?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction:
Kolliphor® EL (K-EL) is among the most useful surfactants in the preparation of emulsions. However, it is associated with low hydrophobic drug loading in the resulting emulsified formulation.
Methods:
In this study, a formulation for intranasal administration of butylidenephthalide (Bdph), a candidate drug against glioblastoma (GBM), was prepared. Physical characteristics of the formulation such as particle size, zeta potential, conductivity, and viscosity were assessed, as well as its cytotoxicity and permeability, in order to optimize the formulation and improve its drug loading capacity.
Results:
The optimized formulation involved the integration of polyethylene glycol 400 (PEG 400) in K-EL to encapsulate Bdph dissolved in dimethyl sulfoxide (DMSO), and it exhibited higher drug loading capacity and drug solubility in water than the old formulation, which did not contain PEG 400. Incorporation of PEG 400 as a co-surfactant increased Bdph loading capacity to up to 50% (v/v), even in formulations using Kolliphor® HS 15 (K-HS15) as a surfactant, which is less compatible with Bdph than K-EL. The optimized Bdph formulation presented 5- and 2.5-fold higher permeability and cytotoxicity, respectively, in human GBM than stock Bdph. This could be attributed to the high drug loading capacity and the high polarity index due to DMSO, which increases the compatibility between the drug and the cell. Rats bearing a brain glioma treated with 160 mg/kg intranasal emulsified Bdph had a mean survival of 37 days, which is the same survival time achieved by treatment with 320 mg/kg stock Bdph. This implies that the optimized emulsified formulation required only half the Bdph dose to achieve an efficacy similar to that of stock Bdph in the treatment of animals with malignant brain tumor.
Introduction
Glioblastoma (GBM), which is classified as a grade IV astrocytoma by the World Health Organization, is a highly aggressive tumor type. Patients diagnosed with GBM have a median survival of <2 years and a progression-free survival of 6.2 to 7.5 months.Citation1 The standard treatment of GBM is surgical resection to remove the maximal amount of tumor, followed by radiotherapy and temozolomide.Citation1,Citation2 However, recurrence has been noted after 6–9 months.Citation3–Citation5 Intracerebral local-release polymer wafers containing n-butylidenephthalide (Bdph), extracted from Angelica sinensis, have shown efficacy against malignant human gliomas.Citation6 However, patients who cannot undergo surgery are not able to receive the required surgical implant for local interstitial drug treatment. The development of a new delivery method for Bdph would be beneficial for patients who cannot undergo surgery.
The blood–brain barrier (BBB) and first-pass effect in the liver are the two major limiting factors in chemotherapy for brain cancer. Intranasal administration is an attractive route for drug delivery to the central nervous system (CNS), as it uses the peripheral olfactory system and peripheral trigeminal system to bypass the BBB and achieve rapid entry into the CNS.Citation7 Intranasal administration provides a noninvasive method to deliver a drug to the brain. Criteria for intranasal drug systems include small volume, low viscosity, small delivered particle size, and appropriate hydrophobic/hydrophilicity.Citation8,Citation9 For example, a viscous liquid or a large particle size would result in fast clearance from nasal mucociliary.Citation9 In order to increase formulation spreadability, which in turn is related to flowability and wettability, it is very important for the drug to be absorbed sufficiently into the nasal mucosa.Citation10 The drug under consideration here, Bdph, is hydrophobic and has a low spreadability. Owing to its high hydrophobicity, it requires emulsification to reach the desired absorption. The addition of immiscible liquids, surfactants, co-surfactants, solvents, or co-solvents to hydrophobic compounds allows the mixture to spontaneously form a nano- or micro-emulsion. Kolliphor® EL (K-EL) and Kolliphor® HS 15 (K-HS15), which have low and high hydrophilic–lipophilic balance (HLB) values, respectively, are two candidate surfactants for emulsified formulations, because they are non-ionic solubilizers with low toxicities. However, there is a drawback in that formulations containing these surfactants show lower Bdph drug encapsulation. Mucoadhesion is also a factor in the choice of surfactant when designing a formulation for intranasal delivery. The use of a polymer with a strong hydrating ability means a stronger interaction of polymer–water interaction compared with the polymer–polymer interaction, which increases mucoadhesion of the emulsion. Polyethylene glycol 400 (PEG 400), the co-surfactant used in our study, is the most commonly used polymer in drug formulations. One application of PEG 400 is as a probe to monitor the permeability of the BBB to a given substance.Citation11 Moreover, PEG 400 has been used in nasal delivery formulations,Citation12 intravenous injections,Citation13 and oral administration.Citation14 Propylene glycol (PG), which is not a polymer but is miscible in water, was also used as a co-solvent in our study to examine the feasibility of nano- or micro-emulsion. The low- and high-polarity solvents ethanol (EtOH) and dimethyl sulfoxide (DMSO), respectively, were used to obtain an optimized formulation. To evaluate the feasibility of intranasal Bdph delivery to treat brain tumors, hydrophobic Bdph was emulsified with a solvent, surfactant, co-solvent, and phosphate-buffered saline (PBS). The emulsified formulations were screened for drug loading, tumor cell cytotoxicity, stability, and permeation through the nasal mucosa. After screening, the candidate emulsified Bdph formulations were further applied intranasally against malignant brain tumor in an animal model.
Material and methods
Emulsification of Bdph
Bdph was purchased from Sigma Aldrich. Bdph emulsions were prepared according to the formulations in . We used EtOH (Sigma Aldrich) and DMSO (Sigma Aldrich) as solvents, Kolliphor® (K-EL) and Kolliphor® HS 15 (K-HS15) as surfactants, and PG and PEG 400 as co-solvent/co-surfactants to prepare the emulsion. The components making up the emulsion were sequentially added into a tube and completely mixed using a vortex mixer to obtain a homogenous emulsified solution. The emulsions were left to stand for one hour at room temperature after mixing prior to analysis.
Table 1 Compositions of n-butylidenephthalide (Bdph) emulsions
Permeation study in an artificial cellulose membrane
The permeation of Bdph emulsion was evaluated across a cellulose membrane in a Franz diffusion cell (customized from San Mei, Taiwan); more details are provided in the Supplementary Information.
Permeation study in nasal mucosa
Human nasal septum quasi-diploid tumor (RPMI 2650) cells purchased from the Bioresource Collection and Research Center (BCRC) were seeded on a cell insert (membrane pore size, 0.4 μm) pre-coated with collagen and cultivated for two days. More details are provided in the Supplementary Information.
Analyses of physical properties of formulations
The Bdph emulsions were reconstituted to a concentration of 200 μg/mL in PBS to analyze the particle size through a nano-droplet size (Malvern Nano-ZS ZEN-3600). The zeta potential and conductivity were measured using a 1:5,000 dilution factor for each formulation in PBS with a zeta potential meter (Malvern Nano-ZS ZEN-3600). To measure the absorption of stock Bdph, the Bdph was dissolved in DMSO to obtain 1% Bdph. The absorbances of Bdph alone and in various emulsions were measured with 1:200 and 1:5,000 dilution factors in PBS at 310 nm using an ELISA reader (Multiskan™ FC Microplate Photometer, Thermo Fisher Scientific Inc.).
Viscosity analysis
The viscosity of the emulsions was analyzed using an AR2000ex system (TA Instruments). The relation between viscosity and shear rate was determined using a cone (40 mm and 4 degrees) and plate mold to measure a 1 mL volume of the each formulation at 25 °C.
MTT assay
GBM8401 cells purchased from BCRC were seeded with 6×104/ml cell solution at a concentration of 100 μL. Stock Bdph dissolved in DMSO, emulsified Bdph, and medium alone (negative control group) were examined by MTT assay. More details are provided in the Supplementary Information.
Orthotropic malignant brain tumor F344 animal model
An orthotropic malignant brain tumor model was developed in Fischer 344 rats via intracranial delivery of 9L gliosarcoma cells with a block tissue.Citation15 Male Fischer F344 rats (230–260 g) were obtained from the National Laboratory Animal Center (Taipei, Taiwan). All of the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of National Dong Hwa University and were used in compliance with the standard operating procedures of the Laboratory Animal Center of National Dong Hwa University. (IACUC 102002, Hualien, Taiwan). The F344 rats were anesthetized with 4% chlorhydrate and placed in a stereotaxic apparatus. The cranium was exposed with a circle of 5 mm diameter by a 0.8 mm burr hole at 3 mm lateral and 5 mm caudal to the bregma. The F344 rats were given intracranial implants of 9L gliosarcoma cells (purchased from BCRC) with a 1×1×1 mm3 block of tissue. The tissue block was obtained from syngeneic F344 rats bearing 9L gliosarcoma cells through subcutaneous injection of 9L cell solution with 1×106 into the backs of rats. Seven days after orthotropic implantation of brain tumors, the rats were randomly grouped into treatment and control groups. The drug was delivered daily for 30 days through a pipette that connected the nasal cavities of the rats to simulate intranasal administration. The survival rate was calculated by recording the survival status of the rats. The rats were sacrificed to inspect and weight the tumor size after 30 days of treatment.
Statistical analysis
All the in vitro results are expressed as averagestandard deviation (SD), obtained from three replicate experiments. The statistical analysis of cytotoxicity and mean survival time in the animal studies was performed using the Student’s t-test and two-tailed tests. A significant difference was considered to exist when the p-value was less than 0.05.
Results
Physical properties of formulations
The emulsions were prepared as described in . All of the emulsion solutions had a white color or homogenous single-layer solution (), indicating that an emulsion solution had been successfully prepared. Kolliphor® EL (K-EL) was more compatible with Bdph than Kolliphor® HS 15 (K-HS15); therefore, most of the successful formulations were prepared with K-EL (). A layer consisting of a yellow oil solution of Bdph separated from the water phase when K-HS15 was employed without PEG 400, indicating that the emulsion failed to form. Moreover, precipitation was observed in the formulation of BN-E containing K-HS15 after 30 days storage at 4 °C.
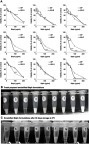
Figure 1 (A) Cell cytotoxicity analysis of emulsified n-butylidenephthalide (Bdph) formulations in GBM8401. Abbreviation BP indicates that Bdph was dissolved in DMSO. The data are expressed as mean ± SD; *p<0.05, **p<0.01, ***p<0.001 versus BP. (B) Stability examination of freshly prepared emulsified Bdph formulations (BN-A (A), BN-B (B), BN-C (C), BN-D (D), BN-E (E), BN-F (F), BN-G (G), BN-H (I), BN-I (I)) and (C) stability examination after 30 days storage at 4 °C. An arrow indicates sedimentation, and a frame line means an insoluble layer was formed. The formulation for each emulsion is given in .
The potential advantages of the self-emulsifying systems include 100% drug entrapment capacity .Citation16 The successful emulsions shown in was a white milk color or colorless, without separation of layers or aggregation, which indicates 100% drug entrapment capacity. The absorbance of Bdph in the formulation increased with increasing percentage of Bdph, suggesting that the emulsion had 100% drug loading capacity (). A high drug loading capacity (up to 50% Bdph) was noted for the emulsions containing PEG 400 as a co-surfactant with BN-D, BN-E, BN-F, and BN-I, but not those containing PG ( and ).
Table 2 Physical characteristic analysis of n-butylidenephthalide (Bdph) emulsions
The mean particle size of the emulsified Bdph formulations was between 34.99 nm and 3.76 μm, while stock Bdph could not be detected, suggesting that only the hydrophobic layer was present in solution. The results showed that formulations with 50% Bdph had particle sizes above 366 nm, whereas emulsions with less than 20% Bdph had particle sizes below 237 nm (). This suggests that Bdph molecules were encapsulated in the emulsion particles, because a higher droplet size was noted in the presence of Bdph.
The formulations showed a slight negative charge on the droplet surface and zeta potential between −2 and −9 mV after emulsification of Bdph (). By contrast, stock Bdph possessed a lower potential of −14.5 mV compared with emulsified Bdph formulations. The conductivity of stock Bdph was 17.10 mS/cm, but those emulsified Bdph formulations were above 17.10 mS/cm, indicating that the emulsified Bdph was an oil in water (O/W)-type formulation.
The results for size, zeta potential, and conductivity all suggest that Bdph was emulsified after mixing with a surfactant and solvent.
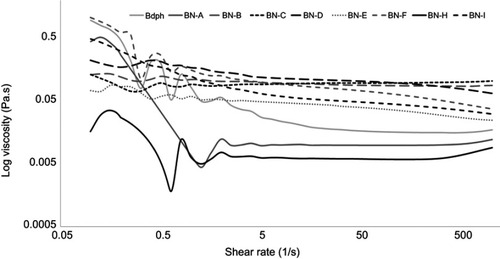
Viscosity
A rheometer was used to analyze the viscosity of emulsified Bdph formulations and stock Bdph. In the analysis, constant shear stress and increased shear rate were applied. All the solutions showed shear thinning characteristics, indicating that they acted as non-Newtonian fluids (). The infinity viscosities of emulsified Bdph formulations ranged from 0.62×10−2 to 10.83×10−2 Pa·s, whereas that of stock Bdph was 1.59×10−2 Pa·s (). A multi-peak was observed for the stock Bdph, as shown in , indicating that a higher shear rate was required for the Bdph to achieve a stable viscosity. By contrast, most of the Bdph emulsification formulations achieved steady-state viscosity at lower shear rates. The spreadability of the Bdph was improved after the drug was emulsified.
Table 3 Viscosity of emulsified n-butylidenephthalide (Bdph) formulations
Cytotoxicity
To evaluate the cytotoxicity of emulsified Bdph formulations in GBM, fresh formulations at concentrations from 0 to 200 μg/ml were prepared and used to treat GBM 8401 cells for 24 hrs. Cell viability was determined by MTT assay and compared with that of untreated cells. The formula to calculate the 50% inhibitory concentration (IC50) was obtained by plotting the drug concentration against cell survival percentage. The value of IC50 for each formulation was obtained using the curve at 50% cell survival (). The IC50 values of the formulations ranged from 32.7 to 150.2 μg/mL (). The BN-B, D, E, F, and I formulations were more cytotoxic than stock Bdph (81.7 μg/mL). Among the formulations, BN-F was the most cytotoxic to GBM8401 cells (32.7 μg/mL).
Table 4 IC 50 of fresh prepared emulsified n-butylidenephthalide (Bdph) formulations in GBM 8401
Stability testing of emulsified Bdph formulations
To investigate the stability of the formulations, their appearance was examined after 30 days storage at 4 °C. The emulsified Bdph formulations (BN-A to I) were homogenous and either transparent or white (). BN-C, D, F, and I had similar appearances after being stored at 4 °C for 30 days. By contrast, a precipitate or insoluble layer appeared in the BN-A, B, E, G, and H formulations after storage under the same conditions ().
In vitro permeation studies
To evaluate the permeation of emulsified Bdph formulations, an artificial cellulose membrane was used in a Franz cell system. The emulsions were collected and examined by ultraviolet-visible light spectroscopy with OD310. The results were analyzed using supplementary Formula (S1) and (S2) and all the calculated results were based on equations with a coefficient of determination (R2) above 0.85 (). The emulsified Bdph (BN-A to BN-I) showed improved flux and permeability coefficient (Peff) compared with stock Bdph, with a lowest flux and Peff of 2.2910−3 μg/cm2s and 0.76
10−6 cm/s, respectively (). The formulations of BN-E, F, and I exhibited the highest permeation flux and Peff above 11.20
10−3 μg/cm2s and 3.57
10−6 cm/s, respectively. By contrast, BN-D, which also showed high drug loading and cytotoxicity, had a lower flux and Peff which are equal to 2.71
10−3 μg/cm2s and 0.98
10−6 cm/s, respectively ().
Table 5 Permeation study of emulsified n-butylidenephthalide (Bdph) formulations through artificial cellulose membranes
A permeation study was further performed in nasal septum squamous cells on a cell insert. All the calculated results were based on equations (). The permeation results in the nasal septum squamous cells showed similar patterns, in that the four emulsified Bdph formulations were higher permeability than stock Bdph, and BN-D exhibited the lowest permeability (). Among the emulsified Bdph formulations, BN-F possessed the highest permeation flux and Peff equaling to 1.6310−3 μg/cm2s and 8.16
10−6 cm/s, respectively ().
Table 6 Permeation study of emulsified n-butylidenephthalide (Bdph) formulations through nasal septum squamous cells
Animal experiment
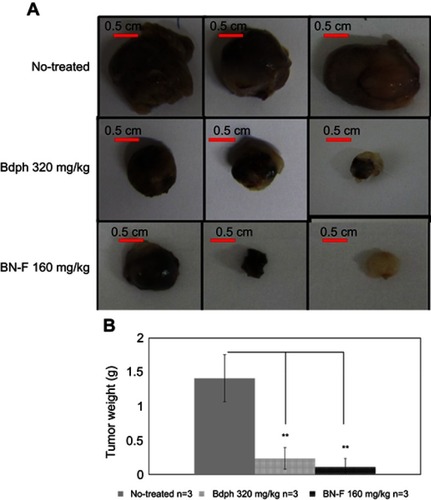
Based on the results of the cytotoxicity and permeation flux studies, BN-F was selected as a candidate formulation for animal studies. The emulsified formulation BN-F consisted of spherical particles with a double layer structure (). The results further confirmed that the hydrophobic drug was emulsified into the surfactant and consolidated by the co-surfactant PEG 400, forming a double layer structure. The emulsified formulation BN-F was used in animal studies to compare its therapeutic efficacy to that of stock Bdph. The drug was delivered intranasally to rats bearing intracerebral malignant brain tumors. An increased survival rate was observed when the rats received Bdph or BN-F, compared with those receiving no treatment (). Using 160 mg/kg BN-F or 320 mg/kg stock Bdph produced a 100% survival rate for at least 37 days (). A decreased survival rate was observed when the dose of BN-F was reduced to 80 mg/kg or that of stock Bdph to 160 mg/kg. The median survival times of animals who received stock Bdph 160 mg/kg and 80 mg/kg BN-F were 31 and above 37 days, respectively (), whereas those who did not receive treatment all died within 26 days ().
Figure 2 (A) Kaplan–Meier survival curves and (B) mean survival times of rats bearing malignant brain tumors after treatment with n-butylidenephthalide (Bdph) formulation. The composition of BN-F is given in . *p<0.05, **p<0.01, ***p<0.0005 compared with the non-treated group.
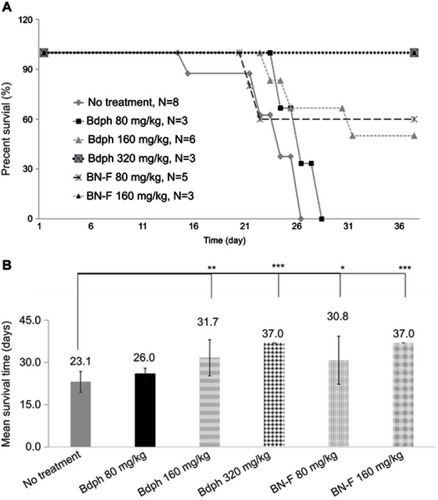
Figure S2 Transmission electron microscopy image of emulsified n-butylidenephthalide (Bdph) formulation BN-F. The white arrows indicate the double layer structure of the BN-F formulation.

The mean survival time of tumor-bearing rats was increased with treatment. The mean survival time of untreated rats was 23.1 days (), compared with 37 days after treatment with 160 mg/kg BN-F and 31.7 days after treatment with 160 mg/kg stock Bdph (). A similar pattern was observed after treatment with 80 mg/kg BN-F and 80 mg/kg stock Bdph, where mean survival times were 30.8 and 26.0 days, respectively (). The survival times of untreated rats bearing tumors were shorter than those of the rats that received treatment, and a proliferating tumor mass was observed in the no-treatment group (). These results suggest that Bdph prolonged the survival rate of animals with malignant brain tumor. Importantly, using an emulsified formulation reduced the required dose of Bdph, as evidenced by the fact that a half dose of BN-F showed equal efficacy to a whole dose of stock Bdph.
Discussion
Biodegradable wafer typeCitation6,Citation17 and liposome-basedCitation18 vehicles for Bdph have previously been employed for treating tumor-bearing animals, but these had <25% drug loading capacity. Here, a 50% Bdph drug loading capacity was achieved with the optimized emulsification of DMSO/K-EL/PEG 400/PBS. More importantly, the emulsified Bdph formulations allowed for a reduction in dosage by 50%.
The surfactant Kolliphor® EL (K-EL) was more compatible with Bdph than Kolliphor® HS 15 (K-HS15). This phenomenon may be related to the HLB values of K-EL (HLB=12–14)Citation19 and K-HS15 (HLB=16).Citation20 Moreover, a double bond structure in the hydrophobic chain of K-EL may confer higher affinity on the aromatic structure of Bdph. Higher drug loading capacities of Bdph were obtained in the formulations BN-D, E, F, and I, all of which contained the co-surfactant PEG 400. A higher drug loading capacity was observed in the presence of PEG 400 but not PG, possibly because PEG 400 could provide a linkage between the hydrophobic and hydrophilic phases. Thus, PEG 400 cooperates with the surfactant to solidify the structure of emulsified Bdph.Citation21 PG, however, is miscible in water and does not provide a connection between the two phases.
A negative correlation between conductive value and oil volume fraction has been demonstrated.Citation22 The higher the percentage of the oil fraction volume, the lower the conductive value, as confirmed by our results. Furthermore, size is an indication of drug loading within a formulation, since the drug is encapsulated in the core of the formulation.Citation23 Higher droplet size was noted in the formulations BN-D, E, F, and I, which also had the highest drug loading (up to 50%).
A higher viscosity occurs when a boundary exists between the two phases. A hydrophobic drug creates a boundary between hydrophobic and hydrophilic phases.Citation24 In the emulsification formulations, higher drug loading was associated with higher viscosity (above 4.05×10–2 Pa·s). However, BN-B and C had lower drug loading (below 17.2%) and had viscosities above 7.55×10−2 Pa·s. This may have been due to the higher percentage of surfactant and hydrophilic PG used in these formulations, and thus a higher viscosity.
A higher drug loading capacity can enhance cytotoxicity.Citation25 The same quantity of drug was applied to GBM8401 in each formulation, but only BN-D, E, F, and I showed greater cytotoxicity than those of BN-A, B, C, G and H, suggesting that their compositions contain more drug per droplet.
A higher drug content in a formulation leads to an increased flux value when the ratio of drug to surfactant is greater than 4.Citation26 The permeation ability of emulsions was correlated with the drug carrier percentage in our studies. To achieve the same drug dose, the formulation with less drug required more excipient than that with higher drug loading. The higher excipient volume may block the membrane and decrease the rate of permeation flux. However, the BN-D formulation, which had the highest drug loading, showed the lowest permeation flux at 2.71×10–3 μg/cm2s, suggesting that higher viscosity decreases the permeation flux.Citation26
In general, evaluation of the stability of a formulation may involve measurement of zeta potential (ZP); ZP-values of ±0–10 mV indicate a highly unstable formulation.Citation27 However, a controversial result of our studies was that the formulations BN-A to -I, the ZP-values of which were less than |0–10| mV, were stable when freshly prepared. Moreover, BN-A and BN-E, which exhibited higher ZP-values than other Bdph emulsions, showed sedimentation after storage at 4 °C for 30 days. The data suggest that hydrophobic/hydrophilic balance might also play a part in stabilization of formulations.Citation28
Only a half dose of emulsified Bdph was required to treat malignant brain tumors. The difference in efficiency between stock Bdph and BN-F for a given dosage can be partly explained by the IC50 values in GBM 8401, which were 81.7 and 32.7 µg/mL for stock Bdph and BN-F, respectively (). Overall, intranasal BN-F showed an therapeutic efficacy owing to its higher cell cytotoxicity in GBM and permeation compared with stock Bdph.
Conclusion
Emulsions of Bdph were screened and characterized in our studies to prepare candidate formulations for intranasal administration. Emulsification resulted in an increase in solubility, as evidenced by conductivity, cell cytotoxicity, and permeation rates. BN-F, which was composed of Bdph/DMSO/K-EL/PEG 400/PBS, was the optimal formulation based on cytotoxicity and permeation rates. BN-F prolonged the survival time of F344 rats with intracerebral 9L glioma tumors using a half dosage of stock Bdph. The results suggest that BN-F delivered intranasally has potential applications in the treatment of GBM.
Abbreviation list
BBB, blood-brain barrier; Bdph, butylidenephthalide; CNS, central nervous system; DMSO, dimethyl sulfoxide; EtOH, ethanol; GBM, glioblastoma; HLB, hydrophilic-lipophilic balance; IC50, 50% inhibitory concentration; K-EL: Kolliphor® EL; K-HS15, Kolliphor® HS 15; PBS, phosphate-buffered saline; Peff: permeability coefficient; PEG 400, Polyethylene glycol 400; PG, propylene glycol; R2, coefficient of determination; SD, standard deviation; TEM: transmission electron microscopy.
Disclosure
The formulation has been patented in the USA (Patent No. US9504751 (B2)). Miss Yu-Shuan Chen reports non-financial support from National Science Council of the Republic of China, Taiwan, during the conduct of the study. Dr Shinn-Zong Lin reports non-financial support from National Science Council of the Republic of China, Taiwan, during the conduct of the study. Dr Tzyy-Wen Chiou reports grants from National Science Council, Taiwan, Republic of China, during the conduct of the study. The authors report no further conflicts of interest in this work.
Acknowledgment
Financial support was received from the National Science Council of the Republic of China, Taiwan (NSC 102-2221-E-259-017) and Ministry of Science and Technology of the Republic of China, Taiwan (MOST 103-2221-E-259-035 and MOST 107-2218-E-303-001-MY3).
Supplementary material
Supplementary material and method
Permeation study in an artificial cellulose membrane
The permeation of Bdph emulsion was evaluated across a cellulose membrane in a Franz diffusion cell (customized from San Mei, Taiwan). The diffusion area of cellulose membrane and the receptor chamber of a Franz diffusion cell were approximately 4.5 cm2 and 23 mL, respectively. An artificial cellulose membrane was cut and immersed into HEPES buffer (118mM NaCl, 1.2 mM MgSO4, 4.8 mM KCl, 5.5 mM D-glucose, 2.5 mM CaCl2, mM Hepes) before the permeation study. The 2 ml of 3 mg/mL Bdph emulsion was added into the donor chamber and HEPES buffer was placed in the receptor chamber before the membrane was immersed into the buffer. A multipoint stirrer was set to 300 rpm, and the whole experiment took place in an incubator at 37 °C. After 10 h, 1 mL of sample was collected from the receptor chamber each hour, after which 1 mL fresh warmed HEPES buffer was added to the receptor chamber. The sampled solution was detected using absorption at 310 nm by ELISA reader. A permeability coefficient (Peff) and flux (J) were obtained using the following supplementary Formula (S1) and (S2).1
V:Capacity of receptor chamber (mL)
C0:Concentration of drug in the donor compartment (mg/mL)
Peff:Permeability coefficient (cm/s)
J:Flux (μg/cm2s)
A:Superficial area of diffusion (cm2)
: Concentration changed over time in a pseudo steady-state (μg/mLs)
Permeation study in nasal mucosa
Human nasal septum quasi-diploid tumor (RPMI 2650) cells were seeded on a cell insert (0.4 μm pores size membrane) pre-coated with collagen and cultivated for 2 days. The cell insert contained monolayer cells placed in a 12 well plate with 1.5 mL HEPES buffer. The emulsified Bdph solution (0.5 mL) dissolved in HEPES buffer were added to the monolayer cells and incubated at 37 °C in 5% CO2. The samples were harvested from the well at 1 hr intervals over 6 hrs and analyzed by UV-VIS spectroscopy. The concentration of Bdph was calculated by the absorption at 310 nm using a standard reference curve. The permeability coefficient (Peff) and Flux were calculated using supplementary Formula (S1) and (S2).
Cultivation of cell lines
GBM 8401, human nasal septum quasidiploid tumor (RPMI 2650), and rat glioma cell lines 9L were purchased from Bioresources Collection and Research Center (BCRC, Taiwan). The GBM8401, RPMI 2650, and 9L cells were cultivated in basal medium of RPMI-1640 (Gibco), Minimum essential medium (Corning), and Dulbecco’s Modified Eagle Medium (Corning), respectively. All the cultural media were provided 10% fetal bovine serum (Gibco) and 1% Penicillin/Streptomycin (100 units/L Penicillin G and 100 μg/L Streptomycin Sulfate, Gibco). The procedures of cell passage were performed following the addition of 0.05% trypsin/EDTA (Biowest, Nuaille, France) to trypsinize the cells, and centrifugation at 240 g for 5 min to harvest the cells. The cellular suspensions were reconstituted in their cultivation medium and incubated at 37 °C in 5% CO2.
MTT assay
GBM8401 Cells (6×103) were seeded in a 96 well for overnight cultivation. Stock Bdph, emulsified Bdph, and medium alone (negative control group) were then added and left to incubate for 24 hrs. Thereafter, the supernatant was removed and 500 μg/mL thiazolyl bluetetrazolium blue (MTT, Sigma, MO, USA) was added to detect mitochondrial activity in any surviving cells. After 1 hr incubation, DMSO was added to dissolve the formazan crystal products. Absorption of the DMSO/formazan solution was detected via enzyme-linked immunosorbent assay (ELISA) reader at 595 nm.
Transmission electron microscopy (TEM)
For TEM observation, the emulsified formulation was diluted 500 times with pure water. Then, 10 μL of the diluted formulation was applied to carbon film-coated 400 mesh copper grids (Electron Microscopy Sciences, Hatfield, PA, USA) for 60 s and extra loaded solution was adsorbed by Kimwipe paper. Grids were negatively stained by incubation with 10 μL of 1.5% phosphotungstic acid for another 60 s. Again, extra loaded solution was adsorbed by Kimwipe paper. The grids of the emulsified formulation were stored in a desiccator for further analysis. Samples were visualized at 120 kV by a transmission electron microscope (JEOL JEM-1200CX II).
Reference
References
- Anjum K, Shagufta BI, Abbas SQ, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed Pharmacother. 2017;92:681–689. doi:10.1016/j.biopha.2017.05.12528582760
- Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2–8. doi:10.1188/16.CJON.S1.2-8
- Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol. 2015;116:294–300. doi:10.1016/j.radonc.2015.07.03226276527
- Fu P, He YS, Huang Q, et al. Bevacizumab treatment for newly diagnosed glioblastoma: systematic review and meta-analysis of clinical trials. Mol Clin Oncol. 2016;4:833–838. doi:10.3892/mco.2016.81627123291
- Roy S, Lahiri D, Maji T, Biswas J. Recurrent Glioblastoma: where we stand. South Asian J Cancer. 2015;4:163–173. doi:10.4103/2278-330X.17595326981507
- Harn HJ, Lin SZ, Lin PC, et al. Local interstitial delivery of z-butylidenephthalide by polymer wafers against malignant human gliomas. Neuro Oncol. 2011;13:635–648. doi:10.1093/neuonc/nor02121565841
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH 2nd. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi:10.1016/j.neuroscience.2004.05.02915262337
- Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982;135:83–88.7130058
- Harris AS, Svensson E, Wagner ZG, Lethagen S, Nilsson IM. Effect of viscosity on particle size, deposition, and clearance of nasal delivery systems containing desmopressin. J Pharm Sci. 1988;77:405–408.3411462
- Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2:215–222. doi:10.4103/2231-4040.9087622247888
- McClung HJ, Sloan HR, Powers P, et al. Early changes in the permeability of the blood-brain barrier produced by toxins associated with liver failure. Pediatr Res. 1990;28:227–231. doi:10.1203/00006450-199009000-000142235119
- van Woensel M, Wauthoz N, Rosiere R, et al. Formulations for Intranasal Delivery of Pharmacological Agents to combat brain disease: a new opportunity to tackle gbm? Cancers (Basel). 2013;5:1020–1048. doi:10.3390/cancers503102024202332
- Li BQ, Dong X, Fang SH, Gao JY, Yang GQ, Zhao H. Systemic toxicity and toxicokinetics of a high dose of polyethylene glycol 400 in dogs following intravenous injection. Drug Chem Toxicol. 2011;34:208–212. doi:10.3109/01480545.2010.50029221314471
- Lin C-F, Hayton WL. Absorption of polyethylene glycol 400 administered orally to mature and senescent rats. Age. 1983;6:52–56. doi:10.1007/BF02431858
- Frazier JL, Wang PP, Case D, et al. Local delivery of minocycline and systemic BCNU have synergistic activity in the treatment of intracranial glioma. J Neurooncol. 2003;64:203–209.14558595
- Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013;2013:848043.24459591
- Huang MH, Chou YW, Li MH, et al. Epigenetic targeting DNMT1 of pancreatic ductal adenocarcinoma using interstitial control release biodegrading polymer reduced tumor growth through hedgehog pathway inhibition. Pharmacol Res. 2018;139:50–61. doi:10.1016/j.phrs.2018.10.01530385365
- Lin YL, Chang KF, Huang XF, et al. Liposomal n-butylidenephthalide protects the drug from oxidation and enhances its antitumor effects in glioblastoma multiforme. Int J Nanomedicine. 2015;10:6009–6020. doi:10.2147/IJN.S8579026451107
- Tang SY, Sivakumar M, Nashiru B. Impact of osmotic pressure and gelling in the generation of highly stable single core water-in-oil-in-water (W/O/W) nano multiple emulsions of aspirin assisted by two-stage ultrasonic cavitational emulsification. Colloids Surf B Biointerfaces. 2013;102:653–658. doi:10.1016/j.colsurfb.2012.08.03623107943
- Shaukat AKK. Tackling the challenges with poorly soluble drugs. J Anal Pharm Res. 2015;1:00001.
- Ma TY, Hollander D, Krugliak P, Katz K. PEG 400, a hydrophilic molecular probe for measuring intestinal permeability. Gastroenterology. 1990;98:39–46.2293598
- Zhang WYY, Lin M, Luo T, Yao C. Electrical conductivity and stability of O/W emulsions. Acta Petrolei Sinica. 2008;24:592.
- Awad TS, Asker D, Romsted LS. Evidence of coexisting microemulsion droplets in oil-in-water emulsions revealed by 2D DOSY (1)H NMR. J Colloid Interface Sci. 2018;514:83–92. doi:10.1016/j.jcis.2017.12.02429245075
- Pal R. Effect of droplet size on the rheology of emulsions. AIChE Journal. 1996;42:3181–3190. doi:10.1002/(ISSN)1547-5905
- Jiang M, Han X, Guo W, et al. Star-shape paclitaxel prodrug self-assembled nanomedicine: combining high drug loading and enhanced cytotoxicity. RSC Adv. 2016;6:109076–109082. doi:10.1039/C6RA23169A
- Hussain A, Haque MW, Singh SK, Ahmed FJ. Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using design expert: part II. Drug Deliv. 2016;23:1242–1253. doi:10.3109/10717544.2015.112447326697777
- Bhattacharjee S. DLS and zeta potential - What they are and what they are not? J Control Release. 2016;235:337–351. doi:10.1016/j.jconrel.2016.06.01727297779
- Laughlin RG. HLB, from a thermodynamic perspective. J Soc Cosmet Chem. 1981;32:371–392.