Abstract
Rosette nanotubes (RNTs) are novel, self-assembled, biomimetic, synthetic drug delivery materials suitable for numerous medical applications. Because of their amphiphilic character and hollow architecture, RNTs can be used to encapsulate and deliver hydrophobic drugs otherwise difficult to deliver in biological systems. Another advantage of using RNTs for drug delivery is their biocompatibility, low cytotoxicity, and their ability to engender a favorable, biologically-inspired environment for cell adhesion and growth. In this study, a method to incorporate dexamethasone (DEX, an inflammatory and a bone growth promoting steroid) into RNTs was developed. The drug-loaded RNTs were characterized using diffusion ordered nuclear magnetic resonance spectroscopy (DOSY NMR) and UV-Vis spectroscopy. Results showed for the first time that DEX can be easily and quickly encapsulated into RNTs and released to promote osteoblast (bone-forming cell) functions over long periods of time. As a result, RNTs are presented as a novel material for the targeted delivery of hydrophobic drugs otherwise difficult to deliver.
Introduction
Since the first hip replacement surgery in 1923, orthopedic implants have been routinely inserted, increasing the quality of life for millions of people.Citation1 A variety of materials have been investigated as bone replacements, such as conventional titanium and its alloys, micron grain sized ceramics (eg, hydroxylapatite or HA) and nanostructured hydrogels (eg, poly-lactic-co-glycolic acid or PLGA).Citation2–Citation5 However, current conventional implants suffer from limited lifetimes, often failing before the end of a patient’s natural life expectancy.Citation6,Citation7 For example, today’s orthopedic implants usually have insufficient initial bone growth on their surface and/or poor maintenance of healthy juxtaposed bone over long periods of time. Such problems caused by poor osteointegration of the implanted device can lead to bone loss, implant loosening, and ultimately implant failure. For example, from October 2005 to December 2006, the number of total hip replacement revisions performed in the US reached 51,345 as a result of instability/dislocation, mechanical loosening and osteolysis.Citation8
To enhance the integration of orthopedic implants with bone forming cells (osteoblasts) and surrounding bone, implants with nanostructured surfaces have received a tremendous amount of recent attention.Citation9,Citation10 An important member of this class of nanomaterials are the rosette nanotubes (RNTs, ).Citation11–Citation13 RNTs are novel, biomimetic, self-assembled, supramolecular structures, whose basic building blocks feature the hydrogen bonding arrays of guanine (G) and cytosine (C) DNA bases. RNTs are produced syntheticallyCitation13 and are assembled under physiological conditions. They have been shown to have low toxicityCitation14,Citation15 (in vitro and in vivo) and have been shown to have anti-cancer properties when functionalized with cell adhesive peptides such as arginine-glycine-aspartic acid-serine-lysine (RGDSK).Citation16
Figure 1 Schematic illustration of the hierarchical self-assembly of RNTs with a lysine side chain. The single unit of K1 (G∧C motif conjugated to a lysine amino acid) self-assembles into a rosette ring (lower left), which stacks up to form a stable RNT (right), referred to as K1 in this report.
Abbreviation: RNT, rosette nanontubes.
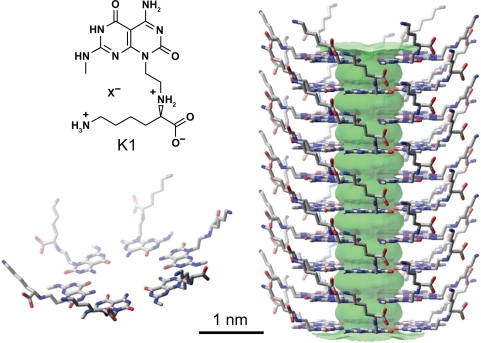
A heteroaromatic bicyclic base functionalized with L-lysine (K–G∧C, ) undergoes a hierarchical self-assembly process under physiological conditions to form a six-membered supermacrocycle maintained by 18 hydrogen bonds. Because of electrostatic forces, base stacking interactions and hydrophobic effects, the rosettes form a stable stack with an 11 Å open channel. L-lysine was chosen to impart chirality, stability, biocompatibility, and water solubility. Thus, each of the RNT building blocks has a lysine chain, and the resulting RNTs (referred to as K1) are covered with lysine amino acids (). The biomimetic, nanostructured nature of RNTs provide a favorable environment for cell adhesion and growth.Citation11,Citation12,Citation17,Citation18
Due to their synthetic accessibility, RNTs have unique advantages for drug delivery applications because: a) they can be chemically conjugated with bioactive peptides such as RGDSK–RNT (RNTs functionalized with Arg-Gly-Asp-Ser-Lys) to improve osteoblast functions on hydrogelsCitation18 and b) they can be loaded with drugs via hydrophobic or stacking interactions.Citation19
As a first step toward the application of RNTs as novel drug delivery devices for orthopedic applications, we investigated the incorporation of dexamethasone (DEX) into RNTs and characterized the drug-loaded RNTs by diffusion ordered nuclear magnetic resonance spectroscopy (DOSY NMR), atomic force microscopy (AFM), and UV–Vis spectroscopy. DEX is known to enhance osteoconductivity and is widely used in bone tissue engineering.Citation20–Citation22 However, this drug interferes with the differentiation of bone marrow-derived stem cells into muscle, fat, cartilage and other tissue-forming cells.Citation23 Therefore, it is important to deliver DEX to the desired site of new bone growth to ensure maximum therapeutic activity and specificity.Citation19
Results of this study showed that DEX was indeed incorporated into RNTs by simply allowing RNTs to self-assemble in solution in the presence of DEX. Subsequent cell studies demonstrated that DEX was released from the RNTs for up to 9 days and had a positive impact on osteoblast functions compared to DEX added to cell culture media alone. This study thus presents a novel drug delivery system where not only is the delivery device itself bioactive, but it can also deliver a variety of drugs for various medical applications.
Materials and methods
Preparation of RNTs
The self-assembling K–G∧C modules were synthesized according to a previously reported strategy.Citation13 They were then assembled into RNTs in distilled water (dH2O) to a final concentration of 100 μg/mL.
1H and DOSY NMR studies
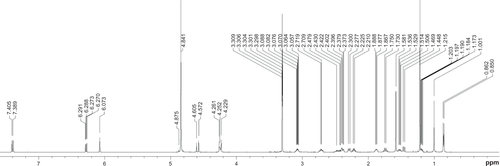
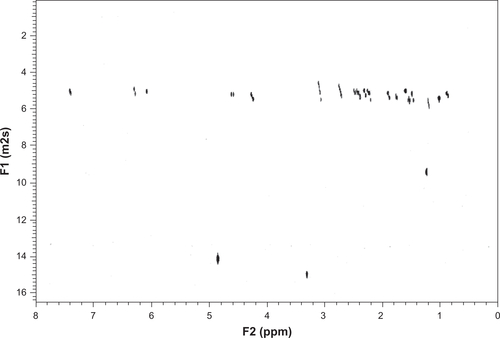
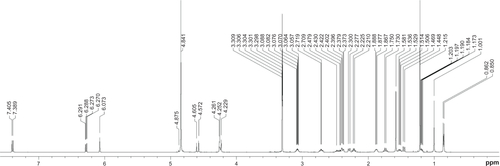
To test the ability of K1 to deliver hydrophobic drugs, 4.07 mg of K–G∧C were dissolved in deuterated methanol (CD3OD, 1 mL) and aged for 1 day at room temperature. Tertbutanol (t-BuOH, 1.3 μL, [Sigma, St Louis, MO] ≥ 99.5% anhydrous) was added as a 1H NMR internal standard to quantify the extent of DEX interaction with K1. DEX (5.11 mg, Sigma, D4902) was then added resulting in a solution composed of DEX:K1 at a molar ratio of 5:3. The solution was aged for 2 days prior to 1H NMR and DOSY NMR investigations. Proton NMR spectra and DOSY were recorded on a Varian Direct Drive VNMRS 600 spectrometer (Agilent Technologies, Santa Clara CA) operating at a magnetic field strength of 14.1 T (600 MHz 1H frequency). A dual broadband probe was used. 1H NMR spectra were acquired at room temperature using a single pulse excitation with a 45° flip angle of 3.6 μ seconds and an acquisition time of 1.7 seconds. The repetition time was 1.0 seconds. It should be noted that as DEX interacts with the RNTs, the corresponding proton integration and diffusion coefficient decrease proportionally as a result of the large relaxation time of the self-assembled RNTs.
Atomic force microscopy (AFM)
AFM experiments were carried out on a Digital Instruments/Veeco Instruments MultiMode Nanoscope IV AFM (Plainview, NY) equipped with an E scanner. Silicon cantilevers (MikroMasch USA, Inc., San Jose, CA) with low spring constants of 4.5 N/m were used in tapping mode with a scan rate of 0.5–1 Hz and amplitude setpoint of 1 V. K1 (20 μL of a 50–250 μg/mL solution) with/without DEX were spin-coated on freshly cleaved mica at 2,000 rpm for 20 seconds and dried in air prior to imaging.
UV-Vis spectroscopy
For UV-Vis experiments (Agilent 8453, Agilent Technologies, Santa Clara, CA), the DEX:K1 (5:3 molar ratio) stock solution containing 4.07 mg of the drug-loaded RNTs in methanol (see above) was diluted to 25 μg/mL with methanol. Solutions of DEX (5 μg/mL) and K1 (25 μg/mL) in methanol were prepared, as well as the same three solutions in dH2O.
DEX loading and release studies
Glass coverslips (Fisher Scientific, Waltham, MA: circular; diameter, 18 mm; thickness, 1 mm) were cleaned with methanol, acetone and water in a sonicator. Three groups were prepared in dH2O: a) glass coverslips were dipped in a water-soluble DEX solution (1 mg/mL, Sigma, St Louis, MO, D2915); b) glass coverslips were first soaked in a K1 solution (100 μg/mL) and after air-drying, they were then immersed into the water-soluble DEX solution (1 mg/mL); and c) glass slides were dipped in a K1 solution (100 μg/mL) with 1 mg/mL water-soluble DEX. Then, all three groups were air-dried and incubated at 37°C in a PBS buffer (3 mL). Aliquots were then taken from the supernatant on a daily basis over a period of 9 days. The DEX solution was mixed with cupric ions in a pH 11 buffer. Because of the reduction of cupric ions, a cuprous cation was produced and spectrophotometrically detected by incorporating with 2-(4-carboxyquinolin-2-yl) quinoline-4-carboxylic acid (Pierce Biotechnology, Rockford, IL). DEX concentrations were quantified by comparing with the standard curve. The accumulated DEX release curve was presented as the result.
Contact angle measurements
To confirm the adsorption of K1 on the glass slides, the contact angles of 100 μL water droplets were measured before and after coating with a K1 solution (100 μg/mL) using a static contact angle meter (KRÜSS, FM40, Hamburg, Germany). An accurate auto pipette was used to ensure that the volume of the sample (20 μL) remained constant across glass slides. The contact angles were measured approximately 30 seconds after the droplets were placed on the surfaces.
Bioactivity
Osteoblasts (bone-forming cells, ATCC, CRL-11372 population numbers <9) were seeded at 1,000 cells/cm2 in well plates and were cultured in Dulbecco’s modified Eagle medium (DMEM) medium (Gibco/BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and 1% penicillin/streptomycin (P/S; Hyclone, Logan, UT) under standard cell culture conditions (37°C, humidified, 5% CO2/95% air environment). DEX-containing samples (10 μL) (see controlled release experiments) and freshly prepared and sterilized DEX (10−8 M) were added to the wells along with the cell culture medium. After the prescribed time period, non-adherent cells were removed by washing twice with phosphate buffered saline (PBS). At the end of 1, 3 and 5 days, osteoblasts were fixed with 10% normal buffered formalin (NBF; Fisher Scientific, Waltham, MA), stained with 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen, Carlsbad, CA), and counted at five random fields of view for each well under a fluorescence microscope (Zeiss Axiovert 200M, Peabody).
Statistical analysis
Data were expressed using the standard error of the mean (SEM). Statistics were performed using a Student’s one-tailed t-test, with P < 0.05 considered statistically significant.
Results
Characterization of Drug-loaded RNT-K1
NMR and DOSY were used to characterize the incorporation between K1 and DEX. Due to their large molecular weight and long relaxation time, K1 and K1-encapsulated DEX were not detectable by 1H NMR spectroscopy. Therefore, the percentage of drug loading could only be inferred from differences in peak heights of the drug relative to an internal standard (t-BuOH). As shown in , at the DEX: K–G∧C molar ratio of 5:3, approximately 32% of DEX was captured by K1. In agreement with this result, a significant change in the diffusion coefficient of DEX was observed in the DOSY spectra ().
Table 1 1H NMR spectroscopy of DEX and RNT-encapsulated DEX
Table 2 DOSY NMR of DEX only and RNT-encapsulated DEX
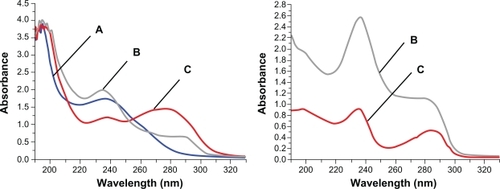
Tapping mode atomic force microscopy (AFM) showed significantly different height profiles for K1 before and after incorporation of DEX (). In addition, the UV-Vis spectra recorded in methanol showed a significant hypochromic effect at 280 nm and a hyperchromic effect around 240 nm. In water, on the other hand, a dramatic hyperchromic effect was observed around 280 and 240 nm suggesting that DEX significantly altered the stacking interaction in K1 ().
Figure 2 Tapping mode AFM height profiles of (A) RNTs and (B) RNT-encapsulated DEX. Average heights measured for RNTs and RNT-DEX complexes were 2.91 ± 0.95 nm and 7.04 ± 0.33 nm, respectively.
Abbreviations: AFM, Atomic force microscopy; RNT, rosette nanotubes; DEX, dexamethasone.
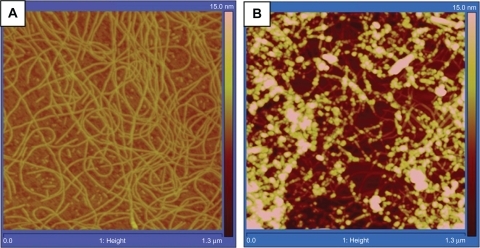
Figure 3 Comparison of UV-Vis absorbance curves in methanol (Left) and water (Right). A) 0.005 mg/mL DEX, B) 0.025 mg/mL DEX-RNTs and C) 0.025 mg/mL RNTs. There is no UV absorbance curve of DEX only in water, because DEX precipitates in water.
Abbreviations: RNT, rosette nanotubes; DEX, dexamethasone.
Drug loading and release experiments
For drug loading experiments, K1 samples with/without incorporation of DEX were prepared on glass slides. To confirm that K1 adhered to the glass slide surfaces, contact angle measurements were carried out. A notable decrease in contact angles (from 54.1 ± 2.1 to 43.9 ± 3.5) for the K1-coated slides was measured confirming that the hydrophilicity of the glass slide surface increased upon K1 adsorption.
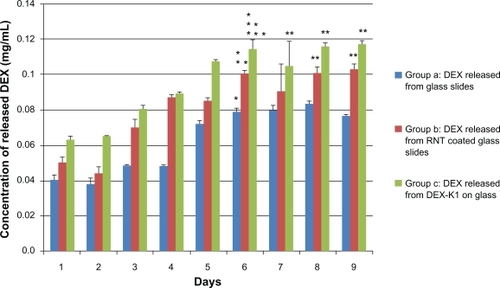
To further test drug loading ability and release kinetics of K1, a release experiment was conducted in a physiological environment: specifically, in water buffer and at 37°C incubation. Two approaches were used to incorporate DEX with K1. The first approach consisted of physically adsorbing K1 onto a glass slide and then dipping the slide in a water-soluble DEX solution. The second approach consisted of assembling K1 in the water-soluble DEX solution and then adsorbing the complex on a glass slide. Results showed that K1 in the first approach retained approximately 24.8% more DEX than the control sample (uncoated glass slide dipped in water-soluble DEX solution) and released DEX up to 6 days. Using the second approach, K1 incorporated approximately 42% more DEX than the control sample ().
Figure 4 Accumulated DEX release curves up to 9 days.
Note: Data are mean ± SEM (n =12). *P < 0.05 compared to DEX concentrations at day 1 to 5; **P < 0.05 compared to group (a) at respective days; ***P < 0.05 compared to group (a) and group (b) at the respective day.
Abbreviations: DEX, dexamethasone; SEM, standard error in the mean.
Drug bioactivity tests
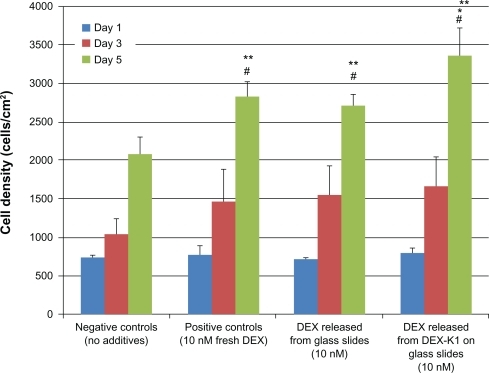
After establishing the ability of K1 to incorporate and slowly release drugs, one important concern is whether the released drugs are still bioactive. To test the bioactivity of the drug, commercially available fresh DEX, along with released DEX from glass slides and from K1 were used as additives separately for osteoblast culture. As shown in , osteoblast densities from the three groups with DEX were higher relative to the negative controls (without DEX). Importantly, DEX released from K1 increased osteoblast density the most.
Figure 5 Osteoblast density cultured with released DEX.
Notes: Data are mean ±SEM (n =9). *P < 0.05 compared to both controls and DEX released from glass slides at day 5; **P < 0.05 compared to negative controls (no additives) at day 5; #P < 0.05 compared to day 1 and 3 results on respective samples.
Abbreviations: DEX, dexamethasone; SEM, standard error in the mean.
Discussion
Traditional drug delivery systems involve chemical or physical modification to bind with specific drugs for their controlled release. Chemical modification aims to establish chemical bonds (such as covalent bonds) between the drug and the carriers. Physical adsorption employs simple procedures, but gives low drug loading and fast release under physiological conditions, thus resulting in limited cell-drug interactions.Citation24,Citation25 However, RNTs have unique design features that make them attractive for drug delivery purposes. Their hydrophobic core resulting from base stacking and self-assembly attracts and entraps small hydrophobic molecules behind a hydrophilic and biocompatible outer surface. Entrapped, water insoluble drugs can thus be delivered under physiological conditions to bone cells.Citation11,Citation12
In this study, DEX was chosen as a model drug because of its wide use in orthopedic applications since it is an anti-inflammatory agent and can control stem cell differentiation into osteoblasts.Citation26,Citation27 DOSY NMR and AFM suggested the incorporation of DEX in K1. Moreover, since UV-Vis spectroscopy showed a significant hyperchromic effect upon DEX encapsulation by K1 in water, any change in K1’s UV-Vis spectrum may be associated with the formation of a stable DEX-K1 complex, possibly via intercalation/stacking interactions.
To further understand how DEX is incorporated into K1 and to determine the DEX loading and release behavior, the DEX-K1 complex was prepared using two different methods: 1) adsorbing DEX onto K1 coated glass slides; and 2) assembling K1 with DEX solution. Results showed that the second method led to a larger amount of DEX loading, and a prolonged release under physiological conditions (37°C in aqueous buffer). These results support the idea that DEX is entrapped inside the hydrophobic core of K1.
Equally important is the biological activity of the released DEX. Since DEX has been reported to increase cell density,Citation22,Citation28 we investigated osteoblast cell cultures with no additives (negative controls), commercially available water-soluble DEX (positive controls), released DEX from uncoated glass slides, and released DEX from the DEX-K1-coated glass slides. Higher osteoblast densities from the three groups with DEX were observed compared to the negative control (without DEX). In addition, DEX-K1-coated glass slides not only exerted the same biological effect as the drug released from DEX-coated glass slides, but also promoted higher osteoblast density, in agreement with earlier reports.Citation11,Citation12,Citation17,Citation18,Citation29
In summary, these results suggest that DEX was incorporated into K1 in the process of self-assembly by hydrophobic and base stacking interactions. Thus, for orthopedic applications, RNTs not only enhance osteoblast adhesion and subsequent functions (such as calcium deposition) as previously shown,Citation11,Citation12,Citation20 but they can also incorporate water-insoluble drugs (such as DEX) and release them in physiological media over an extended period of time.
Conclusions
We have demonstrated that RNTs have unique chemical and physical properties that make them particularly attractive for drug delivery applications. For instance, they can be modified: a) chemically with various peptides to tailor them for specific tissue-engineering applications; b) physically, by drug-entrapment within their core; and c) structurally, by synthetically altering their dimensions (length, diameter) to accommodate a broad range of therapeutic modalities.
The incorporation of DEX into K1 was established by 1H-NMR spectroscopy, DOSY NMR, UV-Vis spectroscopy, AFM, and in vitro osteoblast cultures. This study demonstrated that K1, a member of the RNT family, can encapsulate DEX via a simple self-assembly process, and release it under physiological conditions, over an extended period of time spanning 9 days. The released drug enhanced the established biological activity of K1. We envision, as a result, several applications in drug delivery for the RNT platform.
Acknowledgements
This work was supported by the National Research Council of Canada, the Natural Science and Engineering Research Council of Canada, and the Hermann Foundation. The authors also thank Dr Jae-Young Cho for assistance with AFM measurements.
Supplementary figures
Figure S1 1H spectrum of DEX control (before incorporating with RNTs).
Abbreviations: RNT, rosette nanotubes; DEX, dexamethasone.
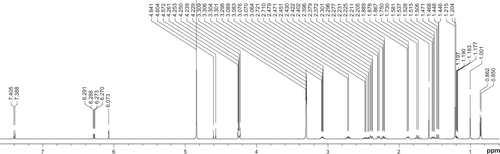
Figure S2 1H spectrum of DEX/RNT composites (after incorporating with RNTs).
Abbreviations: RNT, rosette nanotubes; DEX, dexamethasone.
Disclosure
The authors report no conflicts of interest in this work.
References
- CharnleyJAnchorage of the femoral head prosthesis to the shaft of the femurJ Bone Joint Surg Br196042-B283013855642
- SatoNKuboKYamadaMOsteoblast mechanoresponses on Ti with different surface topographiesJ Dent Res200988981281619767577
- ShiGARenLFWangLZLinHSWangSBTongYQH2O2/HCl and heat-treated Ti-6Al-4V stimulates pre-osteoblast proliferation and differentiationOral Surg Oral Med Oral Pathol Oral Radiol Endod2009108336837519716504
- KuoYCYehCFYangJTDifferentiation of bone marrow stromal cells in poly(lactide-co-glycolide)/chitosan scaffoldsBiomaterials200930346604661319712972
- VidigalGMJrGroismanMGregórioLHSoares GdeAOsseointegration of titanium alloy and HA-coated implants in healthy and 1 animals: a histomorphometric studyClin Oral Implants Res200920111272127719832768
- BalasundaramGWebsterTJNanotechnology and biomaterials for orthopedic medical applicationsNanomedicine (Lond)20061216917617716106
- BalasundaramGWebsterTJAn overview of nano-polymers for orthopedic applicationsMacromol Biosci20077563564217477446
- BozicKJKurtzSMLauEOngKVailTPBerryDJThe epidemiology of revision total hip arthroplasty in the United StatesJ Bone Joint Surg (Am)20099112813319122087
- ChanCKKumarTSLiaoSMuruganRNgiamMRamakrishnanSBiomimetic nanocomposites for bone graft applicationsNanomedicine (Lond)20061217718817716107
- ChoksiANPoonawallaTWilkersonMGNanoparticles: a closer look at their dermal effectsJ Drugs Dermatol20109547548120480790
- ChunALMoralezJGFenniriHWebsterTJHelical rosette nanotubes: a biomimetic coating for orthopedics?Biomaterials200526357304730916023193
- ChunALMoralezJGFenniriHWebsterTJHelical rosette nanotubes: A more effective orthopaedic implant materialNanotechnology200415s234s239
- FenniriHMathivananPVidaleKLHelical rosette nanotubes: design, self-assembly and characterizationJ Am Chem Soc2001123163854385511457132
- JourneayWSSuriSSMoralezJGFenniriHSinghBRosette nanotubes show low acute pulmonary toxicity in vivoInt J Nanomedicine20083337338318990946
- JourneayWSSuriSSMoralezJGFenniriHSinghBLow inflammatory activation by self-assembling Rosette nanotubes in human Calu-3 pulmonary epithelial cellsSmall20084681782318535989
- SuriSARakotondradanyFMylesAJFenniriHSinghBThe role of RGD-tagged rosette nanotubes in the induction of inflammation and apoptosis in human adenocarcinoma cells through the p38 MAPK pathwayBiomaterials200930173084309019250666
- ZhangLChenYRodriguezJFenniriHWebsterTJBiomimetic helical rosette nanotubes and nanocrystalline hydroxyapatite coatings on titanium for improving orthopedic implantsInt J Nanomedicine20083332333318990941
- ZhangLRakotondradanyFMylesAJFenniriHWebsterTJArginine-glycine-aspartic acid modified rosette nanotube-hydrogel composites for bone tissue engineeringBiomaterials20093071309132019073342
- SongSChenYYanZFenniriHWebsterTJSelf-assembled rosette nanotubes for incorporating hydrophobic drugs in physiological environmentsInt J Nanomedicine201110610110721289987
- Guzmán-MoralesJEl-GabalawyHPhamMHEffect of chitosan particles and dexamethasone on human bone marrow stromal cell osteogenesis and angiogenic factor secretionBone200945461762619540373
- BeuleAGSteinmeierEKaftanHEffects of a dexamethasone-releasing stent on osteoneogenesis in a rabbit modelAm J Rhinol Allergy200923443343619671262
- YangLTaoTWangXEffects of dexamethasone on proliferation, differentiation and apoptosis of adult human osteoblasts in vitroChin Med J (Engl)200311691357136014527365
- GrigoriadisAEHeerscheJNAubinJEDifferentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasoneJ Cell Biol19881066213921513384856
- YangLWebsterTJNanotechnology controlled drug delivery for treating bone diseasesExpert Opin Drug Deliv20096885186419637973
- GindyMEPrud’hommeRKMultifunctional nanoparticles for imaging, delivery and targeting in cancer therapyExpert Opin Drug Deliv20096886587819637974
- ShortDJEl MasryWSJonesPWHigh dose methylprednisolone in the management of acute spinal cord injury – a systematic review from a clinical perspectiveSpinal Cord200038527328610822400
- MorimotoDKurodaSKizawaTEquivalent osteoblastic differentiation function of human mesenchymal stem cells from rheumatoid arthritis in comparison with osteoarthritisRheumatology (Oxford)200948664364919398485
- GuerrieroVFloriniJRDexamethasone effects on myoblast proliferation and differentiationEndocrinology19801064119812027188899
- ChenYBilgenBParetaRASelf-assembled rosette nanotube/hydrogel composites for cartilage tissue engineeringTissue Eng Part C Methods20101661233124320184414