Abstract
The present study evaluated whether the magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) could enhance the activity of artesunate (ART), and to explore its potential mechanisms. Cytotoxicity of the copolymer of ART with MNPs-Fe3O4 on K562 cells was detected by MTT assay and the apoptosis rate of K562 cells was measured by flow cytometry. Protein expression levels of bcl-2, bax, bcl-rambo, caspase-3, and survivin in K562 cells were measured by Western blot. After being incubated with the copolymer of ART with MNPs-Fe3O4 for 48 hours, the growth inhibition rate of K562 cells was significantly increased compared with that of K562 cells treated with ART alone (P < 0.05), and the apoptosis rate of K562 cells was increased significantly compared with that of K562 cells treated with ART alone, suggesting that MNPs-Fe3O4 can enhance the activity of ART. Interestingly, the copolymer-induced cell death was attenuated by caspase inhibitor Z-VAD-FMK. Our results also showed that treatment with the copolymer of MNPs-Fe3O4 and ART increased the expression of bcl-2, bax, bcl-rambo, and caspase-3 proteins, and decreased the expression of survivin protein in K562 cells compared with ART treatment alone. These results suggest that MNPs-Fe3O4 can enhance ART-induced apoptosis, which may be related to the upregulation of bcl-rambo and downregulation of survivin.
Introduction
Artesunate (ART), a semi-synthetic derivative of artemisinin extracted from the Chinese herb Artemisia annua, is a safe and effective antimalarial drug. Recently, several studies showed that ART could inhibit the growth of various carcinoma cell linesCitation1–Citation3 and suppress tumor cell proliferation by inducing apoptosis,Citation4 indicating that ART may be a promising novel candidate for leukemia therapy. However, a variety of lines of evidence indicate that ART inhibited cell proliferation in a dose-dependent manner, and a high concentration of ART induced embryo toxicity,Citation5–Citation7 reversible renal damage toxicity,Citation8 and toxicity to bone development,Citation9 which limit the usefulness of ART.
To minimize side effects associated with dosage during chemotherapy, a promising approach is to combine a conventional chemotherapy with new strategies to maximize efficacy of tumor treatment through inducing cell apoptosis. Recent studies demonstrate that nanotechnologies and nanoparticles, which have unique peculiarities such as stable biomolecular absorption, high surface energy, and small sizes comparable to those of biomolecules, are becoming a focus for human medical application, including the delivery of antineoplastic agents to cells and tissues.Citation10,Citation11 We have recently reported that MNPs-Fe3O4, as an anticancer drug deliverer, could enhance the sensitivity of anticancer drugs and reverse the drug resistance of tumor cells.Citation12,Citation13
Apoptosis is the main mechanism of cell death, and is mediated by a cell-intrinsic suicide program, with the relative balance of pro- and anti-apoptotic signaling pathways determining the fate of the cell. There are two main pathways in mammals. One is the death receptor signaling pathway,Citation14 which is regulated by the IAP family including mainly X-IAP, c-IAP1, c-IAP2, KIAP, and survivin. Another pathway is the mitochondrial signaling pathway, in which bcl-2 plays an important role and bcl-2/bax is the key point of cell apoptosis.Citation15 Recently, bcl-rambo, a novel member of bcl-2 family, has been shown to induce apoptosis independent of both bcl-2/bax ratio and the death receptor pathway, but this effect can be specifically blocked by the IAP family.Citation16 The final executor of apoptosis is the caspase family, particularly caspase-3, which is the central proteases for apoptosis.Citation17
Accordingly, the copolymer of ART with MNPs-Fe3O4 may provide a novel clinical candidate for the treatment of leukemia. Here, the sensitivity of K562 cells to this copolymer and its possible underlying mechanisms were investigated.
Materials and methods
Main materials
The following materials were purchased: 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Ameresco, Solon, OH); Z-VAD-FMK (Beyotime Biotechnology); RPMI 1640 medium and fetal calf serum (GIBCO, USA); artesunate (Guilin Pharmaceutical Co., Ltd., China); Annexin V-FITC (Biosea Biotechnology Co., Ltd., Beijing, China); total protein extraction kit P1250 (Applygen Technologies Inc., Beijing, China); BCA protein assay kit (Biosynthesis Biotechnology Co., Ltd., Beijing, China); anti-bcl-2, anti-bax, anti-survivin (Bioworld), anti-bcl-rambo (Biovision), anti-active caspase-3 (Cell Signaling), anti-GAPDH (Santa Cruz, CA), and goat-antirabbit HRP-IgG (Santa Cruz, CA); Western chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA). MNPs-Fe3O4 was kindly supplied by the state Key Laboratory of Bioelectronics, Southeast University, Nanjing, China, and was well distributed in 0.01 M phosphate-buffered saline (PBS) by ultrasound to obtain MNPs-Fe3O4 colloidal suspension. ART at different concentrations (12.5, 25, 50, 75, and 100 μM) was conjugated with MNPs-Fe3O4, at the molar ratios of 25:1, 50:1, and 100:1 by mechanical absorption polymerization at 4°C for 12 hours, as previously reported.Citation18,Citation19
Cell line and cell culture
K562 cells, derived from a patient with chronic myeloid leukemia in blast crisis, had been constantly preserved in our laboratory, and were cultured in a flask in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 incubator.
Cytotoxicity assays
K562 cells (2 × 104 cells per well) in logarithmic growth phase were cultured in 96-well flat-bottomed plates in a triplicate pattern and treated with different concentrations of ART, MNPs-Fe3O4, or the copolymer of ART with MNPs-Fe3O4 in the presence or absence of 40 μM Z-VAD-FMK for 48 hours. MTT (20 μL, 5 mg/mL) was added to each well and incubated for 4 hours. Then, 200 μL of dimethylsulfoxide was added to each well and the plate was vortexed for 10 minutes at 37°C. Finally, the optical density value (A) of each well was measured at a measurement wavelength of 540 nm using a plate reader (Model 550, BIO-RAD, Japan). Cell growth inhibition ratio was calculated as (1 − A540 of experimental well/A540 of blank control well) × 100%. Each assay was repeated at least 3 times.
Apoptosis assay by flow cytometry
Based on the results of cytotoxicity assay, the best combination condition of ART with MNPs-Fe3O4 (100:1 M/M) was used to measure the apoptosis rate. Briefly, after being treated with ART, MNPs-Fe3O4 or the copolymer of MNPs-Fe3O4 and ART for 48 hours, K562 cells were collected, washed twice with ice-cold PBS, and then cells was suspended in 200 μL of binding buffer and 10 μL of Annexin V-FITC for 15 minutes in the dark. Thereafter, 300 μL of binding buffer and 5 μL of propidium iodide were added to each sample. Finally, the cells were analyzed using BD FACSDiva flow cytometry (BD FACS Canto™ II) with Cell Quest software.
Western blot analysis
After K562 cells were treated as mentioned above, total protein was extracted and quantified according to the manufacturer’s protocol. Each equal amount of protein was loaded on sodium dodecyl sulfate polyacrylamide gel, run at 100 volts for 2 hours, and then the protein was transferred to a polyvinylidene fluoride membrane. The membranes were blocked in 5% fat-free milk at room temperature for 2 hours, and the blots were stained with specific primary antibodies, including anti-bcl-2, anti-bax, anti-survivin, anti-bcl-rambo, anti-active caspase-3, and anti-GAPDH antibodies overnight at 4°C. Then, the membranes were washed and incubated with goat-antirabbit HRP-IgG for 1 hour at room temperature, and imaged with a chemiluminescent substrate. Thereafter, the bound immunoglobulins were removed from the membranes by washing twice with restore western blot stripping buffer, and the signal was detected using the ChemiDoc XRs+ (BIO-RAD) with the enhanced chemiluminescence and analyzed by the Image Lab (ECL). GAPDH was used as the internal control.
Statistical analysis
All experiments were repeated 3 times. Data are presented as mean ± standard deviation and analyzed using the SPSS (Release13.0; SPSS Inc, Chicago, IL). One-way analysis of variance (ANOVA), 2-sample t-test, or rank test was performed to illustrate the significant difference between the treated groups and the control. The difference was considered statistically significant at P < 0.05.
Results
Inhibition of cell growth
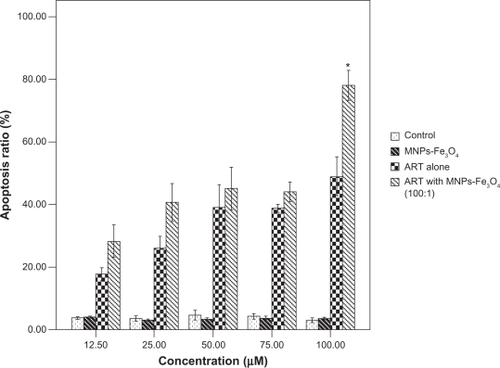
When K562 cells were treated with the copolymer of ART and MNPs-Fe3O4 at the molar ratio of 100:1, the cell viability was decreased significantly compared with that of K562 cells treated with ART alone (0.47 ± 0.11 vs 1.10 ± 0.02) (P < 0.05). Although the cell viability of K562 cells was reduced to 0.66 ±0.03 at a molar ratio of 25:1 and 0.73 ±0.04 at a molar ratio of 50:1, there were no significant differences between the copolymer and ART alone group (P > 0.05), suggesting that the molar ratio of 100:1 was the best condition for copolymerization.
Moreover, when ART at the concentrations of 75, or 100 μM was combined with MNPs-Fe3O4 (M/M = 100:1) cell proliferation was remarkably inhibited, especially at the concentration of 100 μM, compared with ART alone (P < 0.05) (); however there was no significant difference at the concentrations of 12.5, 25, and 50 μM (P > 0.05). The cell growth inhibition in the MNPs-Fe3O4 group was less than that in the ART group with or without MNPs-Fe3O4. In addition, it was noted that MNPs-Fe3O4 alone could slightly inhibit cell proliferation when its concentration was from 0.125 μM to 4 μM, but there was no significant difference (P > 0.05).
Figure 1 The inhibition ratio of K562 treated with ART or the copolymer of ART with MNPs-Fe3O4 (100:1) for 48 hours.
Note: *P < 0.05, compared with the ART alone.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; ART, artesunate.
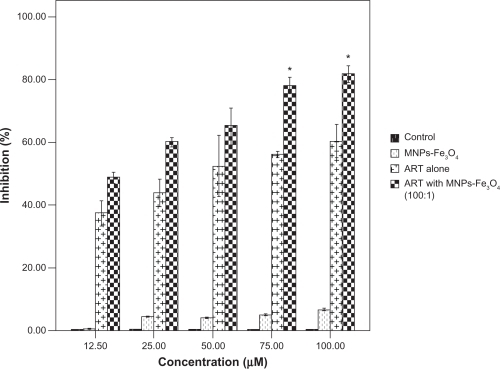
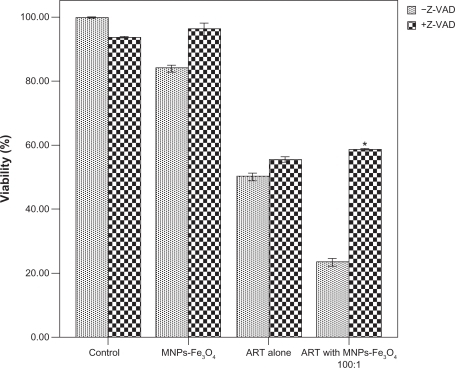
To further verify that caspase activation is necessary for the copolymer-induced apoptosis, K562 cells were treated with the copolymer of ART and MNPs-Fe3O4 in the presence or absence of general caspase inhibitor Z-VAD-FMK. Cell survival rate was measured to see whether the antiproliferative effect of the copolymer was preventable. As shown in , the copolymer-induced cell death was attenuated by Z-VAD-FMK, since cell survival rate in the presence of Z-VAD-FMK was increased more than its absence (58.73 ± 0.37% vs 23.67 ± 1.45%) (P < 0.05), suggesting that in combination with MNPs-Fe3O4, ART-induced apoptosis proceeded via the caspase-dependent pathway.
Figure 2 Effect of 1.0 μM MNPs-Fe3O4, 100 μM ART, and the copolymer of ART with MNPs-Fe3O4 (100:1) on the cell viability in the presence or absence of 40 μM Z-VAD-FMK for 48 hours.
Note: *P < 0.05, compared with the absence of Z-VAD-FMK.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; ART, artesunate.
Enhancement of apoptosis by the copolymer of ART with MNPs-Fe3O4
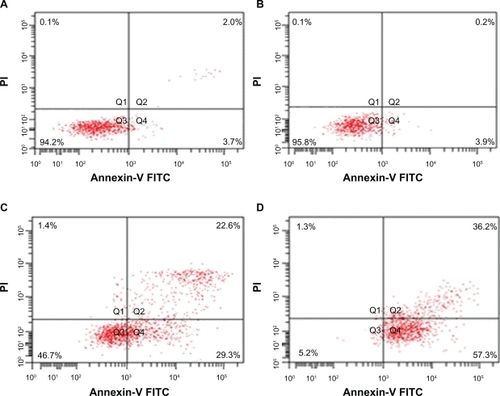
After being incubated with ART, MNPs-Fe3O4, or the copolymer of ART with MNPs-Fe3O4 (M/M = 100:1) for 48 hours, the proportion of apoptotic cells in the copolymer treated group was increased significantly compared with that in 100 μM ART-treated group (78.02 ± 10.83% vs 48.90 ± 14.08%) (P < 0.05) ().
Figure 3 Apoptosis of K562 cells treated with ART or the copolymer of ART with MNPs-Fe3O4 for 48 hours. A) Control; B) 1 μM MNPs-Fe3O4; C) 100 μM ART; D) the copolymer of 100 μM ART with 1 μM MNP-Fe3O4.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; ART, artesunate.
However, there were no significant differences in the apoptosis rate of K562 cells between the copolymer of 12.5 μM ART with 0.125 μM MNPs-Fe3O4-treated group and the 12.5 μM ART-treated group (28.10 ± 11.66% vs 17.64 ± 4.49%); between the copolymer of 25 μM ART with 0.25 μM MNPs-Fe3O4-treated group and the 25 μM ART-treated group (40.54 ± 13.45% vs 25.76 ± 8.80%); between the copolymer of 50 μM ART with 0.5 μM MNPs-Fe3O4-treated group and the 50 μM ART alone group (44.95 ± 13.66% vs 38.98 ± 14.57%); and between the copolymer of 75 μM ART with 0.75 μM MNPs-Fe3O4-treated group and the 75 μM ART alone group (43.9 ± 5.53% vs 38.53 ± 2.10%) (P > 0.05) ( and ). However, the apoptosis rate in all groups was higher than that in both MNPs-Fe3O4 and control groups (P < 0.05). These results to some extent indicate that MNPs-Fe3O4 may enhance ART-induced cell apoptosis.
Expression of bcl-2, bax, bcl-rambo, caspase-3, and survivin
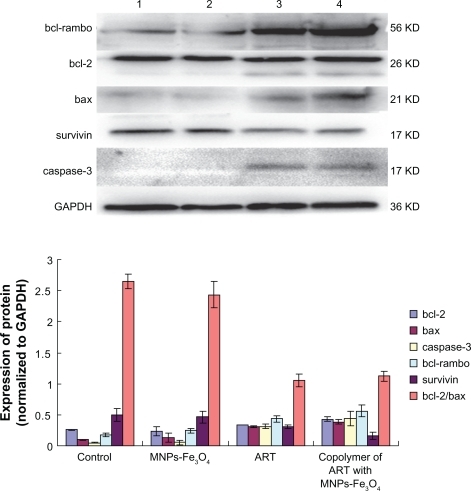
Western blot assay was performed to trace the changes of apoptosis-related genes on treatment with MNPs-Fe3O4, ART, or the copolymer of ART with MNPs-Fe3O4 for 48 hours. It was observed that the expression of survivin was downregulated in the ART group, and this downregulation was even more apparent in the group of copolymer of ART with MNPs-Fe3O4. The levels of bax and bcl-2 were increased in both ART group and the copolymer of ART with MNPs-Fe3O4 group compared with control group and MNPs-Fe3O4 group (see , P < 0.05), but there was no obvious difference in the bcl-2/bax ratio. The levels of bcl-rambo and caspase-3 were upregulated when the ART was combined with the MNPs-Fe3O4 (P < 0.05). These results suggest that the copolymer of ART with MNPs-Fe3O4 triggers changes in the expression levels of apoptosis-related genes in K562 cells, among which upregulation of bcl-rambo and downregulation of survivin are the two major alterations.
Figure 5 Expressions of bcl-2, bax, bcl-rambo, survivin, and caspase-3 protein in K562 cells.
Notes: Lane 1) K562 control; Lane 2) 1 μM MNPs-Fe3O4; Lane 3) 100 μM ART; Lane 4) the copolymer of 100 μM ART with 1 μM MNPs-Fe3O4.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; ART, artesunate.
Discussion
ART could inhibit the proliferation of tumor cells in vitro and in vivo by downregulating the Wnt/β-catenin pathway.Citation4,Citation20 However, toxicity of ART to blood vessels and bone is an obstacle for clinical application of ART, which has limited its wide-spread application. Nowadays, MNP-Fe3O4 as a targeted drug carrier with target orientation and sustained-release properties has been widely studied in medical research. It has been previously reported that MNP-Fe3O4 with anticancer drugs could effectively improve efficiency of chemotherapeutic agents which has a synergistic effect in multidrug resistance.Citation12,Citation13
Our present study demonstrated that the unique MNPs-Fe3O4 could increase the biological effects and decrease the side effects of ART. MTT results revealed that after being combined with MNPs-Fe3O4 (100:1, M/M), ART showed an enhanced cytotoxic effect on K562 cells; the copolymer of 100 μM ART with 1 μM MNPs-Fe3O4 had the most enhanced cytotoxicity among the five groups. Interestingly, the copolymer of ART and MNPs-Fe3O4-induced cell death was attenuated since cell survival rate was increased by Z-VAD-FMK, suggesting that the copolymer of ART and MNPs-Fe3O4-induced apoptosis proceeded via the caspase-dependent pathway.
Apoptosis, as a process of programmed cell death, is normal for the development and health of multicellular organisms.Citation14 Apoptotic obstruction is an important event in a tumor genesis, and apoptosis-oriented treatment is common in cancer treatment. Our present studies show the copolymer of 100 μM ART with 1 μM MNPs-Fe3O4 could significantly induce K562 cell apoptosis (P < 0.05), and the apoptosis induced by ART was dose-dependent, as has been reported in other tumor cells.Citation1–Citation3 These results to some extent indicate that MNPs-Fe3O4 can enhance the ART-induced apoptosis.
Apoptosis is the consequence of a series of precisely regulated events that are frequently altered in tumor cells. ART-induced cell apoptosis is apparently involved in the death receptor pathway and the mitochondria.Citation21–Citation23 The relative ratios of the various bcl-2 family members such as bcl-2/bax could determine how much cellular stress was needed to induce apoptosis.Citation24,Citation25 A recent report showed that bcl-rambo, located in mitochondria, contained conserved bcl-2 homology (BH) motifs 1, 2, 3, 4. But no interaction was found between bcl-rambo with either anti-apoptotic (bcl-2, bcl-Xl, bcl-w, A1, MCL-1, E1B-19K, and BHRF1) or pro-apoptotic (bax, bak, bik, bid, bim, and bad). Its over-expression induces apoptosis by the C-terminal membrane anchor region which was preceded by a unique 250 amino acid insertion containing two tandem repeats. This function was inhibited only by the IAP family.Citation15,Citation16 Our results showed that treatment with the copolymer of ART with MNPs-Fe3O4 upregulated the expression of bcl-2, bax, bcl-rambo and active caspase-3 protein. It is worth noting that expression of bcl-2 in the copolymer-treated group shows an increasing trend (P < 0.05). Although the expression of bax was also increased, the computer-assisted image analysis shows that in combination with MNPs-Fe3O4, the ratio of bcl-2/bax in K562 cells was in a slightly ascendant trend compared with ART alone (P > 0.05), suggesting that MNPs-Fe3O4 enhanced ART-apoptosis through bcl-2/bax-independent mechanism, which differs from the results previously published.Citation24,Citation25
On the other hand, studies showed that Wnt/β-catenin, as an important self-renewal pathway,Citation20 could regulate the transcription of survivin, p53, and c-myc. Overexpression of survivin has been detected in various hematological malignancies, including acute leukemias, myelodysplastic syndrome, chronic myeloid leukemia, and other types of lymphoid malignancies.Citation26–Citation28 Survivin can bind to and potently inhibit caspases, including caspase-3.Citation29
Caspases are synthesized as proenzymes and can be activated by proteolysis at several sites. The active caspase-3 is a heterotetramer of two large and two small subunits.Citation30 In our present study, the downregulation of survivin expression and a significant increase in the expression of active caspase-3 protein were observed at the same time in the K562 cells treated with the copolymer of MNPs-Fe3O4 and ART, suggesting that the potent inhibition of survivin expression could augment the activity of caspase-3, thus substantially enhancing apoptosis of K562 cells induced by the combination of MNPs-Fe3O4 with ART.
Conclusion
In summary, these results showed that MNPs-Fe3O4 could enhance ART-induced apoptosis in K562 cells, which may be related to the upregulation of bcl-rambo and downregulation of survivin. It would be worthwhile evaluating the effects of the copolymer on normal cells in vitro for clinical application.
Acknowledgements
This paper was supported by the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University.
Disclosure
Ying Wang and Yuxiang Han contributed equally to this work. There are no conflicts of interest to report.
References
- EfferthTDunstanHSauerbreyAMiyachiHChitambarCRThe anti-malarial artesunate is also active against cancerInt J Oncol20011876777311251172
- MercerAECoppleIMMaggsJLO’NeillPMParkBKThe role of heme and the mitochondrion in the chemical and molecular mechanisms of Mammalian cell death induced by the artemisinin antimalarialsJ Biol Chem201128698799621059641
- DuJHZhangHDMaZJJiKMArtesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xeno-grafts in vivoCancer Chemother Pharmacol20106589590219690861
- LiLNZhangHDYuanSJTianZYWangLSunZXArtesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathwayInt J Cancer20071211360136517520675
- ChenHHZhouHJWuGDLouXEInhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1Pharmacology2004711915051917
- ClarkRLEmbryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimesterReprod Toxicol20092828529619447170
- WhiteTEBushdidPBRitterSLaffanSBClarkRLArtesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivoBirth Defects Res B Dev Reprod Toxicol20067741342917066416
- LiQXieLHJohnsonTOSiYHaeberleASWeinaPJToxicity evaluation of artesunate and artelinate in Plasmodium berghei-infected and uninfected ratsTrans R Soc Trop Med Hyg200710110411216860356
- ClarkRLWhiteTEA ClodeSGauntIWinstanleyPWardSADevelopmental toxicity of artesunate and an artesunate combination in the rat and rabbitBirth Defects Res B Dev Reprod Toxicol20047138039415617018
- Fernandez-PachecoRValdiviaJGIbarraMRMagnetic nanoparticles for local drug delivery using magnetic implantsMethods Mol Biol200954455956919488723
- DartonNJHallmarkBHanXPalitSSlaterNKMackleyMRThe in-flow capture of superparamagnetic nanoparticles for targeting therapeuticsNanomedicine20084192918093881
- ChenBADaiYYWangXMSynergistic effect of the combination of nanoparticulate Fe3O4 and Au with daunomycin on K562/A02 cellsInt J Nanomedicine2008334335018990943
- ChenBALaiBBChengJDaunorubicin-loaded magnetic nano-particles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivoInt J Nanomedicine2009420120819918366
- JarpeMBWidmannCKnallCAnti-apoptotic versus pro-apoptotic signal transduction: checkpoints and stop signs along the road to deathOncogene199817147514829779994
- OltvaiZNMillimanCLKorsmeyerSJBcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell deathCell1993746096198358790
- KataokaTHollerNMicheauOBcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extensionJ Biol Chem2001276195481955411262395
- HengartnerMOThe biochemistry of apoptosisNature200040777077611048727
- GaoHWangJYShenXZDengYHZhangWPreparation of magnetic polybutylcyanoacrylate nanospheres encapsulated with aclacinomycin A and its effect on gastric tumorWorld J Gastroenterol2004102010201315237424
- WangXZhangRWuCThe application of Fe3O4 nanoparticles in cancer research: a new strategy to inhibit drug resistanceJ Biomed Mater Res A20078085286017072850
- HuYChenYDouglasLLiSBeta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemiaLeukemia20092310911618818703
- LiPCLamERoosWPZdzienickaMZKainaBEfferthTArtesunate derived from traditional Chinese medicine induces DNA damage and repairCancer Res2008684347435118519695
- Hamacher-BradyASteinHATurschnerSArtesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalysed lysosomal reactive oxygen species productionJ Biol Chem20112866587660121149439
- EfferthTSauerbreyAOlbrichAMolecular modes of action of artesunate in tumor cell linesMol Pharmacol20036438239412869643
- EfferthTGiaisiMMerlingAKrammerPHLi-WeberMArtesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cellsPLoS One20072e69317668070
- WuGDZhouHJWuXHApoptosis of human umbilical vein endothelial cells induced by artesunateVascul Pharmacol20044120521215653096
- BadranAYoshidaAWanoYExpression of the anti-apoptotic gene survivin in myelodysplastic syndromeInt J Oncol200322596412469185
- TammIKornblauSMSegallHExpression and prognostic significance of IAP-family genes in human cancers and myeloid leukemiasClin Cancer Res200061796180310815900
- TammIRichterSOltersdorfDHigh expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemiaClin Cancer Res2004103737374415173080
- ShinSSungBJChoYSAn anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7Biochemistry2001401117112311170436
- BhardwajAAggarwalBBReceptor-mediated choreography of life and deathJ Clin Immunol20032331733214601641