?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to develop a mucoadhesive coacervate microparticulate system to deliver viable Lactobacillus rhamnosus cells into the gut for an extended period of time while maintaining high numbers of viable cells within the formulation throughout its shelf-life and during gastrointestinal transit.
Methods
Core coacervate mucoadhesive microparticles of L. rhamnosus were developed using several grades of hypromellose and were subsequently enteric-coated with hypromellose phthalate. Microparticles were evaluated for percent yield, entrapment efficiency, surface morphology, particle size, size distribution, zeta potential, flow properties, in vitro swelling, mucoadhesion properties, in vitro release profile and release kinetics, in vivo probiotic activity, and stability. The values for the kinetic constant and release exponent of model-dependent approaches, the difference factor, similarity factor, and Rescigno indices of model-independent approaches were determined for analyzing in vitro dissolution profiles.
Results
Experimental microparticles of formulation batches were of spherical shape with percent yields of 41.24%–58.18%, entrapment efficiency 45.18%–64.16%, mean particle size 33.10–49.62 μm, and zeta potential around −11.5 mV, confirming adequate stability of L. rhamnosus at room temperature. The in vitro L. rhamnosus release profile follows zero-order kinetics and depends on the grade of hypromellose and the L. rhamnosus to hypromellose ratio.
Conclusion
Microparticles delivered L. rhamnosus in simulated intestinal conditions for an extended period, following zero-order kinetics, and exhibited appreciable mucoadhesion in simulated intestinal conditions.
Introduction
Intake of viable Lactobacillus rhamnosus (LR) cells, at around 107 cfu,Citation1,Citation2 aids in the prevention of intestinal tract illnesses,Citation3 suppresses bacterial infection in renal patients,Citation4 safeguards the urogenital tract by excreting biosurfactants,Citation5 stimulates antibody production, aids the immune system, assists the phagocytic process, helps the body to combat dangerous invasive bacteria, controls food-associated allergic inflammation,Citation6 shortens the duration of diarrhea associated with rotavirus infection,Citation7 and reduces use of antibiotics to treat Helicobacter pylori infection.Citation8
Reported therapeutic benefits are associated with the ability of LR to secret coagulin, a bacteriocin, which is active against a broad spectrum of enteric microbes.Citation1 LR is well tolerated with very rare side effects, and its regular intake can be effective in supplementing and maintaining digestive tract health. Processing conditions during formulation and noncompliance with storage requirements during shipment and storage result in loss of cell viability in the dosage formulation. Acidic conditions in the stomach, various hydrolytic enzymes, and bile salts in the gastrointestinal tract also adversely affect the viability of LR after ingestion.Citation9–Citation14
Nowadays, microparticulate systems have been exploited, not only to reduce loss of cell viability during storage and transport, but also to improve and maintain viable cells arriving in the intestine.Citation9–Citation11 Decreased performance of microparticles is attributable to their short gastric retention time, a physiological limitation which can be improved by coupling mucoadhesion properties to the microparticles through developing mucoadhesive microparticles which will in turn simultaneously improve gastric retention time and bioavailability.Citation15–Citation17 Hypromellose and hypromellose phthalate are safe for human consumption, and because of the good mucoadhesive and release rate-controlling properties of hypromellose, it is preferred in mucoadhesive formulations.Citation16–Citation19 These observations indicate a strong need to develop a dosage form that will deliver LR into the gut with improved gastric retention time and adequate stability during storage and gastrointestinal transit, which can be achieved with extended-release mucoadhesive microparticles.
Materials and methods
Materials
Freeze-dried LR R0011-150 powder was donated by Cipla Limited (Mumbai, India). Hypromellose phthalate (HP-50) was donated by Glenmark Pharmaceuticals Limited (Nasik, India). Different grades of hypromellose, ie, Methocel E5 Premium LV (E5), Methocel E50 Premium LV (E50), and Methocel E10 M Premium CR (E10 M), were donated by Indoco Remedies Limited (Mumbai, India). DeMann Rogosa Sharpe agar media and other analytical grade laboratory chemicals were purchased from HiMedia Laboratories Limited (Mumbai, India).
In-house LR specification compliance test
A number of cell count tests (bacteriological, total aerobic bacteria, coliforms, enterobacteriaceae, other Gram-negative bacteria, yeast, molds), and tests to ensure the absence of contaminants (Escherichia coli, Staphylococcus aureus, and Salmonella), were performed as a compliance to the specifications of the certificate of analysis.
Preparation of mucoadhesive microparticles
Core mucoadhesive microparticles of LR were prepared aseptically with hypromellose employing coacervation and phase separation technique.Citation16,Citation20 Hypromellose 5 g was dissolved in 200 mL of cold deionized water (4°C ± 2°C). Polysorbate-80 2 g was dissolved in this solution under stirring, followed by aseptic filtration using a 0.45 μm PVDF filter membrane (Millipore Corporation, Bedford, MA). A calculated quantity of LR was dispersed in the above solution, sonicated at 20 kHz for 1 minute, and the temperature was raised gradually up to 30°C ± 2°C with stirring at 500 ± 25 rpm for 30 minutes. Acetone 50 mL was added dropwise under stirring and stirred for a further 10 minutes. Microparticles were collected by aseptic filtration of the dispersion with a 10 μm nylon filter (Millipore Corporation), followed by washing three times with sterile water for injection (30°C ± 2°C) and kept in a desiccator for 24 hours. All formulation batches having the composition described in were prepared in triplicate. Aseptic processing was carried out on the bench using a horizontal laminar flow clean air work station (1500048-24-24, Klenzaids Bioclean Devices Ltd, Mumbai, India).Citation16,Citation20
Table 1 Formulation formulae and values of evaluation parameters of all formulation batches
Coating of microparticles
HP-50 solution 200 mL (10% w/w) was prepared with phosphate bufferCitation21 at pH 6.8, and polyethylene glycol 200 4 g and polysorbate-80 2 g was dissolved in it. The solution was filtered aseptically using a 0.45 μm PVDF filter membrane followed by dispersing tare core microparticles in it under stirring at 300 ± 25 rpm, and then 40 mL of propan-2-ol was added dropwise. Stirring was continued for 30 minutes, then the coated microparticles were separated by aseptic filtration, washed three times with sterile water for injection (30°C ± 2°C), and kept in a desiccator for 24 hours, followed by determination of the final weight, aseptically packed in glass vials, and stored in a refrigerator for further use.
Coating stage percent weight gain
From the tare weight (WI) of the dried core microparticles that had been subjected to coating and the tare weight (WF) of the dried coated microparticles, the coating stage percent weight gain value was determined using Equationequation 1(1) .
Percent yield study
Calculation for percent yield values (w/w) of all batches were done using Equationequation 2(2) .
Measurement of viable cell number
Measurement of viable cells in sample was done using the following methods.Citation22
Direct microscopic count using dye exclusion test
A thoroughly mixed cell suspension (2–5 × 105 cells/mL) was aseptically prepared to 1 mL with sterile phosphate buffer pH 6.8. Cell suspension 200 μL was mixed thoroughly with 300 μL of sterile phosphate buffer pH 6.8 and 500 μL of 0.4% Trypan blue solution in a 1.5 mL microfuge tube (creating a dilution factor of 5), and kept aside for five minutes. With a coverslip in place, a small volume of the Trypan blue cell suspension was transferred into the chamber of a hemocytometer using a Pasteur pipette and the chamber was allowed to fill up by capillary action to avoid overfilling or underfilling. All the cells (nonviable cells stain blue and viable cells remain opaque) in the 1 mm center square and the four corner squares were counted under a microscope. The number of viable cells per unit of sample (g or mL) was calculated using Equationequation 3(3) . This is a simple and rapid method that provides an approximate result, and was performed in triplicate.
Viable plate counts
One gram of sample, alternately one mL of sample solution, containing LR was transferred aseptically into a presterilized 10 mL volumetric flask containing 5 mL of sterile saline TS, sonicated at 20 kHz for one minute, and diluted to 10 mL with sterile saline TS. One mL of this suspension was diluted to 10 mL in an autoclaved test tube (25 mm × 150 mm size) with sterile saline TS and mixed thoroughly. Serial dilution was continued until a suitable dilution was achieved (approximately 100 cell/mL). The final dilution tube was allowed to stand in a water bath at 70°C for 30 minutes and was then cooled immediately to about 45°C. Saline TS,Citation21 simulated gastric fluid TS, Citation21 and simulated intestinal fluid TSCitation21 contain inorganic salts but no carbon source, thus LR cells will not proliferate in this media, and remain in a state of stasis until plated on media containing a carbon source.Citation22
DeMann Rogosa Sharpe agar medium was liquefied and cooled to 45°C on a water bath. One mL of sample from the heat-treated final dilution tube was transferred into sterile Petri dishes (six per sample), and 15 mL of molten medium was poured, mixed thoroughly, and then incubated in an inverted position at 40°C for 48 hours after solidification.
Six plates were counted and the average count per plate was calculated. The number of cfu per unit (mL or g) of sample was calculated using Equationequation 4(4) .
Entrapment efficiency
In an aseptic manner, 500 mg of accurately weighed coated microparticles were kept with 25 mL of sterile simulated intestinal fluid in a hermetically sealed sterile glass vial at 4°C ± 2°C for 24 hours. The dispersion was subjected to a viable plate count (ie, a viable spore count value in cfu/g) and entrapment efficiency was calculated using Equationequation 5(5) .Citation16
Morphology
The coated microparticles were mounted on aluminum stubs using double-sided adhesive tape. The stubs were then vacuum-coated with a thin layer of gold and examined with a scanning electron microscope (JSM 5610 LV, Jeol, Tokyo, Japan).Citation23–Citation28
Particle size, size distribution, and zeta potential
The core and coated microparticles were dispersed in deionized water (pH 6.8) and sonicated at 20 kHz for three minutes to get a homogenous dispersion (0.5% w/v). The dispersions were put into a small-volume disposable zeta cell and subjected to particle size study using photon correlation spectroscopy with an inbuilt Zetasizer (Nano ZS, Malvern Instruments, Worcestershire, UK) at 633 nm and 25°C ± 0.1°C. The electrophoretic mobility measured (in mm/sec) was converted to the zeta potential.Citation16,Citation25–Citation30
Flow properties
The flow properties of the coated microparticles were determined from the result of the study parameters, ie, angle of repose, Carr’s index, and the Hausner ratio.Citation16,Citation21
In vitro swelling
An in vitro swelling test of the coated microparticles was conducted in simulated intestinal fluid. The size of the dried microparticles and those after incubation in simulated intestinal fluid for 5 hours were measured using a calibrated optical microscope (CX RIII, Labomed, Ambala, India). Percent swelling value was determined from the diameter of the microparticles at time t (DT) and initial time t= 0 (D0) using Equationequation 6(6) .Citation16
Mucoadhesion
Following institutional animal ethical committee guidelines, the mucoadhesion affinity of the coated microparticles for intestinal mucosa was assessed by the following methods.
Ex vivo mucoadhesive strength
A suspension of coated microparticles in simulated intestinal fluid was prepared, and the number of microparticles per mL (No) was determined by optical microscopy. One mL of this suspension was fed to overnight-fasted albino rats of either gender (in groups of three) which were then sacrificed at hours 0, 4, 8, and 12 to isolate their stomach and intestinal regions. The number of microparticles adhering to the stomach and the intestinal regions (NS) was counted after the regions were cut open longitudinally. Percent adhesive strength value as a measure of ex vivo mucoadhesive strength test was calculated using Equationequation 7(7) .Citation16
In vitro washoff test
A strip of goat intestinal mucosa was mounted on a glass slide, on which a dispersion of accurately weighed microparticles (Wa) in simulated intestinal fluid was uniformly spread and incubated in a desiccator at 90% relative humidity for 15 minutes. The slide was then placed in a cell at an angle of 45°. Simulated intestinal fluid of 37°C ± 0.5°C was circulated at a rate of 1 mL/min to the cell over microparticles adhering to the intestinal mucosa. The weight of washed out microparticles (Wf) in the washings was determined by separation through centrifugation followed by drying at 50°C. The percent mucoadhesion value as a measure of the in vitro washoff test was calculated using Equationequation 8(8) .Citation31
In vitro release
In vitro release studies of the coated microparticles were done using a USP basket apparatus (TDT-06T, Electrolab, Mumbai, India) at 37°C ± 0.5°C and 100 rpm containing 900 mL of sterile dissolution medium, ie, simulated gastric fluid and simulated intestinal fluid with about 1 g of accurately weighed microparticles contained in the basket (wrapped with 100 mesh nylon cloth) of dissolution apparatus. At predetermined time points, 5 mL of dissolution medium was withdrawn for up to 14 hours, with immediate replacement of fresh dissolution medium, subjected to viable cell number determination, and the result was expressed as the percentage of viable LR cells released with respect to the practical viable spore count value.Citation16
In vitro release kinetics, statistical evaluation, and data fitting
A mean value of three determinations at each time point was used to fit an in vitro viable cell release profile of all formulation batches to different kinetic models so as to find their release exponents. The mean value of 12 determinations was used to estimate the difference factor (f1), the similarity factor (f2), and the two indices of Rescigno (ξ1 and ξ2).Citation16,Citation32 Statistical analysis of percent released data and other data were performed using one-way analysis of variance at a significance level of 5% (P < 0.05). In vitro release kinetic studies, statistical evaluation, data fitting, nonlinear least square curve fitting, simulation, and plotting were performed using Excel software (version 2007, Microsoft Software Inc, Redmond, WA) for determining the parameters of each equation.
In vivo probiotic activity
The in vivo probiotic activity of the coated microparticles was evaluated using a mouse enterococci stool colonization method, following institutional animal ethical committee guidelines.Citation16 One milliliter of coated microparticle dispersion (102 cfu/mL) in simulated intestinal fluid was fed to albino mice in groups of six. Stools were collected at 6-hourly intervals for up to 48 hours and subjected to an enterococci colonization density study.
Accelerated stability
Following an International Conference on Harmonization guidelines, coated microparticles from all formulation batches were stored under a range of temperature and humidity conditions (30°C ± 2°C/65% ± 5% relative humidity and 40°C ± 2°C/75% ± 5% relative humidity) in a stability analysis chamber (Darwin Chambers Company, St Louis, MO) and in a refrigerator (2°C–8°C) for an accelerated stability study of up to six months.Citation16,Citation32,Citation33
Results and discussion
The coacervation and phase separation technique described here is a simple, rapid, two-step method, which appears to be suitable for the preparation of coacervate extended-release mucoadhesive microparticles loaded with LR cells. It eliminates exposure of LR cells to high temperatures, organic solvents, and mechanical stress, while maintaining their viability during processing. Temperatures above 20°C and nonaqueous solvents adversely affect and decrease the viability of LR, and this is the reason for commencing developmental processes below 20°C in aqueous medium. Hypromellose is soluble in cold water, with solubility in water decreasing with increasing temperature, and it is insoluble in organic solvents like chloroform, dichloromethane, ether, and acetone.Citation19 Hypromellose has excellent rate-controlling and mucoadhesion properties.Citation16,Citation17,Citation19 Hypromellose phthalate is soluble in aqueous alkali and insoluble in water and propan-2-ol.Citation19 Hypromellose was selected as a mucoadhesive polymer and hypromellose phthalate as a coating polymer because of their aforementioned properties, and both are considered to be safe for human consumption.Citation16–Citation19 Polysorbate-80 was incorporated in the formulation as a dispersing agent for homogeneous dispersion of LR cells, and polyethylene glycol 200 was incorporated into the coating solution as a plasticizer to impart plasticity to the coat and to prevent it from splitting and cracking.
LR cells used complied with certificate of analysis specifications, when tested in accordance with the method of analysis provided by the manufacturer. Coating stage percent weight gain values of the formulation batches were in the range of 10.1%–13.2% w/w.
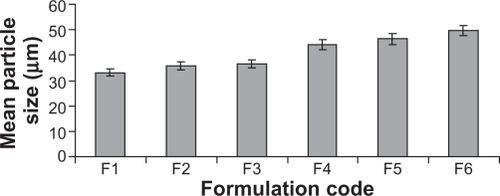
The percent yield value of the formulation batches ranged from 41.24% to 58.18% w/w, which varied according to the grade of hypromellose used, following the order E5 > E50 > E10 M, and an increase in the LR to hypromellose ratio decreased the value, and the highest value was observed for the formulation containing E5 (). A similar trend was also noticed for the entrapment efficiency values that lie between 45.18% and 64.16% cfu/g.
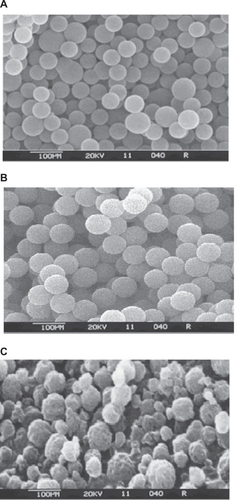
Scanning electron micrographs () of formulations F1, F3, and F5 demonstrate the surface morphology and particle size of the coated microparticles. The microparticles of all formulation batches were spherical in shape with a smooth surface, with the exception of microparticles belonging to formulation F5, the surface morphology of which was found to be coarser and shriveled. A coarser and shriveled surface texture in turn will improve adhesion by having stronger mechanical interactions.Citation17
Figure 1 Scanning electron microscopy photographs of microparticles from formulation batches (A) F1, (B) F3, and (C) F5.
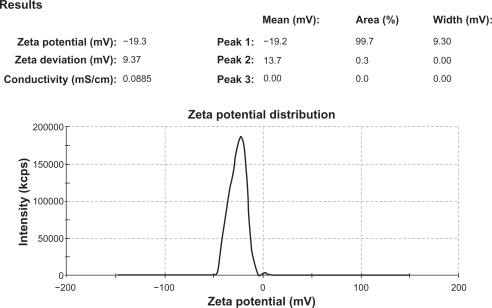
The mean particle size values of all formulation batches were in the range of 33.10–49.62 μm (), which increases with an increase in polymer concentration, while that the grade of hypromellose varied according to mean particle size value in the order of E10 > E50 > E5 M, with the highest value for microparticles prepared with E10 M ( and ). A nearly equal zeta potential of around −19.2 mV was observed for uncoated microparticles of all formulation batches, while coated microparticles had a nearly equal zeta potential at around −11.5 mV. This value is lower than that of the coated microparticles, indicating the presence of hypromellose phthalate on the surface of the microparticles. The zeta potential report for the uncoated microparticles from formulation batch F1 is shown in . The flow properties of the formulation batches lie within the passable and very poor ranges.
The percent swelling value of the formulation batches was 0.82%–1.38%, and decreases with increasing LR to hypromellose ratio. A variation in the grade of hypromellose also decreased the value in the order of E5 > E50 > E10 M, with the highest value for the microparticles prepared with E5 ().
The percent adhesive strength of all formulation batches was 42.61%–73.36%, which decreases with an increase in the LR to hypromellose ratio (). A difference in the grade of hypromellose varied the value in the order of E5 > E50 > E10 M, with the highest value seen for E5. A similar trend was also noticed with the percent mucoadhesion value, which ranged between 44.43% and 75.92% for all formulation batches. These results indicate that the mucoadhesion properties of the microparticles varied according to the grade of hypromellose and the LR to hypromellose ratio, and that microparticles from formulation batch F1 had the highest mucoadhesion affinity with the intestinal mucosa, so may exhibit high gastric retention time in comparison with the other batches.
The in vitro swelling test result, ex vivo mucoadhesive strength determination, and in vitro washoff test result, as a measure of the mucoadhesion affinity of the microparticles reveals that the mechanism of mucoadhesion initially follows the adsorption theoryCitation34,Citation35 and subsequently the diffusion theory.Citation35
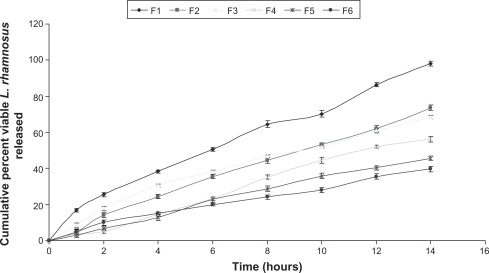
The amount of viable LR cells released from the microparticle system in simulated gastric fluid was negligible, but viable LR cell release was almost regulated and extended in simulated intestinal fluid (), indicating that enteric coating of microparticles competently protects cell viability at acidic pH, prevents cell release at gastric pH, and releases viable LR cells at intestinal pH.
Figure 4 Comparative in vitro release profile of viable Lactobacillus rhamnosus cells from coated microparticles of all formulation batches in simulated intestinal fluid TS, following zero-order kinetics.
The results of the in vitro swelling test, ex vivo mucoadhesive strength determination test, in vitro washoff test, and in vitro release profile study demonstrates that, in the intestine (pH > 5.0), the coating of the microparticle is dissolved, thereby releasing core microparticles. The liberated core microparticles swell in the intestine, resulting in intimate contact between the microparticles and the mucous membrane. The mucoadhesive chains then penetrate into the crevices of the tissue surface and intermingle with ions in the mucus, with formation of hydrogen bonds between the carboxylic groups of the polymer chains (hypromellose) and mucin molecules, leading to adhesion of the microparticles to the mucous membrane lining the intestinal wall.Citation36–Citation38
The kinetic constant and release exponent values of model-dependent approaches () show that the mechanism of viable LR cell release from coated microparticles follows a zero-order kinetics model, because the plot of cumulative percent viable cell release versus time was found to be linear, with the highest regression coefficient (r2) value in comparison with those of the other models. For all formulation batches, the zero-order kinetics model r2 value ranged between 0.9834 and 0.9947. Study of shape parameter values for the Weibull model () reveal that the curve is sigmoid or S-shaped, with upward curvature, followed by a turning point as β exceeded 1.Citation16,Citation32 Study of the location parameter (Td) for the Weibull model () characterizes the time interval necessary to dissolve or release 63.2% of the drug present in the delivery systemCitation16,Citation32 and shows that the Td of the formulation batches ranges from 7.7580 to 26.637 hours and the r2 value from 0.9310 to 0.9670.
Model-independent release exponent values are listed in , and show that for all formulation pairs, ie, the intrapolymer and interpolymer batches, the ξ1 values lie between 0.070 and 0.328, the ξ2 values lie between 0.240 and 0.407, the f1 value lies between 17.00 and 58.00, and the f2 value lies between 24.00 and 68.00, indicating dissimilarity in product performance of the formulation batches.Citation16,Citation32
Table 2 Values of the dissimilarity factor (f1), the similarity factor (f2) and the two indices of Rescigno (ξ1 and ξ2)
A plot of the in vitro viable LR cell release profile following a zero-order kinetics model for all formulation batches in simulated intestinal fluid is shown in , and demonstrates that the rate of viable LR cell release from the microparticles decreased significantly with an increase in the LR to polymer ratio, while variation in the grade of hypromellose influenced the release rate from the microparticles, following the order of E10 > E50 > E5 M.
The in vivo probiotic activity evaluation result shows that oral administration of the extended-release mucoadhesive microparticles of LR from all the formulation batches resulted in statistically significant reductions in the density of enterococci colonization in the stool of albino mice up to 24 hours to 36 hours.
The stability study result shows adequate stability of the microparticles under storage conditions of 30°C ± 2°C/65% ± 5% relative humidity, with no change in color and texture or statistically significant decrease in viable LR cell content with respect to the viable spore count, and also confirms that the LR cells were compatible with the excipients used in the formulation. A statistically significant decrease in viable LR cell content was observed with respect to practical viable spore counts at 40°C ± 2°C/75 ± 5% relative humidity, indicating product instability under these storage conditions.
Extended-release mucoadhesive microparticles from formulation batch F1 was found to be superior to the other prototype formulations because it exhibited the highest values of percent yield, entrapment efficiency, and mucoadhesion affinity, having the ability to protect the viability of LR cells during storage and gastrointestinal transit, and releasing viable LR cells in the gut for an extended period of time, as shown via zero-order kinetics.
Conclusion
These experimental results suggest that this extended-release microparticulate system loaded with LR cells could be prepared by a conventional coacervation and phase separation technique. It has the potential to deliver viable LR cells to the gut for an extended period of time, while maintaining the viability of LR cells during storage and gastrointestinal transit, and could be viewed as an alternative to conventional dosage forms. However, extensive in vivo studies will be required to establish the use of a coacervate extended-release microparticulate system as an alternative to the conventional dosage form of LR.
Acknowledgements
Thanks are extended to Cipla Ltd for the sample of freeze dried LR cells, to Glenmark Pharmaceuticals Ltd for the sample of HP-50, and to Indoco Remedies Ltd for the samples of Methocel.
Disclosure
The author reports no conflicts of interest in this work.
References
- SandersMEMorelliLTompkinsTASporeformers as human probiotics: Bacillus, Sporolactobacillus, and BrevibacillusCompr Rev Food Sci Food Saf20032101110
- KrasaekooptWBhandariBDeethHEvaluation of encapsulation techniques of probiotics for yoghurtInt Dairy J200313313
- ConwayPLGorbachSLGoldinBRSurvival of lactic acid bacteria in the human stomach and adhesion to intestinal cellsJ Dairy Sci1987701123106442
- ManleyKJFraenkelMBMayallBCPowerDAProbiotic treatment of vancomycin-resistant enterococci: a randomised controlled trialMed J Aust200718645445717484706
- SchieszerJAntibiotic UTI prophylaxis slightly betterRenal and Urology News112009
- PeltoLIsolauriELiliusEMNuutilaJSalminenSProbiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjectsClin Exp Allergy1998281474147910024217
- GuandaliniSPensabeneLZikriMALactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trialJ Pediatr Gastroenterol Nutr200030546010630440
- ArmuzziACremoniniFOjettiVEffect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot studyDigestion2001631711173893
- ChandramouliVKailasapathyKPeirisPJonesMAn improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditionsJ Microbiol Methods200456273514706748
- O’RiordanKAndrewsDBuckleKConwayPEvaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storageJ Appl Microbiol2001911059106611851814
- SultanaKGodwardGReynoldsNArumugaswamyRPeirisPKailasapathyKEncapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurtInt J Food Microbiol200062475511139021
- GilliandSESpeckMLInstability of Lactobacillus acidophilus in yogurtJ Dairy Sci19776013941398
- LankaputhraWEVShahNPSurvival of Lactobacillus acidophilus and Bifidobacterium spp in the presence of acid and bile saltsCultured Dairy Products J19953027
- ShahNPProbiotic bacteria: selective enumeration and survival in dairy foodsJ Dairy Sci20008389490710791807
- AsaneGSNirmalSARasalKBNaikAAMahadikMSRaoYMPolymers for mucoadhesive drug delivery system: a current statusDrug Dev Ind Pharm2008341246126618720139
- AlliSMAFormulation and evaluation of Bacillus coagulans-loaded hypromellose mucoadhesive microspheresInt J Nanomedicine2011661962921674019
- ChowdaryKPRRaoYSMucoadhesive microspheres for controlled drug deliveryBiol Pharm Bull2004271717172415516712
- LiCLMartiniLGFordJLRobertsMThe use of hypromellose in oral drug deliveryJ Pharm Pharmacol20055753354615901342
- RoweRCSheskeyPJOwenSCHandbook of Pharmaceutical Excipients5th edLondon, UKPharmaceutical Press2006
- ZhangLLiuYWuZChenHPreparation and characterization of coacervate microcapsules for the delivery of antimicrobial oyster peptidesDrug Dev Ind Pharm20093536937818941970
- United States Pharmacopoeial ConventionUnited States Pharmacopoeia-National Formulary (USP-NF) 2008Rockville, MDUS Pharmacopoeial Convention Inc2007
- TortoraGJFunkeBRCaseCLMicrobiology: An IntroductionIndiaDorling Kindersley (India) Pvt Ltd2006
- MukherjeeBSantraKPattnaikGGhoshSPreparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymersInt J Nanomedicine2008348749619337417
- de AzevedoMBTasicLFattoriJNew formulation of an old drug in hypertension treatment: the sustained release of captopril from cyclodextrin nanoparticlesInt J Nanomedicine201161005101621720512
- SebakSMirzaeiMMalhotraMKulamarvaAPrakashSHuman serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysisInt J Nanomedicine2010552553220957217
- AziziENamaziAHaririanIRelease profile and stability evaluation of optimized chitosan/alginate nanoparticles as EGFR antisense vectorInt J Nanomedicine2010545546120957167
- XieSZhuLDongZWangYWangXZhouWZPreparation and evaluation of ofloxacin-loaded palmitic acid solid lipid nanoparticlesInt J Nanomedicine2011654755521468357
- HaoJFFangXSZhouYFDevelopment and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken designInt J Nanomedicine2011668369221556343
- ThakralNKRayARBar-ShalomDErikssonAHMajumdarDKThe quest for targeted delivery in colon cancer: mucoadhesive valdecoxib microsphereInt J Nanomedicine201161057106821720517
- GuanPLuYQiJEnhanced oral bioavailability of cyclosporine A by liposomes containing a bile saltInt J Nanomedicine2011696597421720508
- VenkateswaramurthyNSambathkumarRPerumalPClarithromycin mucoadhesive microspheres for anti Helicobacter pylori therapy: Formulation and in-vitro evaluationInt J Current Pharm Res201022427
- AlliSMASamantaAMukherjeeBAliSMADehuryGKanungoSHydrophilic polymeric matrix tablet for sustained delivery of levofloxacinInt J Pharm Sci Tech201054055
- SahanaBSantraKBasuSMukherjeeBDevelopment of biodegradable polymer based tamoxifen citrate loaded nanoparticles and effect of some manufacturing process parameters on them: a physicochemical and in-vitro evaluationInt J Nanomedicine2010562163020856837
- MikosAGPeppasNAMeasurement of the surface tension of mucin solutionsInt J Pharm19895315
- JastiBLiXClearyGRecent advances in mucoadhesive drug delivery systemsBusiness Briefing: Pharmtech2003194197
- DucheneDTouchardFPeppasNAPharmaceutical and medical aspects of bioadhesive systems for drug administrationDrug Dev Ind Pharm198814283318
- LeungSHSRobinsonJRPolymer structure features contributing to mucoadhesion IIJ Control Release199012187194
- ChickeringDEMathiowitzEFundamentals of bioadhesionMathiowitzEChickeringDELehrCMNovel Approaches and DevelopmentNew York, NYMarcel Dekker1999