Abstract
Introduction
Targeted intervention to the uterus has great potential for the treatment of obstetric complications (eg, preterm birth, dysfunctional labor, and postpartum hemorrhage) by improving the effectiveness and safety of therapeutic compounds. In particular, targeting the oxytocin receptor (OTR) is a novel approach for drug delivery to the uterus. The aim of this study was to report the complete data set for the pharmaceutical synthesis and in vitro characterization of PEGylated liposomes conjugated with anti-OTR monoclonal antibodies (OTR-Lipo) or atosiban (ATO-Lipo, OTR antagonist).
Methods
OTR-targeted liposomal platforms composed of 1,2-distearoyl-sn-glycero-2-phosphocholine and cholesterol were prepared according to the method of dried lipid film hydration. Ligands were conjugated with the surface of liposomes using optimized methods to maximize conjugation efficiency. The liposomes were characterized for particle size, ligand conjugation, drug encapsulation, liposome stability, specificity of binding, cellular internalization, mechanistic pathway of cellular uptake, and cellular toxicity.
Results
Both OTR-Lipo and ATO-Lipo showed significant and specific binding to OTRs in a concentration-dependent manner compared to all control groups. There was no significant difference in binding values between OTR-Lipo and ATO-Lipo across all concentrations evaluated. In addition, OTR-Lipo (81.61%±7.84%) and ATO-Lipo (85.59%±8.28%) demonstrated significantly increased cellular internalization in comparison with rabbit IgG immunoliposomes (9.14%±1.71%) and conventional liposomes (4.09%±0.78%) at 2.02 mM phospholipid concentration. Cellular association following liposome incubation at 4.05 mM resulted in similar findings. Evaluation of the mechanistic pathway of cellular uptake indicated that they undergo internalization through both clathrin- and caveolin-mediated mechanisms. Furthermore, cellular toxicity studies have shown no significant effect of both liposomal platforms on cell viability.
Conclusion
This study further supports OTRs as a novel pharmaceutical target for drug delivery. OTR-targeted liposomal platforms may provide an effective way to deliver existing therapies directly to myometrial tissue and avoid adverse effects by circumventing non-target tissues.
Background
Targeted drug delivery of nanomedicines has shown to be beneficial for increasing the therapeutic index of compounds by improving drug targeting to specific sites of disease and/or attenuating localization in healthy non-target tissues. Obstetrics is an area of clinical medicine that has recently gained attention for drug targeting. Current treatment for obstetric complications (eg, preterm labor, dysfunctional labor, and postpartum hemorrhage) is challenging, as there are limited effective and safe therapeutic options available.Citation1–Citation5 Preterm labor (birth before 37 weeks of gestation) is the most important cause of perinatal morbidity and mortality,Citation6–Citation8 whereas postpartum hemorrhage represents the most important cause of maternal morbidity and mortality worldwide.Citation9,Citation10 In addition, up to one third of women may require induction of labor, primarily due to concerns for the health of the mother or fetus.Citation11,Citation12
The development of novel strategies to deliver therapeutic agents specifically to the uterus would address the clinical challenge of effectively managing these obstetric complications. Ligand-targeted nanomedicines have shown enormous potential for site-specific delivery of therapeutic compounds to designated cell types, which selectively express or overexpress specific receptors at the site of action. For obstetric complications, the smooth muscle layer of the uterus (myometrium) represents an ideal target for pharmacological interventions by controlling the contractility state of the uterus. In this way, therapeutic agents can be directed to the uterus to inhibit myometrial contractions (tocolysis) for the management of preterm labor or enhance myometrial contractions (uterotonic) for the management of dysfunctional labor and postpartum hemorrhage.Citation13
More specifically, the oxytocin receptor (OTR) is considered one of the most important molecules in the myometrium and thus a promising target for drug delivery. OTR agonists and antagonists have been clinically used to modify the contractility of the uterus; however, the clinical efficacy of the available agents has been disappointing. For example, oxytocin was reported to be effective in ~50% of patients,Citation12 and atosiban (ATO-Lipo, OTR antagonist) showed lack of efficacy over other conventional treatments and no evidence of improving neonatal outcomes.Citation14,Citation15 Despite this, OTR density in the uterus significantly increases with the progression of pregnancy.Citation16,Citation17 Low OTR expression has been reported in early gestation, but this rises significantly over the course of pregnancy to ~12-fold by 37–41 weeks.Citation17 Maximal expression is seen after the onset of labor, which is assumed to mediate an increase in sensitivity of the myo-metrium to oxytocin at term.Citation18,Citation19 Importantly, OTR numbers in the myometrium have also been shown to be upregulated in preterm labor.Citation16,Citation17,Citation20 Therefore, designing a drug delivery system to target this moiety would provide a logical means to enhance local drug delivery to the uterus.
We have recently developed a targeted drug delivery system for the uterus by conjugating anti-OTR poly-clonal antibodies to a liposomal platform.Citation21,Citation22 Liposomes have the advantage of having flexible physicochemical and biophysical properties, which allow easy modifications to address different delivery considerations.Citation23–Citation26 The functionality of the drug delivery system was evaluated on murine and human myometrial tissues, as well as in vivo in a murine model of preterm labor.Citation21 However, the poly-clonal nature of the antibody posed a significant limitation to specificity of binding to OTRs.Citation22 In order to improve the clinical translation of this technology, it is important to determine the effectiveness of other potential anti-OTR ligands for the targeted delivery system – in particular, anti-OTR monoclonal antibodies and atosiban (OTR antagonist). Therefore, the aim of this study was to report the complete data set for the pharmaceutical synthesis and characterization of PEGylated immunoliposomes conjugated with anti-OTR monoclonal antibodies (OTR-Lipo) and atosiban-conjugated PEGylated liposomes (ATO-Lipo), and compare their specific cellular interaction with OTR-expressing myometrial cells in vitro.
Materials and methods
Liposome-related materials
1,2-Distearoyl-sn-glycero-2-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPE-PEG(2000) maleimide), and 1,2-distearoyl-sn-glycero-3-phosphoetha-nolamine-N-[carboxy (polyethylene glycol)-2000] (DSPE-PEG(2000) carboxylic acid) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). N-Succinimidyl-3-(2-pyridyldithio) propionate (SPDP), cholesterol, atosiban, and tris(2-carboxyethyl) phosphine hydrochloride (TCEP) were from Sigma-Aldrich Co. (St Louis, MO, USA). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), CBQCA protein assay kit and N-hydroxysuccinimide (NHS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Rabbit IgG antibody and anti-OTR monoclonal antibody (ab181077) were obtained from Abcam (Cambridge, UK). PD-10 column was from VWR International (Radnor, PA, USA). All other chemicals and solvents were of at least analytical grade.
Cell-related materials
FBS, trypsin-EDTA (1:250), trypan blue solution 0.4%, dansylcadaverine (D4008), chlorpromazine hydrochloride (C8138), genistein (G6649), filipin (F9765), methyl-β−cyclodextrin (C4555), nocodazole (M1404), cytochalasin D (C8273), MTT-based assay kit (CGD1), and all buffer reagents were from Sigma-Aldrich Co. l-Glutamine, DMEM, Gibco® Antibiotic-Antimycotic (penicillin, streptomycin, ampho-tericin), sodium pyruvate, Alexa Fluor™ 488 conjugated cholera toxin subunit B (C34775), and Alexa Fluor 488 conjugated transferrin (T13342) were obtained from Thermo Fisher Scientific. Fluoresbrite® YG carboxylate microspheres (size 1 µm) was from Polysciences Inc. (Warrington, PA, USA). hTERT-immortalized myometrial (hTERT-myo) cells were obtained from Prof Roger Smith (University of Newcastle, Australia). All other reagents were of analytical grade. The POLARstar Optima™ fluorescent plate reader and its corresponding software were obtained from BMG Labtech (Ortenberg, Germany). The Institutional Bio-safety Committee at the University of Newcastle approved all experiments and the use of the cell line.
Preparation of immunoliposomes
Immunoliposomes composed of DSPC and cholesterol (molar ratio 2:1) containing DSPE-PEG(2000) maleimide at 1.5 mol% of DSPC as a coupling lipid were prepared according to the method of dried lipid film hydration in PBS (pH 7.4) as previously described.Citation27 DiI (fluorescent lipophilic dye) was added at 0.2 mol% of DSPC, and drugs were incorporated based on their hydrophilicity. The resulting multi-lamellar dispersions were reduced in size and lamellarity by ultrasonication at 60% amplitude for 5 minutes at 65°C. The activated liposome suspension was immediately mixed with thiolated antibody at room temperature. Thiolated antibodies were prepared by conjugating anti-OTR monoclonal antibodies (25 µg) or non-specific rabbit IgG (25 µg) with SPDP (6.25 mg/mL; SPDP/mAb molar ratio =10:1). PD-10 column equilibrated with distilled water was used to remove excess SPDP and fractions containing pyridyldithiopropionated-Ab (PDP-Ab) conjugates (assessed by absorbance at 280 nm) were lyophilized and stored at 4°C under nitrogen gas. PDP-Ab was reduced with 5 mM TCEP for 5 minutes to produce thiolated-Ab (Ab-SH), and absorbance was checked at 280 nm (protein concentration) and 343 nm (SPDP modification) to ensure stability of the compound. Thiolated antibody was mixed immediately with liposomes for 1 hour at room temperature with stirring in the dark. TLX ultracentrifugation (Optima™) was used to remove unconjugated Ab and non-encapsulated drug (100,000× g; 45 minutes). Liposomes were resuspended in PBS (pH 7.4) and stored at 4°C under nitrogen gas and in the dark, and were used within 2 weeks.
Preparation of atosiban-conjugated liposomes
Liposomes were composed of DSPC, cholesterol, and DSPE-PEG(2000) carboxylic acid at a molar ratio of 2:1:0.06 according to the method of dried lipid film hydration in MES buffer (pH 4.7). The fluorescent lipophilic dye DiI was added at 0.2 mol% of DSPC. Drugs were incorporated based on their hydrophilicity. The resulting multilamellar dispersions were reduced in size and lamellarity by ultrasonication at 60% amplitude for 5 minutes at 65°C. NHS and EDC (molar ratio 2:1) were then added to the liposome suspension and incubated for 15 minutes. Prior to the addition of atosiban, the pH of the liposome suspension was raised to 7.2–7.5 for optimal reaction with amine-containing molecules. The liposome suspension was incubated overnight at room temperature with stirring in the dark. Excess reagents were removed by TLX ultracentrifugation (Optima) (100,000× g; 45 minutes). Liposomes were resuspended in PBS (pH 7.4) and stored at 4°C under nitrogen gas and in the dark and were used within 2 weeks.
Liposome characterization
Size distribution of the liposomes was determined by dynamic laser light scattering (Zetasizer Nano ZS™; ATA Scientific, Taren Point, Australia). Phospholipid concentration was determined indirectly by measuring DiI concentration within liposomes. CBQCA protein assay was used to quantify the amount of antibody associated with the liposomes, using bovine serum albumin for the preparation of the standard curve. Atosiban concentration was evaluated using HPLC.
HPLC analysis
Drug concentration was determined by HPLC using an Agilent Technologies 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA), consisting of an autoinjector, column oven, binary pump and UV-Vis detector. Data were integrated using Agilent Chemstation software. Separation was performed using a Thermo Scientific BDS Hypersil C18 column (150×4.6 mm, 5 µm). Drugs were dissolved in distilled water (hydrophilic drugs) or ethanol (hydrophobic drugs), with subsequent dilutions made in mobile phase for the calibration curves.
HPLC mobile phase and settings for nifedipine
Mobile phase consisted of 60% acetonitrile and 40% 10 mM KH2PO4 buffer (pH 2.5), which was pumped through the column at 1 mL/min. The column was maintained at 25°C and the wavelength of detection was 208 nm.
HPLC mobile phase and settings for salbutamol hemisulfate
Mobile phase consisted of 30% methanol and 70% 0.05 M KH2PO4 buffer (pH 3.5), which was pumped through the column at 1 mL/min. The column was maintained at 25°C and the wavelength of detection was 230 nm.
HPLC mobile phase and settings for atosiban
Mobile phase consisted of 30% acetonitrile and 70% 10 mM KH2PO4 (pH 2.5), which was pumped through the column at 1 mL/min. The column was maintained at 25°C and the wavelength of detection was 220 nm.
Atomic force microscopy (AFM)
AFM was performed on a Cypher Scanning Probe Microscope (Asylum Research; Oxford Instruments, Abingdon, UK). Commercial pyramidal silicon tips (TAPAl300-G; BudgetSensors, Sofia, Bulgaria) with a radius <10 nm, a resonance frequency of ~300 kHz and a nominal force constant of 40 (20–75) N/m were used. All measurements were performed in tapping mode to avoid damage of the sample surface. The scan speed was proportional to the scan size and the scan rate was between 1.95 and 2.44 Hz. The results were visualized in amplitude mode. Liposomes were directly transferred onto a silicon chip by dipping it into the lipo-some suspension. The silicon chip was then dried at room temperature before analysis.
Liposome stability assay
The dialysis technique was used to evaluate the in vitro stability of the liposomal formulations in PBS pH 7.4% and 50% FBS at 37°C over a 48 hours duration, as previously described.Citation22 A modified assay was used to evaluate the true release of each drug from the liposomes without surpassing saturation point, which addressed the potential solubility issues across the dialysis membrane. Drug retention percentage was determined by: Drug retention (%) = 100% − [(Dt/D0) × 100%], where Dt and D0 indicate the amount of drug released from the liposomes at certain intervals and the total amount of drug in the liposome suspension, respectively. Liposome samples were collected at the end of the study and lysed with ethanol for analysis of drug content by HPLC.
Specificity of binding to OTRs on myometrial cells
Specificity of binding was evaluated on hTERT-myo cells as previously described.Citation22 In brief, cells were seeded at 4×104 cells per well in 96-well tissue culture plates in complete medium (DMEM containing 1% l-glutamine, 1% sodium pyruvate, 1% Gibco Antibiotic-Antimycotic, 10% FBS) at 37°C in 5% CO2. At confluence, the plates were exchanged with serum and supplement-free DMEM to avoid binding of serum proteins to the liposome surfaces which might induce agglomeration. For competitive inhibition studies, cells were preincubated with a saturating concentration of anti-OTR monoclonal antibodies for 30 minutes. Liposomes were added to the cells for 1 hour at 4°C to determine exclusively cell binding.Citation28,Citation29 A fluorescent dye (DiI) was incorporated into the phospholipid bilayer as a marker for the liposomes. At the end of incubation, ice-cold PBS was used to wash the cells three times to remove unbound lipo-somes and fluorimetric detection (POLARstar Optima) was used to assess binding at excitation/emission wavelengths corresponding to DiI (549/565 nm). Background reading was assessed with cells incubated in medium alone.
Cellular uptake of OTR-targeted liposomes
Cellular association of OTR-targeted liposomes was assessed on hTERT-myo cells as previously described.Citation22 In brief, cells were seeded in 96-well tissue culture plates (4×104 cells per well) and grown until confluent under the conditions described above. At confluence, the plates were exchanged with serum and supplement-free DMEM prior to the addition of fluorescent-labeled liposomes for 1 hour at 37°C. It should be noted that both receptor binding and internalization takes place at this temperature.Citation28,Citation29 At the end of incubation, ice-cold PBS was used to wash the cells three times before fluorimetric detection to assess extracellularly bound liposomes. Acid wash (0.1 M HCl for 10 minutes)Citation30 was used to release surface-bound fluorescence before lysis of the cells with the addition of 1% Triton X-100 in PBS for 10 minutes at 37°C. Results from the acid wash were expressed as extracellularly bound fluorescence, whereas fluorescence in the lysis fractions was expressed as internal-ized fluorescence. Background reading was evaluated with solubilized cells without prior incubation with liposomes.
Endocytotic mechanisms of OTR-targeted liposomes
Mechanisms of cellular uptake were determined in the presence of specific inhibitory agents for different types of endocytosis, as previously described.Citation22 In brief, hTERT-myo cells were preincubated with specific inhibitory agents for 30 minutes at 37°C and were then incubated with lipo-somes for 1 hour at 37°C. Chlorpromazine hydrochloride and monodansylcadaverine were used as inhibitiors of clathrin-mediated endocytosis, whereas genistein and fili-pin were used to inhibit caveolin-mediated endocytosis. Methyl-β-cyclodextrin is able to inhibit both clathrin- and caveolin-mediated endocytosis by depleting cholesterol. Nocodazole was used to inhibit macropinocytosis, and cytochalasin D was used to inhibit both phagocytosis and macropinocytosis. Positive controls were used together with specific inhibitors to investigate clathrin-mediated endocytosis (Alexa Fluor 488 conjugated transferrin at 120 µg/mL), caveolin-mediated endocytosis (Alexa Fluor 488 conjugated cholera toxin subunit B at 0.6 µg/mL), and phagocytosis and macropinocytosis (Fluoresbrite YG carboxylate microspheres of 1 µm diameter at 100 µg/mL). At the end of incubation, cells were washed three times with ice-cold PBS before fluorimetric detection as described above.
Cellular toxicity
Cell viability following exposure of hTERT-myo cells to liposomes and specific endocytotic inhibitors at various concentrations for 24 and 1.5 hour, respectively, was measured using an MTT-based assay, as previously described.Citation22 Triton X-100 (1% in PBS) was used as a positive control. At the end of incubation, the medium was removed and MTT solution (5 mg/mL MTT in supplemented DMEM) was added to the cultures (final concentration of 0.5 mg/mL) and incubated for 4 hours at 37°C. The medium was then discarded and formazan crystals were solubilized using 150 µL dimethylsulfoxide for 15 minutes under light protection and at room temperature. Absorbance was measured at 550 nm in a microplate spectrophotometer (POLARstar Optima), which is directly proportional to cellular metabolism. Background reading was assessed with untreated cells.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM) or SD. GraphPad Prism 7.01 software was used for statistical analysis. One-way ANOVA with Tukey’s multiple comparison test was used to evaluate differences between formulation groups or between time points (one independent variable). Comparisons between the different formulation groups over various concentrations were made using two-way ANOVA with Tukey’s multiple comparison test (two independent variables). Differences were considered significant when P<0.05.
Results
Dispersion properties of the liposome formulations
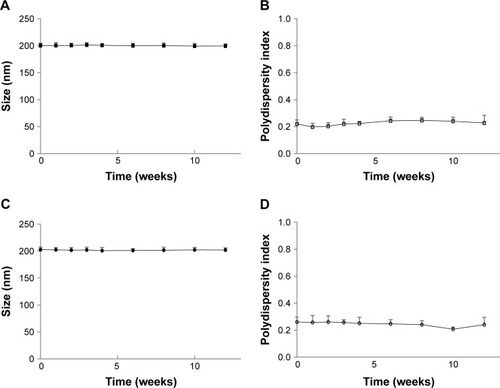
OTR-Lipo and ATO-Lipo had a mean particle size of 201±2.9 and 201±3.8 nm with a polydispersity index of 0.240±0.072 and 0.220±0.056, respectively. The size and polydispersity index of the control and drug-encapsulated liposome formulations were similar (). The choice of particle size was based on the results from our previous studies.Citation21,Citation22 The low polydispersity indexes indicate that the mean particle size is a reasonable indicator of the size of the majority of the particles in the dispersions. All liposome formulations had an approximate neutral net charge, as they were composed of neutral phospholipids. Both nifedipine-loaded liposomes and salbutamol-loaded liposomes had drug encapsulation efficiencies of >98%, which equated to ~4 mg of drug per milliliter of liposome suspension (). Mean atosiban coupling ratio for the ATO-Lipo was 5.970±0.232 µg of peptide per µmol of phospholipid, which equated to ~2,017±78.39 molecules per liposome. Mean antibody coupling ratio for the OTR-Lipo was 1.227±0.023 µg of antibody per µmol of phospholipid. With a starting antibody concentration of 25 µg and a phospholipid concentration of 2.026×10−5 mol, this equated to a conjugation efficiency of >99% (). Experiments were conducted to monitor size and size distribution of the liposome formulations under storage conditions of 4°C in PBS pH 7.4 over 12 weeks to ensure stability over time. The size and polydispersity index of the liposome formulations were stable over this period ().
Table 1 Physicochemical characteristics of liposome formulations (mean ± SD, n=3)
Figure 1 Particle size and polydispersity index of OTR-Lipo (A, B) and ATO-Lipo (C, D) over a period of 12 weeks.
Note: The results are represented as mean ± SD of three independent experiments (P>0.05, ANOVA).
Abbreviations: OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
Stability of drug-encapsulated OTR-targeted liposomes
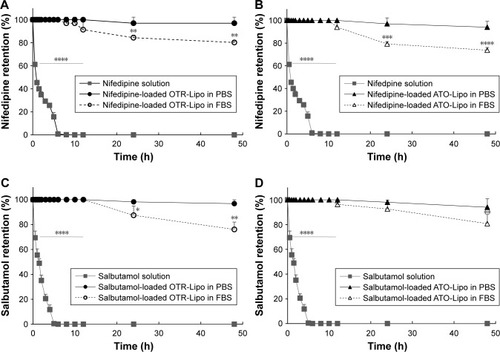
Nifedipine (hydrophobic drug) and salbutamol hemisulfate (hydrophilic drug) were used to evaluate the in vitro stability of OTR-Lipo and ATO-Lipo. Nifedipine is a hydrophobic compound; therefore, its solubility in PBS pH 7.4 was initially assessed to ensure that the parameters of the dialysis assay did not surpass the saturation point for the drug. This determined that 70 µg of nifedipine in 10 mL PBS solution within the dialysis tubing was able to dialyze into 30 mL of the PBS release volume. This was not required for salbutamol as it is readily soluble in an aqueous phase, thereby allowing 1 mg of the drug to be placed in 10 mL PBS solution within the dialysis tubing. Minimal drug release was detected for both salbutamol-loaded OTR-targeted liposomes and nifedipine-loaded OTR-targeted liposomes in PBS at 37°C across the 48 hours duration of the experiment (). In serum, there was a significant difference in stability at 24 and 48 hours compared to baseline values for both the liposome formulations (). This equated to a decrease of 19.7% for nifedipine-loaded OTR-Lipo (P<0.01), 23.9% for salbutamol-loaded OTR-Lipo (P<0.01), 26.2% for nifedipine-loaded ATO-Lipo (P<0.0001), and 19.2% for salbutamol-loaded ATO-Lipo (P<0.01) at 48 hours. Drug release was not limited by the diffusion of free drug through the dialysis membrane, as shown by complete recovery of the drug solutions in the dialysis medium within 6 hours. Therefore, both of the drugs evaluated were able to pass through the cellulose membrane freely.
Figure 2 Stability of nifedipine-loaded OTR-Lipo (A), nifedipine-loaded ATO-Lipo (B), salbutamol-loaded OTR-Lipo (C), and salbutamol-loaded ATO-Lipo (D) in PBS pH 7.4 and 50% FBS at 37°C.
Notes: The data represent the mean ± SD of three independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used to assess drug retention at various time points compared to their respective baseline values (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Abbreviations: OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
Liposomal imaging with AFM
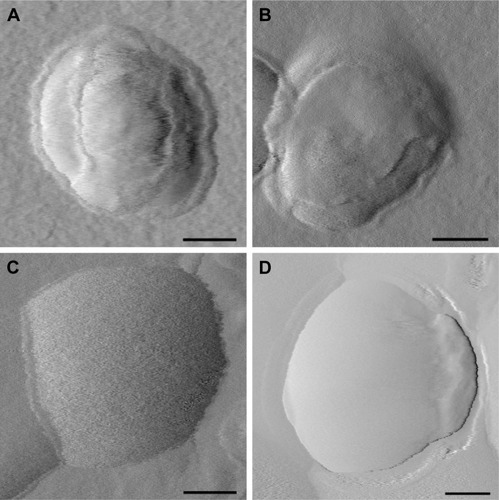
AFM was used to visualize all liposomal formulations when dried to confirm particle size and morphology. For size determination, all visible particles within a representative scan area were individually evaluated. OTR-Lipo, ATO-Lipo, rabbit IgG immunoliposomes, and conventional liposomes showed approximate vesicle sizes of 200 nm (), which corresponds with the results from dynamic laser light scattering. The surface of conventional liposomes was typically smooth (), whereas small surface structures can be detected at the membrane border for OTR-Lipo () and rabbit IgG immunoliposomes () and only slightly for ATO-Lipo (). Surface coverage in the height mode was difficult to evaluate due to collapsed vesicular structure when dried.
Figure 3 Representative AFM images of OTR-Lipo (A), ATO-Lipo (B), rabbit IgG immunoliposomes (C), and conventional liposomes (D).
Note: Scale bars correspond to 50 nm.
Abbreviations: AFM, atomic force microscopy; OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
Specificity of binding
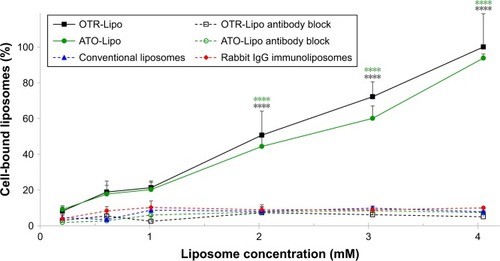
OTR-Lipo and ATO-Lipo bound significantly to hTERT-myo cells in a concentration-dependent manner in comparison with all control groups (P<0.0001) (). There was no significant difference in binding values between OTR-Lipo and ATO-Lipo across all concentrations evaluated (P>0.05). Negative control binding experiments using non-specific rabbit-IgG immunoliposomes and conventional liposomes were assessed to demonstrate the specificity of the binding toward OTR expressing cells. The results showed very low cell binding values across all concentrations evaluated and were not significantly different (P>0.05). In another set of experiments, hTERT-myo cells were pretreated with excess monoclonal antibody directed against OTRs to further demonstrate the specific nature of the OTR-Lipo and ATO-Lipo interaction. shows significant inhibition of binding of both OTR-Lipo and ATO-Lipo to hTERT-myo cells pretreated with excess anti-OTR monoclonal antibody (1 mg/mL) by up to 95.0% (P<0.0001) and 92.1% (P<0.0001), respectively.
Figure 4 Concentration-dependent binding of OTR-Lipo and ATO-Lipo to hTERT-myo cells.
Notes: Conventional liposomes and rabbit IgG immunoliposomes were used as controls. Competitive inhibition of binding of OTR-targeted liposomes was also evaluated following preincubation with excess anti-OTR monoclonal antibody. Liposomes were incubated for 1 hour at 4°C. The results are represented as mean ± SEM of six independent experiments. Two-way ANOVA with Tukey’s multiple comparison test was used to assess intergroup differences at various concentrations (****P<0.0001).
Abbreviations: SEM, standard error of the mean; OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
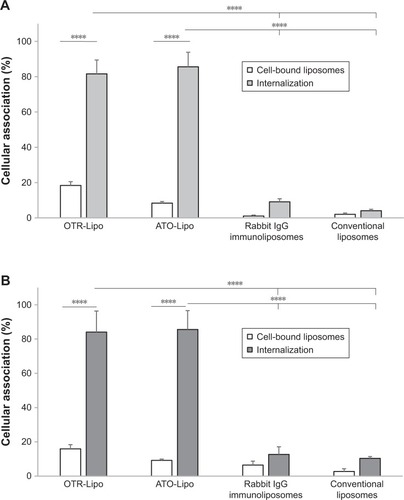
Degree of cellular uptake
OTR-Lipo (81.61%±7.84%) and ATO-Lipo (85.59%±8.28%) demonstrated significantly increased cellular internalization compared with rabbit IgG immunoliposomes (9.14%±1.71%) and conventional liposomes (4.09%±0.78%) at 2.02 mM phospholipid concentration after 1 hour of incubation at 37°C (, P<0.0001). Similar results were shown following liposome incubation at 4.05 mM (), with cell-bound liposomes of 15.95%±2.45% and 9.21%±0.74% and cellular internalization of 84.05%±12.30% and 85.60%±11.00% for OTR-Lipo and ATO-Lipo, respectively. Low levels of cell binding were shown for non-specific rabbit-IgG immunoli-posomes and conventional liposomes at both concentrations, which were not significantly different from cell-bound OTR-Lipo and ATO-Lipo (P>0.05). No significant difference was also detected between cellular binding and uptake for rabbit-IgG immunoliposomes and conventional liposomes (P>0.05).
Figure 5 Cellular association of OTR-Lipo, ATO-Lipo, rabbit IgG immunoliposomes and conventional liposomes by hTERT-myo cells.
Notes: Liposomes were incubated at 2.02 mM (A) and 4.05 mM (B) for 1 hour at 37°C. The results are represented as mean ± SEM of six independent experiments. Two-way ANOVA with Tukey’s multiple comparison test was used to assess intergroup differences in cell binding and internalization, as well as individual group differences between cell binding and internalization (****P<0.0001).
Abbreviations: SEM, standard error of the mean; OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
Mechanisms of cellular uptake
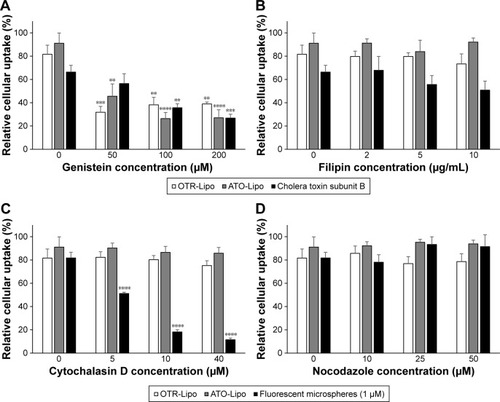
All three pharmacological inhibitors of clathrin-mediated endocytosis (chlorpromazine, monodansylcadaverine, and methyl-β-cyclodextrin) were able to significantly block the internalization of OTR-Lipo and ATO-Lipo (, P<0.0001). As further support of the specificity for inhibition of clathrin-mediated endocytosis, the results also showed significant inhibition of uptake for fluorescent-labeled trans-ferrin with all three compounds (positive control, P<0.0001). However, methyl-β-cyclodextrin is also known to block caveolin-mediated endocytosis, which is supported by significant inhibition of uptake of the positive control – cholera toxin subunit B (, P<0.0001). Hence, further investigations were conducted with more specific pharmacological inhibitors of caveolin-mediated endocytosis (genistein and filipin). shows significant inhibition of cellular uptake for OTR-Lipo (P<0.01), ATO-Lipo (P<0.0001), and fluorescent-labeled cholera toxin subunit B (positive control, P<0.001) with genistein. Filipin did not affect the uptake of both OTR-targeted liposomes or cholera toxin subunit B on hTERT-myo cells (, P>0.05). Furthermore, no significant inhibition of cellular uptake was observed for OTR-Lipo and ATO-Lipo with cytochalasin D (, P>0.05), a pharmacological inhibitor of both phagocyto-sis and macropinocytosis. Cytochalasin D was, however, able to inhibit the uptake of fluorescent microspheres of 1 µm diameter (positive control, P<0.0001). Nocodazole (pharmacological inhibitor of macropinocytosis) was unable to block the cellular uptake of both OTR-targeted liposomes and positive control (fluorescent microspheres) across all concentrations evaluated (, P>0.05).
Figure 6 Cellular uptake of OTR-Lipo and ATO-Lipo following preincubation with various concentrations of pharmacological inhibitors of clathrin-mediated endocytosis (A, B and C).
Notes: Liposomes were incubated at 2.02 mM for 1 hour at 37°C. The results are represented as mean ± SEM of six independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used to assess inhibition of cellular uptake of OTR-targeted liposomes and positive control (transferrin) in the presence of various concentrations of inhibitors compared to their respective baseline values (**P<0.01, ***P<0.001, ****P<0.0001).
Abbreviations: SEM, standard error of the mean; OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
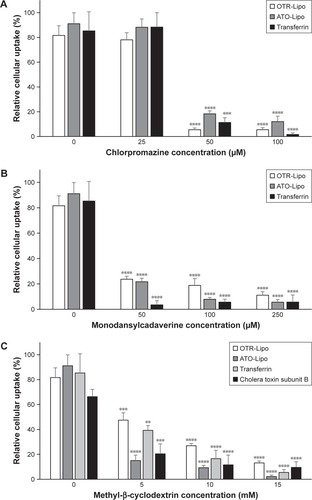
Figure 7 Cellular uptake of OTR-Lipo and ATO-Lipo following preincubation with various concentrations of pharmacological inhibitors of caveolin-mediated endocytosis (A and B) and macropinocytosis and phagocytosis (C and D).
Notes: Liposomes were incubated at 2.02 mM for 1 hour at 37°C. The results are represented as mean ± SEM of six independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used to assess inhibition of cellular uptake of OTR-targeted liposomes and positive control (cholera toxin subunit B or 1 µm microspheres) in the presence of various concentrations of inhibitors compared to their respective baseline values (**P<0.01, ***P<0.001, ****P<0.0001).
Abbreviations: SEM, standard error of the mean; OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
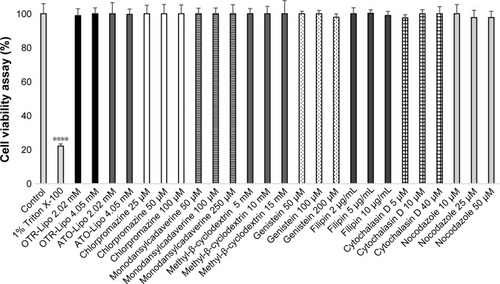
Cellular toxicity
Cell viability studies were conducted following incubation with liposomes and the endocytotic inhibitors to rule out cellular toxicity as a potential reason for decreased lipo-some internalization during these experiments (). The results showed no significant effect of OTR-Lipo and ATO-Lipo on the viability of hTERT-myo cells following 24 hours of incubation at both the concentrations evaluated (2.02 and 4.05 mM) (P>0.05). Furthermore, incubation with the endocytic inhibitors at the concentrations used in the in vitro experiments for 1.5 hour (cellular incubation time) did not impair cell viability (P>0.05). Significant cell death was demonstrated for hTERT-myo cells treated with Triton X-100 (positive control) (P<0.0001).
Figure 8 Cell viability following exposure to OTR-targeted liposomes and specific endocytotic inhibitors at various concentrations for 24 and 1.5 hour, respectively.
Notes: Triton X-100 (1% in PBS) was used as a positive control. Two-way ANOVA with Tukey’s multiple comparison test was used to assess cell viability at various concentrations compared to healthy control cells (****P<0.0001).
Abbreviations: OTR-Lipo, PEGylated immunoliposomes conjugated with anti-oxytocin receptor monoclonal antibodies; ATO-Lipo, atosiban-conjugated PEGylated liposomes.
Discussion
We have engineered liposomal platforms that are able to specifically target OTRs expressed on myometrial cells. OTRs are cell surface receptors containing seven transmembrane domains and belong to the class I family of G protein-coupled receptors.Citation31 They are regulated by changes in receptor expression and desensitization, as well as local changes in oxytocin concentration.Citation11,Citation31–Citation33 The expression of OTRs in the uterus is significantly upregulated during gestation and undergoes rapid downregulation after parturition.Citation11,Citation16,Citation17 Importantly, OTR levels were shown to be maximal and significantly higher after the onset of labor, either preterm or term, than before the onset of labor.Citation17 This tissue-specific regulation of OTR expression is ideal for specific and local accumulation of OTR-targeted nanoparticles to the uterus in obstetric complications such as preterm labor, dysfunctional labor and postpartum hemorrhageCitation21 – in which the management of these conditions is challenging due to limited effective and safe treatment options.Citation1–Citation5 In the uterus, the expression of OTRs is not confined to the myometrium. Chorio-decidual tissue expression of OTRs also increases during parturitionCitation34 and spatial differences exist between OTR expression within uterine tissues, which is suggested to aid fetal decent and passage during labor.Citation11
When anti-OTR monoclonal antibodies or rabbit IgG molecules were conjugated to the liposomes, small surface structures were visible along the membrane border. Based on molecular weights of the targeting ligands, we would not expect to see the conjugation of atosiban with the surface of liposomes as clearly as compared with monoclonal antibodies or IgG molecules. Studies in the literature have attempted to visualize the conjugation of targeting ligands to the surface of liposomes using AFM with varying results.Citation35,Citation36 For example, Bendas et al demonstrated that when antibodies are attached to liposomes via a PEG 2000 anchor, it can cause high protein mobility that affects the ability to image the proteins,Citation36 whereas Anabousi et al reported the detection of small globular structures at the surface of such liposomes.Citation35 Similar to Bendas et al,Citation36 we found it difficult to clearly image the anti-OTR antibodies attached to the liposomes via a PEG 2000 anchor. Data obtained from AFM can also deviate slightly from the results of dynamic laser light scattering because of an interaction of the soft and flexible liposomes with the surface of the silicon chip substrate, which we also observed. This is particularly relevant when the liposomes are dried onto the silicon chip prior to analysis, thereby resulting in liposomes flattening and/or spreading on the silicon support.
Conjugation of OTR targeting ligands to the surface of liposomes increased cellular interaction with myometrial uterine cells. hTERT-immortalized myometrial cells were used to evaluate specificity of OTR targeting, as they exhibit the phenotype and properties of highly differentiated smooth muscle cells, without the high variability of responses seen with primary myometrial cells.Citation37,Citation38 Human myometrial cells express OTRsCitation39–Citation41 and have been reported to acquire additional OTRs in culture,Citation42–Citation44 with upregulation likely due to the presence of fetal bovine serum in the culture medium.Citation45 It should be noted that serum and supplement free media were used in the in vitro cellular association studies to determine binding and cellular uptake of the OTR-targeted liposomal platforms in a reductionist in vitro system, which would mimic the in vivo situation of when the liposomes reached the myometrium of the uterus. We have demonstrated highly specific binding of OTR-Lipo and ATO-Lipo to OTR-expressing hTERT-myo cells using concentration-dependent binding studies, competitive inhibition experiments, and negative control binding studies. No significant difference was detected in binding efficiency between liposomes conjugated with anti-OTR monoclonal antibodies and atosiban. Similarly, Refuerzo et al showed improved attachment, retention, and targeting efficiency with atosiban-conjugated liposomes compared with non-targeted liposomes on smooth muscle cells isolated from pregnant humans and mice.Citation46 The use of monoclonal antibodies is preferential to polyclonal antibodies for translational purposes to improve the specificity of binding. This is due to monoclonal antibodies being able to specifically detect a particular epitope on the antigen, which means they are less likely to cross-react with other proteins.
Cellular association experiments showed significant cellular uptake for both OTR-Lipo and ATO-Lipo following binding, which is necessary for subsequent intracellular processing and release of encapsulated therapeutic agents in myometrial cells. Interestingly, anti-OTR monoclonal antibodies and atosiban in the free drug form typically act by blocking natural ligands from binding to myometrial OTRs on the membrane surface.Citation14,Citation15 However, conjugation of these ligands to the surface of liposomes enhances its uptake into myometrial cells. The cellular interaction of liposomes is highly dependent on several liposomal characteristics including particle size, charge, composition, sterical stabili-zation, and specific properties of the conjugated ligands.Citation23,Citation27 OTR-targeted liposomes themselves are unlikely to impact on OTR desensitization rates, as the concentration of ligand conjugated to the surface of the liposomes is low. Usually much higher doses and continuous dosing of oxytocin are required to cause downregulation of OTRs.Citation47–Citation49
Evaluation of the mechanistic pathway of cellular uptake of the different OTR-targeted liposomal platforms indicates that they both undergo internalization through clathrin- and caveolin-mediated mechanisms. OTRs have previously been shown to congregate with β-arrestin following activation into defined punctuated regions of the plasma membrane, which suggest that they are targeted into clathrin-coated pits for internalization.Citation31,Citation50 OTRs are also highly expressed in the cholesterol-rich, caveolin-containing membrane domains of the plasma membrane.Citation51,Citation52 Furthermore, results from cellular toxicity experiments showed no significant effects of both OTR-targeted liposomal platforms and the endocytotic inhibitors on cell viability at 24 and 1.5 hour (cellular incubation time), respectively. It should be noted that utilization of pharmacological inhibitors is a common approach for studying endocytotic pathways. Generally, a number of pharmacological inhibitors should be evaluated as they can lack specificity for defined pathways and have been shown to be cell type dependent.Citation53 Therefore, a balance is needed between the concentration of inhibitor high enough to inhibit endocytosis but not to induce cytotoxicity.Citation53
OTR-Lipo and ATO-Lipo were shown to be highly stable upon dilution in both an aqueous phase (PBS pH 7.4) and serum (50% FBS). Drug retention was only significantly lower than baseline values when the liposomes were dispersed in serum at 24 and 48 hours. This is likely due to the bioac-tive substance in FBS, including plasma proteins which can lead to opsonization, destabilization, and lipid exchange.Citation23 The amount of encapsulated drug released was between 19.2% and 26.2% at 48 hours. This is unlikely to affect the performance of the OTR-targeted liposomes, as human serum generally contains 7% proteins (50% FBS was used in the experiment), and the expected application for the technology would require good stability in the circulation particularly following the first few hours after initial administration. Therefore, the targeted nanoparticles are suitable for administration to sites of high dilution, which occurs following intravenous administration. Stability against leakage has been achieved by using phospholipids (eg, DSPC) that remain in the solid phase at physiological temperatures and incorporating cholesterol in the lipid bilayer to minimize lipid exchange.Citation54 PEGylation also improves the circulation half-life of the formulation by evading recognition by the immune system.Citation23,Citation55,Citation56 Salbutamol and nifedipine were chosen for the stability study due to their difference in hydrophobicity, which allows evaluation of the delivery system to potentially encapsulate a variety of therapeutic agents. Hydrophobic molecules are inserted into the bilayer membrane, and hydrophilic molecules can be entrapped in the aqueous center.Citation23 In addition, salbutamol and nifedipine are currently used to treat preterm labor and have been associated with significant maternal and fetal adverse effects, due to the high doses needed to achieve therapeutic effects in the uterus.Citation2–Citation4
The functionality of the OTR-targeted liposomal platforms has previously been evaluated on murine and human myometrial tissues as well as in vivo in a murine model of preterm labor.Citation21,Citation46 We have shown that liposomes conjugated with anti-OTR polyclonal antibodies (200 nm) and loaded with salbutamol, nifedipine, or rolipram (tocolytic agents) inhibited myometrial contractions ex vivo, while those loaded with dofetilide (uterotonic agent) increased contraction duration.Citation21 Empty OTR-targeted liposomes and non-targeted control liposomes loaded with these agents had no effect.Citation21 Similar findings have also been demonstrated for ATO-Lipo (124 nm).Citation46 Furthermore, liposomes loaded with indomethacin (tocolytic agent) and conjugated with anti-OTR polyclonal antibodiesCitation21 or atosibanCitation46 significantly reduced the rates of preterm birth in mice compared with non-targeted liposomes and showed no detectable accumulation in the maternal brain or fetus.Citation21,Citation46 Parturition is considered an inflammatory process,Citation23,Citation57 which indicates that the enhanced permeability and retention (EPR) effect may also play a role in liposome accumulation into the uterus. To what extent EPR occurs in parturition as well as in obstetric complications will need to be evaluated in future studies. It should be noted, however, that OTR-targeted liposomes are unlikely to be effective in patients previously exposed to continuous high doses of oxytocin, as this typically leads to desensitization of OTRs for hours or even days,Citation47,Citation48 thereby reducing the targeting effectiveness of the drug delivery system. This phenomenon is accompanied by a downregulation of OTRs at the protein and mRNA level.Citation47,Citation49
Conclusion
This study further supports OTRs as a novel pharmaceutical target for drug delivery. OTR-targeted liposomes may provide an effective way to deliver existing therapies directly to myometrial tissue for the treatment of obstetric complications and avoid adverse effects by circumventing non-target tissues. Achieving targeted delivery of therapeutic agents to the myometrium would potentially increase therapeutic efficacy, decrease the therapeutically effective dose, and/or reduce the risk of systemic side effects. This study shows that when all physicochemical parameters of the liposomes are the same, both OTR-Lipo and ATO-Lipo have similar stability, specificity in OTR binding, degree of cellular uptake, mechanism of endocytotic uptake, and effect on cell viability. Translational development will depend on comprehensive preclinical and clinical studies, as well as other factors specific to the commercialization of nanomedicines.Citation58
In addition to the uterus, OTRs have been reported to be expressed in a multitude of tissues, including breast, pituitary, kidney, ovary, thymus, heart, vascular endothelium, osteo-clasts, myoblasts, pancreatic islet cells, and adipocytes.Citation11,Citation31 However, their clinical relevance in humans has not been fully established. Further studies will be required to quantify the biodistribution of OTR-targeted liposomes in these tissues in relevant animal models of these obstetric complications. OTRs are also structurally similar to the vasopressin receptor subtypes.Citation11,Citation20,Citation59 It will be important to determine the degree of cross-reactivity of the different OTR-targeted liposomal platforms with vasopressin receptors and the potential clinical implications of this interaction with regard to biodistribution of the delivery system. It should be noted that vasopressin receptors (V1a receptors) are also expressed in the uterus and are functionally coupled to myometrial contraction.Citation60,Citation61 Interestingly, OTRs have also been detected in several human cancer tissues and cell lines,Citation31 including human breast cancer,Citation62,Citation63 uterine leiomyoma,Citation41,Citation64 adenocarcinoma of the endometrium,Citation65 neuroblastoma, and glioma.Citation66 Therefore, OTR-targeted drug delivery systems may also be adapted to assist in the treatment of other disease pathologies character-ized by an upregulation of OTR expression.
Author contributions
SH contributed to study concept and design, liposome manufacturing and characterization, liposome imaging, cellular studies, analysis and interpretation of data, and drafting of manuscript. BV contributed to liposome imaging and analysis and interpretation of data. Both authors were involved in revising the article critically for important intellectual content and gave final approval of the version to be published. The authors agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Health and Medical Research Council, Hunter Medical Research Institute, Global Alliance to Prevent Prematurity and Stillbirth, Gladys M Brawn Fellowship, and University of Newcastle. This work was performed in part at the Materials node of the Australian National Fabrication Facility, which is a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australian researchers.
Disclosure
SH has a patent through the University of Newcastle related to the use of targeted liposomes. BV reports no conflicts of interest in this work.
References
- ButwickAJCarvalhoBBlumenfeldYJEl-SayedYYNelsonLMBatemanBTSecond-line uterotonics and the risk of hemorrhage-related morbidityAm J Obstet Gynecol20152125642.e1642.e725582104
- de HeusRMolBWErwichJJAdverse drug reactions to tocolytic treatment for preterm labour: prospective cohort studyBMJ2009338b74419264820
- HaasDMCaldwellDMKirkpatrickPMcintoshJJWeltonNJTocolytic therapy for preterm delivery: systematic review and network meta-analysisBMJ2012345e622623048010
- JørgensenJSWeileLKLamontRFPreterm labor: current tocolytic options for the treatment of preterm laborExpert Opin Pharmacother201415558558824456411
- MousaHABlumJAbou El SenounGShakurHAlfirevicZTreatment for primary postpartum haemorrhageCochrane Database Syst Rev20142CD003249
- LiuLOzaSHoganDGlobal, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysisLancet2015385996643044025280870
- ManuckTARiceMMBailitJLPreterm neonatal morbidity and mortality by gestational age: a contemporary cohortAm J Obstet Gynecol20162151103.e1103.e1426772790
- RomeroRDeySKFisherSJPreterm labor: one syndrome, many causesScience2014345619876076525124429
- Al-ZirqiIVangenSForsenLStray-PedersenBPrevalence and risk factors of severe obstetric haemorrhageBJOG2008115101265127218715412
- KhanKSWojdylaDSayLGülmezogluAMvan LookPFWho analysis of causes of maternal death: a systematic reviewLancet200636795161066107416581405
- ArrowsmithSWraySOxytocin: its mechanism of action and receptor signalling in the myometriumJ Neuroendocrinol201426635636924888645
- BlanchGLavenderTWalkinshawSAlfirevicZDysfunctional labour: a randomised trialBr J Obstet Gynaecol199810511171209442174
- ArrowsmithSKendrickAWraySDrugs acting on the pregnant uterusObstet Gynaecol Reprod Med201020824124724443652
- GoodwinTMPaulRSilverHThe effect of the oxytocin antagonist atosiban on preterm uterine activity in the humanAm J Obstet Gynecol199417024744788116700
- RomeroRSibaiBMSanchez-RamosLAn oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescueAm J Obstet Gynecol200018251173118310819855
- WathesDCBorwickSCTimmonsPMLeungSTThorntonSOxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labourJ Endocrinol1999161114315110194538
- FuchsARFuchsFHussleinPSoloffMSOxytocin receptors in the human uterus during pregnancy and parturitionAm J Obstet Gynecol198415067347416093538
- FuchsARFieldsMJFreidmanSShemeshMIvellROxytocin and the timing of parturition. Influence of oxytocin receptor gene expression, oxytocin secretion, and oxytocin-induced prostaglandin F2 alpha and E2 releaseAdv Exp Med Biol19953954054208713995
- KimuraTTakemuraMNomuraSExpression of oxytocin receptor in human pregnant myometriumEndocrinology199613727807858593830
- AkerlundMTargeting the oxytocin receptor to relax the myometriumExpert Opin Ther Targets200610342342716706682
- PaulJWHuaSIlicicMDrug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptorAm J Obstet Gynecol20172163283.e1283.e1427567564
- HuaSSynthesis and in vitro characterization of oxytocin receptor targeted PEGylated immunoliposomes for drug delivery to the uterusJ Liposome Res2019111
- SercombeLVeeratiTMoheimaniFWuSYSoodAKHuaSAdvances and challenges of liposome assisted drug deliveryFront Pharmacol2015628626648870
- UlrichASBiophysical aspects of using liposomes as delivery vehiclesBiosci Rep200222212915012428898
- MajumderPBhuniaSBhattacharyyaJChaudhuriAInhibiting tumor growth by targeting liposomally encapsulated CDC20siRNA to tumor vasculature: therapeutic RNA interferenceJ Control Release201418010010824556418
- ReddyJAAbburiCHoflandHFolate-targeted, cationic liposome-mediated gene transfer into disseminated peritoneal tumorsGene Ther20029221542155012407426
- HuaSChangHIDaviesNMCabotPJTargeting of ICAM-1-directed immunoliposomes specifically to activated endothelial cells with low cellular uptake: use of an optimized procedure for the coupling of low concentrations of antibody to liposomesJ Liposome Res20112129510520429814
- Lopes de MenezesDEPilarskiLMAllenTMIn vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphomaCancer Res19985815332033309699662
- VoineaMManduteanuIDragomirECapraruMSimionescuMImmunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells-a potential tool for specific drug deliveryPharm Res200522111906191716088429
- EvertsMKoningGAKokRJIn vitro cellular handling and in vivo targeting of E-selectin-directed immunoconjugates and immunoliposomes used for drug delivery to inflamed endotheliumPharm Res2003201647212608538
- ZinggHHLaporteSAThe oxytocin receptorTrends Endocrinol Metab200314522222712826328
- SzukiewiczDBilskaAMittalTKMyometrial contractility influences oxytocin receptor (OXTR) expression in term trophoblast cells obtained from the maternal surface of the human placentaBMC Pregnancy Childbirth20151522026377392
- GimplGFahrenholzFThe oxytocin receptor system: structure, function, and regulationPhysiol Rev200181262968311274341
- TakemuraMKimuraTNomuraSExpression and localization of human oxytocin receptor mRNA and its protein in chorion and decidua during parturitionJ Clin Invest1994936231923238200965
- AnabousiSLaueMLehrC-MBakowskyUEhrhardtCAssessing trans-ferrin modification of liposomes by atomic force microscopy and transmission electron microscopyEur J Pharm Biopharm200560229530315939240
- BendasGKrauseABakowskyUVogelJRotheUTargetability of novel immunoliposomes prepared by a new antibody conjugation techniqueInt J Pharm19991811799310370205
- SharkeyJOlceseJTranscriptional inhibition of oxytocin receptor expression in human myometrial cells by melatonin involves protein kinase C signalingJ Clin Endocrinol Metab200792104015401917726073
- SoloffMSJengYJIliesMImmortalization and characterization of human myometrial cells from term-pregnant patients using a telomerase expression vectorMol Hum Reprod200410968569515243128
- MaggiMPeriABaldiEInterferon-alpha downregulates expression of the oxytocin receptor in cultured human myometrial cellsAm J Physiol19962715 Pt 1E840E8468944670
- TaharaATsukadaJTomuraYPharmacologic characterization of the oxytocin receptor in human uterine smooth muscle cellsBr J Pharmacol2000129113113910694212
- BusnelliMRimoldiVViganòPPersaniLDi BlasioAMChiniBOxytocin-induced cell growth proliferation in human myometrial cells and leiomyomasFertil Steril20109451869187420056210
- MolnárMRigoJJrRomeroRHertelendyFOxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cellsAm J Obstet Gynecol19991811424910411794
- OhmichiMKoikeKNoharaAOxytocin stimulates mitogen-activated protein kinase activity in cultured human puerperal uterine myometrial cellsEndocrinology19951365208220877536662
- PhaneufSEurope-FinnerGNVarneyMMackenzieIZWatsonSPLópez BernalAOxytocin-stimulated phosphoinositide hydrolysis in human myometrial cells: involvement of pertussis toxin-sensitive and -insensitive G-proteinsJ Endocrinol199313634975098386215
- JengYJSoloffSLAndersonGDSoloffMSRegulation of oxyto-cin receptor expression in cultured human myometrial cells by fetal bovine serum and lysophospholipidsEndocrinology20031441616812488330
- RefuerzoJSLeonardFBulayevaNUterus-targeted liposomes for preterm labor management: studies in pregnant miceSci Rep201663471027725717
- VrachnisNMalamasFMSifakisSDeligeoroglouEIliodromitiZThe oxytocin-oxytocin receptor system and its antagonists as tocolytic agentsInt J Endocrinol2011201135054622190926
- PlestedCPBernalALDesensitisation of the oxytocin receptor and other G-protein coupled receptors in the human myometriumExp Physiol200186230331211429648
- PhaneufSRodríguez LiñaresBTambyrajaRLMackenzieIZLópez BernalALoss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labourJ Reprod Fertil20001201919711006150
- OakleyRHLaporteSAHoltJABarakLSCaronMGMolecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*J Biol Chem200127622194521946011279203
- GimplGBurgerKPolitowskaECiarkowskiJFahrenholzFOxytocin receptors and cholesterol: interaction and regulationExp Physiol200085 Spec No:41S-49S
- GimplGFahrenholzFHuman oxytocin receptors in cholesterol-rich vs cholesterol-poor microdomains of the plasma membraneEur J Biochem200026792483249710785367
- VercauterenDVandenbrouckeREJonesATThe use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfallsMol Ther201018356156920010917
- AndersonMOmriAThe effect of different lipid components on the in vitro stability and release kinetics of liposome formulationsDrug Deliv2004111333915168789
- AllenTMLong-circulating (sterically stabilized) liposomes for targeted drug deliveryTrends Pharmacol Sci19941572152207940982
- TorchilinVPImmunoliposomes and PEGylated immunoliposomes: possible use for targeted delivery of imaging agentsImmunomethods1994432442587820455
- NormanJEBollapragadaSYuanMNelsonSMInflammatory pathways in the mechanism of parturitionBMC Pregnancy Childbirth20077Suppl 1S717570167
- HuaSde MatosMBCMetselaarJMStormGCurrent trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and CommercializationFront Pharmacol2018979030065653
- ShojoHKanekoYCharacterization and expression of oxytocin and the oxytocin receptorMol Genet Metab200071455255811136546
- BossmarTAkerlundMFantoniGSzamatowiczJMelinPMaggiMReceptors for and myometrial responses to oxytocin and vasopressin in preterm and term human pregnancy: effects of the oxytocin antagonist atosibanAm J Obstet Gynecol19941716163416427802081
- MaggiMDel CarloPFantoniGHuman myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptorsJ Clin Endocrinol Metab1990704114211542156888
- CassoniPSapinoAFortunatiNMunaronLChiniBBussolatiGOxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase AInt J Cancer19977223403449219843
- ItoYKobayashiTKimuraTInvestigation of the oxytocin receptor expression in human breast cancer tissue using newly established monoclonal antibodiesEndocrinology199613727737798593829
- LeeKHKhan-DawoodFSDawoodMYOxytocin receptor and its messenger ribonucleic acid in human leiomyoma and myometriumAm J Obstet Gynecol19981793 Pt 16206279757961
- CassoniPFulcheriECarcangiuMLStellaADeaglioSBussolatiGOxytocin receptors in human adenocarcinomas of the endometrium: presence and biological significanceJ Pathol2000190447047710699997
- CassoniPSapinoAStellaAFortunatiNBussolatiGPresence and significance of oxytocin receptors in human neuroblastomas and glial tumorsInt J Cancer19987756957009688301
