Abstract
Background
We report herein a novel strategy for the preparation of protein-based nanode-livery vehicles for hydrophobic active pharmaceutical ingredients.
Methods
The procedure consisted of three steps, ie, exposure of hydrophobic residues of a protein to a pH-induced partial unfolding: interaction between hydrophobic residues on the protein and the hydrophobic active pharmaceutical ingredient, and a final step where the structure of the protein was reversed to a native-like state by returning to neutral pH. As proof of concept, the interaction of paclitaxel with partially unfolded states of human serum albumin was evaluated as a potential method for the preparation of water-soluble complexes of the taxane with albumin.
Results
We found that paclitaxel readily binds to pH-induced partially unfolded albumin, leading to the formation of optically clear water-soluble complexes. The complexes thus formed were more stable in solution when the albumin native state was at least partially restored by neutralization of the solution to a pH around 7. It was also observed that the hydrodynamic radius of human serum albumin was only slightly increased after the cycle of pH changes, remaining in a monomeric state with a size according to paclitaxel binding. Furthermore, paclitaxel binding did not affect the overall exposure of charged groups of human serum albumin, as evaluated by its interaction with an ionic exchange resin.
Conclusion
The in vitro biological activity of the complexes formed was qualitatively equivalent to that of a Cremophor®-based formulation.
Introduction
Paclitaxel (Taxol®, Bristrol-Myers Squibb, New York) is a very potent anticancer agent currently approved as first-line treatment for advanced carcinoma of the ovary, adjuvant treatment of breast carcinoma and nonsmall cell lung cancer, and as a second-line treatment for Kaposi’s sarcoma related to acquired immunodeficiency syndrome.Citation1,Citation2 Because of its extremely poor aqueous solubility, paclitaxel has been formulated in ethanol and polyethoxylated castor oil (Cremophor EL®), a vehicle that has been associated with bronchospasm, hypotension, and various hypersensitivity reactions, in particular after rapid administration or 10 minutes after initiating drug infusion.Citation3,Citation4 Premedication with corticosteroids and antihistamines is mandatory to reduce the incidence of serious hypersensitivity reactions. However, milder reactions still occur in 5%–30% of treated patients.Citation5 To avoid the toxicities associated with the cosolvents required for taxane administration, and also to improve the solubility of paclitaxel, a number of alternative formulation strategies have been investigated.Citation6–Citation8 In this context, polyethylene glycol was evaluated as a biocompatible polymer which improves water solubility of paclitaxel. However, the polyethylene glycol–paclitaxel complex thus formed showed a decrease in antitumor activity.Citation3 Other polymers, such as hyaluronic acid, polyglutamic acid, and nucleic acids, have been evaluated as carriers to improve paclitaxel solubility. Although these complexes had a highly stable composition, the biological activity of the pharmaceutical principal was slow.Citation9,Citation10
Proteins have been used as carriers for hydrophobic drugs, especially human albumin.Citation11–Citation14 In this context, Desai et al described a composition for in vivo delivery of water-insoluble drugs, such as paclitaxel.Citation11,Citation15 In their report, paclitaxel was incorporated in a polymeric shell of albumin with a size no bigger than 10 μm, which was substantially crosslinked through disulfide bonds induced in the protein by sonication. Desai et al then described a new formulation to deliver paclitaxel.Citation16 In this case, the agent was delivered in the form of suspended particles coated with a stabilizing protein. The complex of protein with drug is subjected to high shear, in the absence of conventional surfactants or any polymeric core, a procedure that yielded amorphous nanoparticles with a diameter of less than 200 nm. These results were the basis for the development of Abraxane®, a formulation approved by the US Food and Drug Administration for the treatment of metastatic breast cancer. However, the formulation developed has very limited stability upon reconstitution in saline solution (a maximum of 8 hours refrigerated at 2–8°C) and requires the use of a high-pressure homogenizer for its production.
The binding of paclitaxel to human serum albumin (HSA) has been studied using various methodologies.Citation17–Citation19 Most of these studies describe the interaction under physiological conditions and are primarily concerned with the determination of binding constants.Citation20–Citation22 Nevertheless, comparison of the reported data shows significant differences in affinity constant values, because the experimental conditions for carrying out binding experiments were indeed different.
Another important issue is that most of the formulations developed require the use of a percentage of organic solvent in order to deal with the very low solubility of paclitaxel in aqueous solution. The use of different organic solvents to prepare paclitaxel solutions indeed modifies the interaction process because the nature of paclitaxel binding to HSA is mainly hydrophobic. Because unfolding exposes the hydrophobic residues of proteins, we hypothesized that partially unfolded states of HSA could interact with paclitaxel, leading to formation of water-soluble noncovalent complexes. The results reported here show that reversible acid-induced or alkali-induced denatured forms of albumin interact with paclitaxel, forming complexes that remain associated after protein renaturation, allowing paclitaxel delivery in aqueous media.
Materials and methods
Paclitaxel was obtained from Yunnan Smandbet Co Ltd (Kunming, China). Stock solutions of paclitaxel were prepared by dissolving the drug in either ethanol or dimethylsulfoxide in a final concentration of 11.1 mg/mL. Further dilutions in organic solvent were prepared as necessary when assaying for different ratios and concentrations.
HSA 20% (w/v) with sodium caprylate (0.04 M) and N-acetyl tryptophan (0.04 M) as stabilizers was obtained from the Laboratorio de Hemoderivados, National University of Córdoba, Argentina. To remove the excipients, albumin was dialyzed against distilled water or saline solution, and diluted with distilled nonpyrogenic water to the final concentration used in each assay. Defatted albumin was prepared by adsorption of fatty acids onto charcoal as previously described.Citation23 Sodium dodecyl sulfate and urea were from Sigma Chemical Co (St Louis, MO). All other chemicals used were of analytical grade.
Turbidimetry
Optical density at a wavelength of 600 nm was used as a measure of the optical clarity of the samples.
Determination of HSA concentration
Protein concentration was determined using a Coomassie Brilliant blue assay or by direct absorbance at 280 nm, as previously described.Citation24
Determination of paclitaxel concentration
Paclitaxel concentration was measured on a Curosil B C18 column (250 × 3.20 mm ID, particle size 5 μm) and a Curosil guard column (30 × 4.60 mm ID, particle size 5 μm) B C18 supplied by Phenomenex (Torrance, CA). The mobile phase was 60% (v/v) acetonitrile and 40% (v/v) biodistilled water. Flow rate was 0.7 mL/min and the eluent was monitored at 227 nm. Chromatography was performed at ambient temperature (20 ± 2°C).
Protein chromatography
Reaction mixtures and controls were chromatographed on an Äkta Explorer 100 system (GE Healthcare, Barrington, IL) fitted with a Superdex 200 or Mono Q column previously equilibrated with 50 mM phosphate buffer (pH 7.0) and 100 mM NaCl (phosphate-buffered saline). The elution profile was followed using an ultraviolet detector at 280 nm, and the total protein levels were quantified using a Coomassie brilliant blue assay.Citation24 Molecular weight markers in concentrations of 3 mg/mL were run similarly, and fractions analyzed for protein content using the Coomassie brilliant blue assay. HSA was eluted from the anion exchange resin with 50 mM sodium phosphate pH 7.0, 0.5 M NaCl.
Water-soluble paclitaxel noncovalently bound to albumin
Paclitaxel was solubilized in an organic solvent, such as ethanol or dimethylsulfoxide, and the solutions were then centrifuged to remove any potential particles. The solutions were slowly added under gentle agitation to the solution of HSA previously adjusted to the different conditions to be tested. After 1 hour of stabilization at the selected experimental conditions (temperature, pH, and ionic strength), the pH was slowly adjusted to 7.0 ± 0.2 with 1 M Tris-HCl pH 7.0, if necessary, and the paclitaxel precipitated was removed by centrifugation (14,000 × g, 10 minutes) at a series of time intervals. Finally, an aliquot of supernatant (300 μL) was filtered through 0.2 μm and the concentration of paclitaxel in solution (bound and unbound to HSA) was determined by reverse-phase high-pressure liquid chromatography. The amount of insoluble paclitaxel was determined from the precipitates by reverse-phase high-pressure liquid chromatography after its solubilization in ethanol.
Human larynx epithelioma cell culture conditions
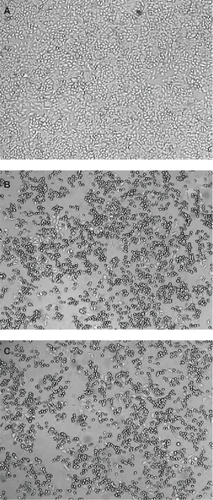
Human larynx epithelioma cells (ATCC CCL-23) were propagated in minimum essential medium supplemented with 10% irradiated fetal bovine serum (Natocor, Córdoba, Argentina), 100,000 IU/L penicillin (Life Technologies, Rockville, MD), and 2 mM L-glutamine (Sigma). Cell monolayers were incubated in the above medium in the presence or absence of different concentrations of paclitaxel: HSA or paclitaxel in Cremophor (control) over 24 hours. Cell viability was assayed by trypan blue exclusion. Cell monolayers were photographed without any staining in an Axiovert 135 M Carl-Zeiss microscope at 50 × (Oberkochen, Germany).
Results and discussion
Solubilization of paclitaxel by interaction with reversibly denatured HSA
It is known that HSA undergoes reversible conformational isomerization with changes in pH of the solution containing the protein.Citation25,Citation26 At neutral pH, HSA assumes the normal form, which abruptly changes to a highly charged fast migrating form at pH values lower than 4.3 because this form moves “fast” on gel electrophoresis. In this condition, albumin is in a compact partially denaturated state, with a significant amount of secondary structure but a largely disordered tertiary structure.Citation27 Upon further reduction in pH to lower than 2.7, the protein structure changes to the fully extended form. The α-helix content decreases to a minimal value of 25%, and HSA is in an expanded form and increases the exposure of its hydrophobic surface.Citation25,Citation26 On the other hand, an increase in pH to 10 also induces a reversible denaturation of HSA that exposes part of its hydrophobic residues.Citation28,Citation29 We hypothesized that these reversible partially denaturated structures could contribute to the binding of a highly hydrophobic molecule such as paclitaxel, leading to the formation of soluble complexes. As shown in , not only does paclitaxel form clearer solutions when incubated with denatured acid than with native HSA, but also a proportion of the complexes thus formed remains soluble even after pH is reversed to 7. The differences between the preparations are also reflected in the dramatic differences in their turbidity which, when measured before clarification by centrifugation, turned out to be a good predictor of the outcome in terms of paclitaxel solubilization (). It is important to stress the fact that all samples were optically clear (OD at 600 nm < 0.1) after centrifugation. Similar results were obtained when defatted or excipient-free HSA was used, suggesting that neither fatty acids nor sodium caprylate or N-acetyl tryptophan interfere with binding of paclitaxel to HSA (data not shown). Incubation at an alkaline pH also rendered optically clear solutions of paclitaxel and HSA ().
Table 1 Effect of pH on the turbidity and solubility of Ptx–HSA complexes
Effect of incubation temperature on binding paclitaxel to HSA
In an attempt to optimize the binding of paclitaxel to partially unfolded albumin, we evaluated the effect of incubation temperature on the formation of water-soluble paclitaxel–HSA complexes with different molar ratios exposing hydrophobic surfaces of HSA by acid pH. As expected, a lowered incubation temperature rendered the system more hydrophobic, and led to clearer solutions and an increased amount of paclitaxel remaining solution ().
Table 2 Effect of incubation temperature on the turbidity and solubility of Ptx–HSA complexes
It was also observed that the mixtures prepared at a low incubation temperature remained clearer and more soluble for at least a week at 4°C, indicating that the complexes formed had greater stability (data not shown). Upon increasing the temperature from 4°C to 37°C, unacceptable formulation mixtures were obtained due to increased precipitation of paclitaxel and HSA. Similar results were obtained at alkaline pH, except that at a pH of 10, the ester bond in paclitaxel was rapidly hydrolyzed at high temperatures (data not shown).
Effect of ionic strength on formation of water-soluble paclitaxel–HSA complexes
Given the zwitterionic character of HSA and the hydrophobicity of paclitaxel, the ionic strength of the aqueous media is almost certainly a variable that will affect the formation of water-soluble complexes between paclitaxel and HSA. First, an increase in ionic strength could reinforce and stabilize the mainly hydrophobic interaction of paclitaxel with HSA. On the other hand, using a high protein concentration, increasing ionic strength could promote interaction among albumin molecules, leading to a decrease in stability with a concomitant increase in turbidity of the solution. Although not statistically significant, a slight improvement in paclitaxel solubility was apparent with the addition of NaCl up to 0.4 M, suggesting that the addition of salt reinforces and stabilizes the interaction of paclitaxel with HSA (). As expected, we found that a gel was formed at a high ionic strength and an acidic pH, and this led to marked precipitation of protein and turbid mixtures (). This effect was even more sensitive to salt concentration when the HSA concentration was 40 mg/mL, at which a gel was formed with 0.6 M NaCl, whereas at 60 mg/mL a gel was formed with 0.4 M NaCl.
Table 3 Effect of ionic strength on the optical clarity and solubility of Ptx–HSA complexes
Chromatography of paclitaxel–HSA complexes
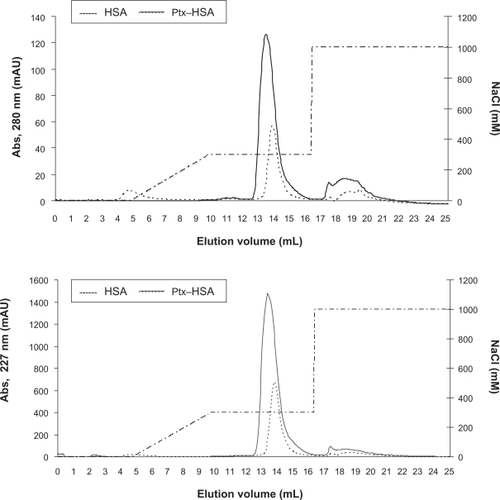
To explore the structural properties of the complexes formed between paclitaxel and acid-denatured HSA, samples of the soluble complexes formed were run on size exclusion and ionic exchange chromatography columns. As determined by reverse-phase high-pressure liquid chromatography analysis of the fractions, paclitaxel coeluted with HSA from a size exclusion resin indicating that there is a physical association between them (data not shown). It was also observed that the elution volume of HSA changed slightly upon binding of paclitaxel, suggesting that it remains monomeric and globular, with a hydrodynamic radius in agreement with that expected for HSA complexed with 4–6 molecules of paclitaxel (). In agreement with this finding, more than 90% of the albumin and paclitaxel were bound and coeluted from an ionic exchange resin, with a profile similar to that of pure HSA ( and ). Altogether, these results confirm that paclitaxel is solubilized in water through a physical interaction with HSA, and also provide evidence suggesting that the complexes formed do not substantially modify the hydrodynamic radius or the net charge of HSA.
Table 4 Binding of Ptx–HSA to anion exchange resin
Figure 1 Size exclusion chromatography of HSA and paclitaxel–HSA. Chromatography on Superdex 200® of HSA (-----) and HSA-paclitaxel. Samples of HSA 5 mg (10 mg/mL) and HSA-paclitaxel 2 mg (10 mg/mL HSA and 1.11 mg/mL paclitaxel) were run on a Superdex 200® column (HR 10/30) at room temperature in phosphate-buffered saline as described in the Materials and methods section.
Abbreviations: HSA, human serum albumin; Ptx, Paclitaxel.
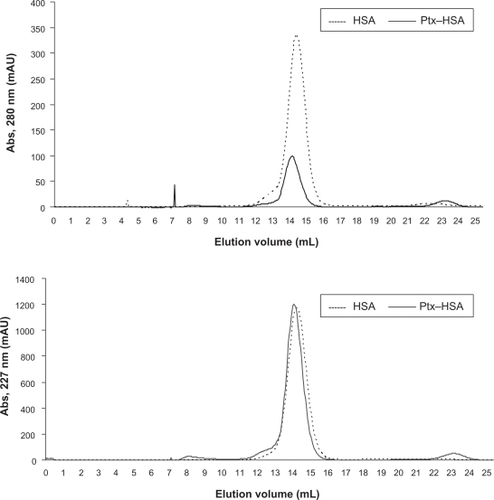
Figure 2 Ionic exchange chromatography of HSA and paclitaxel–HSA complexes. Chromatography on Mono Q® of HSA (-----) and HSA-paclitaxel. Samples of 0.5 mg HSA (1 mg/mL) and 1.25 mg HSA-paclitaxel (5 mg/mL HSA and 0.214 mg/mL paclitaxel) were bound to a Mono Q® column (HR 5/5) at room temperature in 50 mM sodium phosphate (pH 7.0) and eluted with 0.3 M NaCl in 50 mM sodium phosphate (pH 7.0).
Abbreviation: HSA, human serum albumin.
Evaluation of biological activity of water-soluble paclitaxel–HSA complexes
The test used to evaluate the biological activity of the paclitaxel–albumin complex was the inhibition of division of a tumoral cell line. As seen in , control samples show a clear monolayer of human larynx epithelioma cells covering the surface of the Petri dishes. On the other hand, cell samples treated with paclitaxel either in Cremophor or complexed with HSA show few cells, indicating that albumin does not impair the biological effect of paclitaxel on cell division. Furthermore, at least at the dose tested, there was no statistical difference in the number of viable cells between paclitaxel in Cremophor and those complexed with HSA.
Conclusion
Standard formulations of taxanes require use of solvents, such as Cremophor or Tween, which contribute to some of the toxicities associated with paclitaxel-based therapy. To overcome these drawbacks, nanoparticle HSA-bound paclitaxel has been recently approved and is available commercially as Abraxane®. However, this formulation is prepared by high-pressure homogenization of albumin and paclitaxel, leading to the formation of a colloidal suspension with very limited stability. We hypothesized that the exposure of hydrophobic domains of albumin via a partial opening of its structure could improve its interaction with a hydrophobic molecule like paclitaxel. The results obtained confirm that, as previously known, the binding affinity of paclitaxel with native HSA is very low ().Citation15 In agreement with our hypothesis, we found that paclitaxel readily binds to pH-induced partially unfolded HSA, leading to the formation of optically clear water-soluble complexes. The complexes thus formed appear to be more stable in solution when the native state of HSA is at least partially restored by neutralization of the solution to a pH around 7. In agreement with this, we observed that the hydrodynamic radius of HSA is only slightly affected after the cycle of pH changes, and is consistent with paclitaxel binding. Furthermore, paclitaxel binding does not affect the overall exposure of charged groups of HSA, as evaluated by its interaction with an ionic exchange resin. The in vitro biological activity of the complexes formed was qualitatively equivalent to that of a Cremophor-based formulation.
In conclusion, our results not only show that HSA-paclitaxel complexes can be prepared by a simple method that does not require use of high-pressure homogenization, but also open up the possibility to extend this strategy for the design of novel protein-based nanodelivery vehicles for other hydrophobic active pharmaceutical ingredients. Further work is in progress in order to characterize the structure, as well as energetic and dynamic parameters of the complexes formed by the binding of paclitaxel to partially unfolded HSA and to evaluate their activity.
Acknowledgements
This work was supported by a grant from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) and PICT 00696 from FONCYT. IDB and DMB are research staff and VL is a fellow of CONICET.
Disclosure
The authors report no conflicts of interest in this work.
References
- DonehowerRCRowinskyEKGrochowLBLongneckerSMEttingerDSPhase I trial of Taxol in patients with advanced cancerCancer Treat Rep198771117111772891441
- ArbuckSGCanettaROnettoNChristianMCurrent dosage and schedule issues in the development of paclitaxel (Taxol)Semin Oncol19932031398102016
- WeissRBDonehowerRCWiernikPHHypersensitivity reactions from TaxolJ Clin Oncol1990823252327
- FriedlandDGormanGTreatJHypersensitivity reactions from taxol and etoposideJ Natl Cancer Inst19928520367902446
- SzebeniJMuggiaFMAlvingCRComplement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: An in vitro studyJ Natl Cancer Inst1998903003069486816
- SharmaAStraubingerRMNovel taxol formulations: Preparation and characterization of taxol-containing liposomesPharm Res1994118898967937531
- SharmaUSBalasubramanianSVStraubingerRMPharmaceutical and physical properties of paclitaxel (Taxol®) complexes with cyclodextrinsJ Pharm Sci199584122312308801338
- Alkan-OnyukselHRamakrishnanSChaiHBPezzutoJMA mixed micellar formulation suitable for the parenteral administration of taxolPharm Res1994112062127909371
- LeeHJLeeKRParkTGHyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activityBioconjug Chem2008191319132518481885
- SingerJWShafferSBakerBPaclitaxel poliglumex (XYOTAX; CT-2103): An intracellularly targeted taxaneAnticancer Drugs20051624325415711176
- FasanoMCurrySTerrenoEThe extraordinary ligand binding properties of human serum albuminIUBM Life200557787796
- HawkinsMJSoon-ShiongPDesaiNProtein nanoparticles as drug carriers in clinical medicineAdv Drug Deliv Rev20086087688518423779
- MieleESpinelliGPMieleETomaoFTomaoSAlbumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancerInt J Nanomedicine200949910519516888
- SebakSMirzaeiMMalhotraMKulamarvaAPrakashSHuman serum albumin nanoparticles as an efficient noscapine drug delivery system for potencial use in breast cancer: Preparation and in vitro analysisInt J Nanomedicine2010552553220957217
- DesaiNPSoon-ShiongPSandfordPAGrinstaffMWSuslickKSMethods for in vivo delivery of substantially water insoluble pharmacologically active agents and compositions useful therefore United States Patent 5, 439686.881995
- DesaiNPTaoCYangAProtein stabilized pharmacologically active agents, preparation thereof and methods for the use thereof United States Patent 6749868.6152004
- PaálKShkarupinABeckfordLPaclitaxel binding to human serum albumin – automated docking studiesBioorg Med Chem2007151323132917118665
- Trynda-LemieszLPaclitaxel-HSA interaction. Binding sites on HSA moleculeBioorg Med Chem2004123269327515158795
- BertucciCCimitanSRivaAMorazzoniPBinding studies of taxanes to human serum albumin by bioaffinity chromatography and circular dichroismJ Pharm Biomed Anal200642818716413734
- PaálKMüllerJHegedüsLHigh affinity binding of paclitaxel to human serum albuminEur J Biochem20012682187219111277943
- KumarGNWalleUKBhallaKNWalleTBinding of taxol to human plasma, albumin and alpha 1-acid glycoproteinRes Commun Chem Pathol Pharmacol1993803373448102493
- PurcellMNeaultJFTajmir-RiahiHAInteraction of taxol with human serum albuminBiochim Biophys Acta20001478616810719175
- ChenRRemoval of fatty acids from serum albumin by charcoal treatmentJ Biol Chem1967251731816016603
- AusarSFBiancoIDBadiniGDJ Dairy Sci20018436136911233020
- WallevikKHvidtAConformational changes in human serum albumin as revealed by hydrogen-deuterium exchange studiesJ Biol Chem1972247153015355012322
- El KadiNTaulierNLe HuérouJYUnfolding and refolding of bovine serum albumin at acid pH: Ultrasound and structural studiesBiophys J2006913397340416861279
- MuzammilSKumarYTayyabSMolten globule-like state of human serum albumin at low pHEur J Biochem1999266263210542047
- WallevikKReversible denaturation of human serum albumin by pH, temperature, and guanidine hydrochloride followed by optical rotationJ Biol Chem1973248265026554697387
- DockalMCarterDCRükerFConformational transitions of the three recombinant domains of human serum albumin depending on pHJ Biol Chem20002753042305010652284