?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The interaction of the important but often overdosed local anesthetic bupivacaine, its structural analogs 2,6-dimethylaniline, and N-methyl-2,6-dimethylacetanilide, and cocaine, with several electron deficient aromatic moieties were studied primarily by proton NMR and UV-visible spectroscopy. In solution, the anesthetic, its analogs and cocaine are electron donors and form π-π charge transfer complexes with strong aromatic acceptors, as monitored by the upfield changes induced in the NMR chemical shifts (δ) and red-shifted UV-vis wavelength (λmax) absorbance of the acceptors. The equilibrium binding constant, K, was determined from the 1H NMR charge transfer induced chemical shift changes and used to calculate the free energy (ΔG) for complex formation of three acceptor-donor pairs. HPLC results indicate that the concentrations of free bupivacaine, its analogs and of cocaine are reduced from solution via binding to aromatic-functionalized silica.
Introduction
Overdoses of many natural and synthetic chemicals are a problem that must be solved in tandem with the continual development of drugs designed for human comfort and longevity (as well as those intended to inflict harm), but which become toxic when taken in excess. Exposure to excess amounts of some therapeutics can be reversed if general or selective antidotes are available. In emergency situations, it is critical that a patient be quickly stabilized and the availability of an appropriate antidote may be the only pathway to remediation. Thus, it is crucial that materials for use in selectively reversing the adverse effects of overdosed toxins be readily available. These toxins not only include some common street drugs but also prescription therapeutics that can cause death by cardiac arrest if not quickly removed. Three such therapeutics are amiodarone (anti-arrhythmic), amitriptyline (antidepressant), and bupivacaine (local anesthetic), which cause significant morbidity and mortality in humans annually, and for which no specific pharmaceutical antidotes have been developed to date. Technologies based on injectable nanoparticles for in vivo remediation of these therapeutics are currently in development in the author’s laboratories (CitationPartch et al 2003; CitationPowell 2004; CitationLee et al 2005).
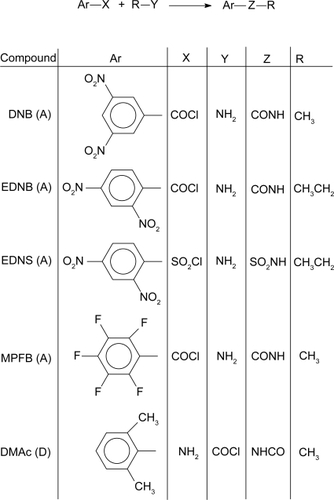
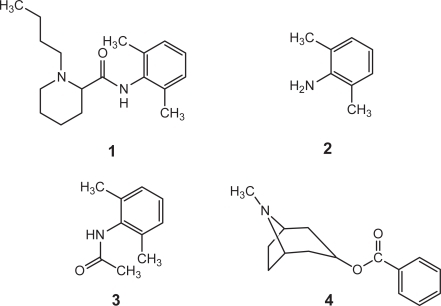
The development of a viable toxin-removal technology has been advanced by the fundamental information obtained in the present work () for the rapid binding of bupivacaine (1) and its analogs 2,6-dimethylaniline and 2,6-dimethylacetanilde, 2, and 3, respectively, and of cocaine, 4 by the acceptor molecules. The structures 1, 2, 3 and 4 show that the molecules are lipophilic in nature, a characteristic common to toxic molecules. 1, 2 and 3 include a benzene ring with two methyl and a nitrogen electron-donating groups,
Charge transfer complexation has been a well-known interaction since the early discoveries made by CitationBenesi and Hildebrand (1949), through the pioneering work of CitationBriegleb (1961) and the valence-bond, and molecular orbital descriptions put forth by CitationMulliken (1952), and CitationDewar and Lepley (1961), respectively. Aromatic interactions are driven by the noncovalent ie, hydrophobic, electrostatic, and hydrogen bonding forces. Although they occur with relatively small binding energies in the range 2–5 kcal/mol, aromatic-aromatic types of electrostatic interaction are one of the principal noncovalent forces governing the fundamental process of molecular recognition between molecules in biology and chemistry (CitationSlifkin 1981; CitationHunter 1994; CitationAdams et al 2001; CitationChujo and Tamaki 2001; CitationSinnokrot et al 2002). For example, it influences the structures of proteins, DNA, host-guest complexes and self-assembled supramolecular architectures (CitationBoal and Rotaldo 2000). The range and significance of charge transfer complex formation in chemical and biological recognition has been illustrated in a recent review (CitationMeyer et al 2003).
Nuclear magnetic resonance has proven valuable for evaluating the binding of antimalarials with heme species (CitationConstantinidis and Satterlee 1988a, Citation1988b), the use of paraquat to probe for certain aspects of polypeptide and protein conformations (CitationVerhoeven et al 1974), and the charge transfer complex formation between trinitrobenzene and a structural analog of the important neurochemical dopamine (CitationDust 1992). Dust’s results suggested to us that π-π charge transfer complex formation might be a means by which selective reversal of the lethal effects of overdosed bupivacaine could be achieved. The method of charge transfer complex formation has not previously been investigated in the context of in vivo toxin removal.
In the present report, the title compound, its analogs 2 and 3, and cocaine, 4, were studied to demonstrate the feasibility of π-π charge transfer complex formation with π-electron deficient aromatic rings. The formation of a π-π charge transfer complex was evaluated by the changes observed in the NMR chemical shifts and UV-vis wavelength of maximum absorbance induced in the aromatic acceptor upon addition of excess amounts of the drugs or analogs. The goal is to employ the concept to develop a common antidote for in vivo application to alleviate overdose toxicity.
Experimental
Materials
The following were obtained from Sigma-Aldrich chemical company, Milwaukee, WI, and used as received: 1,3,5-trinitrobenzene, 1,3,5-tricyanobenzene, 1,3-bis(triflu oromethyl)benzene, 2,4- and 3,5-dinitrobenzoyl chloride, 2,4-dinitrobenzenesulfonyl chloride, 3,5-dinitrobenzyl alcohol, ethylamine, 2,4-, 2,6- and 3,5-dimethylaniline, 3,4-dimethoxytoluene, bupivacaine hydrochloride, tetrahy-drofuran (THF), acetonitrile (HPLC grade), and chloroform-d (CDCl3) and acetonitrile-d3 (CD3CN), the latter two solvents containing 0.03% v/v TMS. Deuterated water (D2O) was obtained from ACROS, New Jersey. Solvent grade acetoni-trile, acetyl chloride, and diethyl ether were obtained from J.T. Baker, Inc., New Jersey. Methylamine, 40% (w/w) aqueous solution, and pentafluorbenzoyl chloride were obtained from Lancaster Synthesis, New Hampshire. Reagent grade acetone was obtained from Pharmco Products, Inc., Connecticut. Nine inch long, 5 mm thin wall NMR sample tubes (Cat. No. 528-PP-9) were obtained from Wilmad Glass Company, Inc., Buena, NJ.
Materials not commercially available to serve as electron acceptors and donors were synthesized. In lieu of 1,3,5-trinitrobenzene, which exhibits a greater π-electron acceptor effect, analogs with the aromatic rings substituted with two nitro groups and a carboxamide or sulfonamide group were studied. The amido functionality provides a handle that can be utilized for subsequent attachment of the π receptor to injectable carrier nanoparticles. One receptor containing a pentafluorophenyl group was studied for variation. 2,6-Dimethylaniline (commercially available) and its acetamide analog, which was synthesized, served as models for the electron-donating portion of the bupivacaine molecule. The syntheses of these molecules are presented in the following section. In the presentation of the NMR results, s, d, t, q, m, and br are singlet, doublet, triplet, quartet, multiplet and broad peak characteristics, respectively. Broad signals are associated with amido hydrogens.
Syntheses of Pi acceptors and donor
N-Methyl-3,5-dinitrobenzamide (DNB)
3,5-Dinitrobenzoyl chloride (5.0 g, 21.3 mmol) was dissolved in 20 mL of ether in a 100 mL round-bottom flask equipped with a stir bar. Aqueous methylamine (3.3 g, 42.5 mmol) was added drop-wise through a funnel with stirring of the benzoyl chloride solution. After all the amine had been added, the reaction was allowed to proceed 12 hours. The precipitate was filtered and washed with water. The water washing was extracted with five 50 mL portions of ether. The ether extracts were combined and dried with anhydrous sodium sulfate, which was removed by filtration. The ether was removed by roto-evaporation. The residue and original precipitate were dried in vacuo at room temperature overnight. Both solids were confirmed to be the same material by melting point and NMR and were combined and recrystallized from toluene to give the product in 87% yield (4.18 g): mp 146–147 °C; NMR (CDCl3): δ 9.16 (t, 1H), 8.96 (d, 2H), 6.48 (s, br, 1H), 3.12 (d, 3H).
N-Ethyl-2,4-dinitrobenzamide (EDNB)
This compound was synthesized by a similar procedure as used for DNB, with the corresponding reagents 2,4-dinitrobenzoyl chloride (5.0 g, 21.3 mmol) and ethyl-amine (1.98 g, 42.6 mmol) dissolved in 20 and 10 mL ether, respectively, and the amine added to the chloride. NMR (CDCl3): δ 8.92 (s, 1H), 8.53 (d, 1H), 7.75 (d, 1H), 5.85 (s, br, 1H), 3.57 (m, 2H), 1.31 (t, 3H).
N-Ethyl-2,4-dinitrobenzenesulfonamide (EDNS)
EDNS was synthesized by a procedure similar to that used for DNB with the corresponding reagents 2,4-dinitrobenzene-sulfonyl chloride (2.66 g) and ethylamine (0.9 g) dissolved in 20 and 10 mL of ether, respectively, and the amine added to the chloride. This solution was refluxed overnight. The resulting solids were filtered on a Buchner funnel and washed consecutively with ether and water then dried at room temperature in vacuo overnight to give the product: mp 95–97 °C; NMR (CDCl3) δ 8.68 (s, 1H), 8.56 (d, 1H), 8.38 (d, 1H), 5.27 (s, br, 1H), 3.25 (m, 2H), 1.21 (t, 3H).
N-Methyl-pentafluorobenzamide (MPFB)
Aqueous methylamine (5.62 g) was added to 50 mL of ether in a round bottom flask with magnetic stirring. Pentafluoro-benzoyl chloride (5 mL) was dripped into the reaction vessel and the reaction allowed to proceed at room temperature overnight. The contents were then transferred to 125 mL separatory funnel, the water layer was removed and the remaining ether layer was washed with five 10 mL portions of water (ether became cloudy after the second washing). The ether layer was dried with anhydrous magnesium sulfate, which was removed by filtration. Evaporation of the ether yielded a solid, mp 85–95 °C. The solid was dissolved in a minimal amount of methylene chloride and extracted with two 15 mL portions of water. The organic layer was dried with magnesium sulfate, filtered, and the methylene chloride removed. The remaining solid was dried in vacuo at room temperature overnight and recyrstallized from toluene to give the product in 55.58% yield (4.53 g): mp 98–99 °C; 1H NMR (CDCl3) δ 6.2 (s, br, 1H), 3.02–3.03 (d, 3H); 19F NMR in CDCl3 showed all three sets of fluorines resonating at δ values of −140.2859 ppm, −150.4915 ppm, and −159.8655 ppm.
2,6-Dimethylacetanilide (DMAc)
2,6-Dimethylaniline (5 g) was dissolved in 20 mL of ether in a round bottom flask. A solution of 10% (w/w) NaOH (15 mL) was added, then 3 mL of acetyl chloride dissolved in 15 mL ether were slowly (∼20 minutes) dripped into the flask and the reaction allowed to take place for 10 hours and resulted in the formation of a precipitate.
The solid was filtered on a Buchner funnel and washed with ether. Recrystallization from toluene gave the product in 70% yield (4.71 g): mp 181–182 °C (lit. 182–184 °C (Aldrich)); NMR (CDCl3) δ 7.08 (m, 3H), 6.70 (s, br, 1H), 2.23 (t, 6H), 1.74 (s, 3H).
Silica functionalized with 3,5-dinitrobenzoyl groups (SiDNB)
Silica (12 nm diameter, 100 mL, 20 wt% in acetone, Nissan Chemical Co.) was placed in a flask and stirred while a solution of 5 g (0.022 mole) 3,5-dinitrobenzoyl chloride dissolved in 50 mL dry THF was added dropwise. The mixture was refluxed for 12 hours during which time the color turned from colorless to light yellow to brown. All solvent was evaporated and the tan solid was treated with 200 mL ethanol, then aqueous sodium bicarbonate and the precipitated solid product collected by centrifugation at 12,000 rpm and washed with ethanol and dried at 110 °C under vacuum to give 7.5 g material; density, 1.809 g mL–1; TEM, 15–20 nm particles and aggregates; TGA 3.6 wt% organics; IR (cm–1), 3095, 1658, 1544, 1346 indicative of aromatic C-H, amide C = O and nitro groups, respectively; SSA 230 m2g–1.
Instrumentation for characterization of reaction products
Nuclear magnetic resonance (NMR)
Proton NMR spectra were recorded on a Varian 300 MHz spectrometer at 25 °C on solutions of donor, acceptor, and of donor-acceptor mixtures following published procedures (CitationHanna and Ashbaugh 1964; CitationFoster and Fyfe 1965; CitationDust 1992; CitationFesik et al 2000). Typically, 1.75 × 10–5 moles of acceptor and sixty times that of donor were dissolved in 1 mL of deuterated solvent, chloroform-d (unless otherwise specified), in a small test tube and transferred quantitatively by pipette to the NMR tube. Experiments meant to provide binding constants were run with constant acceptor concentration (same as above) and the concentration of donor varied such that acceptor to donor ratios ranged from 1:40 to 1:80.
Ultraviolet-visible spectroscopy (UV-vis)
UV-visible spectroscopic measurements were made on a Hewlett Packard 8452A Diode Array Spectrophotometer. These measurements were made in a similar fashion to the NMR experiments in that the acceptor concentration was kept constant while varying the excess concentration of the donor. Typically, 3.5 mL of solvent (or mixture) was run as background for the acceptor in a 1 cm quartz cuvette. An aliquot containing the desired number of moles of the acceptor (typically 1 × 10–5) was then pipetted from a stock solution into the cuvette and mixed thoroughly and rerun to obtain the spectrum for the acceptor. Next, a 3.5 mL solution containing the desired excess moles of donor was run as background for the complex in order that the excess donor did not obscure the spectrum of the complex. An aliquot of the acceptor from the standard solution, containing the predetermined number of moles was then added to the donor solution, thoroughly mixed and its spectrum obtained.
Infrared spectroscopy (IR)
Silica samples were prepared as KBr pellets and examined using a Nicolet Magna 760 spectrometer.
High performance liquid chromatography (HPLC)
In a typical experiment to measure BPC uptake from a liquid (CitationVarshney et al 2004), 1.5% w/v of SiDNB was placed in a phosphate buffer at pH 7.4 containing a known concentration of BPC hydrochloride. The mixture was shaken for 30 minutes at room temperature and then ultrafiltration was carried out via the YM-30 centrifree (Millipore) system using centrifugation at 3000 rpm for 30 minutes. Several 10 μL samples of the filtrate were sequentially injected into an Alliance (Waters Inc., Milliford, MA) HPLC system. The output traces reproducibly indicated less than 5% of the original amount of BPC hydrochloride remained in the liquid. The same amount of cocaine (COC) hydrochloride remained when its solutions were treated with SiDNB. Using less SiDNB in more concentrated BPC hydrochloride solutions resulted in proportionately less amount of drug uptake. For example, 0.05% w/v SiDNB removed 1800 μM amount of drug from a 10,000 μM solution; and, 0.10% w/v SiDNB removed 3800 μM amount. Unmodified silica showed no ability to remove BPC or COC from saline solution.
Results and discussion
Solution measurements
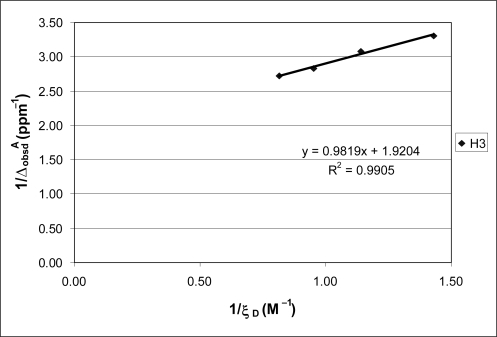
depicts the syntheses and structures of acceptor (A) and donor (D) molecules used in this study. The displacement of charge from donor to acceptor that occurs in the formation of a π-π complex alters electron density thus making the complex amenable to study by nuclear magnetic resonance, a technique quite sensitive to small changes in the electronic environment of a magnetic nucleus. CitationHanna and Ashbaugh (1964) recognized this fact and developed a mathematical expression for use with NMR similar to the CitationBenesi-Hildebrand (1949) UV-Vis equation to evaluate π-π complexes formed between 7,7,8,8-tetracyanoquinodimethane (TCNQ) and various aromatic donors. For the equation to be valid requires that the donor: acceptor concentration ratio be large (eg, Citation20). Plotting the inverse of observed upfield shift in acceptor resonances compared to the values before addition of the donor, versus the inverse of the donor concentration, yields an intercept and slope for calculation of an equilibrium quotient, from which a binding energy for the acceptor-donor complex in question can be obtained.
It was deemed pertinent to repeat Dust’s work using 3,4-dimethoxytoluene as the donor to ascertain our experimental accuracy. The three equivalent aromatic protons of 1,3,5-trinitrobenzene (TNB) appear as a sharp singlet and from an average of five measurements occur at 9.3856 (±0.0022) ppm in CDCl3, which is outside the range of absorption for the solvent (7.2540 ppm) and the donors used, thus satisfying the requirements set forth by CitationHanna and Ashbaugh (1964). Addition of an excess of 3,4-dimethoxytoluene (ratio of acceptor to donor = 1:60) to the solution of TNB caused the TNB proton singlet to shift upfield by 0.187 ppm. Dust obtained a value of 0.183 ppm with the addition of N,N-dimethyl-3,4-dimethoxyphenylethylamine. These values indicate that Dust’s experiment was satisfactorily reproduced in our laboratory.
The upfield chemical shift for acceptor nuclei results from the ring current effect between both aromatic rings, suggesting the existence of ring stacking interactions (CitationIshida et al 1984; CitationHunter et al 2001). The transfer of charge from the donor to the acceptor results in an increase in the π-electron density of the latter leading to an increase in shielding of its aromatic protons and thus to the observation of an upfield shift. It is to be noted that the observed resonance of the acceptor in the complexing medium is an average between free acceptor and that bound in the complex, the protons in each of which have a characteristic precessional frequency (CitationSahai et al 1974), since the complex formation is an equilibrium process.
The electron donating aromatic portion of bupivacaine is an amide analog of 2,6-dimethylaniline. Thus, the NMR spectra were collected for separate mixtures of TNB with each of three isomers, 2,4-, 3,5-, and 2,6-dimethylaniline. The observed upfield changes in the chemical shifts were 0.3086 ppm, 0.2647 ppm, and 0.2995 ppm, respectively. The greater change in the chemical shifts observed for the substituted aniline isomers compared with dimethoxytoluene can be accounted for in terms of the difference between the electron-donating ability of -NH2 and -OCH3. The former substituent is strongly electron donating whereas the latter is only moderately so (CitationHansen 1962).
The next step in the investigation involved the use of aromatic acceptor molecules with a unit for attachment to nanoparticles in the subsequent stage of the planned toxin remediation study. The first acceptor molecule investigated was N-methyl-3,5-dinitrobenzamide (DNB) which exhibits proton area 1 doublet and area 2 triplet resonance lines at 8.9602, 8.9675 ppm, and 9.1680, 9.1745, and 9.1818 ppm, respectively. The addition of the donor caused an upfield shift in each of the resonance lines such that the new resonances appear at 8.9245, 8.9309 ppm and 8.9684, 8.9748, and 8.9822 ppm. The resonance lines of each multiplet were averaged to give a single value for each. Thus, the observed changes in the chemical shifts were calculated to be (doublet) = 0.0412 ppm and (triplet) = 0.1997 ppm.
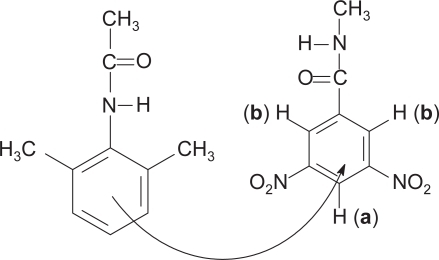
These results not only demonstrate the upfield shift of the resonances but show also that the triplet undergoes a greater change than the doublet. This can be understood in terms of the positions occupied by the protons relative to the electron withdrawing groups in the acceptor molecule, and the realization that the stacking of one ring over another may not be perfectly vertical (CitationDewar and Lepley 1961). depicts the structures of the components and the flow of electron density as the complex is formed between them. Proton a is flanked by the two most electron withdrawing (nitro) groups and come into resonance (triplet) at lower magnetic field strength than proton b (doublet) (a downfield from b) as is observed in the NMR spectrum. This is so since there is less electron density about proton a, as it is diminished there by two neighboring strong electron-withdrawing groups, than about proton b. As electrons are donated to the acceptor (shown by curved arrow), they will be received more so around a than around b since the former is more electron deficient. Upon complex formation, proton a becomes more shielded than b due to the donated electrons, and its resonance shifts upfield to a greater extent.
Figure 2 Depiction of electron flow from 2,6-dimethylacetanilide to the aromatic region containing the electron withdrawing nitro groups of 3,5-dinitrobenzamide in the complex of the two.
When complexed with 2,6-dimethylaniline in CDCl3, the doublet and triplet of DNB underwent upfield shifts of 0.0779 and 0.0874 ppm, respectively. When the donor was the often-overdosed bupivacaine in its free base form, values of 0.0662 ppm (doublet) and 0.2513 ppm (triplet) were obtained. The free base form of bupivacaine was generated by stirring 1.0 g of the hydrochloride salt in 9.45 mL of 0.326M NaOH then extracting with five 10 mL portions of methylene chloride. It was of interest to determine the formation of a π-π complex between therapeutically administered bupivacaine hydrochloride and the acceptor in an aqueous medium. The hydrochloride salt of bupivacaine readily dissolved in water but the acceptor employed in this study did not. Therefore, the formation of the complex was carried out in a 50/50 mixture of D2O and CD3CN. In this solvent system, DNB upfield shift values of 0.0275 ppm (doublet) and 0.0891 ppm (triplet) were observed. The lower values obtained in the more polar solvent system can be explained in terms of a stronger interaction of the ionic donor with this solvent and a weaker interaction of the organic acceptor with this solvent, thereby limiting the interaction between the two solutes and diminishing charge transfer complex formation between them.
The protons on two other nitroaromatic acceptors, N-ethyl-2,4-dinitrobenzamide and N-ethyl-2,4-dinitroben-zenesulfonamide underwent the same upfield shifts when complexed with 2,6-dimethylaniline in chloroform-d. However, only the chemical shifts due to the π-π interaction with the benzamide could be determined when the donor was bupivacaine. The aromatic resonance of the sulfonamide was overlapped by that of bupivacaine and could not be resolved. Three magnetically inequivalent protons exist in both these acceptor molecules and shows the chemical shifts upon complexation.
Table 1 Observed changes in the chemical shifts of N-ethyl-2,4-dinitrobenzamide and N-ethyl-2,4-dinitrobenzenesulfonamide when complexed with 2,6-dimethylaniline (DMA) and bupivacaine (BPC) in chloroform-d
Further variety in the structure of the π-electron receptor was provided by study with tricyanobenzene (TCB), and 1,3-bis(trifluoromethyl)benzene, which are possibly more biologically friendly receptors than those of the nitro type. With 2,6-dimethylaniline, an upfield shift of 0.1699 ppm was observed for the aromatic singlet of TCB. Measurements with 1,3-bis(trifluoromethyl)benzene with the donor DMAc in a molar ratio of 1:20 resulted in an average upfield shift for the acceptor proton-2 resonance signal of 0.0049 ppm. Fluorine NMR resonance shifts of N-methylpentafluoro-benzamide before and after complexation with DMAc were from ppm values of −140.2859, −150.4915, and −159.8655 to −140.6222, −152.1669, and 160.8225, respectively, giving the respective shifts of 0.3363 ppm, 1.6754 ppm, and 0.9570 ppm. These results were encouraging in that they demonstrated the feasibility of formulating a toxin receptor containing a more biocompatible group than the nitro group. As pointed out in a recent review on interactions with aromatic systems in chemical and biological recognition, fluorine-containing compounds have enjoyed a rich history in bioorganic and medicinal chemistry (CitationMeyer et al 2003; CitationThayer 2006). Fluorine has been widely studied in these disciplines as a result of its diverse functions, ranging from an isosteric and isoelectronic replacement of the hydroxy group, to a means of enhancing the metabolic stability of drugs and modifying electronic and physical properties (for example, lipophilicity, acidity, and steric hindrance).
The experimental evaluation of formation constants for π-π charge transfer complexes has often proved troublesome. The UV-vis absorption band of the complex might not be observed if it occurs in a region of intense absorption by the uncomplexed molecular partners or in cases where the extinction coefficient of the charge transfer transitions are much weaker than those of the molecular electronic absorption. Additionally, even though McConnell and coworkers (CitationLandauer and McConnell 1952; CitationLawrey and McConnell 1952) suggested as early as 1952 that complexes with stoichiometries other than 1:1 might coexist in equilibrium with the AD complex, most analyses have been made in lieu of that possibility. Although evidence has been presented that for some donor-acceptor systems higher-order complexes do indeed exist (CitationDeranleau 1969a), for most complexes the stoichiometry is 1:1.
The voluminous reports on charge transfer transitions testify to the ability to experimentally determine the thermodynamic properties associated with these processes. CitationPerson (1965), in giving a critical discussion of the problem, has emphasized that the most accurate values for binding constants of a complex are obtained when the equilibrium concentration of the complex is of the same order of magnitude as the equilibrium concentration of the more dilute component. Such condition can be approximated when the ratio of donor to acceptor is large. CitationDeranleau (1969a; Citation1969b) has discussed considerations that must be given to some aspects of the measurement of formation constants from the standpoint of basic binding theory. He has pinpointed various sources of error and suggested criteria for the reliability of formation constants and extinction coefficients obtained from the usual methods of treatment of weak molecular complexes.
We carried out NMR experiments following methods in the literature (CitationHanna and Ashbaugh 1964; CitationFoster and Fyfe 1965; CitationFesik et al 2000) to determine the binding constants for the π-π complexes formed in this study. These methods involve keeping the concentration of the acceptor constant while varying that of the donor, which is always kept in excess, for example, 1:40, 1:50, 1:70, 1:70, and 1:80. The values of the reciprocal of the upfield shift in resonance of protons on acceptor molecules were then plotted against the reciprocal of the donor concentration. An example is shown in . Similar plots for DNB (H4) complexed with DMA and of EDNB(H3) with DMA yielded R2 values of 0.9922 and 0.9776, respectively. Calculations using the slopes and intercepts of the plotted linear lines yielded the K values shown in , and hence the ΔG values. A set of five measurements with N-methyl-3,5-dinitrobenzamide demonstrates the error associated with measuring the chemical shift changes with a standard deviation of 0.0039 ppm, which is associated with the calculated binding constants.
Table 2 Equilibrium constants and free energies of binding
Only bupivacaine appears to form the complex exothermally. Bupivacaine has a large hydrophobic nonaromatic portion that may contribute to a favorable interaction with the hydrophobic receptor molecule. The negative value of ΔG obtained with this molecule is consistent with thermodynamic quantities characteristic for binding driven by both π-π and classic hydrophobic effects, namely, a large favorable complexation entropy TΔS and a small complexation enthalpy, ΔH (CitationMeyer et al 2003). That the other donors each exhibit a positive ΔG indicates that in the free energy expression, the enthalpy of complexation is large relative to the entropy term. As indicated by the upfield shift observed in these systems, complex formation did occur.
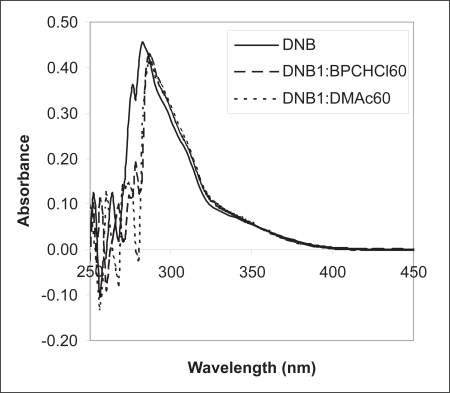
The formation of π-π complexes in these systems was substantiated by evidence obtained by UV-visible spectroscopic measurements. This technique was used to obtain information on the formation of complexes formed between DNB and DMAc and between DNB and bupivacaine hydrochloride (BPCHCl), with an acceptor to donor molar ratio of 1:60 in 50/50 water/acetonitrile, . In the absence of the donor, DNB exhibited a λmax of 278 nm. The addition of the donor caused a red shift in the λmax value to 284 nm. This shift is diagnostic of π-π complex formation. Visibly, mixtures of these donors with acceptors appear red-brown in color due to absorption of green light. However, we were not able to observe such long wavelength absorbance in our studies ().
Dispersion measurements
The goal of this research is to prepare carrier nanoparticles for in vivo reversal of overdoses of exemplary molecules 1 and 4. Encouraged by the positive results from the solution measurements, that pi-pi interactions are favorable between bupivacaine (1) and dinitrobenzene ring receptors, we covalently tethered the latter to silica nanoparticles to obtain proof-of-concept data.
Surface attachment of the 3,5-dinitrobenzoyl moiety to silica was achieved by reaction with the benzoyl chloride reagent by standard procedure. In the present case excess reagent was used, verified by recovery of some in the liquid washes of the solid product, hereafter referred to as SiDNB. This resulted in attaching the maximum number of pi receptor molecules to each particle. Analysis of the solid by TGA and IR verified the desired functionalization had occurred.
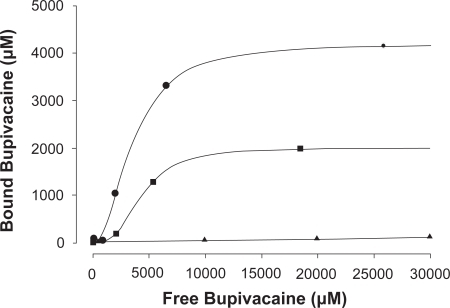
SiDNB proved to be very efficient in removing both bupivacaine (BPC, 1) and cocaine (COC, 4) as hydrochloride salts from saline. HPLC data collected on solutions of each 1 and 4, before and after stirring with sufficient SiDNB showed that uptake of either of the molecules by the particles was over 95% complete within ten minutes. Calculation using weight, density and diameter of the particles, and moles of BPC removed, gave approximately 90 BPC molecules bonded to one as-prepared SiDNB particle. shows the uptake of BPC using less SiDNB per amount of drug, proof that more receptors remove more BPC. The solid collected after mixing with the drug-containing solutions, SiDNB-BPC, exhibited enhanced TGA weight loss and some differences in IR absorbance values compared to SiDNB. The present work has established for the first time that toxins 1 and 4, and simple derivatives of 1, efficiently bind to pi receptor molecules free in solution or attached to carrier silica nanoparticles. This served as precedent for the study reported by this group (CitationLee et al 2005), where oligochitosan microparticles were similarly functionalized, (compare CitationDomszy and Roberts 1985). However, if these chemical antidotes are to be ultimately employed for internal overdose treatment, their in vivo effect on blood cells and organs must first be evaluated. Towards that goal, an isolated guinea pig heart did not exhibit irregular function or damage when exposed to a saline solution of BPC hydrochloride complexed with DNB.
Figure 5 Bupivacaine binding by two amounts of SiDNB: •–0.1%w/v, ▪–0.05%w/v, ▴–0.1%w/v unmodified SiO2. Standard deviation over three runs of each sample was ± 1–3.
Also of concern is the stability to hydrolysis of the ester linkage bonding the dinitrobenzoyl moiety to the silica particle. Two fundamental organic chemical principles oppose each other in this regard. Nitro groups on the aromatic rings of alkyl benzoate esters are known to enhance hydrolysis in both acidic and basic media (Palm 1957, 1990). However, the rate and yield of hydrolysis drops significantly as the size of the alkyl group increases. For example, the rate of hydrolysis of tertiary butyl benzoate at 40 °C is less than half that of methyl benzoate at 25 °C. We have not measured the rate of hydrolytic formation of 3,5-dinitrobenzoic acid from SiDNB but suggest that it will be nil at physiologic conditions due to the massive size of the silica particle to which the precursor is attached. This should ensure that nitroaromatics would not become free molecular species in blood and add to the toxicity already due to overdosed 1 and 4.
Conclusion
The original goal of this research was to demonstrate the formation of charge transfer (π-π) complex between the important but toxic local anesthetic bupivacaine, a π-electron rich aromatic molecule, and an electron deficient aromatic acceptor molecule. This was done in order to establish a proof of concept that the charge transfer phenomenon could be utilized for the removal of the overdosed toxin by attaching the receptor to nanoparticles. By utilizing proton NMR spectroscopy primarily, π-π complexes were shown to form in solution, first between models of bupivacaine and several acceptors, and then between bupivacaine and the dinitrobenzoyl acceptors. The formation of complex was substantiated by UV-visible spectroscopy. The binding energy for bupivacaine complexed with N-ethyl-2,4-dinitrobenzamide was found to be exothermic with a value of ΔG = −395 cal · mol–1 from a binding constant of 1.9558 L · mol–1 determined from the NMR data. This research has shown that the concept of π-π complex formation is a viable method for the development of injectable antidotes for in vivo deployment for the remediation as of overdoses of bupivacaine and other chemical toxins.
Acknowledgements
The authors gratefully acknowledge the funding support provided by the National Science Foundation to the Engineering Research Center at the University of Florida, and of the New York State Science and Technology Foundation for partial funding of the Center for Advanced Materials Processing at Clarkson University. The use of facilities in the Departments of Chemistry at Clarkson University and the State University of New York at Potsdam is gratefully recognized.
References
- AdamsHBlancoJLJChessariG2001Quantitative determination of intermolecular interactions with fluorinated. aromatic ringsChem Eur J73494503
- BenesiHAHildebrandJH1949A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbonsJ Am Chem Soc712703
- BerkeleyPJJrHannaMW19631 N.M.R. Studies of Hydrogen bonding. I. Binary mixtures of Chloroform and Nitrogen basesJ Phys Chem674846
- BoalAKRotelloVM2000Fabrication and self-optimization of multivalent receptors on nanoparticle scaffoldsJ Am Chem Soc122734
- BrieglebG1961Electronen-donator-acceptor-komplexeBerlinSpringer-Verlag
- ChujoyTamakiR2001New preparation methods for organic-inorganic polymer hybridsMRS Bull5389
- ConstantinidisISatterleeJD1988aUV-visible and carbon NMR studies of quinine binding to urohemin I chloride and uroporphyrin I in aqueous solution (I)J Am Chem Soc110927
- ConstantinidisISatterleeJD1988bUV-visible and carbon NMR studies of chloroquine binding to urohemin I chloride and uroporphyrin I in aqueous solutions (I)J Am Chem Soc1104391
- DeranleauDA1969aTheory of the measurement of weak molecular complexes. II. Consequences of multiple equilibriaJ Am Chem Soc91154050
- DeranleauDA1969bTheory of the measurement of weak molecular complexes. I. General considerationsJ Am Chem Soc91154044
- DewarMJSLepleyAR1961n-Complexes. I. Charge transfer spectra on n-complexes formed by trinitrobenzene with Polycyclic Aromatic CompoundsJ Am Chem Soc834560
- DomszyJGRobertsGAF1985Reactions of chitosan: 5. The reaction of chitosan with 2,4 dinitrofluorobenzene and determination of the extent of the reactionInt J Biol Macrommol745
- DustJM1992Charge transfer and electron transfer process in biologically significant systems. 1. Charge transfer complex formation between 1,3,5-trinitrobenzene and N,N dimethyl-3,4-di-O-methyldopamine, a. dopamine analogueCan J Chem70151
- FesikSMedekAHajdukP2000The use of differential chemical shifts for determining the binding site location and orientation of protein-bound ligandsJ Am Chem Soc1221241
- FosterRFyfeC1965Interactions of electron acceptors with bases. XV. Determination of association constants of organic charge-transfer complexes by N.M.R spectroscopyTrans Faraday Soc61512162631
- HannaMWAshbaughAL1964Nuclear magnetic resonance study of molecular complexes of 7,7,8,8-Tetracyanoquinodimethane and aromatic donorsJ Phys Chem684811
- HansenOR1962Hammett series with biological activityActa Chem Scand161593600
- HugginsCMPimentelGC1955Proton magnetic resonance studies of chloroform in solution: evidence for hydrogen bondingJ Chem Phys2371244
- HunterCA1994Meldola lecture. The role of aromatic interactions in molecular recognitionChem Soc Rev23101109
- HunterCALawsonKRPerkinsJ2001Aromatic interactionsJ Chem Soc Perkin Trans2651
- IshidaTIbeSInoueM1984X-Ray crystallographic, spectroscopic, and energy calculation studies of the 3-carbamoyl-1-[3-(indol-3-yl)propyl]pyridinium ion, a charge-transfer interaction model between tryptophan and pyridine coenzymesJ Chem Soc Perkin Trans2297
- LandauerJMcConnellH1952A study of molecular complexes formed by aniline and aromatic nitrohydrocarbonsJ Am Chem Soc741221
- LawreyDMGMcConnellH1952A spectroscopic study of the Benzene-s – trinitrobenzene molecular complex. A criterion for reliability of formation constants of weak complexesJ Am Chem Soc746175
- LeeD-WFlintJMoreyT2005Aromatic-aromatic interaction of amitriptyline: Implication of overdosed drug detoxificationJ Pharm Sci9423738115614810
- MeyerEACastellanoRKDiederichF2003Interactions with aromatic rings in chemical and biological recognitionAngew Chem Int Ed42111210
- MullikenRS1952Molecular compounds and their spectra. III. The interaction of electron donors and acceptorsJ Phys Chem56801
- PalmV1975 1990 Tables of Rates and Equilibrium Constants of Heterolytic Organic Reactions Vol I ; Supp Vol VI(I): Tarta state University Press
- PartchRPowellELeeY-H2003Injectable Nanoparticle Technology for in vivo Remediation of Overdosed ToxinsGogotsiYGUvarovaIVNanostructured Materials and Coatings for Biomedical and Sensor ApplicationsNetherlandsKluwer Academic Publishers2740
- PartchRPowellELeeY-H2006Surface Modification of Dispersed Phases Designed for In Vivo Removal of Overdosed ToxinsSpasicAMHsuJ-PFinely Dispersed Particles Surfactant Science SeriesVol 130Boca RatonCRC Press81332
- PersonWB1965A criterion for reliability of formation constants of weak complexesJ Am Chem Soc872167
- PowellE2004Syntheses, Characterization and Applications of Surface Modified Core Particles for Heightened Physical and Medicinal Properties PhD. Dissertation. Part 2Clarkson UniversityPotsdam, NY13699
- SahaiRLoperGLLinSH1974Investigation of the composition and formation constant of molecular complexesProc Nat Acad Sci USA714149916592155
- SinnokrotMOValeevEFSherrillCD2002Estimates of the Ab Initio limit for π-π interactions: The Benzene dimerJ Am Chem Soc1241088712207544
- SlifkinMA1981The significance of charge transfer interactions in biologyMolecular Interactions2271
- ThayerA2006Fabulous fluorineChem and Eng News842315
- VarshneyMMoreyTEShahDO2004Pluronic microemulsions as nanoreservoirs for extraction of Bupivacaine from normal salineJ Am Chem Soc126510815099093
- VerhoevenJWVerhoeven-SchoffA-MAMassonA1974The use of Paraquat as an NMR and charge-transfer-probe for solvent-exposed aromatic amino-acid side-chainsHelv Chim Acta5725034443291
- WilkinsonGR2001Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, and EliminationHardmanJGLimbirdLEGoodman and Gilman’s The Pharmacological Basis of Therapeutics10th edNew YorkMcGraw-Hill329