Abstract
Anthracycline compounds including daunorubicin are the foundation of many modern chemotherapeutic regimens. However, the side-effects of these compounds can be severe, leading to alopecia, nausea, immune deficiency, and cardiotoxicity. For immunocompromised patients with aggressive Kaposi’s sarcoma (KS), these complications often preclude the completion of appropriate chemotherapeutic regimens. This review focuses on the development and efficacy of liposomal daunorubicin (DaunoXome®; DNX) carriers for the treatment of KS. Encouragingly, DNX demonstrated increased in vivo stability and specificity. As a result, KS patients benefit from higher cumulative chemotherapeutic doses without significant cardiotoxicity. Tumor response to DNX treatment surpasses that of non-encapsulated daunorubicin and is similar to that observed with conventional multi-drug therapies such as ABV (doxorubicin, bleomycin, vincristine). Moreover, some reports indicate the patient quality of life during therapy may improve with DNX treatment. Although the development of DNX represents a significant advance in KS therapy, recent data suggest that additional modification of the liposomal carrier to include pegylation or target specific antibodies may further increase daunorubicin efficacy in the future.
Kaposi’s sarcoma
Originally described in the late nineteenth century, Kaposi’s sarcoma (KS) has become the most prevalent cancer amongst AIDS patients in the US and worldwide (CitationEngels et al 2006). Until recently the etiological agent of this disease remained elusive. Finally, in 1994, Chang and colleagues isolated viral DNA sequences from KS biopsies, resulting in the identification of the Kaposi’s sarcoma-associated herpes virus (KSHV or HHV-8) (CitationChang et al 1994). KSHV is maintained latently in all KS tumors and thus is believed to be the causative agent of the disease (CitationChang et al 1994; CitationCesarman et al 1995; CitationSoulier et al 1995). Several forms of KS are currently recognized, including classic, endemic, iatrogenic (transplant associated), and AIDS-associated (CitationTappero et al 1993). Among the few cases of KS reported in the US prior to the onset of the AIDS epidemic, most were iatrogenic or found in elderly men of Mediterranean descent (classic). In contrast, endemic KS has always been prevalent in Africa, and today has become the most frequently observed pediatric cancer in this population following the rise of the HIV epidemic. AIDS-associated KS first became prevalent worldwide during the 1980s into the early 1990s in men who have sex with men. It has declined following the implementation of highly active anti-retroviral therapy (HAART), as have many other of the original AIDS-defining conditions (CitationEltom et al 2002; CitationCasper 2006; CitationEngels et al 2006). Unfortunately, HAART has had no effect on the incidence of classic, iatrogenic, or endemic KS nor is HAART available to populations who presently experience the highest prevalence of HIV, such as in Africa. More alarming, cases of AIDS-associated KS are now emerging in patients receiving HAART and who maintain adequate immune function (CitationKoon et al 2005; CitationBoshoff 2006; CitationCasper 2006). As the number of HIV-positive individuals continues to grow worldwide, KS is re-emerging as a more aggressive and prominent tumor type.
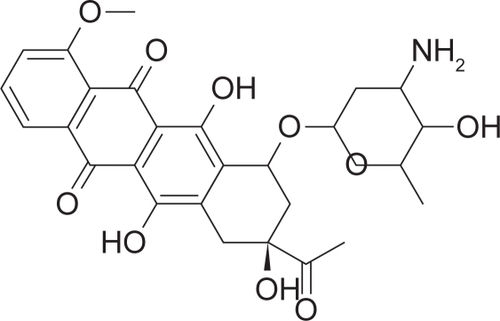
The changing nature of KS is reflected in 5-year survival data reported by the NCI Surveillance Epidemiology and End Results (SEER) program (). As mentioned above, most KS cases in the US prior to 1980 were dermal, minimally aggressive, and often associated with elderly white men. For these patients, KS was rarely life threatening and over 80% survived more than 5 years. During the height of the US HIV epidemic and prior to the onset of HAART, a more aggressive KS tumor emerged. Dramatically, 5-year survival rates in KS patients plummeted from 1985 to 1989. Following the implementation of HAART and the development of KS-specific treatment regimens (interferon alpha, taxol, and combination chemotherapy) the US witnessed a substantial decrease in patient mortality. Yet, even today, less than 50% of all KS patients in the US, ie, with ready access to HAART, reach the 5-year survival mark. The outlook is even worse for the growing number of African American KS patients. Clearly, our ability to treat KS patients both now and in the future is dependent upon the development of novel and efficacious treatment regimens against KS. Restoring immune sufficiency alone does not suffice.
Figure 1 KS survival rates during the rise of the HIV/AIDS pandemic. Data bars represent the percentage of individuals reported by the NCI Surveillance Epidemiology and End Results (SEER) program to have survived 5 years post-diagnosis.
Current KS therapy ranges from watchful waiting to aggressive chemotherapy and is largely dependent upon the lesion location, size, and extent. Localized, non-progressive disease is easily treated through cryotherapy, argon laser excision, local chemotherapeutic injection, or localized radiation. Response rates vary with each of these treatment regimens, but have been reported to reach up to 90% (CitationKrown et al 2004). For AIDS-associated and iatrogenic KS, immune reconstitution is sometimes sufficient to trigger tumor remission. HAART alone caused KS remission in 48%–86% of HIV positive KS patients (CitationLebbe et al 1998; CitationDupin et al 1999; CitationDupont et al 2000; CitationCattelan et al 2001; CitationMurdaca et al 2002; CitationPaparizos et al 2002; CitationWilkinson et al 2002). Similarly, iatrogenic KS can be controlled by limiting the dosage, or switching the type of immunosuppressive therapy employed. However, recent evidence suggests that immune reconstitution is not the sole factor leading to KS regression. Protease inhibitors associated with modern day HAART may also possess anti-KS activities (CitationMonini et al 2004). Moreover, our recent findings along with those reported by Stallone et al suggest that some immunosuppressive drugs like rapamycin may specifically target KSHV-infected tumors (CitationStallone et al 2005; CitationSin et al 2006).
Visceral organ involvement represents the most severe manifestation of KS, and lesions have been reported in the lungs, gastrointestinal tract, lymph nodes, heart, bone, and spleen of affected individuals (CitationKrown et al 2004). For such disseminated or rapidly progressing forms of KS more aggressive therapies must be employed. Classically, a combination of multiple chemotherapeutics has been utilized (eg, CHOP – cyclophosphamide, hydroxydoxorubicin, vincristine/Oncovin®, prednisone; ABV – doxorubicin, bleomycin, vincristine; and BV – bleomycin, vincristine). Alternatively, interferon alpha, paclitaxol, and etoposide effectively treat disseminated KS and are FDA-approved for this purpose (CitationReal et al 1986; CitationLane et al 1988; CitationKrown et al 1993; CitationWelles et al 1998; CitationGill et al 1999; CitationEvans et al 2002). However, the side-effects of chemotherapeutic drug regimens limit their efficacy, especially in immunocompromised KS patients. Anthracycline drugs in particular (eg, doxorubicin, epirubicin, idarubicin, daunorubicin) present a secondary challenge to patient health due to their known cardiotoxic side-effects (CitationElliott 2006). In order to combat these issues, targeted drug delivery systems have been developed for several anthracycline compounds. This review focuses on the pharmaceutical advancements that have aimed to improve both the efficacy and tolerability of daunorubicin in KS treatment.
Daunorubicin
Originally isolated from Streptomyces peucetius varcaesitue, daunorubicin (DNR) has become a mainstay in modern chemotherapy (CitationDimarco et al 1964; CitationBehal 2000). DNR belongs to the anthracycline family of compounds, which hold intrinsic antibiotic and anti-tumor activities. Structurally, anthracyclines contain a daunosamine sugar linked to naphthacene via an O-glycosidic bond (see ) (CitationRobert 1998). Differences in the naphthacene derivative differentiate DNR from other anthracyclines and change the pharmacokinetic properties of the compound (CitationRobert 1998). DNR is believed to function through multiple mechanisms to mediated DNA damage (CitationSinha and Politi 1990). Intercalation of the daunosamin residue into the minor groove of cellular DNA leads to local DNA unwinding (CitationCrooke et al 1978; CitationChaires et al 1982; CitationChaires 1990; CitationNabiev et al 1991; CitationBelloc et al 1992). It is hypothesized that these DNA–DNR complexes serve as a blockade for cellular replication. Alternatively, the activity of topoisomerase II, an enzyme that relieves torsional stress during DNA synthesis, is also significantly inhibited by DNR (CitationTewey et al 1984; CitationFroelich-Ammon and Osheroff 1995). Anthracyclines stabilize interaction between topoisomerase II and cleaved DNA, preventing the enzyme from fully resolving DNA breaks (CitationTewey et al 1984). Finally, cellular metabolism of DNR leads to the formation of free radical species capable of inducing DNA damage (CitationHanda and Sato 1975; CitationDoroshow and Davies 1986; CitationSinha 1989). Thus, through the non-specific targeting of DNA replication, DNR targets all cells with a high proliferation index. Cell cycle progression prior to complete repair of these DNR-induced DNA lesions can result in chromosomal instability or trigger cell death. Since normal cells of the body must also proliferate to maintain homeostasis, significant side-effects including alopecia, nausea, and vomiting are often observed in patients receiving DNR therapy. In order to alleviate some of these adverse side-effects, a liposomal DNR derivative was developed.
Liposomal formulation
Phospholipid spheres were originally discovered and termed liposomes in the 1960s (CitationBangham et al 1965; CitationSessa and Weissmann 1968). Now tailored for use in drug formulations, liposomes can be classified based on lamellarity, size, makeup, and biological distribution (CitationHofheinz et al 2005; CitationNetageri 1993). Unilaminar liposomes, composed of a single lipid bilayer, are frequently used to encapsulate water soluble drugs (CitationHofheinz et al 2005), and can be further classified based on size. Small unilaminar liposomes (SUV) are typically 25–100 nm in diameter, whereas large unilaminar liposomes (LUV) are significantly bigger (100–400 nm) (CitationNetageri 1993). Multilaminar liposomes (MLV) range from 100 nm to over 1 μm in size and contain several concentric lipid bilayers (CitationNetageri 1993). In contrast to SUV and LUV, MLV are used frequently to package lipid soluble drugs (CitationHofheinz et al 2005).
The liposomal structure is hypothesized to stabilize encapsulated drugs in vivo. Liposomes flowing in the blood fail to extravasate intact blood vessels, accumulating instead in areas of discontinuous capillaries, such as tumor tissue (CitationJain 1987; CitationDvorak et al 1988; CitationHuang et al 1992; CitationHobbs et al 1998). In this manner, therapeutic toxicity should be reduced while increasing tumor uptake. Moreover, since KS is a highly vascularized tumor characterized by capillary leakage, this property of liposomal drug formulations may increase therapeutic efficacy. Unfortunately, all liposomes also naturally target the reticuloendothelial system (RES) of the body, which includes the liver, spleen, and bone marrow (CitationPatel and Russell 1988; CitationHuang et al 1992). While circulating in the blood, liposomes bind plasma proteins, immunoglobulins, and complement leading to RES-uptake via opsinization (CitationChonn et al 1992; CitationCullis et al 1998). Extremely large liposomes, like the giant MLVs (>1 μm), are also trapped and concentrated within the small, alveolar capillaries of the lungs (CitationHwang 1987). Certainly, it could be envisioned that liposomal uptake by the RES would have significant advantages for the delivery of potential immunomodulatory therapeutics. However, for most anti-cancer agents this property is undesirable.
Several RES-targeting liposomal drug formulations have been developed using a wide variety of strategies. These drugs have advantages over classic therapies, reducing a number of the toxicities previously associated with anthracycline administration (CitationRoerdink 1987). Some of the first forms of liposomal doxorubicin (LD) employed acidic lipids to draw in the weakly basic drug (CitationAllen 2004). Other approaches establish a liposomal pH gradient, pulling the drug towards the core of each lipid sphere (CitationMayer et al 1986; CitationLi et al 1998). Such technology is utilized in the formulation of therapeutics currently on the market including Myocet® (LD; Medeus Pharma) and Onco TCS® (liposomal vincristine; INEX Parmaceuticals) (CitationEmbree et al 1998; CitationGelmon et al 1999; CitationAllen and Martin 2004). Finally, RES-targeting liposomes can be formulated to contain lipid-soluble drugs within the liposomal membrane. Notably, LEP ETU®, a form of liposomal paclitaxel manufactured by NeoPhar, is manufactured using this technology (CitationTreat et al 2001; CitationAllen and Martin 2004).
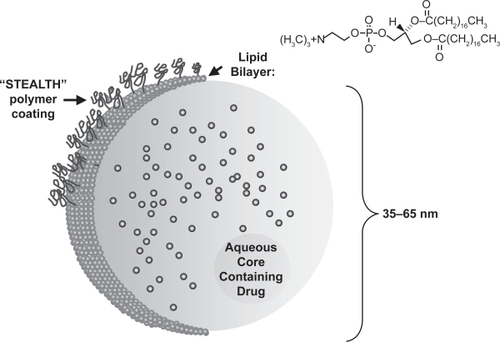
As scientists learn more about the biological properties of liposomes, it has become feasible to develop RES-avoiding drug formulations. Clearly, a reduction in RES uptake provides many pharmacokinetic and biological benefits to any chemotherapeutic. Toxicity, specificity, and plasma half-life are increased by RES avoidance. Several methodologies have been developed that limit RES exposure. Most simply, patients may be subjected to RES saturation with empty liposomes prior to therapeutic administration. This approach limits RES uptake but may have significant, long-term effects on RES function (CitationAbra et al 1980; CitationAllen 1988). Modeling the RES avoidance by erythrocytes, scientists have incorporated polyethylene glycol (PEG) in the lipid bilayer of some liposomes. This “stealth” technology greatly extends the drug half-life through a reduction in RES uptake (reviewed in CitationAllen and Martin 2004). Doxil, a PEG-coated form of liposomal doxorubicin (PLD), is the first FDA-approved KS therapy to employ STEALTH® technology (see ). This approach as well as other RES-avoiding technologies including DaunoXome® (liposomal daunorubicin; DNX) have been highly successful and represent a wave of newly formulated liposomal therapeutics.
Figure 3 Structure of a STEALTH® liposome. A soluble drug component such as daunorubicin is contained within a single lipid bilayer. Addition of polyethylene glycol polymers to the liposomal surface (STEALTH technology) leads to decreased RES uptake and increased drug stability (adapted from CitationAllen and Martin 2004).
Already new technology is being developed to further the targeting specificity of liposomal carriers. Cationic liposomes show high affinity for tumor neovasculature and may demonstrate increased efficacy for highly angiogenic tumor types (CitationThurston et al 1998). In addition, scientists are working to incorporate specific receptors, tumor ligands, and antibodies in future liposomal drug formulations. These modifications should lead to the development of tumor-specific therapies with limited side-effects and maximum efficacy.
Pharmacology and kinetics
DaunoXome®, a liposomal formulation of DNR (DNX), was first licensed in the United Kingdom in 1995 and later approved by the FDA. This liposomal formulation, manufactured by Gilead Pharmaceuticals Inc. (San Dimas, CA), is uniquely formulated to be RES-avoiding. Specifically, DNX is the first liposomal derivative of its kind to be made solely of lipids (CitationAllen and Martin 2004). Composed of three major components: distearoylphosphatidylcholine (DPC), cholesterol, and DNR, DNX liposomes are small (45 nm) and neutrally charged (Gilead Sciences 2000; CitationAllen and Martin 2004). Formulation of DNR into a citrate salt assists in drug encapsulation. Due to the small size and relative neutrality of DNX particles, RES uptake is minimized, leading to prolonged drug circulation (CitationForssen et al 1992). However, in murine model systems, RES absorption of DNX remains 60%–110% higher than that observed during conventional DNR therapy (Gilead Sciences 2000).
Evidence that the pharmacokinetics of DNX would differ greatly from those observed with conventional DNR first came from murine xenograft models. In preclinical trials, mice with lymphosarcoma (P-1798)-derived tumors or mammary adenocarcinoma (MA16C)-derived tumors were treated and monitored for DNX pharmacokinetics (CitationForssen et al 1992). Plasma DNR levels were consistently higher in DNX-treated mice compared with conventional DNR. Clearance of free DNR occurred rapidly (44.9 mL/h) compared with the liposomal formulation (0.195 mL/h). Although the rate of tumor drug accumulation was much higher in mice treated with conventional therapy (ka = 2.64/h) than those receiving DNX (ka = 0.265/h), area under the curve (AUC) values increased 10 times with the liposomal formulation. Differences in pulmonary and cardiac uptake were negligible, suggesting that toxicity to these organs would be limited. As a result, tumor progression as well as mortality significantly decreased in DNX-treated mice. The authors attribute the plasma stability of liposomal DNR to the incorporation of high phase transition temperature of DPC and the addition of cholesterol to the encapsulating lipid bilayer. This stability is required to maintain elevated DNX serum levels long enough to allow for effective tumor uptake. Once entrapped in the fenestrated capillaries of the tumor, DNR becomes bioavailable and functions to effectively limit tumor growth. These tumor-specific targeting properties made liposomal DNR attractive for future clinical studies.
Following the success of DNX in animal models, several clinical trials examined the pharmacokinetic properties of this novel drug formulation in patients. At the time, KS was emerging as a serious threat to the growing number of AIDS patients worldwide. Even though animal models of KS did not become available until recently (CitationAn et al 2006) and, therefore, DNX was never tested preclinically in a bona fide KS-model, there was a strong push to get FDA approval. For this reason, the properties of DNX in KS patients were examined by several groups. The first study, reported in 1995, examined the pharmacokinetics, response, and toxicity of increasing DNX dosages (CitationGill et al 1995). From these data, a dosage between 40 and 60 mg/m2 was shown to be most efficacious. As a result the recommended DNX dosage was set to 40 mg/m2 every 2 weeks, a standard which still holds true today (Gilead Sciences 2000). At these levels, reports of mean plasma AUC range from 114.91 to 120.1 μg h–1 mL–1 (CitationGill et al 1995; CitationFumagalli et al 2000). These data represent an 11- to 12-fold increase over conventional DNR and reflect the stability of the liposomal carrier as previously described in murine model systems (CitationForssen et al 1992; CitationGill et al 1995). Likewise, clearance is significantly reduced in DNX treated patients when compared with those treated with conventional drug (10.5 mL/min vs 233 mL/min, respectively) (CitationAlberts et al 1971; CitationGill et al 1995; CitationFumagalli et al 2000). These two properties combine to stabilize serum DNX, resulting in a half life between 4 and 5.6 h (t1/2 DNR ≈ 0.77 h) (CitationGill et al 1995; CitationFumagalli et al 2000). Peak plasma levels range from 14.8 to 22 μg/mL and far surpass those previously observed with conventional DNR treatment (CitationAlberts et al 1971; CitationGill et al 1995; CitationFumagalli et al 2000). These results suggest that the increased stability of liposomal DNR may provide a mechanism by which high cumulative doses may be administered to patients without serious side-effects. As a result, the efficacy and tolerability of DNX was anticipated to surpass all previous DNR formulations.
Efficacy in KS treatment
Evaluation of the efficacy of an individual KS treatment modality is largely dependent upon the implementation of standard of staging criteria. Unfortunately, many of the initial DNX studies were conducted before the implementation of the Aids Clinical Trials Groups (ACTG) response criteria. In 1989 Krown et al proposed this standard method of staging to assess KS response to clinical trial regimens (CitationKrown et al 1989). The scope of KS involvement is often difficult to assess and may be confounded by other underlying conditions, especially in the context of HIV-AIDS. Determining the extent of visceral disease can be difficult and in the past has been scored mainly upon the severity of patient symptoms. The ACTG criteria have defined a distinct and stringent set of characteristics to classify therapeutic response (see ). Specifically, the appearance of any new lesion or the progression of any KS-related symptom excludes patients from achieving either complete or partial response even if as a whole, the individual experiences a therapeutic benefit (CitationKrown et al 1989). As a result of these stricter criteria, studies conducted using ACTG guidelines show a significant decrease in patient response rates compared with studies conducted using non-standard evaluation criteria (CitationGill et al 1996). Even with the implementation of the ACTG guidelines, many factors may influence the reported success rate of individual trials, including patient CD4 count, treatment history, and concurrent drug administration (CitationPresant et al 1993; CitationGill et al 1995; CitationFumagalli et al 2000; CitationRosenthal et al 2002).
Table 1 Aids Clinical Trials Groups response criteria for AIDS-releated Kaposi’s sarcoma
The first DNX trials focused on the safety and potential therapeutic benefits of liposomal DNR in the treatment of AIDS-associated KS. Three separate phase I/II clinical trials monitored the response of over 115 HIV-positive KS patients (CitationPresant et al 1993; CitationGill et al 1995; CitationTulpule et al 1998). The severity of KS varied greatly among the study participants, but most patients were classified as having highly aggressive disease. Previous treatment with chemotherapy was not used as an exclusion parameter. Once drug safety was established at lower doses (CitationPresant et al 1993; CitationGill et al 1995), study participants were treated every 2 weeks with 40–60 mg/m2 i.v. DNX. Overall response rates (representing those with complete or partial tumor regression), as determined by differential standards, varied from 55% to 60% (CitationPresant et al 1993; CitationGill et al 1995; CitationTulpule et al 1998). Response of pulmonary KS alone was even higher, wherein 75% of study participants experienced complete or partial response according to ACTG standards (CitationTulpule et al 1998). These results, which showed disease regression with few side-effects, provided a rationale to pursue DNX as a novel KS therapy.
The ABV regimen is a classic first-line defense against aggressive or disseminated KS. In clinical trials, the response rates to such combination drug therapy are reported to range from 33% to 88% (CitationKrown et al 2004). To compare the efficacy of DNX with conventional ABV, Gill and colleagues performed a prospective study of 227 HIV-positive KS patients (CitationGill et al 1996). Study participants were randomized to two treatment arms, 40 mg/m2 DNX or ABV (10 mg/m2 doxorubicin, 15U bleomycin, 1 mg vincristine) every 2 weeks. Only patients with advanced KS and no prior systemic chemotherapy were admitted into the trial. All localized treatments had to be discontinued at least 14 days before study enrollment. Surprisingly, ACTG response rates between the two arms of the trial were comparable (25% and 27.9% for DNX and ABV, respectively) with most patients achieving stable disease (58%–62%). No significant difference was observed in time to progression or survival. Complete remission was documented in both DNX- and ABV-treated study participants. Together, these data show that DNX efficacy is not distinguishable from that of conventional, multi-drug ABV therapy.
AIDS-associated KS represents a unique challenge wherein the decision to implement HAART in addition to chemotherapeutic regimens needs to be considered. In fact, in AIDS-associated lymphoma HAART can be delayed until all chemotherapy cycles have been concluded. Even though concomitant administration of HAART and combination-chemotherapy is considered safe and effective (CitationRatner et al 2001), the use of AZT with chemotherapy was previously contraindicated (CitationLim and Levine 2005). Importantly, withholding HAART during chemotherapy is safe, allows for the administration of higher dose therapy, and does not modulate AIDS progression (CitationLittle et al 2003). Whether these lymphoma-specific recommendations also applied to DNX administration in KS was previously unknown.
Protease inhibitors are known to block the activity of cytochrome p450IIIA4 (CYP3A4), which metabolizes anthracycline drugs (Citationvon Moltke et al 1998). Due to this property, the efficacy of anthracyclin therapy in combination with HAART was formerly questioned. The emergence of HAART-resistant HIV strains has since led to the desire to maintain anti-retroviral treatment during the course of anthracycline administration. To determine the ramifications of proteasome inhibitor co-administration, Fumagalli and colleagues examined DNX pharmacokinetics in the presence or absence of HAART (CitationFumagalli et al 2000). Eighteen individuals with rapidly progressing visceral, pulmonary, gastrointestinal, or mucocutaneous KS were enrolled in the study. Of the participants, 39% had undergone prior chemotherapy. Although the pharmacokinetic properties of DNX (delivered at 40 mg/m2) were unaltered by concomitant administration of any nucleoside reverse transcriptase inhibitor (NRTI)-protease inhibitor cocktail, prior anthracycline therapy significantly increased both peak DNX concentration and plasma AUC. These data suggest that prior chemotherapeutic treatments may influence DNX efficacy. Regardless, most study participants (82.3%) achieved either complete or partial response. Together, these findings suggest that HAART does not influence DNX efficacy; however, the effect of anthracyline therapy on the activity of protease inhibitors remains to be addressed.
Although these trials demonstrate that DNX is efficacious in the treatment of AIDS-associated KS, several patient attributes clearly effect therapeutic outcome. Recent reports suggest that prior administration of anthracyline therapy modulates both drug pharmacokinetics and KS response (CitationPresant et al 1993; CitationGill et al 1995; CitationFumagalli et al 2000). Gill and colleagues examined this observation in detail, finding that prior chemotherapy as a whole does not influence patient response (CitationGill et al 1995). However, in study participants previously treated with anthracycline therapies, a significant difference in therapeutic efficacy was observed (p = 0.004) (CitationGill et al 1995). Whether these observations can be attributed to therapeutic resistance or differences in DNX kinetics is unclear; however, it is clear that patient treatment history may influence the choice to begin liposomal DNR treatment. CD4 count also appears to affect DNX efficacy. Patients with a CD4 count below 100 have a significantly lower probability of responding to DNX therapy (p = 0.004), suggesting that control of HIV is essential to successful KS treatment (CitationGill et al 1995). Clearly, additional studies are required to assess the influence of these predisposing factors on DNX efficacy.
Tolerability and toxicity
Production of liposomal anthracycline compounds was pioneered with the goal of producing therapeutics with higher specificity and potency while limiting patient side-effects. A major concern of any anthracycline treatment modality is cardiotoxicity. Both congestive heart failure and cardiomyopathy have been reported to occur in a dose-dependent manner upon DNR treatment (CitationLefrak et al 1973; CitationVon Hoff et al 1979). To prevent the significant complications associated with anthracycline cardiotoxicity, patient left ventricular ejection fraction (LVEF) is closely monitored. In general, individuals demonstrating an LVEF of less than 45% were excluded from all initial DNX studies (CitationGill et al 1996; CitationTulpule et al 1998). Direct comparison of cardiac function in patients treated with DNX vs ABV revealed no significant difference in LVEF during treatment (CitationGill et al 1996). Only one report to date has documented any LVEF decline following DNX administration. Moreover, upon further review of the effected individual’s case history, prior anterior myocardial infarct was noted (CitationGill et al 1996). Thus, although cumulative doses in many trials reached levels of over 600 mg/m2 (CitationGill et al 1995, Citation1996; CitationTulpule et al 1998; CitationRosenthal et al 2002; CitationYoung et al 2004), DNX fails to induce significant cardiotoxicity.
The most prominent DNX side-effect reported in all clinical trials was severe leukopenia, which was observed in 11%–17% of treatment cycles (CitationGill et al 1995; CitationFumagalli et al 2000). Most notably, in one trial, 85% of patients receiving a dose of 60 mg/m2 DNX every 2 weeks experienced grade 3/4 neutropenia (CitationTulpule et al 1998). Moreover, the increased incidence of leukopenia in DNX- vs ABV-treated individuals was statistically significant (p = 0.021) (CitationGill 1996). To combat this adverse effect, hemopoietic growth factors (eg, granulocyte colony-stimulating factor, G-CSF) were administered following 15%–29% of DNX treatment cycles and appeared to be effective in limiting further leukopenia (Fugamalli 2000; CitationRosenthal 2002). Other severe DNX-specific side-effects were not observed.
DNX patients develop alopecia and neuropathy less frequently than those treated with other cytostatic regimens (p < 0.0001) (CitationGill et al 1996). No other significant differences were found between patients treated with liposomal DNR vs conventional chemotherapy (CitationGill et al 1996). Specifically, nausea, fever, and fatigue were similar among treatment regimens (CitationGill et al 1996). In several reports, fever, flushing, and back pain were reported within the first 5 minutes of DNX administration. However, patients were typically able to resume therapy at a slower infusion rate without significant distress (CitationGill et al 1996). summarizes the complications observed in DNX trails for KS. Overall, DNX treatment appears to limit some of the adverse side-effects of conventional chemotherapy (CitationPresant et al 1993; CitationGill et al 1995, Citation1996; CitationFumagalli et al 2000; CitationRosenthal et al 2002).
Table 2 Complications observed in clinical trials of liposomal daunorubicin
Several clinical trials have monitored patient quality of life (QOL) during DNX treatment. During one study, both physical and emotional performance increased over 70% during DNX treatment (CitationPresant et al 1993). A second study comparing DNX with ABV showed that patients within the DNX study arm maintained QOL whereas QOL gradually declined with ABV treatment (CitationGill et al 1996). Although the significance of these data is unclear, the trend suggests that DNX treatment may be more tolerable than conventional chemotherapy, especially during long-term treatment regimens.
Pharmacoeconomics
When considering any new medical intervention, the costs of therapy must be weighed against the potential benefits. For aggressive KS, several treatment options are now available, including conventional chemotherapies (eg, ABV, BV, CHOP), interferon alpha, vinca alkaloids, taxanes, and liposomal anthracyclines (reviewed in CitationKrown et al 2004). In general, liposomal drug formulations are more costly than conventional chemotherapy and are at present unaffordable in the areas of greatest need such as in Africa. However, the potential for these drugs to significantly reduce the adverse effects of anthracyclines make them attractive. To date, no direct comparison of PLD (Doxil®) with DNX has been conducted for KS, yet an indirect comparison can be made assuming that the patient cohorts and response criteria are essentially the equal. In 2008, Bennett and colleagues used data from the DNX vs ABV (CitationGill et al 1996) and PLD vs BV (CitationStewart et al 1998) trials to estimate the cost-effectiveness of each liposomal therapy. While DNX is clearly more affordable upfront (US$538/cycle vs US$1212/cycle with PLD) (CitationBennett et al 1998), the number of cycles required for response as well as the costs associated with G-SCF administration must be included in order to properly compare the two therapies. During DNX treatment a higher number of patients required granulocyte-colony stimulating factor (G-SCF) therapy, in addition an average of 8.6 cycles of DNX vs 4.8 cycles of PLD were necessary for patient response (CitationGill et al 1996; CitationBennett et al 1998; CitationStewart et al 1998). Taking these factors into account, the cost of DNX treatment was still ~US$445 less than PLD (CitationBennett and Calhoun 2004).
In both studies, patient outcome was monitored by ATCG standards at the conclusion of the trial. Response rates for PLD vs DNX were 59% and 25%, respectively (CitationGill et al 1996; CitationStewart et al 1998). Due to this disparity among treatment regimens, the cost-effectiveness ratio (the cost associated with achieving one response) was twice as high for DNX (US$26,483) as for PLD (US$11,976) (CitationBennett et al 1998). In a study of Swedish patients, similar results were obtained, demonstrating that PLD is more cost effective than DNX for the treatment of KS (CitationHjortsberg et al 1999).
Comments and conclusions
Treatment of aggressive KS has long relied upon the activity of combination chemotherapy. Although largely successful, these treatments carry many serious side-effects. The arrival of the liposomal anthracycline formulations provides patients new therapeutic options to combat KS. Herein, we discussed the pharmacokinetics, efficacy, and tolerability of DNX in AIDS-associated KS patients. Although response rates varied between trials, DNX proved superior to conventional DNR treatment and comparable to ABV (CitationGill et al 1996). The liposomal formulation stabilizes DNR, preventing rapid clearance and allowing more time for the drug to accumulate in KS lesions (CitationForssen et al 1992; CitationGill et al 1995). Notably, as observed with other liposomal anthracycline formulations, cardiotoxicity is limited even in the presence of high cumulative DNR doses (≥500 mg/m2) (CitationGill et al 1995; CitationGill et al 1996; CitationTulpule et al 1998). Although DNX costs less per cycle than other liposomal anthracyclines on the market, indirect comparison suggests that PLD is actually more cost-effective due to an increased response rate (CitationBennett and Calhoun 2004). As both prior chemotherapeutic treatment as well as CD4 count have been reported to influence patient response to DNX (CitationGill et al 1995), it may be important to determine patient response in the context of naïve, CD4 high (>100/μL) individuals. In this setting, DNX may show significant promise.
Currently Doxil (PLD) represents the major competitor to DNX therapy. In general, patient response to Doxil requires fewer treatment cycles and thus is more cost effective (CitationGill et al 1996; CitationStewart et al 1998; CitationBennett and Calhoun 2004). It is clear that the pegylated, STEALTH®, formulation of Doxil further limits RES uptake, resulting in longer periods of drug circulation in vivo (CitationAllen and Martin 2004). Whether similar formulations of DNR would prove more efficacious in the treatment of KS is unclear since anthracyclines display virtually identical mechanistic properties. Importantly, each new advance in liposomal technology has increased drug efficacy, specificity and tolerability, leading to better therapeutic options for patients with KS.
Strikingly, the efficacy of DNX has been predominantly evaluated in the context of HIV-positive males. Although there is an urgency to develop more effective drugs for AIDS-associated KS, little is know about the effects of DNX on HIV-negative patients. Previous reports have observed no difference in the response rates of endemic vs epidemic KS to therapy (CitationStein et al 1994; CitationKigula-Mugambe and Kavuma 2005). However, patient HIV status is sure to influence DNX efficacy as CD4 count significantly influences therapeutic outcome (CitationGill et al 1995). Clinical trials have thus far failed to address DNX efficacy in females. In fact, less than 1% of DaunoXome study participants were female (CitationPresant et al 1993; CitationGill et al 1995, Citation1996; CitationTulpule et al 1998; CitationFumagalli et al 2000). These data reflect the predominantly male HIV population at the outset of the AIDS epidemic in the US. To date, however, females account for almost half of the HIV-positive population world-wide (CitationQuinn and Overbaugh 2005; CitationUNAIDS 2006). Over 25% of patients diagnosed with HIV in 2004 were women and this number continues to grow (CitationCDC 2006). In areas where KS is endemic and spread by non-sexual as well as sexual routes, KS is found in women and female children almost as frequently as in men. As women often display differential drug metabolics, it is essential that additional studies be conducted in female KS patients in order to clearly understand and assess DNX efficacy.
Finally, use of universal response criteria, such as those provided by the ATCG or recently proposed (CIT-NEJM), should greatly improve our ability to assess therapeutic efficacy. Proper assessment of visceral disease may require the advent of new technologies to improve KS detection and characterization. Additionally, future development of DaunoXome to decrease RES-uptake may be needed in order to compete with the pegylated anthracycline formulations currently on the market. Still, DNX clearly represents a significant advance in KS chemotherapy, providing multiple benefits over conventional, ABV-like therapies and potentially improving patient QOL.
References
- AbraRMBosworthMEHuntCA1980Liposome disposition in vivo: effects of pre-dosing with lipsomesRes Commun Chem Pathol Pharmacol293493607414053
- AlbertsDSBachurNRHoltzmanJL1971The pharmacokinetics of daunomycin in manClin Pharmacol Ther12961045541137
- AllenTM1988Toxicity of drug carriers to the mononuclear phagocyte systemAdv Drug Deliv Rev25567
- AllenTMMartinFJ2004Advantages of liposomal delivery systems for anthracyclinesSemin Oncol3151515717735
- AnFQFolarinHMCompitelloN2006Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivoJ Virol8048334616641275
- BanghamADStandishMMWatkinsJC1965Diffusion of univalent ions across the lamellae of swollen phospholipidsJ Mol Biol13238525859039
- BehalV2000Bioactive products from StreptomycesAdv Appl Microbiol471135612876796
- BellocFLacombeFDumainP1992Intercalation of anthracyclines into living cell DNA analyzed by flow cytometryCytometry1388051459004
- BennettCLCalhounEA2004Pharmacoeconomics of liposomal anthracycline therapySemin Oncol31191515717744
- BennettCLGolubRMStinsonTJ1998Cost-effectiveness analysis comparing liposomal anthracyclines in the treatment of AIDS-related Kaposi’s sarcomaJ Acquir Immune Defic Syndr Hum Retrovirol1846059715842
- BoshoffCWeissRA2006Kaposi Sarcoma Herpesvirus: New PerspectivesNew YorkSpringer
- CasperC2006Defining a role for antiviral drugs in the treatment of persons with HHV-8 infectionHerpes1342716895654
- CasperCKrownSE2006Presented at: 10th International Conference on Malignancies in AIDS and Other Aquired ImmunodeficienciesBethesda, MA, USA
- CattelanAMCalabroMLGasperiniP2001Acquired immunodeficiency syndrome-related Kaposi’s sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcomeJ Natl Cancer Inst Monogr28444911158206
- Centers for Disease Control and Prevention (CDC)2006HIV/AIDS Surveillence Findings [online] Accessed 16 October 2006. URL: http://www.cdc.gov/hiv/topics/women/surveillance.htm
- CesarmanEChangYMoorePS1995Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomasN Engl J Med3321186917700311
- ChairesJB1990Biophysical chemistry of the daunomycin-DNA interactionBiophys Chem351912022204442
- ChairesJBDattaguptaNCrothersDM1982Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acidBiochemistry213933407126524
- ChangYCesarmanEPessinMS1994Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcomaScience266186597997879
- ChonnASempleSCCullisPR1992Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimesJ Biol Chem26718759651527006
- CrookeSTDuvernayVHGalvanL1978Structure-activity relationships of anthracyclines relative to effects on macromolecular synthesesMol Pharmacol142908642929
- CullisPRChonnASempleSC1998Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivoAdv Drug Deliv Rev3231710837632
- DimarcoAGaetaniMDorigottiL1964Daunomycin: a new antibiotic with antitumor activityCancer Chemother Rep3831814167466
- DoroshowJHDaviesKJ1986Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radicalJ Biol Chem2613068743005279
- DupinNRubin De CervensVGorinI1999The influence of highly active antiretroviral therapy on AIDS-associated Kaposi’s sarcomaBr J Dermatol1408758110354025
- DupontCVasseurEBeauchetA2000Long-term efficacy on Kaposi’s sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92Aids149879310853980
- DvorakHFNagyJADvorakJT1988Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromoleculesAm J Pathol133951092459969
- ElliottP2006Pathogenesis of cardiotoxicity induced by anthracyclinesSemin Oncol33S2716781283
- EltomMAJemalAMbulaiteyeSM2002Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998J Natl Cancer Inst9412041012189223
- EmbreeLGelmonKTolcherA1998Pharmacokinetic behavior of vincristine sulfate following administration of vincristine sulfate liposome injectionCancer Chemother Pharmacol41347529523729
- EngelsEAPfeifferRMGoedertJJ2006Trends in cancer risk among people with AIDS in the United States 1980–2002Aids2016455416868446
- EvansSRKrownSETestaMA2002Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi’s sarcoma: an AIDS Clinical Trials Group clinical studyJ Clin Oncol2032364112149296
- ForssenEACoulterDMProffittRT1992Selective in vivo localization of daunorubicin small unilamellar vesicles in solid tumorsCancer Res523255311596882
- Froelich-AmmonSJOsheroffN1995Topoisomerase poisons: harnessing the dark side of enzyme mechanismJ Biol Chem27021429327665550
- FumagalliLZucchettiMParisiI2000The pharmacokinetics of liposomal encapsulated daunorubicin are not modified by HAART in patients with HIV-associated Kaposi’s sarcomaCancer Chemother Pharmacol4549550110854138
- GelmonKATolcherADiabAR1999Phase I study of liposomal vincristineJ Clin Oncol1769770510080616
- Gilead Sciences, Ltd2000Summary of product characteristics: DaunoXome [online] Accessed 18 October 2006. URL: http://www.gilead.com/pdf/uk/dx_spc.pdf.
- GillPSEspinaBMMuggiaF1995Phase I/II clinical and pharmacokinetic evaluation of liposomal daunorubicinJ Clin Oncol1399610037707129
- GillPSTulpuleAEspinaBM1999Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcomaJ Clin Oncol1718768310561228
- GillPSWernzJScaddenDT1996Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcomaJ Clin Oncol142353648708728
- HandaKSatoS1975Generation of free radicals of quinone group-containing anti-cancer chemicals in NADPH-microsome system as evidenced by initiation of sulfite oxidationGann66437239881
- HjortsbergCPerssonULidbrinkE1999Cost-effectiveness analysis of pegylated-liposomal doxorubicin and liposomal daunorubicin treatments in patients with Kaposi’s sarcomaActa Oncol381063710665764
- HobbsSKMonskyWLYuanF1998Regulation of transport pathways in tumor vessels: role of tumor type and microenvironmentProc Natl Acad Sci USA954607129539785
- HofheinzRDGnad-VogtSUBeyerU2005Liposomal encapsulated anti-cancer drugsAnticancer Drugs1669170716027517
- HuangSKLeeKDHongK1992Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing miceCancer Res525135431394121
- HwangK1987Liposome pharmacokinetics in liposomes: from biophysics to therapeuticsNew YorkMarcel Dekker, Inc
- JainRK1987Transport of molecules across tumor vasculatureCancer Metastasis Rev65595933327633
- Kigula-MugambeJBKavumaA2005Epidemic and endemic Kaposi’s sarcoma: a comparison of outcomes and survival after radiotherapyRadiother Oncol76596216019094
- KoonHBBubleyGJPantanowitzL2005Imatinib-induced regression of AIDS-related Kaposi’s sarcomaJ Clin Oncol23982915572730
- KrownSEMetrokaCWernzJC1989Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology CommitteeJ Clin Oncol7120172671281
- KrownSENorthfeltDWOsobaD2004Use of liposomal anthracyclines in Kaposi’s sarcomaSemin Oncol31365215717737
- KrownSERealFXCunningham-RundlesS1983Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi’s sarcomaN Engl J Med308107166835320
- LaneHCKovacsJAFeinbergJ1988Anti-retroviral effects of interferon-alpha in AIDS-associated Kaposi‘s sarcomaLancet21218222903954
- LebbeCBlumLPelletC1998Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi’s sarcomaAids12F45499619797
- LefrakEAPithaJRosenheimS1973A clinicopathologic analysis of adriamycin cardiotoxicityCancer32302144353012
- LiXHirshDJCabral-LillyD1998Doxorubicin physical state in solution and inside liposomes loaded via a pH gradientBiochim Biophys Acta141523409858673
- LimSTLevineAM2005Recent advances in acquired immunodeficiency syndrome (AIDS)-related lymphomaCA Cancer J Clin5522941260126416020424
- LittleRFPittalugaSGrantN2003Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biologyBlood1014653912609827
- MayerLDBallyMBCullisPR1986Uptake of adriamycin into large unilamellar vesicles in response to a pH gradientBiochim Biophys Acta85712363964703
- MoniniPSgadariCToschiE2004Antitumour effects of antiretroviral therapyNat Rev Cancer48617515516959
- MurdacaGCampelliASettiM2002Complete remission of AIDS/Kaposi’s sarcoma after treatment with a combination of two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitorAids16304511807324
- NabievIRMorjaniHManfaitM1991Selective analysis of antitumor drug interaction with living cancer cells as probed by surface-enhanced Raman spectroscopyEur Biophys J19311161915156
- NetageriGVJenkinsSAParsonsDL1993Liposome drug delivery systemsLancaster, PATechnomic Publishing Company, Inc
- PaparizosVAKyriakisKPPapastamopoulosV2002Response of AIDS-associated Kaposi sarcoma to highly active antiretroviral therapy aloneJ Acquir Immune Defic Syndr30257812045689
- PatelHMRussellNJ1988Liposomes: from membrane model to therapeutic applicationsBiochem Soc Trans16909103224753
- PresantCAScolaroMKennedyP1993Liposomal daunorubicin treatment of HIV-associated Kaposi’s sarcomaLancet341124238098393
- QuinnTCOverbaughJ2005HIV/AIDS in women: an expanding epidemicScience3081582315947174
- RatnerLLeeJTangS2001Chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma in combination with highly active antiretroviral therapyJ Clin Oncol192171811304769
- RealFXOettgenHFKrownSE1986Kaposi’s sarcoma and the acquired immunodeficiency syndrome: treatment with high and low doses of recombinant leukocyte A interferonJ Clin Oncol4544513958767
- RobertJ1998AnthracyclinesGrochowLBAmesMMA clinician’s guide to chemotherapy pharmacokinetics and pharmacodynamicsBaltimore, MDWilliam and Wilkins93174
- RoerdinkFHDaemenTBaker-WoudenbergIAJM1987JohnsonPLlyod-JonesJGTherapeutic utility of liposomes in drug delivery systems: fundamentals and techniquesChichester, EnglandEllis Horwood, Ltd6680
- RosenthalEPoizot-MartinISaint-MarcT2002Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcomaAm J Clin Oncol2557911823698
- SessaGWeissmannG1968Phospholipid spherules (liposomes) as a model for biological membranesJ Lipid Res9310185646182
- SinSHRoyDWangL2007Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signalingBlood10921657317082322
- SinhaBK1989Free radicals in anticancer drug pharmacologyChem Biol Interact692933172659197
- SinhaBKPolitiPM1990AnthracyclinesCancer Chemother Biol Response Modif1145572223402
- SoulierJGrolletLOksenhendlerE1995Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s diseaseBlood861276807632932
- StalloneGSchenaAInfanteB2005Sirolimus for Kaposi’s sarcoma in renal-transplant recipientsN Engl J Med35213172315800227
- SteinMELakierRSpencerD1994Radiation therapy for non-AIDS associated (classic and endemic African) and epidemic Kaposi’s sarcomaInt J Radiat Oncol Biol Phys286136198113104
- StewartSJablonowskiHGoebelFD1998Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study GroupJ Clin Oncol16683919469358
- TapperoJWConantMAWolfeSF1993Kaposi’s sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapyJ Am Acad Dermatol28371958445054
- TeweyKMChenGLNelsonEM1984Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase IIJ Biol Chem259918276086625
- ThurstonGMcLeanJWRizenM1998Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in miceJ Clin Invest1011401139525983
- TreatJDamjanovNHuangC2001Liposomal-encapsulated chemotherapy: preliminary results of a phase I study of a novel liposomal paclitaxelOncology (Williston Park)1544811396365
- TulpuleAYungRCWernzJ1998Phase II trial of liposomal daunorubicin in the treatment of AIDS-related pulmonary Kaposi’s sarcomaJ Clin Oncol163369749779714
- UNAIDS2006 2006 report on the global AIDS epidemic: a UNAIDS 10th anniversary special edition [online]. Accessed 16 October 2006. URL: http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp
- Von HoffDDLayardMWBasaP1979Risk factors for doxorubicin-induced congestive heart failureAnn Intern Med9171017496103
- von MoltkeLLGreenblattDJGrassiJM1998Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavirJ Clin Pharmacol38106119549640
- WellesLSavilleMWLietzauJ1998Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi’s sarcomaJ Clin Oncol161112219508198
- WilkinsonJCopeAGillJ2002Identification of Kaposi’s sarcoma-associated herpesvirus (KSHV)-specific cytotoxic T-lymphocyte epitopes and evaluation of reconstitution of KSHV-specific responses in human immunodeficiency virus type 1-Infected patients receiving highly active antiretroviral therapyJ Virol7626344011861829
- YoungAMDhillonTBowerM2004Cardiotoxicity after liposomal anthracyclinesLancet Oncol565415522651