Abstract
Zinc oxide nanoparticles (ZnO-NPs) were synthesized via a solvothermal method in triethanolamine (TEA) media. TEA was utilized as a polymer agent to terminate the growth of ZnO-NPs. The ZnO-NPs were characterized by a number of techniques, including X-ray diffraction analysis, transition electron microscopy, and field emission electron microscopy. The ZnO-NPs prepared by the solvothermal process at 150°C for 18 hours exhibited a hexagonal (wurtzite) structure, with a crystalline size of 33 ± 2 nm, and particle size of 48 ± 7 nm. The results confirm that TEA is a suitable polymer agent to prepare homogenous ZnO-NPs.
Introduction
Understanding the mechanisms of the human body at the molecular and nanometer scale has improved tremendously, but developments in therapeutic options for treating severe and debilitating diseases such as cancer and autoimmunity have lagged by comparison.Citation1 In this regard, nanomedicine, which is the application of nanotechnology to medical problems, can offer new approaches in therapy. The application of nanotechnology in biology requires further studies for the development of new materials in the nanosize range. These materials have many potential applications in biological science and clinical medicine.Citation2,Citation3
One of the better-known materials that have been widely used for medical applications is zinc oxide nanoparticles (ZnO-NPs). It is not too far from the truth to say that the ZnO is a magic material because of its wide area of applications and flexibility of preparation in different morphologies with different properties. Reflecting the basic properties of ZnO, fine particles of the oxide have deodorizing and antibacterial action, and for that reason are added into various materials including cotton fabric, rubber, and food packaging.Citation4,Citation5 ZnO is widely used to treat a variety of other skin conditions, in products such as baby powder and barrier creams to treat diaper rashes, and in calamine cream, antidandruff shampoos, and antiseptic ointments.Citation6,Citation7 It is also a component in tape (called “zinc oxide tape”) used by athletes as a bandage to prevent soft tissue damage during workouts.Citation8 Therefore, several new routes have been developed to synthesize ZnO-NPs, such as a wet polymerization method, sol-gel, sol-gel combustion, precipitation, hydrothermal, solvothermal, chemical vapor deposition (CVD), microwave assisted, a sonochemical method, and thermal oxidation.Citation9–Citation18 Some of these methods have limitations. For example, it is not easy to control the growth of the ZnO nanostructures in the microwave, sol-gel, and sol-gel combustion methods because of the speed of the reactions. We have tried to develop a better controlled method that is reliable, safe, and cheap.
In this work, a simple solvothermal method was used to prepare the ZnO-NPs. The aim of this research is the preparation of ZnO-NPs with a narrow size distribution that are suitable for medical applications such as in sunscreen protection. Triethanolamine (TEA) was used as a polymerization agent in order to control the morphology of the ZnO-NPs because of its special structure. The reaction mechanism and effect of TEA were investigated.
Experimental
Zinc acetate (Zn(CH3COO)2.2H2O), ethanol, and TEA were used as starting materials. 0.5 M zinc acetate solution was prepared by dissolving 7.68 g of zinc acetate in 35 mL of ethanol. The solution was stirred at 60°C, and then the TEA was added to the solution, all at one time. The molar ratio of TEA/Zn2+ was fixed at 1:1. The solution was stirred at 60°C for 1 hour. After the stirring period, a clear and homogenous solution was obtained. The Zn2+ solution was then aged at room temperature for another hour. The solutions were poured in a stainless steel autoclave in a 50 mL Teflon vessel, and placed in the furnace for 18 hours at 150°C. The sample was then cooled down to room temperature. The formed white precipitates were dispersed in ethanol solution (30% in deionized water). The precipitates were separated by centrifugation of the mixture (4000 rpm for 4 minutes) at room temperature. These washing steps were repeated 3 times to remove the TEA polymers. Subsequently, the white precipitates were dried in an oven at 60°C overnight.
The structure of the prepared ZnO-NPs was characterized by powder X-ray diffraction (XRD, Philips, X’pert, Cu Kα). Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM, Hitachi H-7100 electron microscope) were used to study the morphology of the ZnO-NPs. The UV-vis spectra were recorded over the range of 200–1000 nm by a UV-vis Evolution 300 PC (Thermo Scientific, Japan).
Results and discussion
The mechanism of ZnO-NPs formation
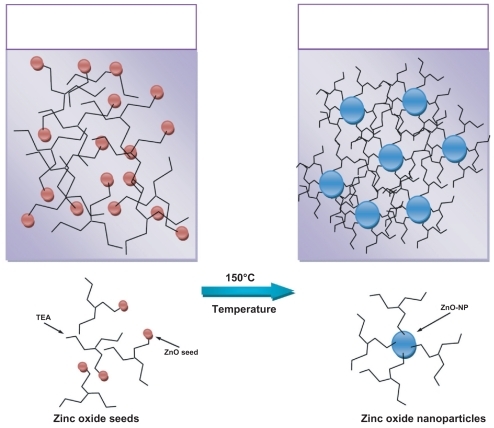
In the solvothermal process, alcohol plays a very important role in contributing the unoccupied oxygen to Zn2+ in order to form ZnO.Citation19 The formed ZnO seeds are attracted to some of the TEA chains because of the ionic–dipolar interaction between the hydrogen atoms in the polymer and oxygen in the ZnO. The ZnO-NPs will grow with the association of the ZnO seeds. On the other hand, some of the TEA chains are attracted to each other by hydrogen-bonding forces. So, the growth of the particles will be eliminated, because the polymer chains do not permit the ZnO seeds to reach each other easily. The complete process is shown in .
Fourier transform infrared spectroscopy (FTIR) analysis
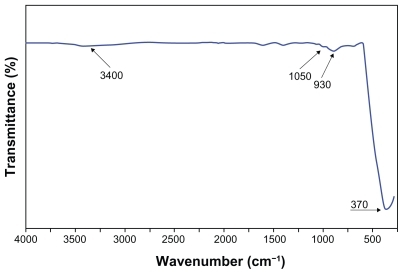
shows the FTIR of the ZnO-NPs prepared by the solvothermal method, in the range of 4000–280 cm−1. A broad absorption band was observed at around 375 cm−1. The band at 375 cm−1 corresponds to the E2 mode of hexagonal ZnO (Raman active).Citation11 There were several small absorption bands at 930, 1050, and 3400 cm−1. These absorption bands were likely related to CO2 (C-O) and H2O (O-H) absorbed from the atmosphere (air), and can therefore be neglected. The FTIR results show the high purity of the obtained ZnO-NPs.
XRD
The XRD pattern of the ZnO-NPs prepared by the solvothermal process at 150°C for 18 hours is shown in . All detectable peaks can be indexed to ZnO wurtzite structure (PDF code no: 00-036-1451). The wurtzite lattice parameters, for example the values of d, the distances between adjacent crystal planes (hkl), were calculated from the Bragg equation, λ = 2d sinθ. The lattice constants a, b, and c; the inter-planar angles, the angle ϕ between the planes (h1k1l1) of spacing d1 and the plane (h2k2l2) of spacing d2; and V, the primary cell volumes, were calculated from the Lattice Geometry equation.Citation20 The (100) and (002) planes were used to calculate the lattice parameters of the prepared ZnO-NPs, and the following values were obtained: d(100) = 0.2787 nm, d(002) = 0.2598 nm, a = b = 0.3218 nm, c = 0.5195 nm, ϕ = 90°, and V = 46.58 nm3.
Figure 3 The X-ray diffraction pattern of the zinc oxide nanoparticles prepared by the solvothermal method at 150°C.
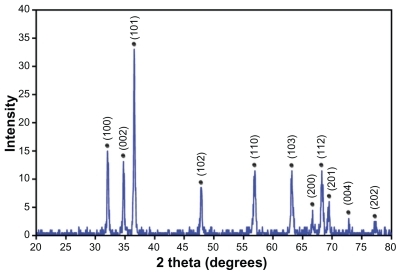
The Scherrer equation, D = (kλ/βhklcosθ), was used to determine the crystalline sizes of the ZnO-NPs where D is the crystalline size in nanometers (nm), λ is the wavelength of the radiation (1.54056 Å for CuKα radiation), k is a constant equal to 0.94, βhkl is the peak width at half-maximum intensity, and θ is the peak position. The (102) plane was chosen to calculate the crystalline size (either plane can be used for this purpose). The crystalline sizes of the ZnO-NPs prepared at 150°C for 18 hours were observed to be 33 ± 2 nm.
Optical properties
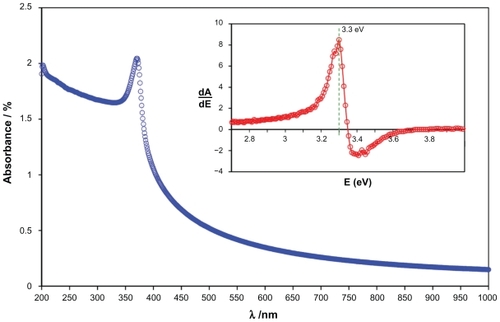
The room temperature UV-vis absorption spectra of ZnO-NPs are shown in . The ZnO-NPs were dispersed in ethanol with concentration of 0.1% wt and then the solution was used to perform the UV-vis measurement. The spectrum reveals a characteristic absorption peak of ZnO at wavelength of 370 nm which can be assigned to the intrinsic band-gap absorption of ZnO due to the electron transitions from the valence band to the conduction band (O2p → Zn3d).Citation11 In addition, this sharp peak shows that the particles are in nanosize, and the particle size distribution is narrow. It is clearly shown that the maximum peak in the absorbance spectrum does not correspond to the true optical band gap of the ZnO-NPs. A common way to obtain the band gap from absorbance spectra is to get the first derivative of the absorbance with respect to photon energy and find the maximum in the derivative spectrum at the lower energy sides.Citation21 The derivative of the absorbance of the ZnO-NPs is shown in the inset of , and it indicates a band gap of 3.3 eV for the ZnO-NPs. The good absorption of the ZnO-NPs in the UV region proves the applicability of this product in such medical application such as sunscreen protectors or as antiseptic ointments.Citation7
Morphology
shows the TEM, SEM, and size distribution of the ZnO-NPs prepared by the solvothermal method at 150°C for 18 hours. The TEM () shows that the ZnO-NPs have grown in a near-hexagonal shape, which demonstrates the good quality of the ZnO-NPs. shows the SEM micrograph of the ZnO-NPs at 150,000X magnification. The SEM figure indicates a homogeneous shape and size for ZnO-NPs. Also, it shows the ZnO-NPs are well dispersed in the powder form. The size histograms of the ZnO-NPs are shown in . The histograms indicate that the main particle sizes of the ZnO-NPs made by the solvothermal method at temperature of 150°C for 6 hours is about 48 ± 7 nm.
Figure 5 The transmission electron micrograph morphology image of zinc oxide nanoparticles (ZnO-NPs) (A), the scanning electron micrograph of the ZnO-NPs (B), and the particle size distribution of the ZnO-NPs (C).
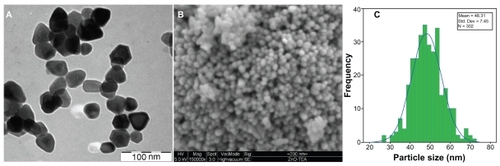
The TEM, SEM, and size distribution results confirm that a narrow size distribution can be obtained for ZnO-NPs prepared by a solvothermal method using TEA as a polymerization agent, compared to some of the other results.Citation10–Citation12
Conclusion
The ZnO-NPs were made successfully by a solvothermal method at the low temperature of 150°C for 18 hours. TEA was used as a polymerization agent to control the growth of the ZnO-NPs because of its special structure that terminates the growth of the ZnO-NPs. The XRD results show that the ZnO-NPs were formed in a hexagonal structure with crystalline size of 33 ± 2 nm. A sharp absorption peak (370 nm) was detected in the UV-vis region that corresponds to an optical band gap of the ZnO-NPs which was found to be 3.3 eV. TEM shows that the ZnO-NPs exhibit a near-hexagonal shape according to their crystalline structure. The ZnO powder was very homogeneous, as shown by SEM. The average particle size of 48 ± 7 nm was obtained for ZnO-NPs from the particle size distribution graph. The results confirm the quality of the ZnO-NPs, which make them suitable for medical applications.
Acknowledgments
This work was supported by the University of Malaya through grants no: UM.C/625/1/HIR/041.
Disclosure
The authors report no conflicts of interest.
References
- HanleyCLayneJPunnooseAPreferential killing of cancer cells and activated human T cells using ZnO nanoparticlesNanotechnology20081929510318836572
- LanoneSBoczkowskiJBiomedical applications and potential health risks of nanomaterials: molecular mechanismsCurr Mol Med2006665166317022735
- GronebergDAGiersigMWelteTPisonUNanoparticle-based diagnosis and therapyCurr Drug Targets2006764364816787165
- PadmavathyNVijayaraghavanREnhanced bioactivity of ZnO nanoparticles– an antimicrobial studySci Technol Adv Mat20089035004
- QunLChenSLJiangWCDurability of nano ZnO antibacterial cotton fabric to sweatJ Appl Polym Scie2007103412416
- AkhavanOGhaderiEEnhancement of antibacterial properties of Ag nanorods by electric fieldSci Technol Adv Mat200910015003
- HardingFBreast Cancer: Cause – Prevention – CureAylesburyTekline Publishing200683
- HughesGMcLeanNRZinc oxide tape: a useful dressing for the recalcitrant finger-tip and soft-tissue injuryArch Emerg Med1988542232273233136
- YingKLHsiehTEHsiehYFColloidal dispersion of nano-scale ZnO powders using amphibious and anionic polyelectrolytesCeram Inter20093511651171
- ZakAKMajidWHAbd DarroudiMYousefiRSynthesis and characterization of ZnO nanoparticles prepared in gelatin mediaMater Lett2011657073
- ZakAKAbrishamiMEMajidWHAbd YousefiRHosseiniSMEffects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion methodCeram Inter201137393398
- WangYZhangCBiSLuoGPreparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactorPowder Technol2010202130136
- LuCHYehCHInfluence of hydrothermal conditions on the morphology and particle size of zinc oxide powderCeram Inter200026351357
- SangkhapromNSupapholPPavarajarnVFibrous zinc oxide prepared by combined electrospinning and solvothermal techniquesCeram Inter201036357360
- YousefiRMuhamadMRZakAKInvestigation of indium oxide as a self-catalyst in ZnO/ZnInO heterostructure nanowires growthThin Solid Films201051859715977
- CaoYLiuBHuangRXiaZGeSFlash synthesis of flower-like ZnO nanostructures by microwave-induced combustion processMater Lett201165160163
- MishraPYadavRSPandeyACGrowth mechanism and photoluminescence property of flower-like ZnO nanostructures synthesized by starch-assisted sonochemical methodUltrason Sonochem20101756056519932043
- XuCHLuiHFSuryaCSynthetics of ZnO nanostructures by thermal oxidation in water vapor containing environmentsMater Lett2011652730
- NiederbergerMPinnaNMetal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and ApplicationLondonSpringer-Verlag2009
- ZakAKMajidWHAbd AbrishamiMEYousefiRSolid State Sci201113251256
- BecerrilMSilva-LopezHZelaya-AngelORev Me Fıs2004506588593