 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Lung cancer is the most common cancer and the leading cause of total deaths worldwide. Its classified into two major types including non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC) based on the origin of abnormal lung cells as well as the smoking status of the patient. NSCLC is the most common and aggressive type of lung cancer representing 80%–85% of all cases.
Purpose
The aim of the study was to present lyotropic liquid crystalline nanoparticles (LCNPs) as promising carriers for co-delivery of the chemotherapeutic agent, pemetrexed (PMX) and the herbal drug, resveratrol (RSV) for effective lung cancer management.
Methods
The proposed PMX-RSV-LCNPs were prepared by hydrotrope method. Hydrophobic ion pairing with cetyl trimethyl ammonium bromide (CTAB) was implemented to increase the encapsulation efficiency of the hydrophilic PMX up to 95%±3.01%.
Results
The tailored PMX-RSV-LCNPs exhibited a particle size of 173±0.26 nm and biphasic release pattern with a relatively initial burst release within first 3–4 hour followed by sustained release up to 24 hours. Moreover, PMX-RSV-LCNPs manifested superior concentration and time dependent cytotoxicity profile against A549 lung cancer cells with IC50 4.0628 µg/mL. Besides, the enhanced cellular uptake profile based on bioadhesive properties of glyceryl monoolein (GMO) as well as energy independent (cholesterol dependent) pattern. In-vivo evaluations against urethane induced lung cancer bearing mice demonstrated the potentiality of PMX-RSV-LCNPs in tumor growth inhibition via inhibition of angiogenesis and induction of apoptosis. The results were supported by histopathological analysis and immunohistochemical Ki67 staining. Moreover, PMX-RSV-LCNPs displayed a promising safety profile via attenuating nephro- and hepatotoxicity.
Conclusion
PMX-RSV-LCNPs elaborated in the current study hold a great promise for lung cancer treatment.
Introduction
Lung cancer is one of the most dreadful cancers for both men and women.Citation1 American Cancer Society estimated that in 2018 lung and bronchus cancers would be responsible for 234,030 new cases which represent 14% of all new cancer cases and 154,050 deaths.Citation2 Lung cancer is mainly classified into non-small-cell lung cancer (NSCLC; major and common form responsible for 85% cases) and small-cell lung carcinoma.Citation3,Citation4 Despite the researcher’s attempts to improve outcomes among lung cancer patients, results are still unsatisfactory.Citation5 For advanced stages of lung cancer, chemotherapy is considered the first line of treatment which is usually administered intravenously via systemic circulation.Citation4,Citation6 However, conventional chemotherapy either used alone or in combination with others faces many obstacles that limit its use. Most of the chemotherapeutic agents have poor aqueous solubility, lack of selectivity, and extensive metabolism via first-pass effect which in turn require higher doses to achieve the therapeutic level that subsequently affect tumor cells as well as normal healthy cells.Citation7–Citation9
Alimta® (pemetrexed, PMX, Eli Lilly and Company, Indianapolis, Indiana, USA), a promising Food and Drug Administration (FDA)-approved chemotherapeutic agent as monotherapy for the maintenance treatment of NSCLC, is also considered as the first-line treatment in combination with cisplatin.Citation10 PMX disodium (C20H19N5Na2O6) is a white freely water-soluble crystalline powder with a molecular weight of 471.37 g/mol.Citation11 PMX is a potent multi-targeted antifolate agent that exerts its action by disrupting de novo biosynthesis of thymidine and purine nucleotides essential for cell replication. The use of PMX, either as monotherapy or in combination, is accompanied with many undesirable adverse effects including bone marrow suppression manifested as anemia, neutropenia, leukopenia, and thrombocytopenia as well as nephro- and hepatotoxicities.Citation12–Citation16 Moreover, PMX is rapidly eliminated and unchanged by renal tubular secretion and to a lesser extent by glomerular filtration. In addition, it undergoes limited hepatic metabolism with terminal half-life between 2 and 5 hours.Citation12 Therefore, encapsulating PMX in a suitable drug delivery system seems to be a promising approach to surmount PMX pharmaceutical obstacles and improve its clinical utility.
Recently, the use of herbal drugs has magnetized the researchers’ attention based on their great tendency in sensitizing tumor cells and imparting synergistic activity to chemotherapeutic agents.Citation17–Citation19 Among promising herbal drugs, resveratrol (RSV) proved its great capability in protecting against cancer.Citation20 RSV (3,5,4′-trihydroxystilbene) is a poly-phenolic phytoalexin produced in various plants in response to stress.Citation21,Citation22 RSV has several activities including antioxidant, anti-inflammatory, proapoptotic, antiproliferative, antiangiogenesis, and chemopreventive properties.Citation21–Citation24 In addition, it plays a vital role in overcoming multidrug resistance (MDR) associated with most of the chemotherapeutic agents. This was attributed to its ability to prohibit P-glycoprotein (P-gp) activity, thus potentiating intracellular accumulation of coadministered chemotherapeutic payloads.Citation25,Citation26A promising synergistic combination of RSV and PMX was previously reported by Chen et al,Citation27 where PMX significantly decreased the expression of ERCC1, phospho-p38 MAPK protein levels, and the DNA repair capacity, thus enhancing the RSV-induced cytotoxic effect on NSCLC cells and proposing the potential outcomes of coadministering PMX and RSV for the treatment of lung carcinoma. However, RSV has many limitations including instability, poor water solubility, low bioavailability, and short biological half-time. In addition, rapid and extensive metabolism hindered the anticancer activity of RSV even in high doses.Citation28 Therefore, optimizing a nanoscale drug delivery system encapsulating both drugs could be of prime importance for enhanced lung cancer treatment.
Recently, researchers pay great attention to lyotropic liquid crystalline nanoparticles (LCNPs) as new lipid-based drug delivery systems for cancer therapy. They are self-assembled structures formed when several polar lipids including Glyceryl monooleate (GMO), phytantriol, glycolipids, and glycerates were dispersed in water in the presence of stabilizers. LCNPs possess bicontinuous lipid bilayer enclosing water channels. Their unique multi-compartmental structure enables them to efficiently deliver hydrophilic, hydrophobic, or amphiphilic molecules.Citation29–Citation31 The biocompatibility, biodegradability, bio-adhesion, and prolonged drug release characteristics make LCNPs suitable carriers for cancer therapy. Their inherent small particle size (PS) facilitates passive targeting via enhanced permeation and retention effect.Citation32–Citation35 Recently, Freag et alCitation30 successfully formulated surface-modified PEGylated aloe-emodin-loaded LCNPs (AE-polyethylene glycol [PEG]-LCNPs) for breast cancer treatment. The LCNPs investigated in their study demonstrated improved hemocompatibility, increased serum stability, and enhanced cytotoxicity and cellular uptake in breast cancer cells compared to free drug.
In view of the abovementioned information, the current study focused, for the first time, on the fabrication of LCNPs for the combined delivery of both PMX and RSV. The proposed formulation was evaluated in vitro and in vivo for their efficacy in lung cancer treatment. In vitro cytotoxicity and cellular uptake studies were performed using A549 lung carcinoma cells. In addition, in vivo antitumor activity of PMX-RSV-LCNPs was evaluated in urethane-induced lung cancer-bearing mice.
Materials and methods
Materials
PMX (purity 98%) and RSV were purchased from Baoji Guokang Bio-Technology Co., Ltd (Baoji, People’s Republic of China). Peceol® (GMO) was kindly provided by Gattefosse (Saint-Priest, France). Human A549 lung cancer cell line was purchased from Egyptian Organization for Biological Products and Vaccines (VACSERA Holding Company, Giza Governorate, Egypt). Poloxamer-407 (P407), cetyltrimethylam-monium bromide (CTAB), DMEM, FBS, and MTT were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Caspase 3 (CASP-3) ELISA Kit was purchased from WKEA Med Supplies Corp. (Chaoyang District, Changchun, Jilin, People’s Republic of China). Vascular endothelial growth factor (VEGF) ELISA kit, RayBio® (catalog number: ELR-VEGF-001) was purchased from RayBio Tech Inc. (Norcross, GA, USA). All other reagents and chemicals were of analytical grade.
Methodology
Preparation of PMX-RSV-loaded LCNPs
PMX-RSV-LCNPs were fabricated via the hydrotrope method.Citation36 Briefly, RSV (10 mg) was dissolved in an isotropic mixture of 200 mg GMO and 0.18 mL absolute ethanol. The resulting isotropic solution was added dropwise onto 2 mL of P407 solution (0.5% w/v) containing 10 mg PMX under magnetic stirring. The mixture was equilibrated at room temperature for 24 hours and then dispersed into another 6 mL of P407 solution (0.5% w/v) followed by homogenization at 13,000 rpm for 3 minutes to finally obtain the LCNP dispersion.
Different formulation variables including the type of hydrotrope (ethanol, PEG 400, propylene glycol), concentration of ethanol, P407 concentration, and percentage loading of PMX and RSV were investigated. The influence of different variables on PS, size distribution, zeta potential, and encapsulation efficiency of the prepared LCNPs was investigated.
For cellular uptake studies, fluorescent-labeled LCNPs were prepared, where 0.08% w/w coumarin-6 (with respect to lipid) was added to the isotropic mixture of GMO and absolute ethanol and then processed as mentioned earlier.
Preparation of ion-paired PMX-RSV-loaded LCNPs
To increase the hydrophobicity of PMX and improve its lipid solubility, in situ hydrophobic ion pairing (HIP) of PMX with the cationic surfactant CTAB was performed following the previously reported protocol.Citation37,Citation38 Briefly, 34 mg of CTAB was added with 10 mg PMX into P407 solution prior to the addition of the isotropic (GMO and ethanol) mixture and then processed as mentioned earlier. Optimization of the in situ ion-pairing process involved the investigation of different PMX:CTAB molar ratios of 1:2 and 1:4 on the % entrapment efficiency (% EE) of PMX.
Development of novel HPLC method for simultaneous determination of PMX and RSV
A novel, accurate, precise, and sensitive HPLC method was developed and validated for simultaneous determination of both RSV and PMX using Agilent 1260 Infinity HPLC system. Details of the test are provided in Supplementary materials.
Characterization of LCNPs
EE of PMX and RSV
The %EE of different LCNP formulations was determined using the dialysis bag method following a protocol previously reported by Swarnakar et alCitation39 Briefly, 1 mL of LCNP dispersion was transferred to dialysis bag. The bags were then placed in 50 mL PBS and subjected to centrifugation (Model 3 K-30; Sigma Laboratory Refrigerated Centrifuge, Osterode am Harz, Germany) at 500 rpm for 30 minutes to separate free PMX and RSV. The procedure was optimized and validated in-house for complete separation of free drugs from the formulation. The actual concentrations of PMX and RSV entrapped within LCNPs represent the difference between the initial amounts added during LCNP fabrication and free unentrapped drug separated after centrifugation (EquationEquation 1(1) ). The concentrations of PMX and RSV were quantified using HPLC at λmax=225 and 306 nm, respectively.
PSand zeta potential
The mean PS, polydispersity index (PDI), and zeta potential (δ-potential) of LCNP formulations were measured via dynamic light scattering (DLS) and electrophoretic mobility technique using Zetasizer (Nano ZS/ZEN3600 Zetasizer; Malvern Instruments, Malvern, UK). The samples were diluted with distilled water (1:50) to obtain final experimental values in triplicate at room temperature. Results were shown as mean size ± SD.Citation40
Study of the phase behavior of LCNPs
Investigation of the phase behavior of both placebo and dual drug-loaded formulations was performed using a polarizing microscope. Micron-sized liquid crystalline particles were fabricated as described earlier but without the size reduction homogenization step. After that, a drop of the dispersion was placed onto a slide and imaged under polarizing light microscope (Zeiss Axiovert 40 MAT microscope fitted with camera) at magnification power of 100× to evaluate the occurrence of birefringence.Citation41
Transmission electron microscopy (TEM)
The morphology of ion-paired PMX-RSV-LCNPs was examined via TEM (Jeol JEM-2100; JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.Citation42 After sample dilution with distilled water (1:20), a drop of the sample was placed onto a carbon-coated copper grid and left for air-drying. Then, the films were visualized under TEM and photographed.
In vitro release study
In vitro drug release profile from different LCNP formulations was performed using the dialysis bag method.Citation43 Samples investigated encompassed free aqueous solution of PMX, free ethanolic solution of RSV, PMX-RSV-LCNPs (F8), and ion-paired PXM-RSV-LCNPs (F10). Samples were added into a sealed dialysis bag (molecular weight cutoff [MWCO]: 12–14 kDa). The dialysis bags were placed in 100 mL phosphate buffer, pH 7.4, and incubated at 37°C with a shaking speed of 100 rpm in shaking water bath. Aliquots (2 mL) were withdrawn at different time intervals (0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours) followed by compensation with the same volume of fresh release medium. The samples were filtered using 0.45 µm Millipore filter (Millipore®; Thermo Fisher Scientific, Waltham, MA, USA) and then quantified for their PMX and RSV content using validated HPLC method as mentioned earlier.
In vitro anticancer study
Cell culture
A lung carcinoma cell line A549 was used for examining in vitro cytotoxicity of the proposed formulations. For the propagation of A549 cells, American Type Culture Collection (ATCC) complete growth medium (Ham’s F12K medium with 2 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 90%; FBS, 10%) was utilized. The cells were grown in 75 mL flasks in an atmosphere of 5% CO2 and 100% relative humidity and subcultured two to three times per week.
In vitro cytotoxicity
In vitro cytotoxicity of free PMX, free RSV, free combined PMX/RSV solution, blank LCNPs, and ion-paired PMX-RSV-LCNPs (F10) was evaluated in A549 lung cancer cells using MTT assay.Citation44 Details of the test are provided in Supplementary materials.
In vitro cellular uptake
A549 lung cancer cells were seeded on CELL view™ slide at a density of 3,000 cells/well and left overnight to allow enough time for cell attachment. Cells were incubated with fresh medium containing free coumarin-6 dye and coumarin-6-loaded LCNPs at the concentration of 0.08% w/w coumarin-6 (with respect to lipid). Details of the test are provided in Supplementary materials.
In vivo study
Animals
The guidelines developed by the Animal Care and Use Committee, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt, and in accordance with regulations of the National Research Council’s guide for the care and use of laboratory animals were adopted for in vivo study. Twenty-eight Balb/c male mice (15±5 g, 3–4 weeks) were housed in stainless steel cages in four groups (seven mice per group). The animals were kept under standard environmental conditions (23°C±1°C, 55%±5% humidity and 12 hours/12 hours light/dark cycle) and maintained with free access to standard laboratory diet (balanced nutrient of chow and water). Lung cancer was induced within mice via intraperitoneal injection of chemical carcinogen and urethane (ethyl carbamate, 1 g/kg) followed by post-dose of urethane (0.5 g/kg) after 15 days. The mice were left for 12–16 weeks to ensure complete induction of lung cancer. After that, experimental model of mice was sacrificed to evaluate tumor incidence and precancerous lesions prior to exposing rest of mice to treatment. The excised lungs were stained with H&E and undergone histopathological and immunohistochemical investigations.
Evaluation of antitumor efficacy
After tumor induction, 28 mice were classified randomly into four groups, 7 mice each. The groups encompassed as follows: positive control group (untreated tumor-bearing mice), negative control group (healthy mice treated with saline), free PMX/RSV-treated group, and ion-paired PMX/RSV-LCNP (F10)-treated group. All animals were administered their formulation via intravenous (IV) route into the tail vein. Animals of the last two groups were injected with equivalent concentration of PMX and RSV (0.94 mg/kg) twice per week for 21 days. Free PMX/RSV mixture was dissolved in cosolvent (dimethyl sulfoxide/PEG 400/saline 0.5/4.5/5 v/v/v). At 25th day, the mice were sacrificed and the excised lungs were weighted and divided for tumor growth biomarker measurement as well as histopathological and immunohistochemical examinations.
Evaluation of tumor growth biomarkers
The excised parts of lung tumors were carefully weighed and homogenized in cold PBS using tissue homogenizer to make a final 40% tissue homogenate. This homogenate was centrifuged at 1,700 rpm at 4°C for 10 minutes, and the supernatants were divided into separate small aliquots and stored at -80°C for further quantitative determination of tumor growth biomarkers. The degree of angiogenesis was determined via detecting the level of the angiogenic VEGF using “VEGF ELISA Kit” (RayBio Tech Inc.), while the induction of apoptosis was confirmed by determining the level of tissue CASP-3 using “caspase-3 (CASP-3) ELISA Kit” (WKEA Med Supplies Co.). All the markers were quantified according to the manufacturer’s protocol.
Histopathological studies
A part of excised lung tumors was preserved and fixed with 10% neutral formalin for 24 hours, then stained with hematoxylin for 5 minutes and eosin for 2 minutes (H&E), dehydrated in alcohol, and mounted in Canada balsam before microscopical examination. The tumors obtained from different groups were examined in ten random sections (×40) for histopathological neoplastic changes. Moreover, the average number of microscopic metastatic lung foci and their diameter were determined.
Immunohistochemical analysis
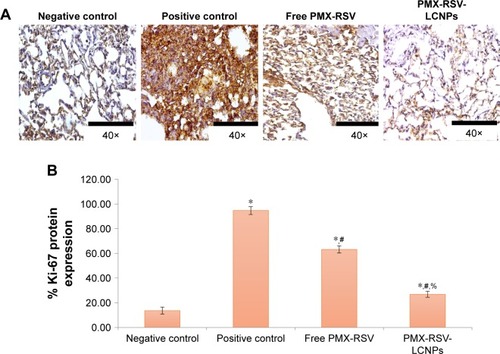
The previously conserved excised lungs in formalin and embedded in paraffin as blocks were cut and rinsed using xylene and then rehydrated before staining steps. Methanolic solution of hydrogen peroxide (7%) was added for 20 minutes, then left for 5 minutes in heated citrate phosphate buffer (pH 6.0) using microwave then immersed in milk/distilled water (5%) for 45 minutes. Individual treatment of sections was performed using Ki-67 antibody at 4°C overnight. After that, the sections were washed and retreated with a secondary anti-mouse biotinylated antibody in a dark room for 1 hour. Nuclear staining was carried out to localize cellular Ki-67 immunoreactivity. Each tumor sample was examined for Ki-67 expressions in ten random sections (×40) in which the number of positive cells/total was calculated. Moderate-to-strong brownish cellular cytoplasmic staining was accounted for positive, whereas unstained or faint stained cellular cytoplasm was accounted for negative.
Statistical analyses
Statistical analysis of in vitro results was carried out using Student’s t-test (P≤0.05; GraphPad Prism version 3.02; GraphPad Software, Inc., La Jolla, CA, USA). However, the results of the in vivo antitumor activity were analyzed using IBM SPSS Software package (version 20; IBM Corporation, Armonk, NY, USA). Statistical significance between the groups was analyzed using ANOVA and Tukey’s multiple comparison tests. Statistical significance was judged at the 5% level.
Results and discussion
Preparation of ion-paired PMX-RSV-LCNPs
The aim of the current study was to develop lyotropic LCNPs for the co-delivery of PMX and RSV. Thanks to the unique structure of LCNPs which facilitate incorporation of different types of drugs, both hydrophilic (PMX) and lipophilic (RSV) drugs were successfully loaded in the LCNPs. Herein, the hydrotrope method, previously developed by Spicer et al,Citation36 was adopted for the fabrication of LCNPs in which a hydro-trope was used to increase the aqueous solubility of poorly soluble GMO lipid. The dropwise addition of this hydro-tropic mixture to water containing P407 resulted in gradual decrease in the solubility of GMO resulting in instantaneous formation of stable liquid crystalline particles in a nanometric range. In addition, the hydrotrope method guaranteed the stability of the thermosensitive drug RSV bypassing the drastic conditions accompanied by conventional method including elevated temperature and pressure. The composition of the prepared LCNP formulations (F1–F10) is summarized in . As given in the table, PMX (F8) demonstrated relatively low %EE due to its anionic nature and high aqueous solubility (log P 1.54) that could result in its poor entrapment into lipid-based systems and subsequent partitioning into the continuous aqueous phase. Generally, sustained release of water-soluble drugs from nanocarrier delivery systems remains a challenging task. Many attempts have been exploited to overcome this challenge including chemical cross-linking and HIP.Citation45,Citation46 Therefore, in the current study, the HIP technique was applied to enhance the hydrophobicity of PMX and enhance its EE.Citation38 HIP technique is simply performed by the interaction between ionic drug and the opposite ionic head group of a fatty acid, surface-active agents, or other amphiphilic molecules at suitable pH without the modification of their chemical structures. In the current study, CTAB was selected as a counter-cationic surface-active agent for in situ formation of HIP with PMX. CTAB was added to the aqueous phase containing PMX, and the influence of different PMX:CTAB molar ratios on the %EE was investigated. As summarized in , :4 PMX:CTAB molar ratio (F10) showed the highest %EE of PMX (95%), so it was selected as an optimum ratio for further studies.
Table 1 Composition and physicochemical characterization of placebo and PMX-RSV-loaded LCNPs
Physicochemical characterization of LCNPs
PS
Effect of different hydrotropes
The effect of different hydrotropes including propylene glycol, PEG 400, and absolute ethanol on the quality attributes of the prepared formulations was investigated (F1–F3). The concentration of hydrotropes represented 70% w/w of the amount of GMO.Citation47 As summarized in , ethanol (F3) possessed a great tendency in reducing the viscosity of GMO which in turn facilitates prompt diffusion of the hydrotropic mixture into aqueous phase. This was evidenced by significant (P<0.05) decrease in both PS and PDI compared to (F1, propylene glycol; F2, PEG 400). Therefore, ethanol was selected as the suitable hydrotrope for further investigations.
Effect of ethanol concentration
The influence of other ethanol concentrations (F4, 50; F5, 100% w/w relative to total lipid) on PS and PDI was investigated. As summarized in , it was obvious that increasing solvent concentration was associated with significant reduction in PS and PDI (P<0.05). Increasing ethanol concentration beyond 70% w/w revealed insignificant decrease (P>0.05) in the PS. Therefore, ethanol at the concentration of 70% w/w was chosen as an optimum hydrotrope concentration which resulted in promising PS of 167 nm and PDI of 0.2 (F3).
Effect of stabilizer concentration
P407 is a triblock copolymer widely used in stabilizing LCNPs. This hydrophilic nonionic surfactant has a unique capability in adsorbing to the surface of LCNPs, thus inhibiting their aggregation. In addition to its great tendency in stabilizing internal structure of LCNPs through preventing transition to other mesophase structures, herein, the influence of three different concentrations of P407 (0.25%, 0.5%, and 1% w/v) was screened (F3, F6–F7). As summarized in , increasing P407 concentration from 0.25% to 0.5% w/v relative to total dispersion volume was associated with obvious reduction in PS and PDI. Increasing P407 concentration beyond 0.5% w/v resulted in insignificant reduction in PS (F3, 167 nm; F7, 150 nm). Therefore, 0.5% w/v P407 concentration was selected as the optimum concentration for additional investigations. Our results were in good agreement with Freag et alCitation47 who reported that 0.5% w/v P407 concentration relative to total dispersion volume was quite sufficient to stabilize LCNPs and maintain their internal structure.
Effect of percentage drug loading
In an attempt to minimize the dose of cytotoxic PMX and reduce its associated adverse effects, PMX and RSV were successfully loaded within LCNPs at the weight ratio of 1:1. The characteristic absorption bands of PMX and RSV () on the validated HPLC analysis () with good linearity ranging from 0.4 to 2 mg% PMX and RSV concentration () proved that both PMX and RSV were successfully loaded into LCNPs and the drug carrier was effective for the co-delivery of the two drugs. It was obviously observed that CTAB modification significantly increased %EE of PMX (). The average %EE of PMX and RSV in the optimized ion-paired nanoformulation was 95% and 98%, respectively. Physicochemical characterization results indicated that loading RSV and PMX did not affect the physicochemical properties of LCNPs; it was found that PMX-RSV-loaded LCNPs possess almost similar PS and PDI to blank LCNPs.
Zeta potential
All LCNPs that were formulated without CTAB (F1–F8) showed a negatively charged zeta potential around -34 mV although GMO is a neutral lipid, which could be explained by adsorption of free hydroxyl groups on the surface of LCNPs in addition to free oleic acid found in commercially available GMO.Citation48 A significant alteration in zeta potential upon the addition of CTAB was detected. As summarized in , ion-paired PMX-RSV-LCNPs (F9–F10) displayed a positively charged zeta potential which is sufficient for the colloidal stability and preventing aggregation.
Phase behavior
Polarized light microscope is commonly utilized to study the liquid crystalline phases depending on their textures. The hexagonal phases exhibit a fan-like texture, whereas the cubic phases show a dark diffraction pattern when viewed under a polarizing light microscope. Herein, both blank and PMX-RSV-loaded LCNPs demonstrated a dark diffraction patterns when examined under polarizing microscope reflecting the isotropic nature of the formulations.Citation41
Transmission electron microscopy
The surface morphology of optimized ion-paired PMX-RSV-LCNPs was visualized via TEM. As depicted in , LCNPs displayed spherical particles with an average PS of 180 nm which was in agreement with the measurements obtained by DLS.
Figure 1 Physicochemical characterization of optimized LCNPs.
Notes: (A) Transmission electron micrograph displaying morphology of ion-paired PMX-RSV-LCNPs (F10). (B) In vitro release of PMX and RSV from PMX-RSV-LCNPs (F8) and ion-paired PMX-RSV-LCNPs (F10) in comparison to free PMX and RSV solution in PBS (pH 7.4) at 100 rpm and 37°C using the dialysis bag diffusion method. (C) The influence of different CTAB:PMX molar ratios on the release of PMX from ion-paired LCNPs in PBS (pH 7.4) at 100 rpm and 37°C using the dialysis bag diffusion method.
Abbreviations: CTAB, cetyltrimethylammonium bromide; LCNPs, liquid crystalline nanoparticles; PMX, pemetrexed; RSV, resveratrol.
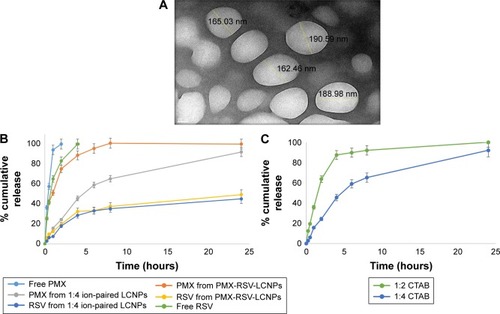
In vitro release study
In vitro release behavior of each free RSV, free PMX, PMX-RSV-LCNPs (F8), and ion-paired PMX-RSV-LCNPs (F9–F10) was examined in commonly utilized release media PBS (pH =7.4). As shown in , both free RSV and PMX exhibited burst release profile, a relatively rapid and entire release was observed after 2 and 4 hours for PMX and RSV, respectively. However, after incorporation of drugs into LCNPs, a sustained release behavior of both drugs was observed. The release behavior of RSV was characterized by a biphasic release pattern where a relatively initial burst release within first 3–4 hours was observed followed by sustained release up to 24 hours. The initial burst release could be explained via relatively large surface area-to-volume ratio provided by LCNPs, while the continuous sustained release of drugs from PMX-RSV-LCNPs could be referred to the diffusion of both drugs from the lipid core of bicontinuous bilayer of the liquid crystalline structure.Citation49 To check the effect the HIP process on the release behavior of PMX (), two formulations of modified PMX-RSV-LCNPs with two different concentrations of CTAB (F9–F10) were compared to unmodified PMX-RSV-LCNPs (F8). Compared to unmodified LCNPs, CTAB-modified LCNPs possess an additional controlled release of PMX and upon increasing the concentration of CTAB (F10), more sustained release behavior from regular lipid channels was observed. This may be attributed to the extra hydrophobic nature imparted to LCNPs by CTAB. Obviously, the proposed ion-paired PMX-RSV-LCNPs provided an acceptable sustained release pattern of PMX up to 24 hours. This sustained drug release behavior is an essential prerequisite to guarantee constant and controlled release of anticancer drugs within tumor cells which in turn reduce their systemic cytotoxicity.
In vitro cytotoxicity studies
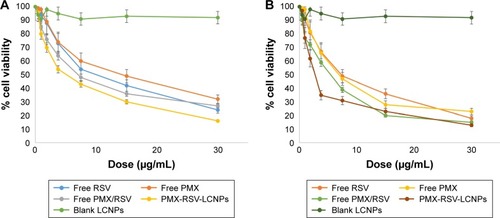
The cytotoxic influence of free RSV, free PMX, free RSV/PMX mixture, blank LCNPs, and ion-paired PMX-RSV-LCNPs (F10) on A549 lung cancer cell line was determined using MTT assay (). Better therapeutic outcomes for resistant tumor cells were mainly achieved via combinational therapy based on two or more anticancer agents. Coadministering of herbal drugs with potent anticancer drugs hold great promise, particularly, PMX and RSV have been reported for their in vitro cytotoxic activity on lung cancer cells.Citation27 Furthermore, the combined doses of PMX and RSV loaded into PMX-RSV-LCNPs were precisely determined by their dose dependency and relative amount of PMX vs RSV to A549 cells, to maximize the synergistic antitumor efficacy. Blank LCNPs demonstrated good cytocompatibility with insignificant cytotoxicity on A549 cancer cells after 24 hours () and 48 hours (; % cell viability >85%). RSV and PMX were evaluated at concentration ranged from 0.468 to 30 µg/mL. Free PMX and RSV exhibited a dose-dependent cytotoxic efficacy on the lung cancer cells. To check the synergistic activity between both drugs, the combined IC50 was calculated and is summarized in . As summarized in the table, free RSV/PMX mixture exhibited a lower IC50 value (4.5 µg/mL) compared to each free PMX and free RSV (IC50: 7.9 and 8.4 µg/mL, respectively). It was clearly observed that PMX-RSV-LCNPs had a significant superior cytotoxic activity on lung cancer cells compared to free RSV/PMX mixture. This superiority in the cytotoxicity of LCNP formulations could be attributed to the enhanced internalization of LCNPs facilitated by both bioadhesive property of GMO and energy-independent (cholesterol-dependent) uptake mechanism associated with LCNPs resulting into higher intracellular concentration of both RSV and PMX.Citation50 It is worth to mention that the cytotoxicity of PMX-RSV-LCNPs was more evident following 48-hour incubation than 24 hours which could be ascribed to the sustained release behavior of RSV and PMX imparted by LCNPs.
Table 2 IC50 of free drugs and optimized LCNPs after 24 and 48 hours using Compusyn® software
Figure 2 In vitro cytotoxicity of ion-paired PMX-RSV-LCNPs (F10) compared to blank LCNPs, free PMX, free RSV, and free PMX/RSV cosolvent on A549 NSCLC cell line at the concentration of 0.468–30 µg/mL expressed as % cell viability after (A) 24 hours and (B) 48 hours.
Abbreviations: LCNPs, liquid crystalline nanoparticles; NSCLC, non-small-cell lung cancer; PMX, pemetrexed; RSV, resveratrol.
Cellular uptake of optimized LCNPs
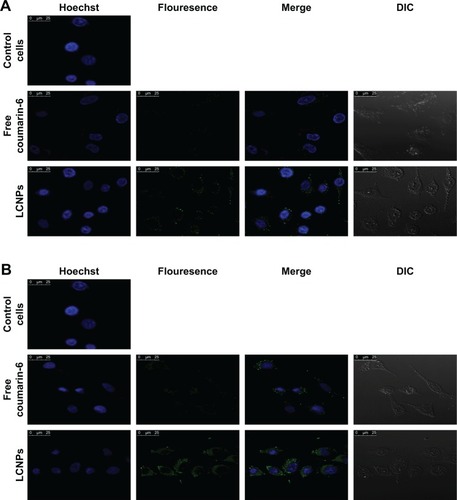
The qualitative cellular uptake study of the proposed LCNPs was carried out on A549 cell line using confocal microscopy. Coumarin-6 was used as a model hydrophobic fluorescent dye which could be easily entrapped within lipid bilayer of LCNPs. To assess the entrapment and retention of dye in LCNPs, the coumarin-6-loaded LCNPs were placed in centrifugal ultrafilter (Vivaspin® 6, molecular weight cutoff [MWCO]100 kDa; Vivaproducts, Inc, Littleton, MA). The ultrafilters were then centrifuged at 4,000 rpm for 30 minutes. The fraction of dye released (unentrapped) during 24 hours with all formulations was within 1%–2%, suggesting a good entrapment. Untreated A549 cells were used to exclude cellular autofluorescence. A549 cells were incubated with free coumarin-6 and coumarin-6-loaded LCNPs for 4 () and 24 hours (). As shown in the figure, LCNPs demonstrated superior cellular uptake compared to free dye. The increased intracellular concentration after 24 hours confirmed the time-dependent cellular uptake of LCNPs. To guarantee that ion-paired LCNPs were taken up and internalized into A547 and not only adsorbed onto the surface of cellular membrane, their uptake was evaluated using z-stack images. The z-direction image () displayed the cross-sectional uptake of coumarin-6-loaded LCNPs in which they were traveled from the apical surface of cellular membrane toward the basolateral membrane represented from z-0 to z-29. Image of z-29 represents maximum intensity projections of whole A549 cells. The fluorescence images manifested that LCNPs with a size of 173 nm move rapidly across the cellular membrane, distribute within the cytosol, successively move across different cellular planes, and localize in the perinuclear region. The video displaying z-stacks confirmed particle localization within the cells mainly within the cytoplasm and seldom dispersed in the nuclei was included as provided in Supplementary materials. The enhanced cellular uptake of coumarin-6-loaded LCNPs could be attributed to GMO bioadhesive and membrane-fusing features in addition to the unique structure of LCNPs in which water channels surrounded by bicontinuous lipid bilayer offer hydrophilic–hydrophobic pattern which facilitates interaction with hydrophobic cholesterol on cell surface.Citation50 In addition, it is worth mentioning that nanoparticle size, shape, and surface charge could possibly affect their interaction with the cellular membrane. It was previously reported that NPs with an average size between 200 and 350 nm could successfully distribute to different tissues, whereas NPs <70 nm were instantly excreted by the kidneys. Moreover, the positively charged surface imparted by CTAB could further facilitate cellular uptake by binding to the anionic surface proteoglycans via adsorptive-mediated endocytosis.Citation51 On the other hand, free coumarin-6 entered the cell via simple diffusion resulted in rapid saturation of intracellular region prohibiting further entry of free dye by time unlike LCNPs which entered via endocytosis resulted in gradual release of free dye thus evading intracellular saturation. These findings were in accordance with MTT assay in which optimized PMX/RSV-loaded LCNPs displayed higher cytotoxic effect than free PMX/RSV mixture.
Figure 3 Confocal microscopy images demonstrating cellular uptake of coumarin-6-loaded LCNPs relative to free coumarin-6 and control cells after incubation with A549 lung cancer cell line for (A) 4 hours and (B) 24 hours in which the first channel of nuclei is represented by blue stain of Hoechst, the second channel of free coumarin-6 or coumarin-6-loaded LCNPs is represented by green fluorescence, third channel of merge is represented by overlay between two channels, and finally the fourth channel of DIC is represented by the light microscopic image of A549 cells.
Abbreviations: LCNPs, liquid crystalline nanoparticles; DIC, differential interference contrast.
In vivo antitumor activity
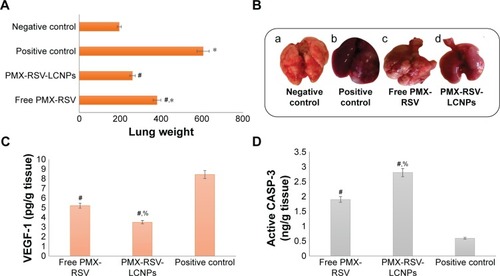
The purpose of this study was to investigate the antitumor efficacy and systemic toxicity of the synergistic combination (PMX and RSV) administered intravenously either in free form or loaded within the optimized LCNPs. This study was carried out over 25 days on Balb/c male urethane-induced lung cancer-bearing mice. The antitumor efficacy of PMX-RSV-LCNPs (F10) was evaluated via both macroscopic and microscopic examination after lung cancer induction and/or PMX-RSV-LCNP treatment. The average lung weight of tumor-bearing mice treated with free PMX/RSV mixture (380 mg±10) was significantly lower than the positive control group (605±25) (P<0.05). However, the mice treated with PMX-RSV-LCNPs showed the best reduction in average lung weight (260±15 mg) with insignificant difference to that of the negative control group (195±10 mg), confirming the great potentiality of optimized ion-paired PMX-RSV-LCNPs (F10) in improving the therapeutic outcomes (). shows the representative images of excised lungs from different mice groups where excised lungs of positive control group showed malignant surface lesions characterized by a massive number of lung adenomatous foci, while lungs obtained from PMX-RSV-LCNP (F10)-treated group significantly possess normal physiological features with significantly reduced malignant surface lesions.
Figure 4 In vivo antitumor activity.
Notes: (A) Average weight of excised lungs of mice measured at the end of treatment course. (B) Representative images of excised lung tumors displaying differences among mice groups after 3 weeks of exposing to different treatments; (a) negative control, (b) positive control, (c) free PMX-RSV mixture, and (d) ion-paired PMX-RSV-LCNPs (F10). The level of the tumor biomarkers measured using ELISA in tumor homogenate including (C) VEGF and (D) CASP-3 in urethane-induced lung cancer-bearing mice treated with free PMX-RSV and ion-paired PMX-RSV-LCNPs (F10) compared to untreated positive control. *P<0.05 vs negative control, #P<0.05 vs positive control, %P<0.05 vs free PMX/RSV mixture.
Abbreviations: CASP-3, caspase 3; LCNPs, liquid crystalline nanoparticles; PMX, pemetrexed; RSV, resveratrol; VEGF, vascular endothelial growth factor.
Tumor biomarkers
To determine the therapeutic efficacy of intravenously administered free PMX/RSV mixture and optimized ion-paired PMX-RSV-LCNPs (F10), tumor biomarkers were assessed. As depicted in , the level of VEGF in lungs treated with ion-paired PMX-RSV-LCNPs (F10; 3.5 pg/g) was significantly (P<0.05) lower than those treated with free PMX/RSV mixture (5.21 pg/g). The FDA-approved multi-targeted antifolate PMX plays a major role in decreasing growth rate of tumor cells which subsequently minimizes the need for vascularization and angiogenesis and decreases the tumor burden.Citation52 Moreover, the chemopreventive polyphenolic RSV was reported as antiangiogenic factor which reduces VEGF secretion from cocultured A549.Citation53 Thanks to the synergistic effect of our chemo–herbal combination (PMX-RSV), the developed nanocarriers succeeded to develop additional control for tumor growth.Citation27
These results were in agreement with CASP-3 level () that is activated in the apoptotic cells in which lungs treated with ion-paired PMX-RSV-LCNPs (F10) showed significantly higher active CASP-3 (2.8 ng/g tissue) than those treated with free PMX/RSV (1.9 ng/g tissue) (P<0.05).
Histopathological and immunohistochemical analysis
As depicted in , the H&E-stained lung sections revealed normal physiological features of negative control mice in which normal alveoli were lined with single epithelial tissues, whereas the positive urethane-treated control group exhibited various preneoplastic to neoplastic lesions extending from epithelial hyperplasia to adenoma (blue arrow). In addition to hemorrhage and inflammatory cells, infiltration represented by white and black, respectively. Free PMX/RSV mixture-treated mice were associated with reduction in his-topathological profile of neoplastic transformation, while the ion-paired PMX-RSV-LCNP (F10)-treated group displayed an outstanding amelioration in the carcinogenic histopathological profile. It was observed that, compared to the positive control groups, lungs harvested from lung cancer-bearing mice treated with IV injection of free PMX/RSV mixture resulted in the reduced number of adenomatous foci while those harvested from mice treated systemically with optimized ion-paired PMX-RSV-LCNPs (F10) displayed the best reduction in both number and diameter of lung adenomatous foci (). Furthermore, the immunohisto-chemical analysis revealed the reduced expression of the proliferation marker Ki-67 protein indicating the superiority of ion-paired PMX-RSV-LCNPs (F10) in attenuating proliferation of urethane-induced adenomas ().
Figure 5 (A) Histopathological analysis of H&E-stained excised lung sections of mice treated with free PMX-RSV and ion-paired PMX-RSV-LCNPs (F10) in comparison to positive control and negative control groups. (B) Average number of foci in the excised lungs, (C) The diameter of lung foci (mm).
Notes: #P<0.05 vs positive control, %P<0.05 vs Free PMX/RSV mixture.
Abbreviations: LCNPs, liquid crystalline nanoparticles; PMX, pemetrexed; RSV, resveratrol.
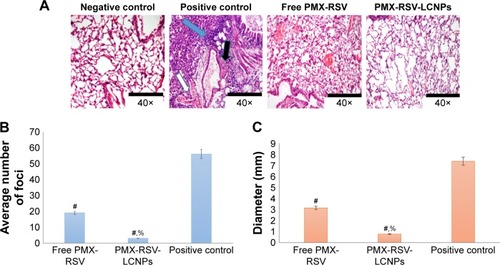
Figure 6 Immunohistochemical analysis: (A) Ki-67 staining. (B) % expression of Ki-67 of excised lung sections of urethane-induced lung cancer-bearing mice received free PMX-RSV and ion-paired PMX-RSV-LCNPs (F10) in comparison to untreated positive control.
Note: *P<0.05 vs negative control, #P<0.05 vs positive control, %P<0.05 vs free PMX-RSV mixture.
Abbreviations: LCNPs, liquid crystalline nanoparticles; PMX, pemetrexed; RSV, resveratrol.
Safety index
It was observed that positive control mice suffered from gradual loss in body weight of along the whole experimental duration which could be attributed to lung tumor invasion and malfunction. Compared to the free combined PMX/RSV group, the PMX-RSV-LCNP (F10) group showed less changes in total body weight which suggested lower adverse effects of PMX-RSV-LCNPs (). Strikingly, PMX-RSV-LCNP (F10)-treated mice maintained the well-organized alveolar structure of lung tissues which significantly improved their survival rates up to 60% during the experimental period compared to only 30% survival rate of mice treated with free PMX/RSV mixture (). The death of mice was based on remarkable disruption of alveolar structure resulted from lung damage of urethane-treated mice.Citation54,Citation55
Figure 7 Assessment of safety index of optimized LCNPs.
Notes: (A) Average body weights of urethane-induced lung cancer-bearing mice treated with free PMX-RSV and ion-paired PMX-RSV-LCNPs (F10) for 3 weeks compared to untreated positive control. (B) Survival rate curve among treated groups in comparison to positive and negative control groups. H&E-stained kidney (C) and liver (D) of different treatment groups.
Abbreviations: IV, intravenous; LCNPs, liquid crystalline nanoparticles; PMX, pemetrexed; RSV, resveratrol.
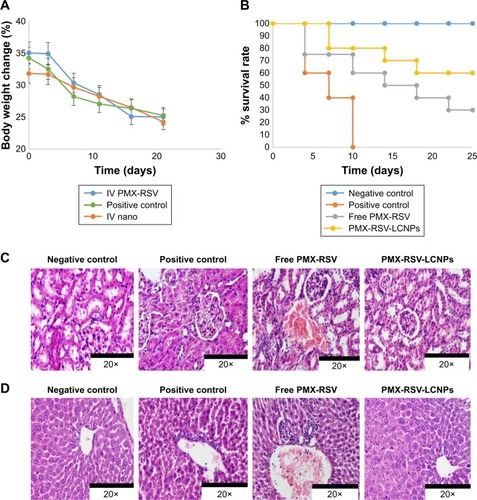
PMX is well known for its hepato- and nephrotoxicity. However, RSV plays an important role in attenuating toxicity associated with PMX as it exerts hepato- and nephroprotective effect.Citation56–Citation59 Our proposed LCNPs enabled the delivery of the synergistic combination of RSV and PMX which played an important role in improving treatment outcomes based on their capability in killing cancerous cells in addition to remarkable reduction in the dosages of PMX which alleviates the toxicity concerns of the proposed PMX-RSV-LCNP formulation at the systemic level. Compared to untreated positive control or mice treated with free PMX/RSV mixture, optimized nanoformulation induced the least damage to the kidney and liver as demonstrated in , respectively. Conclusively, the improved therapeutic outcomes of urethane-induced lung cancer-bearing mice receiving PMX-RSV-LCNPs could be attributed to the great tendency of LCNPs in protecting these payloads from premature release in blood stream and reaching other organs which in turn maximize accumulation of synergistic combination (PMX/RSV) in cancer tissue. In addition, the leaky vasculature within tumor site and impaired lymphatic drainage facilitate penetration and retention of the proposed LCNPs with nanometric PS of 173 nm. Obviously, PMX-RSV-LCNPs could efficiently target and suppress lung cancers which in turn enhanced survival rates and remarkably minimized systemic side effects as compared to free PMX/RSV.
Conclusion
In this study, we shed light on the potential of LCNPs for the co-delivery of promising synergistic combination of FDA-approved PMX and herbal RSV for lung cancer treatment. PMX-RSV-LCNPs were prepared via simple hydro-trope method. In situ HIP was carried out using CTAB to improve encapsulation efficiency and to guarantee extended release behavior of hydrophilic PMX up to 24 hours. This can interestingly minimize the dose of chemotherapeutic PMX and overcome MDR. The proposed dual drug-loaded LCNPs manifested promising cytotoxic effects as well as enhanced cellular uptake against A549 lung cancer cells. The superior in vivo antitumor efficacy of ion-paired PMX-RSV-LCNPs was demonstrated in terms of reduced lung weights, reduced number of lung adenomatous foci and their corresponding diameter, lower angiogenic biomarker (VEGF), and higher apoptotic biomarker (CASP-3). Furthermore, they are associated with an improved histopathological and immunohistochemical profile. Strikingly, the proposed system reduced hepato- and nephrotoxicity commonly associated with PMX. Collectively, the ion-paired PMX-RSV-LCNPs offer a promising avenue for lung cancer treatment via combining the merits of chemotherapeutic and herbal drugs.
Summary points
This work has been directed to the preparation of LCNPs for the co-delivery of chemo–herbal drug combination of RSV and PMX for effective lung cancer treatment.
In situ ion-pairing approach was utilized to alter the solubility characteristics of PMX and enhance its EE into the lipidic matrix. LCNPs co-loaded with RSV and ion-paired PMX were successfully tailored with PS around 173 nm and high %EE for both drugs.
Sustained and continuous drug release behavior up to 24 hours was successfully achieved. Such sustained release pattern is preferred to decrease the systemic cytotoxicity associated with PMX.
PMX-RSV-LCNPs demonstrated enhanced cytotoxicity on A549 lung cancer cell line as determined by MTT assay as well as superior cellular uptake attributed to the bioadhesive and membrane-fusing features of GMO.
The in vivo antitumor efficacy of PMX-RSV-LCNPs was evaluated on lung cancer-bearing mice. PMX-RSV-LCNPs showed a superior antitumor efficacy evidenced by reduced lung weights, diminished number of lung adenomatous foci as well as their diameter, lower level of the angiogenic biomarker (VEGF), and higher apoptotic biomarker (CASP-3) level.
PMX-RSV-LCNPs demonstrated an improved histopathological and immunohistochemical profile compared to free drugs.
In vivo toxicity study revealed that the proposed PMX-RSV-LCNP system reduced hepato- and nephrotoxicity associated with free PMX.
In summary, LCNPs gathered the privileges of both chemotherapeutic and herbal drugs which could offer a new potential avenue for lung cancer treatment.
Acknowledgments
This work was supported by the research grant (No 5731) from Science and Technology Development Fund (STDF), Ministry of Scientific Research, Egypt.
Supplementary materials
Methodology
HPLC method for the assay of pemetrexed (PMX) and resveratrol (RSV)
A novel, accurate, precise, and sensitive HPLC method was developed and validated for simultaneous determination of both RSV and PMX using Agilent 1260 Infinity HPLC system equipped with a quaternary pump, an autosampler, vacuum degasser, diode array detector, and Agilent Chemstation data-processing system. The HPLC analysis was carried out with an Inertsil® ODS-3 reversed-phase column (250 × 4.6 mm, 5 µm; GL Sciences Inc, Torrance, CA, USA). The column was maintained at room temperature. For chromatographic elution, the injection volume was 20 µL. A step gradient was utilized for elution in which the mobile phase consisted of acetonitrile: dibasic phosphate buffer (pH adjusted to 4.9 using orthophosphoric acid) with the following ratio 15:85 to 6 minutes then converted into 50:50 gradually over 0.5 minutes, and the flow rate was 1.5 mL/min. Total run time was 10 minutes; PMX was eluted at 4.8 minutes and RSV at 9.8 minutes. PMX was detected at 225 nm, while RSV was detected at 306 nm.
Evaluation of in vitro cytotoxicity
In vitro cytotoxicity of free PMX, free RSV, free combined PMX/RSV solution, blank liquid crystalline nanoparticles (LCNPs), and ion-paired PMX-RSV-LCNPs was evaluated in A549 lung cancer cells using MTT assay.Citation1 Briefly, 1,000–5,000 cells/well were seeded in 96-well culture plates containing 100 µL of cell line-specific medium and incubated at 37°C in a 5% CO2 atmosphere. The cells were then treated with each formulation at concentrations ranged from 0.468 to 30 µg/mL of each PMX and/or RSV and incubated at 37°C for 24 and 48 hours. Then, cells were subjected to the MTT analysis for cell viability determination. The optic density was measured at 490 nm on a microplate reader with control wells containing only cell culture medium. The viability data were analyzed in GraphPad Prism, and the IC50 value for each system was calculated. Each IC50 value was calculated from n=6 viability measurements per concentration. IC50 was calculated after 24 and 48 hours.
In vitro cellular uptake
A549 lung cells were seeded on CELL view™ slide at a density of 3,000 cells/well and left overnight to allow enough time for cell attachment. Cells were incubated with fresh medium containing free coumarin-6 dye and coumarin-6-loaded LCNPs at the concentration of 0.08% w/w coumarin-6 (with respect to lipid). After incubation for 4 and 24 hours, the medium was removed and cells were rinsed twice using PBS to eliminate any extracellular residues. Then, cells were fixed using 4% paraformaldehyde solution in dark. The nuclei were stained using 2-(4-ethoxyphenyl)-6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazole (Hoechst AG, Frankfurt, Germany) containing DPX (DePeX [Distrene 80: A commercial polystyrene, a plasticizer, e.g., dibutyl phthalate and xylene]) as mounting medium. Finally, the intracellular uptake of optimized LCNPs into A549 cell line was visualized via confocal laser scanning microscopy (DMi8; Leica Microsystems, Wetzlar, Germany) using the following filter set: excitation wavelength, 450 nm; emission wavelength, 505 nm. All obtained confocal images were analyzed using Leica AF software. Moreover, the internalization of particles within each section of the cells at various cell depths was visualized using three-dimensional cellular images per time (z-stacks), where serial sections were taken across 30 fields and stacked with cross-sectional slices (25 µm) perpendicular to the plane of the cell monolayer midpoint (z-axis).Citation2,Citation3
Results and discussions
HPLC method for the assay of PMX and RSV
Several attempts were carried out for simultaneous quantitation of PMX and RSV in mixture. Isocratic elution using different proportions of mobile phases incluwding methanol, acetonitrile, acidified water, and buffer at different ratios was not enough to provide satisfactory separation for the mixture’s components. Therefore, gradient elution was applied to separate peaks of both drugs.Citation4 In the literature, various methods were reported for individual quantitation of PMX and RSV in formulations and biological fluids.Citation4–Citation8 The optimized mobile phase composed of HPLC grade acetonitrile: dibasic phosphate buffer (pH adjusted to 4.9 using orthophosphoric acid) with the following ratio 15:85 to 6 minutes and then converted into 50:50 gradually over 0.5 minutes; flow rate was 1.5 mL/min. A rapid, sensitive, simple, and efficient method was developed for simultaneous determination of RSV and PMX in stock solution and nanoformulations. The principal peak obtained with the standard solution was at 225 nm which was selected as the λmax of the PMX, whereas the major peak of RSV was detected at λmax 306 nm. A good linearity was shown by the calibration curve obtained from 0.4 to 2 mg% PMX and RSV concentration (). The coefficient of determination (R2) of 0.9997 was obtained from the linear regression analysis of the data (peak area vs concentration). Selected chromatograms were displayed (), in which retention time of PMX was 4.427 minutes and of RSV was 11.87 minutes so that the total run time was 12 minutes.
As summarized in , the HPLC method was validated according to International Conference on Harmonization (IHC) guidelines for intra- and inter-day precision and accuracy. The low values of relative standard deviation (RSD) as well as % error of mean <2 confirmed the precision and accuracy of the developed HPLC method. Limit of detection was 0.2 mg%, while the limit of quantitation was 0.4 mg%. The proposed assays were also successfully applied to evaluate the entrapment efficiency (EE) of the novel LCNPs for the co-delivery of PMX and RSV for lung cancer.
Figure S1 HPLC calibration curve of PMX and RSV in methanol at λmax 225 and 306 nm, respectively.
Abbreviations: PMX, pemetrexed; RSV, resveratrol.
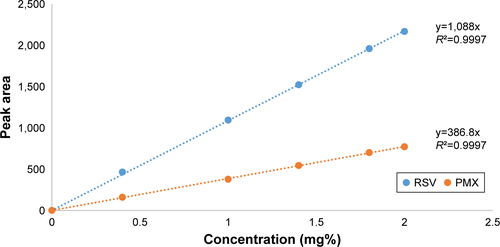
Figure S2 Selected chromatogram of (A) PMX and (B) RSV displaying retention time at their corresponding λmax.
Abbreviations: PMX, pemetrexed; RSV, resveratrol.
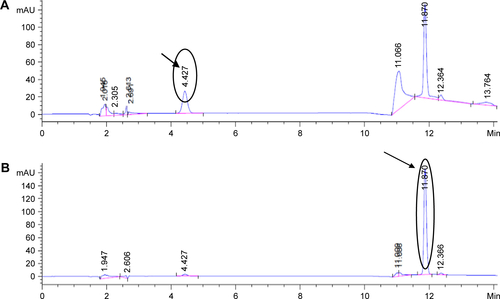
Figure S3 Live uptake of ion-paired LCNPs in A549 cells in which three-dimensional time laps images distinguishing between cystolic and perinuclear accumulation of 178 nm LCNPs of 30 z-stacks inside the cells in the z-direction along the total depth measured (z-stack =30 slices at 25 µm per slices).
Note: The individual stacks were assembled as video displaying the oscillation of green fluorescence along the cross-sectional internalization of LCNPs from the apical to basolateral cellular membrane represented from z-0 to z-29.
Abbreviation: LCNPs, liquid crystalline nanoparticles.
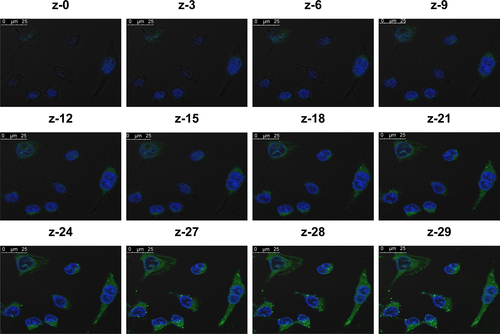
Table S1 Intra- and inter-day precision and accuracy of PMX and RSV (n=5 for each concentration)
References
- KhattabSNNaimSEAEl-SayedMDesign and synthesis of new s-triazine polymers and their application as nanoparticulate drug delivery systemsNew J Chem2016401195659578
- YadavKSJacobSSachdevaGSawantKKIntracellular delivery of etoposide loaded biodegradable nanoparticles: cytotoxicity and cellular uptake studiesJ Nanosci Nanotechnol20111186657666722103065
- TeskačKKristlJThe evidence for solid lipid nanoparticles mediated cell uptake of resveratrolInt J Pharm20103901616919833178
- El-LakanySAElzoghbyAOElgindyNAHamdyDAHPLC methods for quantitation of exemestane-luteolin and exemestane-resveratrol mixtures in nanoformulationsJ Chromatogr Sci20165481282128927130876
- RivoryLPClarkeSJBoyerMBishopJFHighly sensitive analysis of the antifolate pemetrexed sodium, a new cancer agent, in human plasma and urine by high-performance liquid chromatographyJ Chromatogr B Biomed Sci Appl2001765213514011767306
- SinghGPaiRSPanditVDevelopment and validation of a HPLC method for the determination of trans-resveratrol in spiked human plasmaJ Adv Pharm Technol Res20123213013522837962
- WarnerAPiranerIWeimerHWhiteKDevelopment of a purity control strategy for pemetrexed disodium and validation of associated analytical methodologyJ Pharm Biomed Anal2015105465425527981
- KatsagonisAAtta-PolitouJKoupparisMAHPLC method with UV detection for the determination of trans-resveratrol in plasmaJ Liquid Chromatogr Relat Technol200528913931405
Disclosure
The authors report no conflicts of interest in this work.
References
- AbdelazizHMGaberMAbd-ElwakilMMInhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregatesJ Control Release201826937439229180168
- SmithRAAndrewsKSBrooksDCancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screeningCA Cancer J Clin201767210012128170086
- American Cancer SocietyWhat Is Non-Small Cell Lung Cancer?Atlanta, GAAmerican Cancer Society2016
- ZappaCMousaSANon-small cell lung cancer: current treatment and future advancesTransl Lung Cancer Res20165328830027413711
- MidthunDEEarly detection of lung cancerF1000Res20165739
- American Cancer SocietyTreatment Choices for Non-Small Cell Lung Cancer, by StageAtlanta, GAAmerican Cancer Society2017
- ChidambaramMManavalanRKathiresanKNanotherapeutics to overcome conventional cancer chemotherapy limitationsJ Pharm Pharm Sci2011141677721501554
- Chemotherapy And Selective Toxicity [webpage on the Internet]All Answers Ltd.2013 Available from: https://www.ukessays.com/essays/biology/chemotherapy-and-selective-toxicity-biology-essay.php?vref=1Accessed December 28, 2018
- ElzoghbyAOElgoharyMMKamelNMImplications of protein- and Peptide-based nanoparticles as potential vehicles for anticancer drugsAdv Protein Chem Struct Biol20159816922125819280
- CohenMHJohnsonJRWangYCSridharaRPazdurRFDA drug approval summary: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancerOncologist200510636336815967829
- PubChem [homepage on the Internet]Compound Database; CID=446556 Available from: https://pubchem.ncbi.nlm.nih.gov/compound/446556Accessed December 3, 2017
- AdjeiAAPharmacology and mechanism of action of pemetrexedClin Lung Cancer20045Suppl 2S51S5515117425
- ChattopadhyaySMoranRGGoldmanIDPemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applicationsMol Cancer Ther20076240441717308042
- FuldADDragnevKHRigasJRPemetrexed in advanced non-small-cell lung cancerExpert Opin Pharmacother20101181387140220446853
- HoCDaviesAMSanghaRSPhase I/II trial of pemetrexed plus nab-paclitaxel in advanced solid tumor patients with emphasis on non-small cell lung cancerInvest New Drugs20133161587159124013936
- MahmudFJeonOCAlamFOral pemetrexed facilitates low-dose metronomic therapy and enhances antitumor efficacy in lung cancerJ Control Release201828416017029908222
- El-FarSWHelmyMWKhattabSNBekhitAAHusseinAAElzoghbyAOPhytosomal bilayer-enveloped casein micelles for code-livery of monascus yellow pigments and resveratrol to breast cancerNanomedicine201813548149929376765
- KabaryDMHelmyMWElkhodairyKAFangJYElzoghbyAOHyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinomaColloids Surf B Biointerfaces201816918319429775813
- SabraSAElzoghbyAOSheweitaSASelf-assembled amphiphilic zeinlactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancerEur J Pharm Biopharm201812815616929689288
- ElzoghbyAOEl-LakanySAHelmyMWAbu-SerieMMElgindyNAShell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapyNanomedicine (Lond)201712242785280529094642
- DonnellyLENewtonRKennedyGEAnti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanismsAm J Physiol Lung Cell Mol Physiol20042874L774L78315180920
- BishayeeACancer prevention and treatment with resveratrol: from rodent studies to clinical trialsCancer Prev Res (Phila)20092540941819401532
- VaroniEMLo FaroAFSharifi-RadJIritiMAnticancer molecular mechanisms of resveratrolFront Nutr20163827148534
- HamzaRZEl-ShenawyNSAnti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male ratsToxicol Rep2017439940728959665
- ElzoghbyAEditorial (Thematic issue: nanocarriers based on natural polymers as platforms for drug and gene delivery applications)Curr Pharm Des201622223303330427165162
- ElzoghbyAFreagMMamdouhHElkhodairyKZein-based nanocarriers as potential natural alternatives for drug and gene delivery: Focus on cancer therapyCurr Pharm Des201723355261527128641543
- ChenRSKoJCChiuHCPemetrexed downregulates ERCC1 expression and enhances cytotoxicity effected by resveratrol in human nonsmall cell lung cancer cellsNaunyn Schmiedebergs Arch Pharmacol2013386121047105923912706
- SummerlinNSooEThakurSQuZJambhrunkarSPopatAResveratrol nanoformulations: challenges and opportunitiesInt J Pharm2015479228229025572692
- AngelovaAAngelovBMutafchievaRLesieurSCouvreurPSelf-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug deliveryAcc Chem Res201144214715621189042
- FreagMSElnaggarYSAbdelmonsifDAAbdallahOYStealth, bio-compatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: in vitro and in vivo studiesInt J Nanomedicine201611114799481827703348
- LiuRWangSFangSLiquid crystalline nanoparticles as an ophthalmic delivery system for tetrandrine: development, characterization, and in vitro and in vivo evaluationNanoscale Res Lett201611125427188974
- ElzoghbyAOAbd-ElwakilMMAbd-ElsalamKElsayedMTHashemYMohamedONatural polymeric nanoparticles for brain-targeting: implications on drug and gene deliveryCurr Pharm Des201622223305332326845323
- ElzoghbyAOHemasaALFreagMSHybrid protein-inorganic nanoparticles: from tumor-targeted drug delivery to cancer imagingJ Control Release201624330332227794493
- GaberMMedhatWHanyMSaherNFangJYElzoghbyAProtein-lipid nanohybrids as emerging platforms for drug and gene delivery: challenges and outcomesJ Control Release2017254759128365294
- SabraSAbdelmoneemMAbdelwakilMSelf-assembled nano-carriers based on amphiphilic natural polymers for anti-cancer drug delivery applicationsCurr Pharm Des201723355213522928552068
- SpicerPTHaydenKLLynchMLOfori-BoatengABurnsJLNovel process for producing cubic liquid crystalline nanoparticles (Cubosomes)Langmuir2001171957485756
- DevrimBBozkirADesign and evaluation of hydrophobic ion-pairing complexation of lysozyme with sodium dodecyl sulfate for improved encapsulation of hydrophilic peptides/proteins by lipid-polymer hybrid nanoparticlesJ Nanomed Nanotechnol201561259
- MeyerJDManningMCHydrophobic ion pairing: altering the solubility properties of biomoleculesPharm Res19981521881939523302
- SwarnakarNKThankiKJainSBicontinuous cubic liquid crystalline nanoparticles for oral delivery of doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicityPharm Res20143151219123824218223
- ElzoghbyAOMostafaSKHelmyMWElDemellawyMASheweitaSASuperiority of aromatase inhibitor and cyclooxygenase-2 inhibitor combined delivery: hyaluronate-targeted versus PEGylated protamine nanocapsules for breast cancer therapyInt J Pharm20175291–217819228663087
- SwarnakarNKThankiKJainSBicontinuous cubic liquid crystalline nanoparticles for oral delivery of doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicityPharm Res20143151219123824218223
- ElgindyNElkhodairyKMolokhiaAElzoghbyABiopolymeric nanoparticles for oral protein delivery: design and in vitro evaluationJ Nanomed Nanotechnol20110203110
- ElzoghbyAOMostafaSKHelmyMWEldemellawyMASheweitaSAMulti-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapyPharm Res20173491956196928643236
- KhattabSNAbdel NaimSEEl-SayedMDesign and synthesis of new s-triazine polymers and their application as nanoparticulate drug delivery systemsNew J Chem2016401195659578
- ElzoghbyAOVranicBZSamyWMElgindyNASwellable floating tablet based on spray-dried casein nanoparticles: near-infrared spectral characterization and floating matrix evaluationInt J Pharm20154911–211312226095913
- FreagMSHyaluronate-lipid nanohybrids: fruitful harmony in cancer targetingCurr Pharm Des201723355283529128552066
- FreagMSElnaggarYSAbdelmonsifDAAbdallahOYLayer-by-layer-coated lyotropic liquid crystalline nanoparticles for active tumor targeting of rapamycinNanomedicine (Lond)201611222975299627785978
- DeshpandeSVenugopalERamagiriSBellareJRKumaraswamyGSinghNEnhancing cubosome functionality by coating with a single layer of poly-ε-lysineACS Appl Mater Interfaces2014619171261713325184793
- JainVSwarnakarNKMishraPRPaclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapyBiomaterials201233297206722022809646
- DeshpandeSSinghNInfluence of cubosome surface architecture on its cellular uptake mechanismLangmuir201733143509351628325047
- Harush-FrenkelODebottonNBenitaSAltschulerYTargeting of nanoparticles to the clathrin-mediated endocytic pathwayBiochem Biophys Res Commun20073531263217184736
- LiQYanoSOginoHThe therapeutic efficacy of anti vascular endothelial growth factor antibody, bevacizumab, and pemetrexed against orthotopically implanted human pleural mesothelioma cells in severe combined immunodeficient miceClin Cancer Res200713195918592517908988
- SahinEBaycuCKoparalATBurukoglu DonmezDBekturEResveratrol reduces IL-6 and VEGF secretion from co-cultured A549 lung cancer cells and adipose-derived mesenchymal stem cellsTumour Biol20163767573758226687643
- LuoTLoira-PastorizaCPatilHPPEGylation of paclitaxel largely improves its safety and anti-tumor efficacy following pulmonary delivery in a mouse model of lung carcinomaJ Control Release2016239627127515664
- WuJDengCMengFZhangJSunHZhongZHyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancerJ Control Release2017259768228027947
- KitadaMKoyaDRenal protective effects of resveratrolOxid Med Cell Longev20132013117
- WangYJiangYFanXHepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathwaysToxicol Lett20152362828925956474
- ChenWMShawLHChangPJHepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitroExp Ther Med20161141231123827073428
- OsmanAMTelitySADamanhouriZAChemosensitizing and nephroprotective effect of resveratrol in cisplatin-treated animalsCancer Cell Int2015151625709558
