 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
One of the most attractive properties of quantum dots is their potential to extend the opportunities for fluorescent and multimodal imaging in vivo. The aim of the present study was to clarify whether the composition and structure of organic coating of nanoparticles are crucial for their application in vivo.
Methods
We compared quantum dots coated with non-crosslinked amino-functionalized polyamidoamine (PAMAM) dendrimers, quantum dots encapsulated in crosslinked carboxyl-functionalized PAMAM dendrimers, and silica-shelled amino-functionalized quantum dots. A multimodal fluorescent and paramagnetic quantum dot probe was also developed and analyzed. The probes were applied intravenously in anesthetized animals for visualization of brain vasculature using two-photon excited fluorescent microscopy and visualization of tumors using fluorescent IVIS® imaging (Caliper Life Sciences, Hopkinton, MA) and magnetic resonance imaging.
Results
Quantum dots coated with non-crosslinked dendrimers were cytotoxic. They induced side effects in vivo, including vasodilatation with a decrease in mean arterial blood pressure and heart rate. The quantum dots penetrated the vessels, which caused the quality of fluorescent imaging to deteriorate. Quantum dots encapsulated in crosslinked dendrimers had low cytotoxicity and were biocompatible. In concentrations <0.3 nmol quantum dots/kg bodyweight, these nanoparticles did not affect blood pressure and heart rate, and did not induce vasodilatation or vasoconstriction. PEGylation (PEG [polyethylene glycol]) was an indispensable step in development of a quantum dot probe for in vivo imaging, based on silica-shelled quantum dots. The non-PEGylated silica-shelled quantum dots possessed low colloidal stability in high-salt physiological fluids, accompanied by rapid aggregation in vivo. The conjugation of silica-shelled quantum dots with PEG1100 increased their stability and half-life in the circulation without significant enhancement of their size. In concentrations <2.5 nmol/kg bodyweight, these quantum dots did not affect the main physiological variables. It was possible to visualize capillaries, which makes this quantum dot probe appropriate for investigation of mediators of vasoconstriction, vasodilatation, and brain circulation in intact animals in vivo. The multimodal silica-shelled quantum dots allowed visualization of tumor tissue in an early stage of its development, using magnetic resonance imaging.
Conclusion
The present study shows that the type and structure of organic/bioorganic shells of quantum dots determine their biocompatibility and are crucial for their application in imaging in vivo, due to the effects of the shell on the following properties: colloidal stability, solubility in physiological fluids, influence of the basic physiological parameters, and cytotoxicity.
Introduction
One of the most attractive properties of quantum dots is their potential to extend the opportunities for fluorescent and multimodal imaging in vivo. The high quantum yield (over 50%) and large excitation/emission Stokes shift (up to 300 nm) allow minimization of tissue autofluorescence, especially when using quantum dots with deep-red or near-infrared emission. The high intensity of multiphoton excited fluorescence of quantum dots and the possibility for time-resolved detection of fluorescence additionally increase the signal-to-background ratio in the living organism. Using quantum dots it is possible to visualize complex structures, cells, and even molecular targets at significantly greater depth in vivo, in comparison with that achieved by conventional organic fluorophores.Citation1–Citation3 The chemically functionalized surface of quantum dots, encapsulated in silica or other organic/bioorganic shells, also opens up an opportunity for development of multimodal probes, applicable in two or more imaging techniques, eg, magnetic resonance imaging/optical, positron emission tomography/optical, and positron emission tomography/magnetic resonance imaging/optical).Citation1–Citation4
In the development of quantum dot probes for in vivo multimodal imaging, it is necessary to follow a few rules.Citation4 The most important are that the quantum dot nanocrystal has to be encapsulated in a bioinert and biocompatible shell to isolate it from the environment and to minimize its toxicity, and that the capsulated quantum dot nanocrystal has to be conjugated with biocompatible substances (usually polymers), decreasing its rate of accumulation in the liver and spleen and increasing the half-life in the bloodstream, facilitating access of quantum dots to the respective target and its visualization, and preventing opsonization and induction of a nonspecific immune response.Citation5 The bioinert polymer shells may also increase the water solubility of quantum dots, which facilitates their excretion from the organism through renal filtration and decreases their toxicity.Citation6,Citation7
The idea of encapsulation of nanoparticles in biocompatible shells and their conjugation with biocompatible polymers emerged in the mid 1990s in the context of development of water-soluble paramagnetic and ferromagnetic nanoparticles for bioanalyses,Citation8,Citation9 as a result of earlier experience in the synthesis of biocompatible polymers for drug encapsulation and delivery.Citation10–Citation12 The earliest and most frequently used polymers for encapsulation of nanoparticles are polyethylene glycol (PEG) and dextran, as well as silica/silicagel shells.Citation8,Citation9 Later, biocompatible dendrimers and diblock/triblock copolymers were included in the composition.Citation13,Citation14
Dextran and PEG make the nanoparticles hydrophilic and increase their half-life in the circulation. However, in some cases, the dextran can induce an acute immune response as anaphylaxis.Citation15 Currently, PEG or PEG derivatives are the polymers most commonly used to stabilize nanoparticles and to extend their half-life in the bloodstream due to the “sterically stabilizing effect of PEG”.Citation16 PEG forms a protective hydrophilic layer on the surface, preventing interaction between blood lipoproteins and cells (eg, macrophages) and significantly delays accumulation of the nanoparticles in the liver and spleen.Citation5 This is an essential condition for development of contrast nanoprobes for imaging diagnostics, because it enables a longer scan time and accurate localization and visualization of the target of interest (tissues, cells, or expression of specific receptors or molecules). Therefore, PEGylation seems to be an indispensable step in the development of quantum dot probes for in vivo diagnostic imaging. The longer half-life of PEGylated quantum dots in the circulation also contributes to their effectiveness in target-specific diagnostic imaging. Penetration of quantum dots into the vasculature and their localization in target tissue is known as the “enhanced permeability and retention effect.” There are also other polymers, including poly(oxazoline), poly(glycerol), and poly-N-vinylpyrrolidone, which have a sterically stabilizing action on nanoparticles.Citation17,Citation18
In the present study, we demonstrate the importance of the chemical nature and structure of the organic/bioorganic shells of quantum dots for their application in in vivo diagnostic imaging. Several quantum dot probes were developed and their biocompatibility was investigated and compared in experimental animals, using conventional physiological tests and two-photon excited fluorescent microscopy.
Materials and methods
Quantum dots and quantum dot probes
CdSe/ZnS quantum dots were synthesized using the methods described by Peng and PengCitation19 and Zlateva et al.Citation20 Nanocrystals were characterized by ultraviolet light/ultraviolet and fluorescent spectroscopy, X-ray analysis (Rigaku RINT-2100, Tokyo, Japan), and high-resolution transmission electron microscopy (JEOL JEM-3010, Tokyo, Japan).
The concentration of quantum dots was calculated using the method of Yu et al, with an extinction coefficient of 1 × 105/cm/M.Citation21 Quantum dots were additionally coated with silica as described by Bakalova et al,Citation22 polyamidoamine (PAMAM) dendrimers as described by Wu et alCitation23 (with a slight modification of using the second-generation PAMAM-C12 dendrimer [Sigma-Aldrich, St Louis, MO] instead of octylamine-modified polyacrylic acid), and the second-generation PAMAM-C12 dendrimer (Sigma-Aldrich) crosslinked by a PAMAM-succinamic acid dendrimer (Sigma-Aldrich), with N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride used as a zero-length crosslinker.
The conjugation of silica- or dendrimer-coated quantum dots with PEG was carried out using the method described in Hermanson.Citation24 Methyl-PEGn-NHS esters or methyl-PEGn amines were used for conjugation with amino-functionalized or carboxy-functionalized quantum dots, respectively. The coated quantum dots and quantum dot-PEG conjugates were purified by spin ultrafiltration and kept at 4°C. The amino-functionalized silica-shelled quantum dots were also conjugated with DOTA-phenyl isothiocyanate (DOTA-Bn-NCS/gadolinium, Macrocyclics Company, Dallas, TX) and PEGylated using methods described by Hermanson.Citation24 The quantum dot/DOTA/PEG conjugates were purified by dialysis using a Slide-A-LyzerR (Pierce Biotechnology Inc, Rockford, IL). The multimodal quantum dots were kept at 4° C. Their T1 and T2 contrast properties were characterized using 7 Tesla magnetic resonance imaging (Kobelco, Kobe, Japan).Citation25 All reagents used in this study were of analytical or high-pressure liquid chromatographic grade.
Animals and experimental models
Sprague-Dawley rats weighing 325 ± 15 g were used for investigation of the biocompatibility of the quantum dot probes and their usefulness for fluorescent imaging in vivo. Balb6 nude mice (22 ± g) were used for investigation of the usefulness of quantum dot probes for multimodal imaging of experimental tumors in vivo. Colon26 cells (1 × 105 in 10 μL phosphate-buffered saline, pH 7.4) were inoculated subdermally in the left/right hindpaw. All measurements were performed five or ten days after inoculation, when the tumor was 1–2 mm or 5–6 mm in size, respectively.
All experiments were conducted in accordance with the guidelines of the Physiological Society of Japan and were approved by the Animal Care and Use Committee of the National Institute of Radiological Sciences, Chiba, Japan.
Two-photon imaging of brain circulation in vivo
The rat was anesthetized with isoflurane (Abbott, Tokyo, Japan) 4% for induction, 2% for surgery, and 1.4% during measurements. Tracheal intubation was performed, and the femoral vein was catheterized for drug administration. The tail artery was catheterized for blood pressure monitoring and blood sampling. The rat was fixed in a stereotactic frame, the parietal bone was thinned to translucency over the somatosensory cortex (3 mm × 3 mm), and the dura was kept intact. Body temperature was maintained at 37°C, and systemic arterial blood pressure was periodically monitored at the tail artery. Heart rate and end-tidal gas levels were monitored continuously. To visualize the brain microcirculation, the quantum dot probe was injected (intravenously as a single dose, with an injected volume of 0.3 mL, containing different quantum dot concentrations), and the brain area was excited by an 810 nm pulse laser (Maitai HP, Spectra-Physics, Irvine, CA) under microscope (SP5, Leica) equipped with a 10× objective lens (0.30 numerical aperture). The vessel diameter was then determined by measuring the width of the vessel image projected across the 15 Z images (up to nearly 0.3 mm from the cortical surface) in each vessel segment. The experimental scheme is shown in .
IVIS® imaging in vivo
The mouse was anesthetized with 1.5% isoflurane (Abbott). The tail veil was catheterized for drug administration and the mouse was fixed in the camera of the IVIS® imaging system (Caliper Life Sciences, Hopkinton, MA). Body autofluorescence was registered using an excitation filter of 450 ± 30 nm and an emission filter of 650 nm. The quantum dot probe was injected (intravenously as a single dose of 1.6 nmol/kg bodyweight, 100 μL volume) and body fluorescence was registered at a series of time intervals over 90 minutes. The data were analyzed by Living ImageR software (version 2.50.1, Xenogen Corporation, Alameda, CA).
Magnetic resonance imaging in vivo
Magnetic resonance imaging measurements were performed on a 7.0 Tesla horizontal magnet (Kobelco and Jastec, Tokyo, Japan) interfaced to a Bruker Avance I console (Bruker BioSpin, Karlsruhe, Germany) and controlled with ParaVision 4.0.1 (Bruker BioSpin). The animal (a Balb6 mouse) was anesthetized using isoflurane 1.5% and held in a body cradle (Rapid Biomedical, Rimpar, Germany) in the prone position. Rectal temperature was continuously monitored and automatically controlled at 36.5 ± 0.5°C using a nonmagnetic temperature probe (FOT-M and FTI-10, FISO Technology, Olching, Germany) and an electric temperature controller (E5CN, Omron, Kyoto, Japan) during magnetic resonance imaging measurements. The tail vein was catheterized using a polyethylene tube (PE-10, Becton-Dickinson, Franklin Lakes, NJ) for drug administration. The mouse was then placed in a proton volume radiofrequency resonator (Bruker BioSpin) with a surface radiofrequency receiver (Rapid Biomedical) previously warmed using a body temperature controller (Rapid Biomedical). The resonator units, including the mouse, were placed in the magnet bore. Magnetic resonance imaging was performed using the following parameters: T1-weighted imaging; spin echo sequence with fat suppression preparation pulse; repetition time 400 msec; echo time 9.574 msec; field of view 32 × 32 mm; matrix size 256 × 256; slice thickness 1.0 mm; spatial resolution 125 × 125 × 1000 μm; four averages; and a total scan time of six minutes and 50 seconds. The magnetic resonance images were obtained before and after injection of the quantum dot probe (intravenously, single dose of 1 μmol/kg bodyweight, 100 μL volume) using the same gain level. The magnetic resonance imaging data were reconstructed by ParaVision and analyzed using MRVision image analysis software (version 1.5.8, MRVision Co, Winchester, MA).
Flow-cytometric cell viability assay
The cell suspension (5 × 105 cells/mL) was incubated with quantum dots at a series of concentrations over 24 hours in a humidified atmosphere and washed three times with phosphate-buffered saline. Five hundred microliters of the same cell suspension was incubated with 20 μL of propidium iodide (Sigma-Aldrich) for 10 minutes at room temperature. The cells were washed three times by phosphate-buffered saline, using centrifugation (1000 × g/10 minutes), and finally resuspended in 500 μL of phosphate-buffered saline. The propidium iodide entered the dead cells and did not enter the live ones. The cell suspension was subjected to flow cytometric analysis, using a Beckman Coulter-Epics XL flow cytometer (Fullerton, CA), operated in accordance with the manufacturer’s recommendations after fine adjustments for optimization of the measurements. The forward-scattered and side-scattered parameters were adjusted to accommodate the inclusion of viable and dead cells within the acquisition data. No cells were excluded from the analysis, and about 10,000 cells were counted. Data were collected and analyzed using XL System II software.
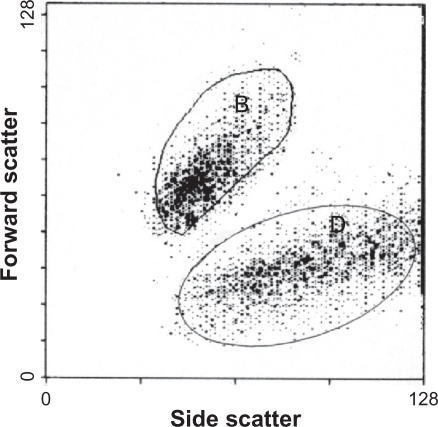
The results were presented as a dot plot of cell fluorescence with quadrant markers drawn to distinguish viable (nonlabeled) and dead (propidium iodide-labeled) cells ().
Figure 1 A flow cytometric histogram of cell fluorescence with quadrant markers, drawn to distinguished viable (nonlabeled) from dead (propidium iodide-labeled) cells. Quadrant B shows viable cells and quadrant D shows dead cells.
Cell viability after treatment with quantum dots was calculated by the following equation:
where Bp is the number of viable cells in quadrant B of the sample, treated by quantum dots; Dp is the number of dead cells in quadrant D of the sample, treated by quantum dots; Dc is the number of dead cells in quadrant D of the control sample (not treated with quantum dots). Quantum dot485 probes were used in all flow cytometric analyses.
Mitochondrial potential assay
The mitochondrial potential was analyzed using the method described by Wong and CortopassiCitation26 using 48-well plates and tetramethylrhodamine ethyl ester as a fluorescent marker (TMRE, Immunochem Diagnostic Technologies Ltd, Truro, Nova Scotia). Briefly, 50 nM of TMRE was added to 500 μL of cell suspension (1 × 106 cells/mL), and the fluorescence was measured using a BioRad microplate reader (BioRad Laboratories, Hercules, CA) equipped with a temperature controller. The temperature of the cell suspension was maintained at 37°C. The fluorescence of each well was detected over two cycles every five minutes at λex = 540 nm and λem = 575 nm. A quantum dot485 probe was added to each well and the fluorescence detection was continued over seven cycles every five minutes. The data were normalized to the autofluorescence of the quantum dot and the cell suspension was not treated with TMRE (baseline fluorescence).
Results and discussion
Biocompatibility of dendrimer-shelled quantum dots
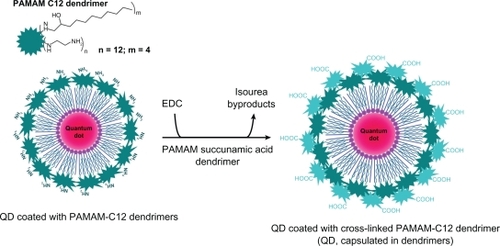
Two quantum dot probes were developed using PAMAM dendrimers as an organic shell (). For the first probe, a quantum dot nanocrystal was coated with a non-crosslinked second generation PAMAM C12 dendrimer). The carbohydrate chains (C12) of dendrimer interact with the hydrophobic coordinating ligands of the quantum dot via van der Waals interactions. As a result, the surface of this dendrimer-coated quantum dot was amino-functionalized and positively charged. The second quantum dot probe was developed from the first one through a crosslink between the surface amino groups of the PAMAM C12 dendrimer and the carboxyl groups of the second-generation PAMAM succinamic acid dendrimer, with formation of amide bonds (). Thus, in the second quantum dot probe, the nanocrystal was capsulated in crosslinked PAMAM dendrimers, and the surface of the nanoparticles was carboxyl-functionalized and negatively charged.
Figure 2 Model structure of quantum dot probes, consisting of CdSe/ZnS quantum dots coated with non-crosslinked PAMAM dendrimers or encapsulated in crosslinked PAMAM dendrimers.
Abbreviations: PAMAM, polyamidoamine; EDC, 1-ethyl-3(3-dimethylamino propyl)-carbodiimide; QD, quantum dot.
The zeta potential of amino-functionalized dendrimer-coated quantum dots was about +7.0 mV at pH 7.4, and for carboxyl-functionalized dendrimer-capsulated quantum dots was about −18.7 mV at pH 7.4. Both measurements were made in phosphate-buffered saline. Both quantum dot probes were characterized with excellent solubility in distilled water, and low-salt and high-salt physiological fluids (eg, buffers, saline solution, phosphate-buffered saline, cell cultured medium, plasma). The quantum dot probes were used for in vivo fluorescent imaging of the brain circulation in anesthetized rats, using two-photon excited fluorescent microscopy.
shows two-photon excited fluorescent images of brain vasculature after intravenous injection of dendrimer-coated quantum dots525. The images were obtained at λex = 810 nm with an emission filter of 525 ± 50 nm, in a horizontal scanning plane at a depth of 150 μm from the surface of the skull.
Figure 3 Two-photon excited fluorescent images of brain vasculature in horizontal planes (at 150 μm depth from brain surface) after intravenous injection of QD525 (coated with non-crosslinked PAMAM dendrimers [A] or capsulated in crosslinked dendrimers [B] in the anesthetized rat).
Notes: All images were obtained at λex = 810 nm and a mean laser power of about 2.7 W. Detectors: filter/band-pass 525/50 nm (via 560/10 nm beam splitter).
Abbreviations: PAMAM, polyamidoamine; QD, quantum dot.
![Figure 3 Two-photon excited fluorescent images of brain vasculature in horizontal planes (at 150 μm depth from brain surface) after intravenous injection of QD525 (coated with non-crosslinked PAMAM dendrimers [A] or capsulated in crosslinked dendrimers [B] in the anesthetized rat).Notes: All images were obtained at λex = 810 nm and a mean laser power of about 2.7 W. Detectors: filter/band-pass 525/50 nm (via 560/10 nm beam splitter).Abbreviations: PAMAM, polyamidoamine; QD, quantum dot.](/cms/asset/7c85b828-d6e7-41ff-beda-1d1a7085c955/dijn_a_17995_f0003_b.jpg)
In the case of quantum dots coated with non-crosslinked dendrimers, turbidity of the images increased during the scan time (). This impeded measurement and calculation of the average diameter of the arterioles and venuoles. Concentration of the injected quantum dot probe was 0.3 nmol quantum dots/kg bodyweight. Increasing the concentration also made it difficult to define the outlines of the large blood vessels (arteries and veins). Intravenous injection of this quantum dot probe changed the basic physiological characteristics of the animal (). Mean arterial blood pressure and heart rate decreased, accompanied by vasodilatation.
Table 1 Physiological characteristics of rats before and after intravenous injection of QDs (coated with noncrosslinked PAMAM dendrimers)
PAMAM C12 dendrimers (without quantum dots) had similar effects (see for supplementary information). Even low concentrations of the PAMAM C12 dendrimer (0.1 nmol/kg bodyweight injected intravenously in the rat) decreased blood pressure and heart rate and induced vasodilatation.
In the case of quantum dots encapsulated in crosslinked dendrimers, the turbidity of the fluorescent images was negligible (). The images did not change during the scan time. The large and small blood vessels were well outlined and it was easy to measure their average diameter. This quantum dot probe did not affect mean arterial blood pressure or heart rate () even one hour after injection.
Table 2 Physiological characteristics of rats before and after intravenous injection of QDs (encapsulated in crosslinked PAMAM dendrimers)
The dendrimer-encapsulated quantum dots were also PEGylated using PEG1100. However, PEGylation did not significantly increase the half-life of the quantum dots in the brain circulation (elimination half-life was approximately 65 minutes for the non-PEGylated probe and approximately 70 minutes for the PEGylated probe), and is not an obligatory step for dendrimer-coated quantum dots.
These results demonstrate the importance of the nature and structure of the organic shell of quantum dots for their application in vivo. The positively charged quantum dot probe, consisting of quantum dots coated with non-crosslinked PAMAM dendrimers, caused side effects in vivo, accompanied by poor quality of the fluorescent images. This quantum dot probe is not appropriate for visualization of the brain vasculature and investigation of the biological phenomena related to the brain circulation. Turbidity of the images after injection of this probe could be explained by its transepithelial and transendothelial penetration. Kitchens et alCitation27 have reported that the cationic PAMAM dendrimers (generation 0–4) are highly permeable for epithelial and endothelial cells and penetrate easily and rapidly through the blood vessels. This property of PAMAM dendrimers is widely used in development of drug delivery systems.Citation28–Citation31 Quantum dots coated with noncrosslinked PAMAM dendrimers were also cytotoxic (), and their application for in vitro imaging analyses was limited.
Table 3 Effect of CdSe/ZnS QD485 (coated with noncrosslinked PAMAM dendrimers) on cell viability in vitro (% from control)
PAMAM C12 dendrimers had similar cytotoxic effects in vitro, while positively charged water-soluble quantum dots, prepared by direct surface exchange of hydrophobic coordinating ligands with cysteamine, were noncytotoxic in concentrations up to 500 nM and at 24 hours’ incubation time (see and for supplementary information). This is in agreement with data reported in 2004 by Derfus et al.Citation32 The authors found that noncoated CdSe/ZnS quantum dots were not cytotoxic in concentrations up to 1 μM, even after ultraviolet irradiation.
The encapsulation of quantum dots in negatively charged crosslinked dendrimers decreased their cytotoxicity and abolished the side effects on physiological variables in vivo. The encapsulated quantum dots did not show cytotoxicity in concentrations up to 500 nM (data not shown), whereas the quantum dots coated with non-crosslinked dendrimers were highly cytotoxic in concentrations >50 nM.
The cytotoxicity of quantum dots coated with non-crosslinked dendrimers can be explained with the intrinsic instability of the micelle type of dendrimer coat. The micelle structure is a result of weak hydrophobic interactions, and its partial destruction in the cell suspension is highly possible. Thus, cytotoxic monomers can be released into the intracellular and extracellular environment. Several groups have also shown that cationic PAMAM dendrimers are cytotoxic in their monomer form.Citation33–Citation38
The micelle type of organic coat could also be destroyed in the bloodstream, and the monomers can have side effects on blood pressure and heart rate (). It has been established in vitro that the cationic PAMAM dendrimers modulate the release of nitric oxide, one of the major vasodilators in vivo,Citation39 while the anionic PAMAM-COOH dendrimers significantly reduce this process.Citation36 In our study, we observed vasodilatation of the brain arterioles and venuoles after injection of quantum dots coated by non-crosslinked positively charged dendrimers (). The quantum dots encapsulated in crosslinked carboxyl-functionalized dendrimers did not manifest such effects (). The encapsulated organic coat is stable because of covalent bonds, and release of monomers is impossible without a specific metabolic pathway.
It is widely accepted that the cytotoxicity of dendrimers is due to their high cellular permeability and colocalization in the mitochondria and lysosomes.Citation35,Citation40 We investigated the effect of both quantum dot probes on the mitochondrial potential (Δψm) of living cells (). The mitochondrial potential is one of the major markers for induction of apoptosis. For this purpose, we used the quantum dot485 and TMRE (λem = 575 nm) as a fluorescent sensor. The fluorescence of TMRE changes proportionate to mitochondrial potential. Loss of the mitochondrial potential is accompanied by a significant decrease or total loss of TMRE fluorescence.Citation26 The kinetic curves in show that quantum dots coated with non-crosslinked PAMAM dendrimers induce depolarization of the mitochondrial membrane and loss of the mitochondrial potential in living cells. The quantum dots encapsulated in crosslinked dendrimers did not affect the mitochondrial potential.
Figure 4 (A) Dynamics of tetramethylrhodamine ethyl ester (TMRE) fluorescence of Jurkat cells in the absence (control) or presence of dendrimer-coated QD485 100 nM. (B) Hypothetical mechanism of cytotoxicity of QD, coated with non-crosslinked polyamidoamine dendrimers.
Abbreviations: Apaf-1, apoptotic peptidase-activating factor 1; QD, quantum dot.
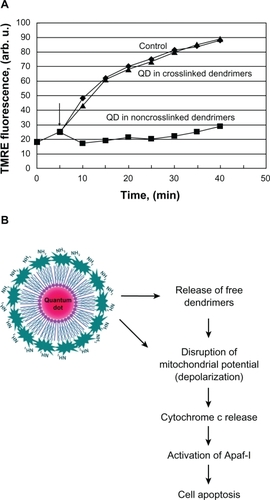
It is widely accepted that loss of mitochondrial potential induces expression of cytochrome c and apoptotic peptidase-activating factor 1. This leads to activation of caspases, induction of apoptosis, and cell death.Citation41–Citation43 This molecular mechanism could explain, at least partially, the cytotoxic effect of quantum dots coated with non-crosslinked PAMAM dendrimers ().
Biocompatibility of silica-shelled quantum dots
Silica materials are one of the most bioinert and biocompatible materials for coating. It has been found that silica nanoparticles with diameters below 50 nm do not activate macrophagesCitation44–Citation46 and should not induce an immune response after intravenous injection. However, silica shells do not provide sufficiently high solubility of quantum dots in high-salt physiological fluids, such as saline solution, phosphate-buffered saline, blood, and plasma.Citation47
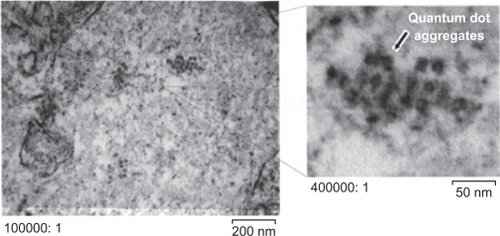
In 2006, we developed silica-shelled single CdSe/ZnS quantum dots with an average diameter of 17.42 ± 2.12 nm, a size distribution of about 12%, and a quantum yield of 30%–45% (depending on the size of the silica-shelled quantum dots, and measured in distilled water, ).Citation48 However, without PEGylation, these amino-functionalized nanoparticles possessed good colloidal stability in distilled water and low-salt buffer, but not in saline solution, phosphate-buffered saline, or body fluids. For example, if mice were inoculated intranasally with non-PEGylated silica-shelled quantum dots, we observed a lot of aggregates in the nasal mucosa (). Therefore, PEGylation and stabilization were necessary before these nanoparticles could be applied for imaging in vivo and especially in the case of intravenous and intranasal administration. The PEGylated silica-shelled quantum dots possessed high colloidal stability and did not aggregate in high-salt physiological fluids (eg, 100 mM phosphate-buffered saline and plasma).
Figure 5 Model structure of QD probe, consisting of silica-shelled QD conjugated with PEG.Citation48
Note: Reprinted with permission from Zhelev Z, Ohba H, Bakalova R. Single quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracers. J Am Chem Soc. 2006;128:6324–6325. Copyright 2006 American Chemical Society.
Abbreviations: QD, quantum dot; PEG, polyethylene glycol.
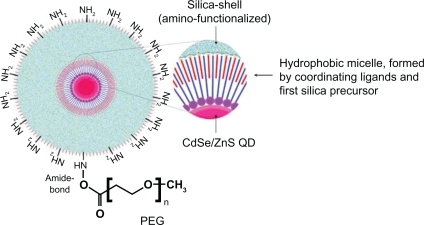
Figure 6 Electron micrographs of nasal mucosa one day after intranasal inoculation of amino-functionalized silica-shelled quantum dots (dissolved in distilled water, 30 drops per animal, single dose).
Abbreviation: QD, quantum dot.
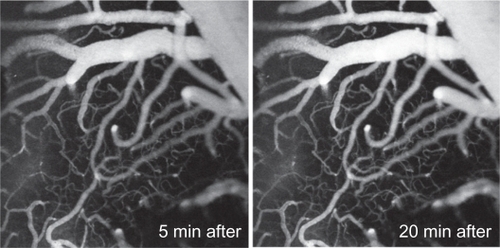
PEG1100-grafted silica-shelled quantum dots injected intravenously into the rat and brain vasculature were visualized using two-photon excited fluorescent microscopy (). The images were obtained at λex = 810 nm and an emission filter of 525 ± 50 nm, with scanning in the horizontal plane at a depth of 150 μm from the surface of the skull. The fluorescent contrast was constant during 30 minutes of scanning. The PEG1100-grafted silica-shelled quantum dots gave clear images even at high concentrations (2.5 nmol quantum dots/kg bodyweight, ). On the other hand, 0.2 pmol quantum dots/kg bodyweight was enough to visualize the large-sized and middle-sized blood vessels at a depth of 150 μm. Increasing the concentration of the quantum dot probe, it was possible to visualize the arteries and veins at a depth of 600–700 μm. These quantum dots did not affect blood pressure or heart rate, and did not provoke vasodilatation or vasoconstriction up to 60 minutes after injection (). The PEGylated silica-shelled quantum dots were noncytotoxic in concentrations up to 500 nM, as determined in cultured cells ().
Table 4 Physiological characteristics of rats before and after intravenous injection of PEG1100-grafted silica-shelled QDs
Table 5 Effect of silica-shelled CdSe/ZnS QDs (conjugated with PEG1100) on cell viability in vitro (% from control)
Figure 7 Two-photon excited fluorescent images of brain vasculature in horizontal planes (at 150 μm depth from the brain surface) after intravenous injection of PEG1100-grafted silica-shelled QD525 (2.5 nmol/kg bodyweight) in the anesthetized rat. The images were obtained at λex = 810 nm and a mean laser power of about 2.7 W. Detectors: filter/band-pass: 525/50 nm (via a 560/10 nm beam splitter).
Abbreviations: QD, quantum dot; PEG, polyethylene glycol.
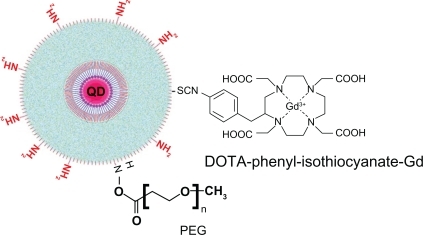
The PEG1100-grafted silica-shelled quantum dots655 were conjugated with DOTA/Gd3+, and the probe was applied for multimodal imaging of cancer in mice. This is a classical scheme for the multimodal quantum dot probes developed by other researchers ().Citation1,Citation4
Figure 8 Model structure of PEGylated multimodal silica-shelled quantum dots.Citation48
Note: Reprinted with permission from Zhelev Z, Ohba H, Bakalova R. Single quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracers. J Am Chem Soc. 2006;128:6324–6325. Copyright 2006 American Chemical Society.
Abbreviations: PEG, polyethylene glycol; DOTA, tetraazacyclododecanetetraacetic acid.
The fluorescent and magnetic resonance imaging characteristics of our multimodal quantum dots are shown in . The fluorescent quantum yield was about 40% in high-salt buffer. The longitudinal and transverse relaxation times (T1 and T2) were about 290 msec and 120 msec, respectively.
Table 6 Spectral characteristics of PEG1100-grafted multimodal silica-shelled QDs
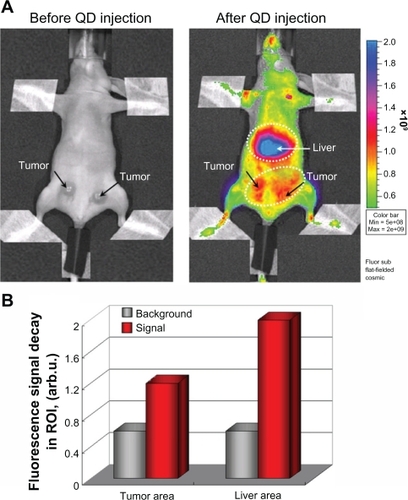
The fluorescent images in show that the multimodal silica-shelled quantum dots visualized the tumor by angiogenesis. The experimental tumor was developed within 10 days after inoculation of Colon32 cells in the left and right hind paws of mice. Fluorescence intensity in the tumor area was approximately three times higher than that in the surrounding tissues (). Maximum fluorescence intensity was detected in the liver area. It seems that these nanoparticles accumulated predominantly in the liver area within 90 minutes of their injection into the tail vein. Other authors have also reported that polymer-coated quantum dots accumulate predominantly in the liver, partially in the spleen, lung, and kidney, and are almost never observed in the heart and brain.Citation49
Figure 9 (A) Fluorescent imaging of tumor by angiogenesis in the anesthetized mouse injected intravenously with PEG1100-grafted multimodal QD655 (1.6 nmol/kg bodyweight, single dose). Dashed lines indicate liver area and tumor area (including angiogenic network). The images were obtained 15 minutes after injection using an IVIS® imaging system, with excitation at 450 ± 30 nm and emission at 650 nm (DsRed filter). The images were obtained on day 10 after inoculation (1 × 105 cells in 10 μL). (B) Fluorescence intensity in tumor area, liver area, and other parts of the body (background fluorescence) after intravenous injection of PEG1100-grafted multimodal QD655 (1.6 nmol/kg bodyweight, single dose). Data were calculated from the images in A.
Abbreviations: QD, quantum dot; PEG, polyethylene glycol.
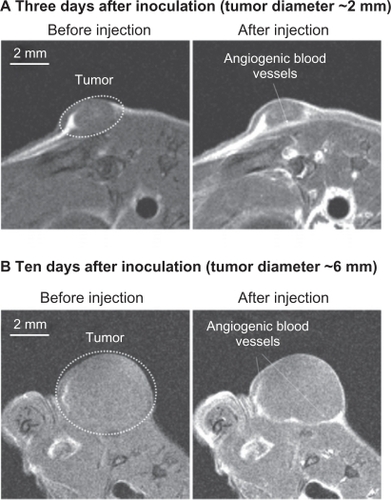
The paramagnetic characteristics of the quantum dot probe also enabled visualization of tumors using magnetic resonance imaging (). The short longitudinal relaxation time (T1) of gadolinium in the silica spheres allowed visualization of the tumor on the third day after inoculation, when tumor size was about 2 mm in diameter and the angiogenic network was not well developed.
Figure 10 T1-weighted magnetic resonance images of colon cancer in the anesthetized mouse injected intravenously with PEG1100-grafted multimodal QD655 embedded with gadolinium (1 μmol/kg bodyweight, single dose). Dashed lines indicate tumor area (including angiogenic vasculature). The images were obtained immediately after injection, using 7.0 Tesla magnetic resonance imaging. Imaging parameters: spin echo sequence with fat suppression preparation pulse; repetition time 400 msec; echo time 9.574 msec; field of view 32 × 32 mm; matrix size 256 × 256; slice thickness 1.0 mm; number of averages 4.
Abbreviations: QD, quantum dot; PEG, polyethylene glycol.
Conclusion
The data presented here show that the type and structure of organic/bioorganic shells of quantum dots are crucial for their colloidal stability and solubility in physiological fluids, and their biocompatibility and cytotoxicity. All these parameters have to be identified and controlled before application of organic-coated quantum dots for in vivo imaging. CdSe/ZnS quantum dots, coated with non-crosslinked PAMAM dendrimers and amino-functionalized, are cytotoxic and nonbiocompatible. These quantum dots have side effects after intravenous administration in healthy animals, ie, decreased blood pressure and heart rate, as well as vasodilatation. Presumably this is as a result of instability of the dendrimer coat in the bloodstream and release of free monomers, inducing cytotoxic mechanisms in the organism. These quantum dots are not appropriate for in vivo imaging of vasculature structure, because the nanoparticles seem to penetrate the vessels, affecting the quality of the images.
CdSe/ZnS quantum dots encapsulated in crosslinked carboxyl dendrimers have low cytotoxicity and comparatively good biocompatibility in concentrations up to 0.3 nmol/kg bodyweight. This quantum dot probe is appropriate for in vivo imaging. PEGylation is not required to increase the half-life of dendrimer-coated quantum dots in the bloodstream.
However, PEGylation is an indispensable step in development of quantum dot probes for in vivo imaging, based on silica-shelled quantum dots. Non-PEGylated and amino-functionalized silica-shelled quantum dots have low colloidal stability in high-salt physiological fluids, which is accompanied with rapid aggregation. The aggregates could induce activation of macrophages and a nonspecific immune response in vivo. The conjugation of silica-shelled quantum dots with PEG1100 increases their half-life in the circulation without significant enhancement of their size. PEG1100-grafted silica-shelled quantum dots are appropriate for visualization of blood vasculature and circulation in vivo, using two-photon excited fluorescent microscopy. In concentrations up to 2.5 nmol/kg bodyweight injected intravenously, these quantum dots do not affect physiological variables, eg, blood pressure, heart rate, and blood vessel diameter. Using these quantum dots, it was possible to visualize capillaries, which makes them appropriate for investigation of mediators of vasoconstriction, vasodilatation, and brain circulation in intact animals in vivo.
It should be noted that our results are related to use of quantum dots for structural fluorescent imaging of blood vessels and tumors. Quantum dot probes with sensing properties also have potential for functional imaging diagnostics in vivo.Citation50
Acknowledgements
We thank Dr Kazuto Masamoto, Center for Frontier Science and Engineering, University of Electrocommunications, Japan, for his help with the two-photon fluorescent imaging, and Ms Sayaka Shibata, Molecular Imaging Center, National Institute of Radiological Sciences, Chiba, Japan, for her assistance with the animal magnetic resonance imaging experiments. This research is partially funded by the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program),” initiated by the Council for Science and Technology Policy (CSTP), as well as by the Grand-in-aid “Kakenhi.”
Supplementary data
Figure S1 Experimental scheme of imaging of two-photon excited fluorescence in rats.
Abbreviation: QD, quantum dot.
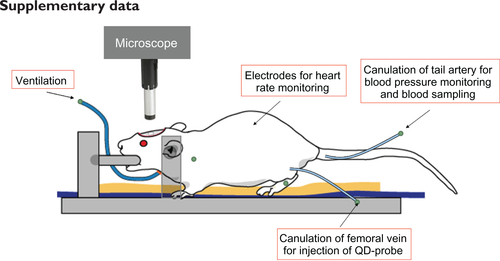
Table S1 Physiological characteristics of rats before and after intravenous injection of polyamidoamine C12 dendrimer 0.1 nmol/kg bodyweight
Table S2 Effect of PAMAM C12 dendrimers on cell viability in vitro (% from control)
Table S3 Effect of water-soluble amino-functionalized CdSe/ZnS QD485 without additional organic shell on cell viability in vitro (% from control)
Disclosure
The authors report no conflicts of interest in this work.
References
- MichaletXPinaudFFBentolilaLAQuantum dots for live cells, in vivo imaging, and diagnosticsScience200530753854415681376
- LarsonDRZipfelWRWilliamsRMWater-soluble quantum dots for multiphoton fluorescence imaging in vivoScience20033001434143712775841
- KosakaNMcCannTEMitsunagaMChoykePLKobayashiHReal-time optical imaging using quantum dot and related nanocrystalsNanomedicine (Lond)2010576577620662647
- BakalovaRZhelevZAokiIKannoIDesigning quantum dot probesNat Photonics20071487489
- RombergBHenninkWEStormGSheddable coatings for long-circulating nanoparticlesPharm Res200825557117551809
- HagensWIOomesAGDe JongWHCasseeFRSipsAJWhat do we need to know about kinetic properties of nanoparticles in the bodyRegul Toxicol Pharmacol20074921722917868963
- LongmireMChoykePLKobayashiHClearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveatsNanomedicine2008370371718817471
- AllermannEBrasseurNBenrezzakOPEG-coated poly(lactic acid) nanoparticles for the delivery of xehadecafluoro zinc phthalocyanine to EMT-6 mouse mammary tumoursJ Pharm Pharmacol1995473823877494187
- LerouxJCDeJaeghereFAnnerBDoelkerEGumyRAn investigation of the role of plasma and serum opsonins on the internalization of biodegradable poly(D,L-lactic acid) nanoparticles by human monocytesLife Sci1995576957037637541
- HellerJControlled release of biologically active compounds from bioerodible polymersBiomaterials1980151576258660
- BucklesRGBiomaterials for drug delivery systemsJ Biomed Mater Res1983171091286826569
- LaurencinCTPierre-JacquesHMLangerRToxicology and biocompatibility considerations in the evaluation of polymeric materials for biomedical applicationsClin Lab Med1990105495702253450
- SidorovSNBronsteinLMValetskyPMStabilization of metal nanoparticles in aqueous medium by polyethyleneoxide-polyethyleneimine block copolymersJ Colloid Interface Sci199921219721110092347
- CrooksRMZhaoMSunLChechikVYeungLKDendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and application to catalysisAcc Chem Res20013418119011263876
- Van der KlauwMMWilsonJHStrickerHDrug-associated anaphylaxis: 20 years of reporting in the Netherlands (1974–1994) and review of the literatureClin Exp Allergy199626135513639027435
- OtsukaHNagasakiYKataokaKPEGylated nanoparticles for biological and pharmaceutical applicationsAdv Drug Deliv Rev20035540341912628324
- WoodleMCControlling liposome blood clearance by surface-grafted polymersAdv Drug Deliv Rev19983213915210837640
- TorchilinVPMicellar nanoparticles: Pharmaceutical perspectivesPharm Res20072411617109211
- PengZAPengXFormation of high-quality CCdTe, CdSe, and CdS nanocrystals using CdO as precursorJ Am Chem Soc20012318318411273619
- ZlatevaGZhelevZBakalovaRKannoIPrecise size-control and synchronized synthesis of six colours of CdSe quantum dots in a slow-increasing temperature gradientInorg Chem2007466212621417602608
- YuWWQuLGuoWPengXExperimental determination of the excitation coefficient of CdTe, CdSe and CdS nanocrystalsChem Mater20031528542860
- BakalovaRZhelevZAokiIOhbaHKannoISilica-shelled single quantum dot micelles as imaging probes with dual or multimodalityAnal Chem2006785925593216906742
- WuXLiuHHaleyKNTreadwayJAImmunofluorescent labelling of cancer marker Her2 and other cellular targets with semiconductor quantum dotsNat Biotechnol200321414612459735
- HermansonGTBioconjugate TechniquesNew York, NYAcademic Press1996
- BakalovaRZhelevZAokiIMultimodal silica-shelled quantum dots: Direct intracellular delivery, photosensitization, toxic, and microcirculation effectsBioconjug Chem2008191135114218494515
- WongACortopassiGAHigh-throughput measurement of mitochondrial membrane potential in a neural cell line using a fluorescence plate readerBiochem Biophys Res Commun200229875075412419317
- KitchensKMEl-SayedMEHGhandehariHTransepithelial and endothelial transport of poly(amidoamine) dendrimersAdv Drug Deliv Rev2005572163217616289433
- MedinaSHEl-SayedMEDendrimers as carriers for delivery of chemotherapeutic agentsChem Rev20091093141315719534493
- NadjwadeBKBechraHMDerkarGKManviFVNadjwadeVKDendrimers: Emerging polymers for drug-delivery systemsEur J Pharm Sci20093818519619646528
- PaleosCMTzivelekaLASideratouZTsiourvasDGene delivery using functional dendritic polymersExpert Opin Drug Deliv20096273819236206
- SamadAAlamMISaxenaKDendrimers: A class of polymers in the nanotechnology for the delivery of active pharmaceuticalsCurr Pharm Des2009152958296919754372
- DerfusAMChanWCWBhatiaSNProbing the citotoxycity of semiconductor quantum dotsNano Lett200941118
- KolhatkarRBKitchensKMSwaanPWGhandehariHSurface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeabilityBioconjug Chem2007182054206017960872
- NamNYHahnHJNamKEvaluation of generation 2, 3 and 4 arginine modified PAMAM dendrimers for gene deliveryInt J Pharm200836319920518718514
- ThomasTPMajorosIKotlyarAMullenDHollMMBakerJRJrCationic poly(amidoamine) dendrimer induces lysosomal apoptotic pathways at theoretically relevant concentrationsBiomacromolecules2009103207321419924846
- WangWXiongWWanJSunXXuHYangXThe decrease of PAMAM dendrimer-induced cytotoxicity by PEG-ylation via attenuation of oxidative stressNanotechnology20092010510319417510
- WangBNavathRSRomeroRKannanSKannanRAnti-inflammatory and anti-oxidant activity of anionic dendrimer-N-acetyl cysteine conjugates in activated microglial cellsInt J Pharm200937715916819463931
- YellepeddiVKKumarAPalakurthiSSurface modified poly(amidoamine) dendrimers as diverse nanomolecules for biomedical applicationsExpert Opin Drug Deliv2009683585019637972
- StankoNASchoenfischMNDendrimers as a scaffold for nitric oxide releaseJ Am Chem Soc20061288265827116787091
- SaovapakhiranAD’EmanueleAAttwoodDPennyJSurface-modification of PAMAM dendrimers modulates the mechanism of cellular internalizationBioconjug Chem20092069370119271737
- AdamsJMCorySApoptosomes: Engines of caspase activationCurr Opin Cell Biol20021471572012473344
- AnichiniAMortariniRSensiMZanonMAPAF-1 signalling in human melanomaCancer Lett200623816817916095810
- CaroppiPSinibaldiFFiorucciLSantucciRApoptosis and human diseases: Mitochondrion damage and lethal role of released cytochrome c as proapoptotic proteinCurr Med Chem2009164058406519754424
- ChoiJBurnsAAWilliamsRMCore-shell silica nanoparticles as fluorescent labels for nanomedicineJ Biomed Opt20071206400718163823
- ChoMChoWSChoiMThe impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticlesToxicol Lett200918917718319397964
- WatersKMMasielloLMZangarRCMacrophage responses to silica nanoparticles are highly conserved across particle sizesToxicol Sci200910755356919073995
- DubertretBSkouridesPNorrisDJNoireauxVBrivanlouAHLibchaberAIn vivo imaging of quantum dots encapsulated in phospholipid micellesScience2002981759176212459582
- ZhelevZOhbaHBakalovaRSingle quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracersJ Am Chem Soc20061286324632516683790
- ChoiHSLiuWMisraPRenal clearance of quantum dotsNat Biotechnol2007251165117017891134
- BakalovaRFluorescent molecular sensors and multi-photon microscopy in brain studiesBrain Res Bull20077315015317499649