Abstract
Aprepitant, a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors, is the active ingredient of EMEND® which has recently been approved by the FDA for the prevention of chemotherapy-induced nausea and vomiting (CINV). Aprepitant undergoes extensive metabolism, primarily via CYP3A4 mediated oxidation. It is eliminated primarily by metabolism and is not renally excreted. The apparent terminal half-life in humans ranged from 9 to 13 hours. Early development studies led to the development of a nanoparticle formulation to enhance exposure and minimize food effects. Two large randomized trials accruing 1099 patients studied the effect in patients receiving cisplatin of adding aprepitant to ondansetron and dexamethasone on day 1 then to dexamethasone on days 2 and 3 to control delayed emesis. The complete response of no vomiting and no rescue medication overall from days 1 to 5 improved from 48% to 68% (p < 0.001), a 13% improvement in acute emesis but a 21% improvement in delayed emesis with the improvement from 51% to 72% (p < 0.001). Similarly, 866 patients treated with cyclophosphamide plus either doxorubicin or epirubicin, received either ondansetron, dexamethasone, and aprepitant on day 1 followed by aprepitant on days 2 and 3 or ondansetron and dexamethasone on day 1 and dexamethasone on days 2 and 3. The overall complete response rate over 5 days was better for the aprepitant group 50.8% vs 42.5% (p=0.015). Complete responses were reported in more patients taking aprepitant in both the acute (76% vs 69%, p=0.034) and delayed (55% vs 49%, p=0.064) phases of vomiting. There were no clinically relevant differences in toxicity by adding aprepitant and improvements in the quality of life of patients on chemotherapy were recorded.
Introduction
Cytotoxic chemotherapy drugs can cause acute nausea and vomiting in the first 24 hours, and then delayed emesis between 2 and 6 days. Cisplatin is the example of a drug with high emetic potential, which means that 90% or more of patients will vomit if they don’t receive prophylactic antiemetics. Cisplatin is often used to test the efficacy of new antiemetic agents. Antiemetics are best given prior to the initial course of therapy because patients who vomit after chemotherapy can develop anticipatory emesis prior to subsequent cycles of chemotherapy (CitationAntiemetic Subcommittee MASCC 1998).
The understanding of the role of the 5 hydroxytryptamine3 receptors, predominantly in the small bowel, as the mediators of acute cytotoxic induced emesis led to a major breakthrough in the control of chemotherapy-induced emesis with the introduction of the 5 hydroxytrytamine3 antagonist, ondansetron, which revolutionized the treatment of acute post-chemotherapy emesis. Ondansetron in combination with dexamethasone controlled acute cisplatin induced emesis in over 80% patients but control of the delayed emesis only approached 50% (CitationRoila et al 1996). However, after the introduction of the 5HT3 antagonists patients were still listing nausea and vomiting in their top three side-effects (Citationde Boer-Dennert et al 1997). Clinicians underestimate the incidence of delayed emesis by up to 30% (CitationGrunberg et al 2004).
Nanoparticle formulation development
Early preclinical studies showed less than dose proportional increases in systemic exposure in Beagle dogs at oral doses of 2 and 32 mg/kg with suspensions of micronized aprepitant (mean size 5 microns). A similar dose–exposure relationship was observed in early clinical studies using tablet formulations made of micronized bulk drug. In addition, a significant, positive food effect on absorption was seen in healthy, young, male volunteers who had been given a high fat breakfast prior to dosing. These early clinical data suggested that the projected efficacious human dose would be relatively high and development of a more bioavailable formulation could potentially reduce the dose. Formulation efforts to develop a more bioavailable formulation that eliminates/minimizes food effect on absorption were crucial for the future of the program.
Aprepitant is a basic compound with a pKa value of 9.7 within the pH range from 2 to 12 (). It is a white to off-white, crystalline, non-hygroscopic solid with a melting point of 254°C. Early salt form screening was conducted but all salts examined showed rapid disproportionation in water and poor chemical stability. The free base form of the molecule was chosen for development based on superior physicochemical properties. The free base aqueous solubility (3–7 μg/ml) is very low in the pH range of 2–10, and increases to 0.13 mg/mL at pH 1.0. The compound has a log P value of 4.8 at pH 7.0, suggesting a relatively high lipophilicity, the potential for dietary fat and bile to solubilize the drug in vivo, and potentially reasonable permeability. Particle size reduction methodologies could increase the available surface area to enhance the rate of solubilization and thereby increase exposure. Preclinical studies in dogs were conducted to examine the relationship between particle size and exposure.
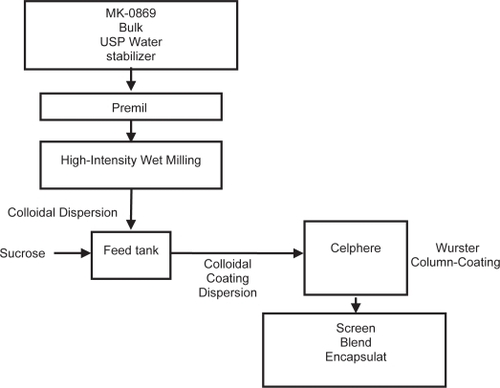
In vivo dog studies of suspensions of aprepitant indicated a 3x increase in exposure as the particle size was reduced from 5 microns to less than 150 nanometers. In the nanomilling process, the slurry of drug particles in an aqueous polymeric solution is recirculated through a stirred media mill filled with polymeric media. The energy imparted by colliding media results in breakage of drug particles. The recirculation process is continued until the desired drug particle size is achieved. The resulting colloidal dispersion of nanometer drug particles is then converted into a solid-dosage form and then processed to form a tablet or a capsule dosage form ().
Solid dosage form process optimization was based on bioavailability, processing, and capabilities of the two lead processes. The column-coating processed formulations had marginally higher exposure compared with a spray-dried processed formulations. The spray-drying process produced a hygroscopic powder due to the formation of amorphous sucrose, one of the key ingredients in the formulation. In addition, the spray-dried powder may require further agglomeration processing to enhance its flow characteristics and the effect of manufacturing scale on the physical properties of the spray dried powder would need to be investigated. Column-coated beads, on the other hand, had good flow characteristics due to their large size (greater than the substrate mean size of 600 μm) and nearly spherical shape. The column-coated beads size would remain the same with scale up since the weight gain (or the composition) would remain the same. Hence, the aprepitant redispersion characteristics of the coated beads would be independent of column coating scale. Both processes have comparable processing time, but the spray drying process is a semi-continuous process compared with batch processing with the column coating process. Based on these considerations, the column-coating-processed formulation was chosen as the lead for further investigation in human clinical studies. The column coating process produced drug-coated beads which are lubricated with 1% sodium lauryl sulfate and filled into gelatin capsules. The coating contains drug-to-sucrose ratio of 1:1, drug-to-hydroxypropyl cellulose (HPC)-SL ratio of 5:1, and coat weight gain of 100 weight percent.
The influence of drug particle size on bioavailability in humans was investigated at a 100-mg dose. The human clinical studies confirmed the 3–4x increase in bioavailability with the nanoparticle formulation compared with the alpine milled (mean 5 micron) drug formulation. In addition, the food effect at 100-mg dose was minimal with the nanoparticle formulation (approximately 40%) compared with the 3–4x food effect observed with the 5 micron drug formulation.
Nanoparticle formulation development focuses on established polymeric stabilizers to maintain size reductions. The addition of other surface active agents, sodium lauryl sulfate, provides viscosity reduction to increase process efficiencies. Redispersants are needed to further stabilize nanoparticle colloidal suspensions to drying and freezing. Processing of the dried material into a final dosage form is driven by gains in yields and dosage form elegance. Nanoparticle formulations provide possibilities to decrease food effects and dosage form potency to further benefit patients.
Clinical trials with cisplatin
When the first of the neurokinin1 (NK1) receptor antagonists, aprepitant, was introduced it improved the control of acute emesis when added to ondansetron and dexamethasone but its most dramatic effects were seen in the delayed phase of cisplatin-induced emesis. Subsequently it was tested with the combination of an anthracycline and doxorubicin ().
Table 1 Key randomized studies with aprepitant
Two large randomized trials, one in South America and the other in Europe, North America, and Australia randomized a total of 1099 patients to study the effect of adding aprepitant to ondansetron and dexamethasone (CitationHesketh et al 2003; CitationPoli-Bigelli et al 2003). Both studied patients receiving their first ever cycles of high-dose cisplatin specified as > 70 mg/m2 over ≤3 hours. The patients on the control arms of both studies received intravenous ondansetron 32 mg 30 minutes before cisplatin and oral dexamethasone 20 mg on day 1 followed by oral dexamethasone 8 mg twice daily from days 2 to 4. The arms where aprepitant was added received oral aprepitant 125 mg one hour before cisplatin, then intravenous ondansetron 32 mg 30 minutes before cisplatin, with oral dexamethasone 12 mg on day 1, oral aprepitant 80 mg and oral dexamethasone 8 mg once daily on days 2 and 3, and on day 4 one dose of oral dexamethasone 8mg.
The complete response of no vomiting and no rescue medication overall from days 1 to 5 improved from 48 to 68% (p<0.001), a 13% improvement in acute emesis but a 21% improvement in delayed emesis with the improvement from 51% to 72% (p<0.001).
The overall complete response rate (CR) of no emesis in the South American trial was 62.7% for the aprepitant group vs 43.3% for the control (p<0.001). In the international trial the CR rate was 72.7% for the aprepitant arm and 52.3% for the control arm (p<0.001). For acute emesis the CR rates were 82.8% vs 68.4% (p<0.001) and 89.2% vs 78.1% respectively, favoring the aprepitant groups. In the delayed phase of emesis the difference between the groups was greater, 67.7% vs 46.8% (p<0.001) in the South American study and 74.4% vs 55.8% (p<0.001) in the international trial. The differences achieved by aprepitant were sustained over the 5 days post chemotherapy. As has been previously reported in other studies, the control of vomiting was superior to the control of nausea. The efficacy of the triple antiemetic therapy (ondansetron, dexamethasone, and aprepitant) persisted over 6 courses of chemotherapy. No differences in response rates were found in different age groups in both the acute and delayed phases of emesis and the improvement of the quality of life was similar for older and younger patients.
Given that it had been common practice to continue the 5HT3 receptor antagonist with dexamethasone into the delayed phase of emesis, a subsequent reported study randomized 489 patients receiving high dose cisplatin to either a control arm of ondansetron and dexamethasone for 4 days or aprepitant, ondansetron, and dexamethasone only on day 1 post chemotherapy followed by aprepitant and dexamethasone on days 2–3 and dexamethasone alone on day 4 (CitationAapro et al 2005). During the first 5 days post chemotherapy, the 3-day aprepitant regimen provided a superior complete response rate (72% vs 61%, p=0.003). Compared with a regimen using 4 days of ondansetron, the regimen using 3 days of aprepitant provided a 9% improvement in protection from nausea and vomiting on day 1 (88% vs 79%, p=0.005) and an 11% improvement on days 2 through 5 (74% vs. 63%, p=0.004). Again there was no significant difference in toxicity between the arms.
Clinical trials with cyclophosphamide and an anthracycline
In a study exploring the use of aprepitant with regimens which do not contain cisplatin, and arguably cause more moderate emesis, 866 patients treated with cyclophosphamide plus either doxorubicin or epirubicin, received either ondansetron, dexamethasone, and aprepitant on day 1 followed by aprepitant on days 2 and 3 or ondansetron and dexamethasone on day 1 and ondansetron days 2 and 3 (CitationWarr et al 2005). All agents were given orally in this study. The overall complete response rate over 5 days was better for the aprepitant group 50.8% vs 42.5% (p=0.015). Complete responses were reported in more patients taking aprepitant in both the acute (76% vs 69%, p=0.034) and delayed (55% vs 49%, p=0.064) phases of vomiting although the delayed phase difference did not reach statistical significance because nausea was not as well controlled as vomiting in this phase. No differences were seen between the groups in the use of rescue medication, which suggests that nausea was not as well controlled as vomiting. The question remains of whether these results could have been improved by adding dexamethasone in the delayed phase of emesis.
The improvement in efficacy in the aprepitant arm was demonstrated over 4 cycles of chemotherapy with the complete response rates in comparison with the control arm being 53.8% vs. 39.4% in cycle 2, 54.1% vs. 39.3% in cycle 3 and 55% vs. 38.4% in cycle 4 for a cumulative percentage improvement (p=0.017) (CitationHerrstedt et al 2005).
Doxorubicin and cyclophosphamide were also given with cisplatin to 80 patients on the control arm and 81 on the aprepitant arm of the two large studies reported above, testing the efficacy of adding aprepitant to antiemetic regimens in patients receiving cisplatin. The improvement seen by adding aprepitant was greater in the arms where doxorubicin and cyclophosphamide were added to cisplatin than when the combination included other agents, with a 33% improvement compared with 20% for the whole study population (CitationGralla et al 2005).
Tolerability and quality of life
Aprepitant is very well tolerated. The benefit in the two large trials of adding aprepitant to ondansetron and dexamethasone for cisplatin-induced emesis was seen with only small differences in side-effects between the two arms of the study (CitationHesketh et al 2003; CitationPoli-Bigelli et al 2003). The most common additional reports with the aprepitant combination were hiccups (4.6%), asthenia/fatigue (2.9%), constipation (2.2%), headache (2.2%), anorexia (2.0%), and increased ALT (2.8%). The only appreciable difference in tolerability in the doxorubicin and cyclophosphamide trial was a higher rate of constipation in patients on the active control regimen (18.0% vs 12.3%) but more dyspepsia in those on the aprepitant regimen (8.4% vs 4.9%) (CitationWarr et al 2005).
Chemotherapy-induced emesis is associated with a significant deterioration in global quality of life (CitationOsoba et al 1997). Patients who have nausea and vomiting report experiencing more fatigue, anorexia, and insomnia. Controlling nausea and vomiting has been found to more globally affect a patient’s quality of life. To measure the impact of the control of emesis on quality of life, the Functional Living Index Emesis (FLIE) was used in the above studies. Logistic regression analysis showed that more patients in the aprepitant groups reported minimal or no impact of chemotherapy-induced emesis on daily life compared with those on just a 5HT3 antagonist and dexamethasone (74.7% vs 63.5% in the South American study, 70.4% vs 64.3% in the international study). In the Warr et al study more patients on aprepitant reported minimal or no impact of CINV on daily life, as measured by the FLIE questionnaire (63.5% v 55.6%; p= 0.019).
Drug interactions
Aprepitant is primarily metabolized by the p-450 isoenzyme CYP3A4 cytochrome system and therefore has the potential to interact with a range of other drugs (Sanchez et al 2005). The metabolic profile of aprepitant over a 2-week period of time can be described as an inhibitor, then an inducer, and then no effect on CYP3A4 after 2 weeks (CitationShadle et al 2004). Of relevance is the interaction with dexamethasone which doubles the plasma concentration of the steroids (CitationMcCrea et al 2003). This is why the doses of dexamethasone in the large cisplatin trials were decreased in the aprepitant arms so the plasma concentrations would be equivalent in both arms of the studies. Interactions are more likely with co-administered oral medications. No interactions with ondansetron or palonosetron have been recorded (CitationBlum et al 2003; CitationShah et al 2005). In the limited studies of possible interactions with cytotoxics, no interactions have been found with docetaxel and there was no obvious problem with the seven different drugs co-administered with cisplatin in the two large cisplatin trials (CitationNygren et al 2005). In a small study, aprepitant inhibited both cyclophosphamide and thiotepa metabolism, but the effects of these interactions was small compared with the total variability (Citationde Jong et al 2005). More data are needed studying possible interactions with oral cytotoxics. There is a decrease in the concentration of co-administered ethinyl oestradiol in studies of long-term use, so patients are advised to use additional barrier contraceptive methods (CitationOlver 2004). A significant induction of CYP2C9 metabolism of warfarin by aprepitant has been found which necessitates close monitoring of clotting studies in the 7–10 days after aprepitant (CitationDepre et al 2005).
Guidelines
These results have led the Multinational Association for Symptom Control in Cancer to recommend that the triple drug regimen of a 5HT3 receptor antagonist, dexamethasone, and aprepitant be used to prevent acute emesis from chemotherapy with high emetic potential, and then to continue with aprepitant and dexamethasone on days 2 and 3 to prevent delayed emesis (CitationThe Antiemetic Subcommittee MASCC 2006). With anthracycline and cyclophosphamide combinations the same recommendation is made with the three drugs on day 1 to prevent acute emesis with aprepitant or dexamethasone to prevent delayed emesis.
Conclusions
Having made a major advance in the control of acute post-chemotherapy emesis with the introduction of the 5HT3 receptor antagonists in combination with dexamethasone, adding the oral NK1 receptor antagonist aprepitant improves the control of the acute phase of emesis, but also has a particular impact on the control of delayed emesis. In practice, patients receiving chemotherapy of high emetic potential are best treated prophylactically with the three drugs to control the acute phase and then two further days of aprepitant with dexamethasone for the delayed phase. Similar recommendations can be applied to patients receiving chemotherapy of moderate emetic potential. The addition of aprepitant adds little to the toxicity of the antiemetic combination and not only improves the control of emesis, but in turn improves the quality of life of the patients receiving chemotherapy.
References
- AaproMSchmollHJPoli-BigelliS2005Comparison of aprepitant combination regimen with 4-day ondansetron + 4-day Dexmethasone for prevention of acute and delayed nausea/vomiting after cisplatin chemotherapy [abstract]Proc Am Soc Clin Oncol23:no. 8007.
- Antiemetic Subcommittee of the Multinational Association of supportive care in Cancer (MASCC)1998Prevention of chemotherapy - and radiotherapy-induced emesis: Results of the Perugia Consensus ConferenceAnn Oncol910229
- BlumRAMajumdarAMcCreaJ2003Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjectsClin Ther2514071912867217
- de Boer-DennertMde WitRSchmitzPI1997Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonistsBr J Cancer761055619376266
- de JongeMEHuitemaADHoltkampMJ2005Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolismCancer Chemother Pharmacol563708[Epub 2005 Apr]. 15838656
- DepreMVan HeckenAOeyenM2005Effect of aprepitant on the pharmacokinetics and pharmacodynamics of warfarinEur J Clin Pharmacol613416Epub 2005 Jun 28. 15983826
- GrallaRJde WitRHerrstedtJ2005Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomized clinical trialsCancer104864815973669
- GrunbergSMDeusonRRMavrosP2004Incidence of chemotherapy-induced nausea and emesis after modern antiemeticsCancer1002261815139073
- HerrstedtJMussHBWarrDG2005Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapyCancer10415485516104039
- HeskethPJGrunbergSMGrallaRJ2003The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patient receiving high-dose cisplatin. The aprepitant protocol 052 study groupJ Clin Oncol2141121914559886
- McCreaJBMajumdarAKGoldbergMR2003Effects of the neurokinin 1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisoloneClin Pharm Ther741724
- NygrenPHandeKPettyKJ2005Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patientsCancer Chemother Pharmacol5560916[Epub 2005 Feb 19]. 15723220
- OlverIN2004Aprepitant in antiemetic combinations to prevent chemotherapy induced nausea and vomitingInt J Clin Prac582016
- OsobaDZeeBWarrD1997Effect of postchemotherapy nausea and vomiting on health-related quality of life The Quality of Life and Symptom Control Committee of the National Cancer Institute of Canada Clinical Trials GroupSupport Care Cancer5307139257427
- Poli-BigelliSRodrigues-PereiraJCaridesAD2003Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin AmericaCancer973090812784346
- RoilaFTonatoMBallatoriE1996Comparative studies of various antiemetic regimensSupport Care Cancer4270808829304
- SanchezRIWangRWNewtonDJ2004Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitantDrug Metab Dispos32128792[Epub 2004 Aug 10]. 15304427
- ShadleCRLeeYMajumdarAK2004Evaluation of potential effects of aprepitant on cytochrome P450 3A4 and 2C9 activityJ Clin Pharmacology44215223
- ShahAKHuntTLGallagherSC2005Pharmacokinetics of palonosetron in combination with aprepitant in healthy volunteersCurr Med Res Opin2159560115899109
- The Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC)2006Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus ConferenceAnn Oncol1720816314401
- WarrDGHeskethPJGrallaRJ2005Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapyJ Clin Oncol2328223015837996