Abstract
Nanotechnology has tremendously influenced gene therapy research in recent years. Nanometer-size systems have been extensively investigated for delivering genes at both local and systemic levels. These systems offer several advantages in terms of tissue penetrability, cellular uptake, systemic circulation, and cell targeting as compared to larger systems. They can protect the polynucleotide from a variety of degradative and destabilizing factors and enhance delivery efficiency to the cells. A variety of polymeric and non-polymeric nanoparticles have been investigated in an effort to maximize the delivery efficiency while minimizing the toxic effects. This article provides a review on the most commonly used nanoparticulate systems for gene delivery. We have discussed frequently used polymers, such as, polyethyleneimine, poly (lactide-co-glycolide), chitosan, as well as non-polymeric materials such as cationic lipids and metallic nanoparticles. The advantages and limitations of each system have been elaborated.
Introduction
The field of gene-based medicine has witnessed great progress since the first somatic gene therapy performed in 1990. Several thousand patients have been involved in clinical trials all over the world with majority focusing on cancer (67%), followed by vascular diseases (8.9%) and monogenic diseases (8.6%) (http://www.wiley.co.uk/genetherapy/clinical). Gene therapy works on the basic concept that the delivery of polynucleotides to the cells will alter the expression of a given protein resulting in therapeutic benefit. Gene therapy involves delivering polynucleotides such as DNA, RNA, anti-sense oligonucleotides and small interfering RNA, either locally or systemically. Although Vitravene, an antisense oligonucleotide-based product, is the only gene delivery product approved so far by US-FDA, there are several other products in late stages of clinical trials. Gendicine, an adenovirus encoding tumor suppressor p53 gene, developed by SiBiono GeneTech Co., Ltd., was recently approved by China’s state food and drug administration, for the treatment of head and neck squamous cell carcinoma.
Delivering functional polynucleotides into cells is the first and most critical step towards efficient gene therapy. The administration of naked DNA resulted in local transient expression in skeletal muscle tissue (CitationWolff et al 1990). Efficient transfection levels have also been obtained on direct application of naked DNA to the liver (CitationHickman et al 1994). To obtain systemic effect with the injection of naked DNA is difficult, however, as the intravenous injection of naked DNA results in low levels of gene expression in all major organs.
In order to enhance uptake of genes into cells, they have to be delivered using a carriers or vectors. The vectors can be broadly classified as viral and non-viral. Viral vectors account for nearly 75% of all clinical trials conducted so far (http://www.wiley.co.uk/genetherapy/clinical). They are essentially viruses that have been stripped of their gene for replication while preserving their ability to transfect cells. The gene of interest is then incorporated into the viral genome. Most commonly used viral vectors are retrovirus, adenovirus, adeno-associated virus and herpes simplex virus (CitationRobbins and Ghivizzani 1998; CitationWalther and Stein 2000)). Virus based approaches are highly efficient as viruses have a highly evolved and specific mechanism for inserting their genome into that of host cell. Despite the high efficiency of viral vectors, their use has been limited by their pathogenicity, immunogenicity and potential for insertional mutagenesis.
Incidences of severe adverse reactions using viral vectors during clinical trials have caused a gradual shift towards non-viral vectors. Non-viral vectors mostly include use of polymers and lipids to deliver genetic material intracellularly protecting it from extracellular and intracellular degradative enzymes and blood components (CitationDe Laporte et al 2006). Non-viral vectors are mostly non-immunogenic, less expensive to produce, relatively safer, and can carry higher amounts of genetic material as compared to viruses. However, transfection efficiency using non-viral vectors remains lower as compared to viral vectors.
Challenges associated with non-viral gene delivery
Non-viral vectors face a multitude of barriers at systemic, tissue and cellular levels that prevent efficient gene delivery to the nucleus of cells. A significant portion of DNA is lost at each step, resulting in a several fold decrease in expression of the encoded protein. Most non-viral delivery vectors display colloidal instability which results in aggregation of the complexes thereby hindering cellular internalization. Systemic delivery of charged vector may result in interaction with blood components which may lead to opsonisation by the reticulo-endothelial system (CitationLiu and Huang 2002). Problems such as steric instability and rapid plasma clearance have been partly overcome by shielding the surface charge of the vectors (CitationKommareddy et al 2005). Properties of vector such as size, shape, and surface characteristics can also have a major impact on its pharmacokinetic properties and delivery efficiency.
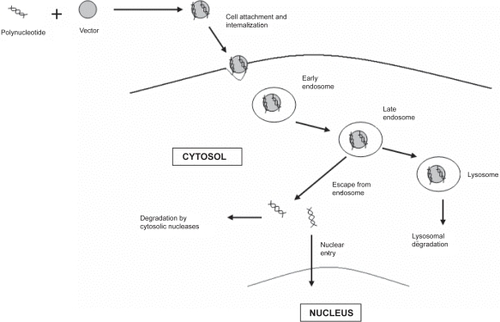
Non-viral vectors, also encounter several extracellular and intracellular barriers before reaching its final destination, the nucleus (CitationRuponen et al 2003; CitationWiethoff and Middaugh 2003). Typical pathway of a nanoparticulate vector inside the cell is depicted in . The presence of nucleases in the extracellular compartment may lead to extensive degradation of the DNA. Cellular internalization is another major barrier as DNA is a polyanionic molecule with a large hydrodynamic volume which makes its entry through the negatively charged plasma membrane extremely difficult. Following uptake into the cell, the vector may become trapped within the endosomal compartment, from which it must escape. Failure to do so may result in degradation of DNA by lysosomal degradative enzymes. Surviving passage through the cytoplasm is a crucial step, because of the presence of cytosolic endonucleases, which may further lead to DNA fragmentation. Lastly, the DNA has to traverse the nuclear envelope to get transcribed. In order to enhance gene therapy, specialized design features are required for delivery vector to overcome each of these barriers, and ensure efficient DNA delivery to the nucleus.
Nanoparticulate vectors for polynucleotide delivery
Nanotechnology has been at the forefront of drug and gene delivery in the last few years. Several types of nanometer scale devices such as nanoparticles, nanospheres, nanotubes, nanogels and molecular conjugates are being investigated (CitationLemieux et al 2000; CitationBianco 2004; CitationRavi Kumar et al 2004; CitationHeidel et al 2005; CitationMurakami and Nakashima 2006). Sub-micron size of delivery systems confers distinct advantages as compared to large sized systems such as higher and deeper tissue penetrability, greater cellular uptake, greater ability of cross blood-brain barrier, and greater ability to target specific cell types (CitationKreuter et al 1995; CitationDavis 1997; CitationVinogradov et al 2002; CitationVogt et al 2006). Although this review, for most part, deals with polymeric nanoparticles, commonly used non-polymeric nanovectors have also been discussed briefly. A summary of all the nanoparticulate vectors discussed in the review is presented in .
Table 1 Polymeric and non-polymeric nanoparticulate vectors for gene delivery
Polymeric nanoparticles
Polymeric nanoparticles or nanospheres are the most commonly used type of nano-scale delivery systems. They are mostly spherical particles, in the size range of 1–1000 nm, carrying the genetic material of interest. The mechanism of incorporation of polynucleotides into polymeric nanoparticles depends on the nature of the polymer. Most cationic polymers have the ability to condense plasmid DNA into nanometer size complexes (polyplexes). DNA can also be entrapped into the polymeric matrix or can be adsorbed or conjugated on the surface of nanoparticles. A variety of natural and synthetic polymers have been used for gene delivery. In this section, we have discussed the most extensively used polymers for nanoparticle-based gene delivery.
Poly lactide-co-glycolide (PLGA) and Poly lactic acid (PLA)
Biodegradable polyesters, PLGA and PLA are amongst the most commonly used polymers for delivering drugs and biomolecules. They consist of units of lactic acid and glycolic acid connected through ester linkage. These biodegradable polymers undergo bulk hydrolysis thereby providing sustained delivery of the therapeutic agent. The rate of degradation of polymer, and the release rate of drug, can be controlled by changing the polymer molecular weight and copolymer composition. The degradation products, lactic acid and glycolic acid, are removed from the body through citric acid cycle (CitationShive and Anderson 1997). The release of therapeutic agent from these polymers occurs by diffusion and polymer degradation.
PLGA and PLA nanoparticles are mostly matrix-type systems prepared by emulsification followed by evaporation of the organic phase (CitationRosca et al 2004). Several studies have shown higher uptake of nanoparticles into cells as compared to larger size particles (CitationDesai et al 1996; CitationDesai et al 1997; CitationDelie et al 2001; CitationPanyam et al 2002). Nanoparticles have the ability to escape endo-lysosomal compartments and release the therapeutic molecule inside the cell at a sustained rate (CitationPanyam et al 2002; CitationKim and Martin 2006). The release pattern can be modulated according to the dosing requirement by changing various formulation parameters. The poly-nucleotide can be incorporated in PLGA/PLA nanoparticles by either entrapment into the polymeric matrix (CitationRibeiro et al 2005) or by surface-adsorption by using a cationic polymer or surfactant (CitationKim et al 2005). PLGA and PLA nanoparticles have been used for the delivering plasmid DNA (CitationCohen et al 2000), siRNA (CitationYuan et al 2006), and aptamers (CitationCheng et al 2007).
Cationic polymers
Cationic polymers by virtue of their positive charge can efficiently condense the anionic polynucleotides into nano-meter range complexes (polyplexes) thereby masking their negative charge (CitationDe Smedt et al 2000; CitationZhang et al 2004). Polynucleotides are polyanionic molecules with a large hydrodynamic diameter which presents a significant barrier towards efficient cellular uptake. Cationic polymers, apart from condensing it to a several fold smaller size, also provide a net positive charge to the complex which helps in attachment on cellular membrane. Also, most cationic polymers bear amine groups that are protonable at acidic pH. Thus, once inside the endosome, these polymers accept proton thereby resisting a drop in pH. This causes influx of counterions (chloride ions) resulting in osmotic swelling and subsequent rupture of endosome. This phenomenon, first explained by Behr, is known as proton sponge effect (CitationBehr 1997). The efficiency of polyplexes has been found to be dependent on the ratio of nitrogen atoms of the polymer to the phosphate groups present in the polynucleotide (N/P ratio) (CitationGebhart and Kabanov 2001). A variety of natural and synthetic cationic polymers have been used for gene delivery. Some of the most commonly used cationic polymers have been discussed below.
Polyethylenimine (PEI)
Polyethyleneimine is the most commonly used cationic polymer and is widely regarded as a gold standard, amongst non-viral vectors, in order to compare transfection efficiencies. Transfection efficiency of PEI has been found to be dependent on a multitude of factors such as molecular weight, degree of branching, N/P ratio, complex size etc (CitationThomas et al 2005). PEI has a high density of protonable amino groups, every third atom being amino nitrogen, which imparts it a high buffering ability at practically any pH. Hence, once inside the endolysosomal compartment, PEI can efficiently destabilize the endosome releasing the polynucleotide in the cytoplasm. PEI can also be used in combination with PLGA/PLA for preparing matrix-type nanoparticles to deliver DNA by adsorption/complexation on the surface (CitationKim et al 2005). Toxicity of PEI, however, has been a concern. Acute toxicity has been observed in several studies both in vitro and in vivo (CitationChollet et al 2002; CitationMoghimi et al 2005). Investigators have studied conjugation of polyethylene glycol (PEG) to PEI to form diblock or triblock copolymers to reduce PEI-associated toxicity (CitationPark et al 2005; CitationZhong et al 2005; CitationChoi et al 2006). PEG also shields the positive charge of the polyplexes, thereby providing steric stability to the complex. Such stabilization prevents non-specific interaction with blood components during systemic delivery (CitationKursa et al 2003).
Polymethacrylates
Polymethacrylates are cationic vinyl-based polymers that possess the ability to condense polynucleotides into nano-meter size particles. Several polymethacrylates such as poly [2-(dimethylamino) ethyl methacrylate] (DMAEMA) and its co-polymers have been used for polynucleotide delivery. Presence of protonable tertiary amine groups in their structure provides buffering ability similar to that of PEI. A range of Polymethacrylates, differing in molecular weights and chemical structures, have been evaluated for their potential as gene delivery vector (CitationDubruel et al 2003; CitationDubruel et al 2004). Polymethacrylates containing only tertiary amine groups were found to be similar to PEI in terms of transfection efficiency while displaying much better biocompatibility profile (CitationDubruel et al 2004). Nanoparticles with a methacrylate core and PEI shell prepared via graft copolymerization have also been employed lately for gene delivery (CitationLi et al 2002; CitationFeng et al 2006). Such conjugation resulted in nanoparticles with a higher transfection efficiency and lower toxicity as compared to PEI alone. We have recently formulated cationic nanoparticles with commercially available polymethacrylate Eudragit® E100 in combination with PLGA/PLA using cationic surfactant, cetyltrimethyl-ammonium bromide (CTAB), and achieved much improved transfection efficiency as compared to PLA/CTAB and PLGA/CTAB nanoparticles (Unpublished data).
Poly-L-Lysine (PLL)
Poly-l-lysine is amongst the first cationic polymers investigated for gene delivery. It is a biodegradable polymer synthesized by polymerization of N-carboxy-anhydride of lysine (CitationZhang et al 2004). Biodegradability is a highly desirable property for gene delivery applications in vivo. Poly-l-lysine is able to form nanometer size complexes with polynucleotides owing to the presence of protonable amine groups on the lysine moiety. Complexation of PLL with polynucleotide results in formation of toroidal or rod-shaped polyplexes less than 100 nm in size (CitationKwoh et al 1999). Although PLL polyplexes have shown good cellular uptake, their transfection efficiency is several folds lower than PEI polyplexes (CitationMerdan et al 2002). Possible reason for this may be reduced endosomal escape due to lack of amine groups that can be protonated at acidic pH (CitationAkinc and Langer 2002). Resultant lack of buffering ability leads to degradation of polyplexes by lysosomal enzymes. Conjugation of PLL with poly (ethylene glycol (PEG) has been performed to shield charge on the surface thereby providing in vivo stability, delaying body clearance, and protecting DNA from nuclease degradation (CitationKatayose et al 1998; CitationKwoh et al 1999). Enhanced transfection efficiency has been observed by conjugating the PLL polyplexes with membrane disruptive peptides and fusion peptides (CitationWagner et al 1992; CitationLee et al 2002). Delivery of siRNA using PLL has also been reported (CitationMoriguchi et al 2005).
Poly (β-amino ester)s
Poly (β-amino ester) (PBAE) is a novel, charge inducible, biodegradable, and non-toxic polymer. PBAE polyplexes have been prepared and optimized in terms of polymer molecular weight, polymer end groups, complex size, and DNA/polymer ratio (CitationLynn and Langer 2000; CitationAkinc et al 2003). The polyplexes displayed transfection efficiencies comparable to PEI and commercial lipid-based transfection reagents and were able to transfect a variety of cell types (CitationAkinc et al 2003). The complexes displayed significantly low cytotoxicity as compared to PEI polyplexes in vitro (CitationLynn and Langer 2000). PBAE in combination with PLGA was used for DNA vaccination and led to significant enhancement in transfection efficiency (CitationLittle et al 2004; CitationLittle et al 2005). PBAE/PLGA microparticles showed high immunogenic potential and led to a significant reduction of tumor size in mice (CitationLittle et al 2004). PBAE/PLGA particles were also able to delay the release of plasmid DNA for several days similar to PLGA particles thereby prolonging bioavailability (CitationLittle et al 2005).
Chitosan
Chitosan, a naturally derived polycation, is amongst most widely investigated polymers for gene delivery. Mucoadhesive property of Chitosan makes it suitable for oral and nasal delivery of DNA (CitationFerrari et al 1997). Chitosan can deliver polynucleotides, both, by encapsulation and by complexation, forming positively charged particles (CitationBozkir and Saka 2004a; CitationKoping-Hoggard et al 2004). Chitosan nanoparticles efficiently protect DNA from nuclease degradation (CitationBozkir and Saka 2004b). Chitosan nanoparticles have also been evaluated for siRNA delivery (CitationHoward et al 2006; CitationKatas and Alpar 2006). Transfection efficiency of chitosan polyplexes is found to be dependent on charge ratio, pH, cell type, molecular weight of chitosan, and degree of deacetylation (CitationSato et al 2001; CitationKoping-Hoggard et al 2004; CitationLavertu et al 2006). Transfection efficiency of optimized chitosan polyplexes was similar to PEI but the onset of expression was found to be slower than PEI which may be due to slower endosomal escape of chitosan nanoparticles (CitationKoping-Hoggard et al 2001). Recently, oligosaccharides derived from chitosan were evaluated for gene delivery after conjugation with stearic acid (CitationHu et al 2006). These nanoparticles displayed transfection efficiency comparable to that of commercial transfection reagent, Lipofectamine™ 2000, at higher time points post-transfection, while showing low cytotoxicity. Chitosan polyplexes physically combined with PLGA microparticles have also been evaluated for sustained gene delivery (CitationYun et al 2005).
Cationic liposomes
Liposomes are spherical vesicles made of phospholipids used to deliver drugs or genes inside the cells. They can range in size from 20 nm to a few microns. The use of cationic liposomes to deliver DNA into cells was first reported in 1987 (CitationFelgner et al 1987). Since then, liposomes have been routinely used in gene delivery research. Positively charged liposomes combine with negatively charged polynucleotides to form liposome/polynucleotide complexes (lipoplexes). Although the positive charge of liposomes is a requirement for preparing lipoplexes, neutral lipids dioleoylphosphatidyl-ethanolamine is also commonly incorporated. It facilitates endosomal destabilization apart from providing structural stability to the liposome. Liposomes destabilize the lipid bilayer membranes by promoting the formation of non-bilayer lipid structures (CitationHafez et al 2001). Cellular entry of lipoplexes is shown to be primarily via clathrin-mediated endocytosis (CitationDass and Burton 2003). Liposomes have also been employed for cell-targeting using a variety of targeting ligands (CitationTalsma et al 2006; CitationTorchilin 2006). Although a variety of high-efficiency liposomes are commercially available for transfecting cells in vitro and in vivo, their toxicity is still a concern. Several lipoplex formulations have caused moderate to severe toxicities in animal models (CitationStewart et al 1992; CitationTousignant et al 2000). At cellular level, lipoplexes have been reported to cause cell shrinking, reduced mitoses, and vacuolization of cytoplasm (CitationLappalainen et al 1994).
Gold nanoparticles
Gold nanoparticles have lately been investigated as an alternative to lipid-mediated and polymer-mediated gene delivery. Gold nanoparticles can be easily prepared, display low toxicity, and the surface can be modified using various chemical techniques. The gold nanoparticles functionalized using quarternary ammonium chains can efficiently transfect mammalian cells (CitationSandhu et al 2002). Up to 8 times higher transfection efficiency compared to PEI was observed using optimized formulations. Systemic delivery of plasmid DNA using PEG-modified gold nanoparticles resulted in improved stability and increased circulation time of DNA in the blood (CitationKawano et al 2006). Surface functionalization of gold nanoparticles using a PEG spacer also resulted in rapid cellular uptake and internalization (CitationShenoy et al 2006).
Magnetic nanoparticles
Delivery of polynucleotides using magnetic nanoparticles, also known as magnetofection, has been reported (CitationScherer et al 2002). In this technique, superparamagnetic iron oxide nanoparticles are prepared, and are coated with a polyelectrolyte such as PEI, to produce particles in size range of 400-1000 nm. Surface coating of PEI facilitates complexation of DNA on the surface. These nanoparticles are then delivered to the cells under the influence of a magnetic field. The mechanism of cellular uptake of magnetofectins was shown to be analogous to PEI polyplexes (CitationHuth et al 2004). Magnetofection for 10 minutes resulted in up to several hundred-fold increase in protein expression, compared to standard transfection, using a variety of transfection vectors in different cell lines (CitationScherer et al 2002). Another study reported an increase in transfection efficiency in primary airway epithelial cells using megetofection (CitationGersting et al 2004).
A recent study, investigating non-viral gene transfer in vivo has, however, reported a decrease in transfection efficiency using magnetic nanoparticles as compared to non-magnetic particles (CitationXenariou et al 2006). The versatility and potential applications of this technique thus remain to be seen.
Conclusions
The ultimate goal of gene therapy is to deliver genes with high transfection efficiency to specific cells without causing any adverse effects. Viral vectors are efficient at delivering genes but are inherently unsafe. Nanotechnology-based gene delivery vectors have shown tremendous potential at overcoming physiological and biochemical barriers towards efficient gene delivery. Vector modifications at molecular level have enabled scientists to develop a variety of nanosystems with high efficiency, high specificity and low toxicity. With increase in the information available about cellular and molecular mechanisms involved in the uptake and transport of these carriers inside the cells, there is great hope for the future of nanoparticulate systems for gene delivery.
References
- AkincALangerR2002Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal traffickingBiotechnol Bioeng785503812115119
- AkincAAndersonDGLynnDM2003Synthesis of poly (beta-amino ester)s optimized for highly effective gene deliveryBioconjug Chem149798813129402
- BehrJP1997The Proton Sponge -A Trick to Enter Cells the Viruses Did Not ExploitCHIMIA51346
- BiancoA2004Carbon nanotubes for the delivery of therapeutic moleculesExpert Opin Drug Deliv1576516296720
- BozkirASakaOM2004aChitosan nanoparticles for plasmid DNA delivery: effect of chitosan molecular structure on formulation and release characteristicsDrug Deliv111071215200009
- BozkirASakaOM2004bChitosan-DNA nanoparticles: effect on DNA integrity, bacterial transformation and transfection efficiencyJ Drug Target12281815512779
- ChengJTeplyBASherifiI2007Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug deliveryBiomaterials288697617055572
- ChoiHSOoyaTYuiN2006One-pot synthesis of a polyrotaxane via selective threading of a PEI-b-PEG-b-PEI copolymerMacromol Biosci6420416761273
- CholletPFavrotMCHurbinA2002Side-effects of a systemic injection of linear polyethylenimine-DNA complexesJ Gene Med4849111828391
- CohenHLevyRJGaoJ2000Sustained delivery and expression of DNA encapsulated in polymeric nanoparticlesGene Ther7189690511127577
- DassCRBurtonMA2003Modified microplex vector enhances transfection of cells in culture while maintaining tumour-selective gene delivery in-vivoJ Pharm Pharmacol55192512625863
- DavisSS1997Biomedical applications of nanotechnology—implications for drug targeting and gene therapyTrends Biotechnol15217249183864
- De LaporteLCruz ReaJSheaLD2006Design of modular non-viral gene therapy vectorsBiomaterials279475416243391
- DelieFBertonMAllemannE2001Comparison of two methods of encapsulation of an oligonucleotide into poly(D, L-lactic acid) particlesInt J Pharm214253011282232
- DesaiMPLabhasetwarVAmidonGL1996Gastrointestinal uptake of biodegradable microparticles: effect of particle sizePharm Res131838458987081
- DesaiMPLabhasetwarVWalterELevyRJ1997The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependentPharm Res141568739434276
- De SmedtSCDemeesterJHenninkWE2000Cationic polymer based gene delivery systemsPharm Res171132610751024
- DubruelPChristiaensBVanlooB2003Physicochemical and biological evaluation of cationic polymethacrylates as vectors for gene deliveryEur J Pharm Sci182112012659932
- DubruelPChristiaensBRosseneuM2004Buffering properties of cationic polymethacrylates are not the only key to successful gene deliveryBiomacromolecules53798815002997
- FelgnerPLGadekTRHolmM1987Lipofection: a highly efficient, lipid-mediated DNA-transfection procedureProc Natl Acad Sci USA847413172823261
- FengMLeeDLiP2006Intracellular uptake and release of poly(ethyleneimine)-co-poly(methyl methacrylate) nanoparticle/pDNA complexes for gene deliveryInt J Pharm3112091416442245
- FerrariFRossiSBonferoniMC1997Characterization of rheological and mucoadhesive properties of three grades of chitosan hydrochlorideFarmaco5249379372602
- GebhartCLKabanovAV2001Evaluation of polyplexes as gene transfer agentsJ Control Release734011611516515
- GerstingSWSchillingerULausierJ2004Gene delivery to respiratory epithelial cells by magnetofectionJ Gene Med69132215293350
- HafezIMMaurerNCullisPR2001On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acidsGene Ther811889611509950
- HeidelJMishraSDavisME2005Molecular conjugatesAdv Biochem Eng Biotechnol9973916568887
- HickmanMAMaloneRWLehmann-BruinsmaK1994Gene expression following direct injection of DNA into liverHum Gene Ther51477837711140
- HowardKARahbekULLiuXDamgaardCK2006RNA Interference in Vitro and in Vivo Using a Novel Chitosan/siRNA Nanoparticle SystemMol Ther144768416829204
- HuFQZhaoMDYuanH2006A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studiesInt J Pharm3151586616632285
- HuthSLausierJGerstingSW2004Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transferJ Gene Med69233615293351
- KatasHAlparHO2006Development and characterisation of chitosan nanoparticles for siRNA deliveryJ Control Release2006725
- KatayoseSKataokaK1998Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(L-lysine) block copolymerJ Pharm Sci8716039519147
- KawanoTYamagataMTakahashiH2006Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulsesJ Control Release111382916487614
- KimISLeeSKParkYM2005Physicochemical characterization of poly(L-lactic acid) and poly(D,L-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrierInt J Pharm2982556215941631
- KimDHMartinDC2006Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug deliveryBiomaterials273031716443270
- KommareddySTiwariSBAmijiMM2005Long-circulating polymeric nanovectors for tumor-selective gene deliveryTechnol Cancer Res Treat46152516292881
- Koping-HoggardMTubulekasIGuanH2001Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivoGene Ther811082111526458
- Koping-HoggardMVarumKMIssaM2004Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomersGene Ther1114415215269712
- KreuterJAlyautdinRNKharkevichDA1995Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles)Brain Res67417147773690
- KursaMWalkerGFRoesslerV2003Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transferBioconjug Chem142223112526712
- KwohDYCoffinCCLolloCP1999Stabilization of poly-L-lysine/ DNA polyplexes for in vivo gene delivery to the liverBiochim Biophys Acta14441719010023051
- LappalainenKJaaskelainenISyrjanenK1994Comparison of cell proliferation and toxicity assays using two cationic liposomesPharm Res111127317971713
- LavertuMMethotSTran-KhanhN2006High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylationBiomaterials2748152416725196
- LeeHJeongJHParkTG2002PEG grafted polylysine with fusogenic peptide for gene delivery: high transfection efficiency with low cytotoxicityJ Control Release792839111853938
- LemieuxPVinogradovSVGebhartCL2000Block and graft copolymers and NanoGel copolymer networks for DNA delivery into cellJ Drug Target89110510852341
- LiPZhuJMSunintaboonP2002New route to amphiphilic core-shell polymer nanospheres: graft copolymerization of methyl methacrylate from water-soluble polymer chains containing amino groupsLangmuir1886416
- LittleSRLynnDMGeQ2004Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccinesProc Natl Acad Sci U S A1019534915210954
- LittleSRLynnDMPuramSV2005Formulation and characterization of poly (beta amino ester) microparticles for genetic vaccine deliveryJ Control Release1074496216112767
- LiuFHuangL2002Development of non-viral vectors for systemic gene deliveryJ Control Release782596611772466
- LynnDLangerR2000Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNAJ Am Chem Soc122107618
- MerdanTKunathKFischerD2002Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experimentsPharm Res19140611883640
- MoghimiSMSymondsPMurrayJC2005A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapyMol Ther11990515922971
- MoriguchiRKogureKAkitaH2005A multifunctional envelope-type nano device for novel gene delivery of siRNA plasmidsInt J Pharm3012778516019173
- MurakamiHNakashimaN2006Soluble carbon nanotubes and their applicationsJ Nanosci Nanotechnol6162716573065
- PanyamJZhouWZPrabhaS2002Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene deliveryFASEB J1612172612153989
- ParkMRHanKOHanIK2005Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriersJ Control Release1053678015936108
- Ravi KumarMHellermannGLockeyRF2004Nanoparticle-mediated gene delivery: state of the artExpert Opin Biol Ther412132415268657
- RibeiroSHussainNFlorenceAT2005Release of DNA from dendriplexes encapsulated in PLGA nanoparticlesInt J Pharm2983546015979263
- RobbinsPDGhivizzaniSC1998Viral vectors for gene therapyPharmacol Ther8035479804053
- RoscaIDWatariFUoM2004Microparticle formation and its mechanism in single and double emulsion solvent evaporationJ Control Release992718015380636
- RuponenMHonkakoskiPRonkkoS2003Extracellular and intracellular barriers in non-viral gene deliveryJ Control Release932131714636726
- SandhuKKMcIntoshCMSimardJM2002Gold nanoparticle-mediated transfection of mammalian cellsBioconjug Chem133611792172
- SatoTIshiiTOkahataY2001In vitro gene delivery mediated by chitosan. effect of pH, serum, and molecular mass of chitosan on the transfection efficiencyBiomaterials2220758011432586
- SchererFAntonMSchillingerU2002Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivoGene Ther9102911857068
- ShenoyDFuWLiJ2006Surface functionalization of gold nanoparticles using hetero-bifunctional Poly (ethylene glycol) spacer for intracellular tracking and deliveryInt J Nanomedicine151816467923
- ShiveMSAndersonJM1997Biodegradation and biocompatibility of PLA and PLGA microspheresAdv Drug Deliv Rev2852410837562
- StewartMJPlautzGEDel BuonoL1992Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in miceHum Gene Ther3267751643147
- TalsmaSSBabenseeJEMurthyN2006Development and in vitro validation of a targeted delivery vehicle for DNA vaccinesJ Control Release112271916549219
- ThomasMGeQLuJJ2005Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivoPharm Res223738015835742
- TorchilinVP2006Recent approaches to intracellular delivery of drugs and DNA and organelle targetingAnnu Rev Biomed Eng83437516834560
- TousignantJDGatesALIngramLA2000Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in miceHum Gene Ther11249351311119421
- VinogradovSVBronichTKKabanovAV2002Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cellsAdv Drug Deliv Rev541354711755709
- VogtACombadiereBHadamS200640 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skinJ Invest Dermatol12613162216614727
- WagnerEPlankCZatloukalK1992Influenza virus hemagglutinin HA–2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicleProc Natl Acad Sci U S A89793481518816
- WaltherWSteinU2000Viral vectors for gene transfer: a review of their use in the treatment of human diseasesDrugs602497110983732
- WiethoffCMMiddaughCR2003Barriers to nonviral gene deliveryJ Pharm Sci922031712532370
- WolffJAMaloneRWWilliamsP1990Direct gene transfer into mouse muscle in vivoScience247146581690918
- XenariouSGriesenbachUFerrariS2006Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivoGene Ther2006 Jun 1; [Epub ahead of print]
- YuanXLiLRathinaveluA2006SiRNA drug delivery by biodegradable polymeric nanoparticlesJ Nanosci Nanotechnol62821817048488
- YunYHJiangHChanR2005Sustained release of PEG-g-chitosan complexed DNA from poly(lactide-co-glycolide)J Biomater Sci Polym Ed1613597816372401
- ZhangSXuYWangB2004Cationic compounds used in lipoplexes and polyplexes for gene deliveryJ Control Release1001658015544865
- ZhongZFeijenJLokMC2005Low molecular weight linear polyethylenimine-b-poly(ethylene glycol)-b-polyethylenimine triblock copolymers: synthesis, characterization, and in vitro gene transfer propertiesBiomacromolecules63440816283777