocosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trialJAMA2010304171903191121045096
- SallowaySSperlingRKerenRA phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer diseaseNeurology201177131253126221917766
- KayeJDekoskySTWilliamsonJDGinkgo biloba for prevention of dementia: a randomized controlled trialArch Neurol200966565265419433666
- DekoskySTWilliamsonJDFitzpatrickALSnitzBEO’MearaESCarlsonMCGinkgo biloba for prevention of dementia: a randomized controlled trialJAMA2008300192663267019066386
- YallapuMMNageshPKJaggiMChauhanSCTherapeutic applications of curcumin nanoformulationsAaps J20151761341135626335307
- KankalaRKTsaiPYKuthatiYWeiPRLiuCLLeeCHOvercoming multidrug resistance through co-delivery of ROS-generating nano-machinery in cancer therapeuticsJ Mater Chem B20175715071517
- KankalaRKLiuCGChenAZOvercoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactorsACS Biomater Sci Eng201731024312442
- ComogluTArisoySAkkusZBNanocarriers for effective brain drug deliveryCurr Top Med Chem201717131490150628017157
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol200721275176018654426
- YehYCCreranBRotelloVMGold nanoparticles: preparation, properties, and applications in bionanotechnologyNanoscale2012461871188022076024
- MurphyCJGoleAMStoneJWGold nanoparticles in biology: beyond toxicity to cellular imagingAcc Chem Res200841121721173018712884
- HuangXEl-SayedMAGold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapyJ Adv Res2010111328
- LohseSEMurphyCJApplications of colloidal inorganic nanoparticles: from medicine to energyJ Am Chem Soc201213438156071562022934680
- JanaNREarhartCYingJYSynthesis of water-soluble and functionalized nanoparticles by silica coatingChem Mater2007192150745082
- NagahamaKUtsumiTKumanoTMaekawaSOyamaNKawakamiJDiscovery of a new function of curcumin which enhances its anticancer therapeutic potencySci Rep201663096227476814
- ManjuSSreenivasanKGold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cellsJ Colloid Interface Sci2012368114415122200330
- DeySSreenivasanKConjugating curcumin to water soluble polymer stabilized gold nanoparticles via pH responsive succinate linkerJ Mater Chem B201535824833
- PalmalSMaityARSinghBKBasuSJanaNRInhibition of amyloid fibril growth and dissolution of amyloid fibrils by curcumin-gold nanoparticlesChemistry201420206184619124691975
- JayaramDTShankarBHRamaiahDEffective amyloid defibrillation by polyhydroxyl-substituted squaraine dyesChem Asian J201510122689269426289494
- ChandraBMaityBKDasAMaitiSFluorescence quenching by lipid encased nanoparticles shows that amyloid-β has a preferred orientation in the membraneChem Commun2018545677507753
- PeppasNABuresPLeobandungWIchikawaHHydrogels in pharmaceutical formulationsEur J Pharm Biopharm2000501274610840191
- SoniGYadavKSNanogels as potential nanomedicine carrier for treatment of cancer: a mini review of the state of the artSaudi Pharm J201624213313927013905
- YcYFunkeWReactive microgels by emulsion polymerization of unsaturated polyester resinsAngew Makromol Chem19821031187202
- LiangFWFunkeWCross-linking self-emulsifying copolymerization of an unsaturated polyester and styreneMacromolecules1996292786508655
- VinogradovSVBronichTKKabanovAVNanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cellsAdv Drug Deliv Rev200254113514711755709
- KabanovAVVinogradovSVNanogels as pharmaceutical carriers: finite networks of infinite capabilitiesAngew Chem Int Ed Engl200948305418542919562807
- WeiXSenanayakeTHBohlingAVinogradovSVTargeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibitionMol Pharm20141193112312225072100
- JiangZDongXLiuHWangYZhangLSunYMultifunctionality of self-assembled nanogels of curcumin-hyaluronic acid conjugates on inhibiting amyloid β-protein fibrillation and cytotoxicityReact Funct Polym20161042229
- ManjuSSreenivasanKConjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stabilityJ Colloid Interface Sci2011359131832521492865
- WangYJPanMHChengALStability of curcumin in buffer solutions and characterization of its degradation productsJ Pharm Biomed Anal19971512186718769278892
- KreuterJNanoparticles – a historical perspectiveInt J Pharm2007331111017110063
- CouvreurPPolyalkylcyanoacrylates as colloidal drug carriersCrit Rev Ther Drug Carrier Syst1988511203293806
- X-YLuD-CWuZ-JLiG-QChenPolymer nanoparticlesVillaverdeAProgress in Molecular Biology and Translational ScienceMassachusettsAcademic Press2011299323
- ShiveMSAndersonJMBiodegradation and biocompatibility of PLA and PLGA microspheresAdv Drug Deliv Rev199728152410837562
- WaghelaBNSharmaADhumaleSPandeySMPathakCCurcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cellsPLoS One2015102e011752625692854
- YangXLiZWangNCurcumin-encapsulated polymeric micelles suppress the development of colon cancer in vitro and in vivoSci Rep201551032225980982
- GouMMenKShiHCurcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivoNanoscale2011341558156721283869
- MulikRSMönkkönenJJuvonenROMahadikKRParadkarARApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture modelMol Pharm20107381582520230014
- MathewAFukudaTNagaokaYCurcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s diseasePLoS One201273e3261622403681
- Djiokeng PakaGDogguiSZaghmiANeuronal uptake and neuroprotective properties of curcumin-loaded nanoparticles on SK-N-SH cell line: role of poly(lactide-co-glycolide) polymeric matrix compositionMol Pharm201613239140326618861
- DogguiSSahniJKArseneaultMDaoLRamassamyCNeuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell lineJ Alzheimers Dis201230237739222426019
- PakaGDRamassamyCOptimization of curcumin-loaded PEG-PLGA nanoparticles by GSH functionalization: investigation of the internalization pathway in neuronal cellsMol Pharm20171419310627744707
- BanghamADStandishMMWatkinsJCDiffusion of univalent ions across the lamellae of swollen phospholipidsJ Mol Biol19651312382525859039
- SessaGWeissmannGIncorporation of lysozyme into liposomes. A model for structure-linked latencyJ Biol Chem197024513329533015459633
- GregoriadisGThe carrier potential of liposomes in biology and medicine (second of two parts)N Engl J Med197629514765770785256
- JainSJainVMahajanSCLipid based vesicular drug delivery systemsAdv Pharm201420147112
- LazarANMourtasSYoussefICurcumin-conjugated nanoliposomes with high affinity for Aβ deposits: possible applications to Alzheimer diseaseNanomedicine20139571272123220328
- MourtasSCanoviMZonaCCurcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptideBiomaterials20113261635164521131044
- SummerlinNSooEThakurSQuZJambhrunkarSPopatAResveratrol nanoformulations: challenges and opportunitiesInt J Pharm2015479228229025572692
- RichardTPawlusADIglésiasMLNeuroprotective properties of resveratrol and derivativesAnn N Y Acad Sci20111215110310821261647
- FrozzaRLBernardiAHoppeJBNeuroprotective effects of resveratrol against Aβ administration in rats are improved by lipid-core nanocapsulesMol Neurobiol20134731066108023315270
- LoureiroJAndradeSDuarteAResveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s diseaseMolecules2017222277
- NevesARQueirozJFReisSBrain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein EJ Nanobiotechnology20161412727061902
- HeXPDengQCaiLFluorogenic resveratrol-confined graphene oxide for economic and rapid detection of Alzheimer’s diseaseACS Appl Mater Interfaces2014685379538224702005
- AnsariMAAbdulHMJoshiGOpiiWOButterfieldDAProtective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s diseaseJ Nutr Biochem200920426927518602817
- NdayCMHalevasEJacksonGESalifoglouAQuercetin encapsulation in modified silica nanoparticles: potential use against Cu(II)-induced oxidative stress in neurodegenerationJ Inorg Biochem2015145516425634813
- SunDLiNZhangWDesign of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s diseaseColloids Surf B Biointerfaces201614811612927591943
- BianchettiARozziniRTrabucchiMEffects of acetyl-L-carnitine in Alzheimer’s disease patients unresponsive to acetylcholinesterase inhibitorsCurr Med Res Opin200319435035312841930
- HudsonSTabetNAcetyl-L-carnitine for dementiaCochrane Database Syst Rev200322CD003158
- PengYSunJHonSL-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer’s diseaseJ Neurosci201030248180818920554868
- PittJThornerMBrautiganDLarnerJKleinWLProtection against the synaptic targeting and toxicity of Alzheimer’s-associated Aβ oligomers by insulin mimetic chiro-inositolsFASEB J201327119920723073831
- CascellaMBimonteSMuzioMRSchiavoneVCuomoAThe efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practiceInfect Agent Cancer2017123628642806
- TurnerRSThomasRGCraftSA randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer diseaseNeurology201585161383139126362286
- YangGWangYTianJLiuJPHuperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trialsPLoS One201389e7491624086396
