Abstract
Background:
Nanotechnology explores a variety of promising approaches in the area of material sciences on a molecular level, and silver nanoparticles (AgNPs) are of leading interest in the present scenario. This review is a comprehensive contribution in the field of green synthesis, characterization, and biological activities of AgNPs using different biological sources.
Methods:
Biosynthesis of AgNPs can be accomplished by physical, chemical, and green synthesis; however, synthesis via biological precursors has shown remarkable outcomes. In available reported data, these entities are used as reducing agents where the synthesized NPs are characterized by ultraviolet-visible and Fourier-transform infrared spectra and X-ray diffraction, scanning electron microscopy, and transmission electron microscopy.
Results:
Modulation of metals to a nanoscale drastically changes their chemical, physical, and optical properties, and is exploited further via antibacterial, antifungal, anticancer, antioxidant, and cardioprotective activities. Results showed excellent growth inhibition of the microorganism.
Conclusion:
Novel outcomes of green synthesis in the field of nanotechnology are appreciable where the synthesis and design of NPs have proven potential outcomes in diverse fields. The study of green synthesis can be extended to conduct the in silco and in vitro research to confirm these findings.
Introduction
Nanotechnology offers fields with effective applications, ranging from traditional chemical techniques to medicinal and environmental technologies. AgNPs have emerged with leading contributions in diverse applications, such as drug delivery,Citation31 ointments, nanomedicine,Citation37 chemical sensing,Citation41 data storage,Citation47 cell biology,Citation54 agriculture, cosmetics,Citation60 textiles,Citation17 the food industry, photocatalytic organic dye–degradation activity,Citation64 antioxidants,Citation66 and antimicrobial agents.Citation68
Despite the contradictions reported on the toxicity of AgNPs,Citation69 its role as a disinfectant and antimicrobial agent has been given considerable appreciation. The available documented dataCitation73,Citation74 and the interest of the community in this field prompted us to work on plant-mediated green synthesis and biological activities of AgNPs.
Different types of nanoparticles
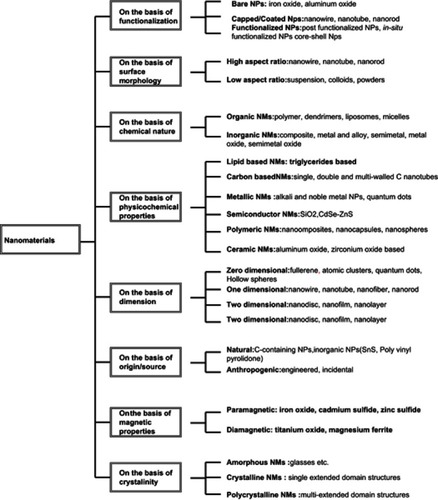
Some distinctive reported forms of nanoparticles (NPs) are core–shell NPs,Citation76 photochromic polymer NPs,Citation78 polymer-coated magnetite NPs,Citation80 inorganic NPs, AgNPs, CuNPs,Citation82 AuNPs,Citation85 PtNPs,Citation86 PdNPs,Citation88 SiNPs,Citation89 and NiNPs,Citation91 while others are metal oxide and metal dioxide NPs, such as ZnONPs,Citation94 CuO NPs,Citation95 FeO,Citation97 MgONPs,Citation100 TiO2 NPs,Citation102 CeO2 NPs,Citation103 and ZrO2 NPs.Citation104 Each of these has an exclusive set of characteristics and applications, and can be synthesized by either conventional or unconventional methods. An extensive classification of NPs is provided in .Citation105–Citation111
Nanoparticle synthesis
Comprehensive approaches available for NP synthesis are bottom-up and top-down.Citation112 The latter approach is immoderate and steady, whereas the former involves self-assembly of atomicsize particles to grow nanosize particles. This can be achieved by physical and chemical means,Citation113 as summarized in . However, ecofriendly green syntheses are economical, and proliferate and trigger stable NP formation, as shown in .
Table 1 Chemical and physical synthesis of AgNPs
Green approach (biological/conventional methods)
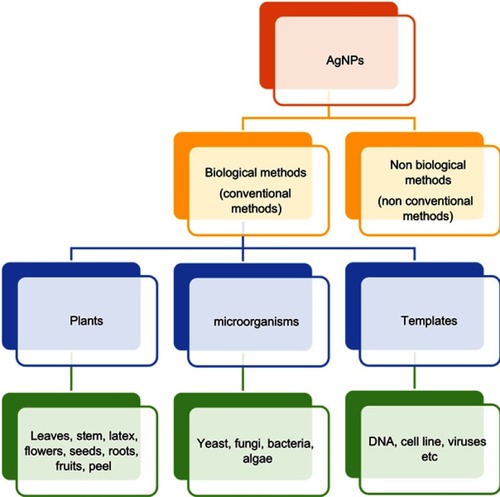
The surging popularity of green methods has triggered synthesis of AgNPs using different sources, like bacteria, fungi, algae, and plants, resulting in large-scale production with less contamination. Green synthesis is an ecofriendly and biocompatible process,Citation119 generally accomplished by using a capping agent/stabilizer (to control size and prevent agglomeration),Citation120 plant extracts, yeast, or bacteria.Citation121
Green synthesis using plant extracts
In contrast to microorganisms, plants have been exhaustively used,as apparent from . This is because plant phytochemicals show greater reduction and stabilization.Citation122 Eugenia jambolana leaf extract was used to synthesize AgNPs that indicated the presence of alkaloids, flavonoids, saponins, and sugar compounds.Citation123 Bark extract of Saraca asoca indicated the presence of hydroxylamine and carboxyl groups.Citation124 AgNPs using leaves of Rhynchotechum ellipticum were synthesized, and the results indicated the presence of polyphenols, flavonoids, alkaloids, terpenoids, carbohydrates, and steroids.Citation125 Hesperidinwas used to form AgNPs of 20–40 nm.Citation126 Phenolic compounds of pyrogallol and oleic acid were reported to be essential for the reduction of silver salt to form NPs.Citation127 Pepper-leaf extract acts as a reducing and capping agent in the formation of AgNPs of 5–60 nm.Citation128 Fruit extracts of Malus domestica acted as a reducing agent. Similarly, Vitis vinifera,Citation39 Andean blackberry,Citation129 Adansonia digitata,Citation130 Solanum nigrum,Citation131 Nitraria schoberiCitation132 or multiple fruit peels have also been reported for AgNP synthesis.Citation133 Combinations of plant extracts have also been reported.Citation134 Some other reductants used for AgNO3 are polysaccharide,Citation135 soluble starch,Citation136 natural rubber,Citation137 tarmac,Citation138 cinnamon,Citation25 stem-derived callus of green apple,Citation25 red apple,Citation139 egg white,Citation140 lemon grass,Citation141 coffee,Citation142 black tea,Citation143 and Abelmoschus esculentus juice.Citation144 Besides these, an extensive diagram representing different parts of different plant leaves, eg, peel, seed, fruit, bark, flower, stem, and root, also used in nanoformulations, is given in . Green synthesis is economical and innocuous.Citation30,Citation38,Citation150
Table 2 Plant-mediated synthesis of AgNPs
Biosynthesis using microorganisms
Bacteria-mediated synthesis of AgNPs
Microorganisms like fungi, bacteria, and yeast are of huge interest for NP synthesis; however, the process is threatened by culture contamination, lengthy procedures, and less control over NP size. NPs formed by microorganisms can be classified into distinct categories, depending upon the location where they are synthesized.Citation183 Otari et al synthesized AgNPs intracellularly using Actinobacteria Rhodococcussp. NCIM 2891.Citation184 Kannan et al biosynthesized AgNPs using Bacillus subtillus extracellularly.Citation185 provides some illustrative examples of the synthesis of AgNPs using different bacterial strains.
Table 3 Bacteria-mediated synthesis of AgNPs
Alga-mediated synthesis of AgNPs
A diverse group of aquatic microorganisms, algae have been used substantially and reported to synthesize AgNPs. They vary in size, from microscopic (picoplankton) to macroscopic (Rhodophyta). AgNPs were synthesized using microalgae Chaetoceros calcitrans, C. salina, Isochrysis galbana, and Tetraselmis gracilisCitation199 Cystophora moniliformis marine algae were used by Prasad et al as a reducing and stabilizing agent to synthesize AgNPs.Citation200 illustrates some examples of the micro and macro-algae used for AgNPs synthesis.
Table 4 Alga-mediated synthesis of AgNPs
Fungus-mediated synthesis of AgNPs
Extracellular synthesis of AgNPs using fungi is also a viable alternative, because of their economical large-scale production. Fungal strains are chosen over bacterial species, because of their better tolerance and metal-bioaccumulation property. gives some of the fungal strains used for AgNP synthesis.
Table 5 Fungus-mediated synthesis of AgNPs
Synthesis from miscellaneous sources
Nanotechnology has placed DNA on a recent drive to be used as a reducing agent.Citation215 Strong affinity of DNA bases for silver make it a template stabalizerCitation216 AgNPs were synthesized on DNA strands and found to be possibly located at N7 guanine and phosphate.Citation217 Another attempt was made with calf-thymus DNA to synthesize AgNPs.Citation218 Similarly, silver-binding peptides were identified and selected using a combinatorial approach for NP synthesis.Citation219
Bioactivities
Antibacterial activity of AgNPs
As a broad-spectrum antibiotic, silver is highly toxic to bacteria. It has been of great interest for the past couple of years, due to its wide spectrum of pharmacological activities, with applications in the fields of agriculture, textiles, and especially medicine. Some attributed contributions are given in .
Table 6 Antibacterial activities of AgNPs
Antifungal activity of AgNPs
Resistant pathogenic activities of bacteria and fungi have increased invasive infections at an alarming rate. Ultimately, the subsequent need is to find more potent antifungal agents. provides some examples from the literature that have reported antifungal properties of green synthesized AgNPs.
Table 7 Antifungal properties of AgNPs
Anticancer activity of AgNPs
The paramount need of today is the synthesis of effective anticancer treatment, as cardiovascular at the top most; cancer is the second most leading cause of human dysphoria. Therefore the synthesis of anticancer agents is of the utmost necessity. AgNPs also possess substantial anticancer activities,Citation239 as shown in .
Table 8 Anticancer property of AgNPs
Anti-inflammatory activity of AgNPs
AgNPs of 20–80 nm were synthesized using Sambucus nigra (blackberry) extract. The NPs were characterized using ultraviolet-visible and Fourier-transform infrared spectroscopy and X-ray diffraction, and further investigations were carried out for anti-inflammatory effects, both in vitro and in vivo, against Wister rats.Citation177
Antiviral activity of AgNPs
Multidimensional biological activities of AgNPs provide significant antiviral potentiality. HEPES buffer was used to synthesize NPs of 5–20 nm. Postinfection antiviral activity of AgNPs was evaluated using Hut/CCR5 cells using ELISA. AgNPs inhibited HIV1 retrovirus 17%–187% more than the reverse-transcriptase inhibitor azidothymidine triphosphateCitation245 Polysulfone-incorporated AgNPs manifested antiviral and antibacterial activity. This was attributed to the release of sufficient silver ions from the membrane, acting as an antiviral agent.Citation246
Cardioprotection
The medicinal herb neem (Millingtonia hortensis) has been used to synthesize AgNPs, and showed significant cardioprotective properties in rats.Citation178
Wound dressing
anotechnology has contributed significantly in the area of wound healing, as healing is attributed to increased anti-inflammatory and antimicrobial activity. A cotton fabric treated with NPs of size 22 nm exhibited potent healing power.Citation247 Another advance in this area was made with the impregnation of AgNPs into bacterial cellulose for antimicrobial wound dressing. Acetobacter xylinum (strain TISTR 975) was used to produce bacterial cellulose, which was immersed in silver nitrate solution. It was effective against both Gram-positive and Gram-negative bacteria.Citation248 The performance of a polymer is increased by the introduction of inorganic NPs. In this regard, polyurethane solution containing silver ions was reduced by dimethylformamide using electrospinning. Collagen was introduced to increase its hydrophilicity. This collagen sponge incorporatingd AgNPs had enhanced wound-healing ability in an animal model.Citation249 Most recently, Jacob et al biosynthesized nanoengineered tissue impregnated with AgNPs, which significantly prevented borne bacterial growth on the surface of tissue and could help in controlling health-associated infections.Citation250
Conclusion
Nature has its own coaching manners of synthesizing miniaturized functional materials. Increasing awareness of green chemistry and the benefit of synthesis of AgNPs using plant extracts can be ascribed to the fact that it is ecofriendly, low in cost, and provides maximum protection to human health. Green synthesized AgNPs have unmatched significance in the field of nanotechnology. AgNPs cover a wide spectrum of significant pharmacological activities, and the cost-effectiveness provides an alternative to local drugs. Besides plant-mediated green synthesis, special emphasis has also been placed on the diverse bioassays exhibited by AgNPs. This review will help researchers to develop novel AgNP-based drugs using green technology.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Prasad R, Swamy VS. Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J Nanopart. 2013;2013:6. doi:10.1155/2013/431218
- Isaac R, Sakthivel G, Murthy C. Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J Nanotechnol. 2013;2013:6. doi:10.1155/2013/906592
- Jagtap UB, Bapat VA. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind Crops Prod. 2013;46:132–137.
- Phanjom P, Borthakur M, Das R, Dey S, Bhuyan T. Green synthesis of silver nanoparticles using leaf extract of Amaranthus viridis. Int J Nanotechnol Appl. 2012;6:53–59.
- Ghoreishi SM, Behpour M, Khayatkashani M. Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary application in electrochemistry. Phys E. 2011;44(1):97–104.
- Suresh G, Gunasekar PH, Kokila D, et al. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim Acta A. 2014;127:61–66.
- Santhoshkumar T, Rahuman AA, Bagavan A, et al. Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculata and Rhipicephalus (Boophilus) microplus. Exp Parasitol. 2012;132(2):156–165.22750410
- Heydari R, Rashidipour M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): synthesis and in vitro cytotoxic effect on MCF-7 cells. Int J Breast Cancer. 2015;2015:6.
- Rajeshkumar S. Synthesis of silver nanoparticles using fresh bark of Pongamia pinnata and characterization of its antibacterial activity against gram positive and gram negative pathogens. Resour-Effic Technol. 2016;2(1):30–35. doi:10.1016/j.reffit.2016.06.003
- Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract. Korean J Chem Eng. 2008;25(4):808–811. doi:10.1007/s11814-008-0133-z
- Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 2016;58:36–43. doi:10.1016/j.msec.2015.08.018
- Sharma K, Kaushik S, Jyoti A. Green synthesis of silver nanoparticles by using waste vegetable peel and its antibacterial activities. J Pharm Sci Res. 2016;8(5):313.
- Moteriya P, Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotechnol. 2017;45(8):1556–1567. doi:10.1080/21691401.2016.126187127900878
- Rao NH, Lakshmidevi N, Pammi S, Kollu P, Ganapaty S, Lakshmi P. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater Sci Eng C. 2016;62:553–557. doi:10.1016/j.msec.2016.01.072
- Saddal SK, Telang T, Bhange VP, Kopulwar A, Santra S, Soni M. Green synthesis of silver nanoparticles using stem extract of Berberis aristata and to study its characterization and antimicrobial activity. J Pharm Res. 2018;12(6):840.
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45(7):1065–1071. doi:10.1016/j.procbio.2010.03.024
- Bharathi D, Josebin MD, Vasantharaj S, Bhuvaneshwari V. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J Nanostruct Chem. 2018;8(1):83–92. doi:10.1007/s40097-018-0256-7
- Baghizadeh A, Ranjbar S, Gupta VK, et al. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J Mol Liq. 2015;207:159–163. doi:10.1016/j.molliq.2015.03.029
- Rivera-Rangel RD, González-Muñoz MP, Avila-Rodriguez M, Razo-Lazcano TA, Solans C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf A. 2018;536:60–67. doi:10.1016/j.colsurfa.2017.07.051
- Dar P, Waqas U, Hina A, et al. DBiogenic synthesis, characterization of silver nanoparticles using multani mitti (Fullers Earth), tomato (Solanum lycopersicum) seeds, rice husk (Oryza sativa) and evaluation of their potential antimicrobial activity. J Chem Soc Pak. 2016;38(4):665–674.
- Aboutorabi SN, Nasiriboroumand M, Mohammadi P, Sheibani H, Barani H. Biosynthesis of silver nanoparticles using safflower flower: structural characterization, and its antibacterial activity on applied wool fabric. J Inorg Organomet Polym Mater. 2018;28(6):2525–2532. doi:10.1007/s10904-018-0925-5
- Karthiga P. Preparation of silver nanoparticles by Garcinia mangostana stem extract and investigation of the antimicrobial properties. Biotechnol Res Innovation. 2018;2(1):30–36. doi:10.1016/j.biori.2017.11.001
- Baskaran C, Ratha Bai V. Green synthesis of silver nanoparticles using Coleus forskohlii roots extract and its antimicrobial activity against Bacteria and Fungus. Int J Drug Dev Res. 2013;5(1):1–10.
- Swamy MK, Akhtar MS, Mohanty SK, Sinniah UR. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim Acta A. 2015;151:939–944.
- Saliem AH, Ibrahim OM, Salih SI. Biosynthesis of silver nanoparticles using cinnamon zeylanicum plants bark extract. مجلة الكوفة للعلوم الطبية البيطرية|Kufa J Vet Med Sci. 2016;7(1):51-63.
- Nazeruddin G, Prasad N, Prasad S, Shaikh Y, Waghmare S, Adhyapak P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind Crops Prod. 2014;60:212–216.
- Selvakumar P, Sithara R, Viveka K, Sivashanmugam P. Green synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in blood compatibility. J Photochem Photobiol B. 2018;182:52–61.29604554
- Bharathi D, Kalaichelvan P, Atmaram V, Anbu S. Biogenic synthesis of silver nanoparticles from aqueous flower extract of Bougainvillea spectabilis and their antibacterial activity. J Med Plants. 2016;4:248–252.
- Sreelakshmy V, Deepa M, Mridula P. Green synthesis of silver nanoparticles from glycyrrhiza glabra root extract for the treatment of gastric ulcer. J Dev Drugs. 2016;5(2):152.
- Tamileswari R, Haniff Nisha M, Jesurani S, et al. Synthesis of silver nanoparticles using the vegetable extract of raphanus sativus (Radish) and assessment of their antibacterial activity. Int J Adv Technol Eng Sci. 2015;3(5):207–212.
- Basu S, Samanta HS, Ganguly J. Green synthesis and swelling behavior of Ag-nanocomposite semi-IPN hydrogels and their drug delivery using Dolichos biflorus Linn. Soft Mater. 2018;16(1):7–19.
- Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int. 2013;2013:5.
- Gavade SM, Nikam G, Dhabbe R, Sabale S, Tamhankar B, Mulik G. Green synthesis of silver nanoparticles by using carambola fruit extract and their antibacterial activity. Adv Nat Sci. 2015;6(4):045015.
- Mittal AK, Kaler A, Banerjee UC. Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron dauricum. Nano Biomed Eng. 2012;4(3):118–124.
- Upadhyay P, Mishra SK, Purohit S, Dubey G, Singh Chauhan B, Srikrishna S. Antioxidant, antimicrobial and cytotoxic potential of silver nanoparticles synthesized using flavonoid rich alcoholic leaves extract of Reinwardtia indica. Drug Chem Toxicol. 2018;42(1):1–11.
- Rajagopal T, Jemimah IAA, Ponmanickam P, Ayyanar M. Synthesis of silver nanoparticles using Catharanthus roseus root extract and its larvicidal effects. J Environl Biol. 2015;36(6):1283.
- Carabineiro S. Applications of gold nanoparticles in nanomedicine: recent advances in vaccines. Molecules. 2017;22(5):857.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A. 2011;79(3):594–598.
- Roy K. ‘Green’synthesis of Silver Nanoparticles by Using Grape (Vitis Vinifera) Fruit Extract: Characterization of the Particles & Study of Antibacterial Activity. 2012.
- Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng C. 2016;58:44–52.
- Roy K, Biswas S, Banerjee PC. Synthesis of Silver nanoparticles by using grape (Vitis vinifera) fruit extract; characterization of the particles and study of antibacterial activity. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2013 ;4(1):1271–1278.
- Singh J, Dhaliwal AS. Novel green synthesis and characterization of the antioxidant activity of silver nanoparticles prepared from nepeta leucophylla root extract. Anal Lett. 2018;52(2):1–18.
- Mishra MP, Padhy RN. Antibacterial activity of green silver nanoparticles synthesized from Anogeissus acuminata against multidrug resistant urinary tract infecting bacteria in vitro and host-toxicity testing. J Appl Biomed. 2018;16(2):120–125.
- Baharara J, Namvar F, Ramezani T, Hosseini N, Mohamad R. Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules. 2014;19(4):4624–4634.24739926
- Ahmad N, Sharma S, Rai R. Rapid green synthesis of silver and gold nanoparticles using peels of Punica granatum. Adv Mater Lett. 2012;3(5):376–380.
- Mukunthan K, Balaji S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Intl J Green Nanotechnol. 2012;4(2):71–79.
- Kaur R, Singh J, Tripathi S. Incorporation of inorganic nanoparticles into an organic polymer matrix for data storage application. Current Appl Phys. 2017;17(5):756–762.
- Raut Rajesh W, Lakkakula Jaya R, Kolekar Niranjan S, Mendhulkar Vijay D, Kashid Sahebrao B. Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci. 2009;5(1):117–122.
- Mata R, Nakkala JR, Sadras SR. Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria alba (frangipani) flower extract. Mater Sci Eng C. 2015;51:216–225.
- Benakashani F, Allafchian A, Jalali SAH. Green synthesis, characterization and antibacterial activity of silver nanoparticles from root extract of Lepidium draba weed. Green Chem Lett Rev. 2017;10(4):324–330.
- Velayutham K, Rahuman AA, Rajakumar G, et al. Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac J Trop Med. 2013;6(2):95–101.23339909
- Kahrilas GA, Wally LM, Fredrick SJ, Hiskey M, Prieto AL, Owens JE. Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustainable Chem Eng. 2013;2(3):367–376.
- Gao Y, Huang Q, Su Q, Liu R. Green synthesis of silver nanoparticles at room temperature using kiwifruit juice. Spectrosc Lett. 2014;47(10):790–795.
- Abdal Dayem A, Hossain MK, Lee SB, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci. 2017;18(1):120.
- Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18(10):105104.
- Gogoi N, Babu PJ, Mahanta C, Bora U. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C. 2015;46:463–469.
- Kumar D, Kumar G, Agrawal V. Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall. bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol Res. 2018;117(2):377–389.29250727
- Bankar A, Joshi B, Kumar AR, Zinjarde S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A. 2010;368(1):58–63.
- Ulug B, Turkdemir MH, Cicek A, Mete A. Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim Acta A. 2015;135:153–161.
- Fukui H. Development of new cosmetics based on nanoparticles In: Naito M, Yokoyama T, Hosokawa K, Nogi K, editors. Nanoparticle Technology Handbook. 3rd ed. Elsevier; 2018:845–877. ISBN: 978-0-444-64110-6
- Sasikala A, Rao ML, Savithramma N, Prasad T. Synthesis of silver nanoparticles from stem bark of Cochlospermum religiosum (L.) Alston: an important medicinal plant and evaluation of their antimicrobial efficacy. Appl Nanosci. 2015;5(7):827–835.
- Erosa MSD, Díaz MMC, Lazcano TAR, Rodríguez MÁ, Aguilera JAR, Del Pilar González-Muñoz M. Aqueous leaf extracts of Cnidoscolus chayamansa (Mayan chaya) cultivated in Yucatán México. Part II: uses for the phytomediated synthesis of silver nanoparticles. Mater Sci Eng C. 2018;91:838–852.
- Vijayan R, Joseph S, Mathew B. Green synthesis of silver nanoparticles using Nervalia zeylanica leaf extract and evaluation of their antioxidant, catalytic, and antimicrobial potentials. Part Sci Technol. 2018;36:1–11.
- Fathima JB, Pugazhendhi A, Oves M, Venis R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J Mol Liq. 2018;260:1–8.
- Cruz D, Falé PL, Mourato A, Vaz PD, Serralheiro ML, Lino ARL. Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena). Colloids Surf B. 2010;81(1):67–73.
- Sharma P, Bhatt D, Zaidi M, Saradhi PP, Khanna P, Arora S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol. 2012;167(8):2225–2233.22692847
- Ashokkumar S, Ravi S, Velmurugan S. Green synthesis of silver nanoparticles from Gloriosa superba L. leaf extract and their catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:388–392.23860402
- Zhang J, Si G, Zou J, Fan R, Guo A, Wei X. Antimicrobial Effects of Silver Nanoparticles Synthesized by Fatsia japonica Leaf Extracts for Preservation of Citrus Fruits. J Food Sci. 2017;82(8):1861–1866.28727146
- McGillicuddy E, Murray I, Kavanagh S, et al. Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ. 2017;575:231–246.27744152
- Ashokkumar S, Ravi S, Kathiravan V, Velmurugan S. Synthesis, characterization and catalytic activity of silver nanoparticles using Tribulus terrestris leaf extract. Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:88–93.24231743
- Kumar PSM, MubarakAli D, Saratale RG, et al. Synthesis of nano-cuboidal gold particles for effective antimicrobial property against clinical human pathogens. Microb Pathog. 2017;113:68–73.29056495
- Philip D, Unni C, Aromal SA, Vidhu V. Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta A. 2011;78(2):899–904.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346.20818017
- Vijayan SR, Santhiyagu P, Ramasamy R, et al. Seaweeds: a resource for marine bionanotechnology. Enzyme Microb Technol. 2016;95:45–57.27866626
- Veerasamy R, Xin TZ, Gunasagaran S, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15(2):113–120.
- Chaudhuri RG, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012;112(4):2373–2433.22204603
- Philip D. Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim Acta A. 2011;78(1):327–331.
- Zhu M-Q, Zhu L, Han JJ, Wu W, Hurst JK, Li AD. Spiropyran-based photochromic polymer nanoparticles with optically switchable luminescence. J Am Chem Soc. 2006;128(13):4303–4309.16569006
- Arunachalam KD, Annamalai SK, Hari S. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int J Nanomedicine. 2013;8(3):1307–1315.23569372
- Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116(9):5338–5431.27109701
- Singh P, Kim YJ, Yang DC. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif Cells Nanomed Biotechnol. 2016;44(8):1949–1957.26698271
- Dhoble SM, Kulkarni NS. Investigation of in vitro and in vivo atifungal property of biologically synthesized copper nanoparticles (CuNP) against rhizoctonia solani a phytopathogen of soyaabean (Glycine max, L. Merrill). Int J Eng Sci Generic Res. 2018;4(5):17–30.
- Wang C, Mathiyalagan R, Kim YJ, et al. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int J Nanomedicine. 2016;11:3691.27570451
- Payne JN, Waghwani HK, Connor MG, et al. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front Microbiol. 2016;7:607.27330535
- Hubbuch J. Novel self patent gold nanoparticles for antineoplastic activity: poster presented at: posters-at-capitol wisteren kenpucky university; march 3; 2016: United state of America. Available from:https://digitalcommons.murraystate.edu/postersatthecapitol/2016/WKU/10/
- Kumar PV, Kala SMJ. Green Synthesis, Characterisation and Biological Activity of Platinum Nanoparticle Using Croton Caudatus Geisel Leaf Extract. Int J Recent Res Aspects. (Special Issue:Conscientious Computing Technologies). 2018. 608-612
- Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett. 2012;76:18–20.
- Molaei R, Farhadi K, Forough M, Hajizadeh S. Green Biological Fabrication and Characterization of Highly Monodisperse Palladium Nanoparticles Using Pistacia Atlantica Fruit Broth. J Nanostruct. 2018;8(1):47–54.
- Liong M, Lu J, Tamanoi F, Zink JI, Nel A. Mesoporous silica nanoparticles for biomedical applications. Google Patents; 2018.
- Kesharwani J, Yoon KY, Hwang J, Rai M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: hypothetical mechanism involved in synthesis. J Bionanosci. 2009;3(1):39–44.
- Woodard A, Xu L, Barragan AA, Nava G, Wong BM, Mangolini L. On the non-thermal plasma synthesis of nickel nanoparticles. Plasma Processes Polym. 2018;15(1):1700104.
- Zayed MF, Eisa WH, Shabaka A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta A. 2012;98:423–428.
- Karuppiah M, Rajmohan R. Green synthesis of silver nanoparticles using Ixora coccinea leaves extract. Mater Lett. 2013;97:141–143.
- Raja A, Ashokkumar S, Marthandam RP, et al. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J Photochem Photobiol B. 2018;181:53–58.29501725
- Uozumi Y, Kim K. Synthesis of Imidazo [1, 2-a] pyrimidines by A3-Coupling on CuO Nanoparticles. Synfacts. 2018;14(08):0883.
- Baharara J, Ramezani T, Hosseini N, Mousavi M. Silver Nanoparticles Synthesized Coating with Zataria Multiflora Leaves Extract Induced Apoptosis in HeLa Cells Through p53 Activation. Ijpr. 2018;17(2):627.29881420
- Muthukumar H, Mohammed SN, Chandrasekaran N, Sekar AD, Pugazhendhi A, Matheswaran M. Effect of iron doped Zinc oxide nanoparticles coating in the anode on current generation in microbial electrochemical cells. Int J Hydrogen Energy. 2018.
- Yakop F, Abd Ghafar SA, Yong YK, et al. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif Cells Nanomed Biotechnol. 2018:1–9.
- Fard SE, Tafvizi F, Torbati MB. Silvernanoparticles biosynthesisedusingCentella asiaticaleaf extract: apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. 2018;12(7):994–1002. doi:10.1049/iet-nbt.2018.506930247143
- Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B. 2019;190:86–97. doi:10.1016/j.jphotobiol.2018.11.01430504053
- Jha AK, Prasad K. Green synthesis of silver nanoparticles using Cycas leaf. Int J Green Nanotechnol. 2010;1(2):P110–P117. doi:10.1080/19430871003684572
- Goutam SP, Saxena G, Singh V, Yadav AK, Bharagava RN, Thapa KB. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem Eng J. 2018;336:386–396. doi:10.1016/j.cej.2017.12.029
- Sisubalan N, Ramkumar VS, Pugazhendhi A, et al. ROS-mediated cytotoxic activity of ZnO and CeO 2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ Sci Pollut Res. 2017:25(11):10482-10492.
- Fathima JB, Pugazhendhi A, Venis R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb Pathog. 2017;110:245–251. doi:10.1016/j.micpath.2017.06.03928666841
- Larson JK, Carvan MJ, Hutz RJ. Engineered nanomaterials: an emerging class of novel endocrine disruptors. Biol Reprod. 2014;91(1):20. doi:10.1095/biolreprod.114.12143424899576
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–1074. doi:10.3762/bjnano.9.9829719757
- Pachapur V, Brar SK, Verma M, Surampalli RY. Nanomaterials in the Environment. In: Nano-Ecotoxicology of Natural and Engineered Nanomaterials for Animals and Humans. American society of civil engineering library. 2015:421-437.
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2017. doi:10.1016/j.arabjc.2017.05.011
- Ranjit K, Baquee AA. Nanoparticle: an overview of preparation, characterization and application. Int. Res. J. Pharm.. 2013;4(4):47–57.
- Bhatia S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae. Springer International Publishing. HolderSpringer International Publishing. Switzerland. 2016;1:33-93.
- Sanjay SS, Pandey AC. A brief manifestation of nanotechnology In: Ashutosh Kumar Shukla, editor. EMR/ESR/EPR Spectroscopy for Characterization of Nanomaterials. Springer; 2017:47–63.
- Arole V, Munde S. Fabrication of Nanomaterials by Top-down and Bottom-up Approaches- An overview. J Adv Appl Sci Technol. 2014;1(2):89–93.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262. doi:10.1016/j.nano.2009.07.00219616126
- Ali SW, Rajendran S, Joshi M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr Polym. 2011;83(2):438–446. doi:10.1016/j.carbpol.2010.08.004
- Tejamaya M, RöMer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46(13):7011–7017. doi:10.1021/es203859622432856
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. doi:10.1128/AEM.02218-0617261510
- Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22):225103. doi:10.1088/0957-4484/18/49/495102
- Dubas ST, Kumlangdudsana P, Potiyaraj P. Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf A. 2006;289(1):105–109. doi:10.1016/j.colsurfa.2006.04.012
- Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi:10.1016/j.jare.2015.02.00726843966
- Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–13941. doi:10.1021/ja029267j14611213
- Daphne J, Francis A, Mohanty R, Ojha N, Das N. Green Synthesis of Antibacterial Silver Nanoparticles using Yeast Isolates and its Characterization. Res J Pharm Technol. 2018;11(1):83–92. doi:10.5958/0974-360X.2018.00016.1
- Mohamad NAN, Arham NA, Jai J, Hadi A. Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Advanced Materials Research. 2014;832: 350-355.
- Gomathi S, Firdous J, Bharathi V, et al. Phytochemical screening of silver nanoparticles extract of Eugenia jambolana using Fourier infrared spectroscopy. Int J Res Pharm Sci. 2017;8(3):383–387.
- Banerjee P, Nath D. A phytochemical approach to synthesize silver nanoparticles for non-toxic biomedical application and study on their antibacterial efficacy. Nanosci Technol. 2015;2(1):1–14.
- Hazarika D, Phukan A, Saikia E, Chetia B. Phytochemical screening and synthesis of silver nanoparticles using leaf extract of Rhynchotechum ellipticum. Int J Pharm Pharm Sci. 2014;6(1):672–674.
- Stephen A, Seethalakshmi S. Phytochemical synthesis and preliminary characterization of silver nanoparticles using hesperidin. J Nanosci. 2013;2013:5. doi:10.1155/2013/126564
- Martínez-Bernett D, Silva-Granados A, Correa-Torres S, Herrera A. Chromatographic analysis of phytochemicals components present in mangifera indica leaves for the synthesis of silver nanoparticles by AgNO3 reduction. Paper presented at: Journal of Physics: Conference Series Vol. 687; 2016.
- Mallikarjuna K, Sushma NJ, Narasimha G, Manoj L, Raju BDP. Phytochemical fabrication and characterization of silver nanoparticles by using Pepper leaf broth. Arabian J Chem. 2014;7(6):1099–1103. doi:10.1016/j.arabjc.2012.04.001
- Kumar B, Smita K, Cumbal L, Debut A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J Biol Sci. 2017;24(1):45–50. doi:10.1016/j.sjbs.2015.09.00628053570
- Kumar CMK, Yugandhar P, Savithramma N. Biological synthesis of silver nanoparticles from Adansonia digitata L. fruit pulp extract, characterization, and its antimicrobial properties. J Intercult Ethnopharmacol. 2016;5(1):79. doi:10.5455/jice.27069729
- Malaikozhundan B, Vijayakumar S, Vaseeharan B, et al. Two potential uses for silver nanoparticles coated with Solanum nigrum unripe fruit extract: biofilm inhibition and photodegradation of dye effluent. Microb Pathog. 2017;111:316–324. doi:10.1016/j.micpath.2017.08.03928867634
- Rad MS, Rad JS, Heshmati GA, Miri A, Sen DJ. Biological synthesis of gold and silver nanoparticles by Nitraria schoberi fruits. Open J Adv Drug Delivery. 2013;1(2):174–179.
- Naganathan K, Thirunavukkarasu S. Green way genesis of silver nanoparticles using multiple fruit peels waste and its antimicrobial, anti-oxidant and anti-tumor cell line studies. Paper presented at 2nd International Conference on Mining, Material and Metallurgical Engineering: IOP Conference Series: Materials Science and Engineering191; 2017. doi:10.1088/1757-899X/191/1/012009
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79. doi:10.1007/s00449-008-0224-618438688
- Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr Res. 2004;339(15):2627–2631. doi:10.1016/j.carres.2004.08.00515476726
- Vigneshwaran N, Nachane R, Balasubramanya R, Varadarajan P. A novel one-pot ‘green’synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341(12):2012–2018. doi:10.1016/j.carres.2006.04.04216716274
- Guidelli EJ, Ramos AP, Zaniquelli MED, Baffa O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim Acta A. 2011;82(1):140–145. doi:10.1016/j.saa.2011.07.024
- Shameli K, Ahmad MB, Zamanian A, et al. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int J Nanomedicine. 2012;7:5603. doi:10.2147/IJN.S3063123341739
- Umoren S, Obot I, Gasem Z. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci. 2014;5:907–914.
- Lu R, Yang D, Cui D, Wang Z, Guo L. Egg white-mediated green synthesis of silver nanoparticles with excellent biocompatibility and enhanced radiation effects on cancer cells. Int J Nanomed. 2012;7:2101. doi:10.2147/IJN.S30631
- Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP. Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011;3(3):189–194. doi:10.1007/BF03353671
- Nadagouda MN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008;10(8):859–862. doi:10.1039/b804703k
- Begum NA, Mondal S, Basu S, Laskar RA, Mandal D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf B. 2009;71(1):113–118. doi:10.1016/j.colsurfb.2009.01.012
- Pande N, Jaspal DK, Ambekar J. Ecofriendly synthesis and applications of silver nanoparticles. J Chem Pharm Res. 2014;6(9):403–410.
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4(1):29. doi:10.1186/2228-5547-4-29
- Sulaiman GM, Mohammed WH, Marzoog TR, Al-Amiery AAA, Kadhum AAH, Mohamad AB. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac J Trop Biomed. 2013;3(1):58–63. doi:10.1016/S2221-1691(13)60024-623570018
- Mousavi B, Tafvizi F, Zaker Bostanabad S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif Cells Nanomed Biotechnol. 2018;46(11):1–12. doi:10.1080/21691401.2018.1430697
- Kumara V, Vermab S, Choudhuryc S, Tyagid M, Chatterjee S, Variyara PS. Biocompatible silver nanoparticles from vegetable waste: its characterization and bio-efficacy. Int J Nano Matl Sci. 2015;4(1):70–86.
- Sathishkumar M, Sneha K, Won S, Cho C-W, Kim S, Yun Y-S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B. 2009;73(2):332–338. doi:10.1016/j.colsurfb.2009.06.005
- Ojha S. Green Synthesis of Metallic Nanoparticles Using Leaf Extract of Selected Silkworm Host Plants and Their Applications. Indian institute of technology Guwahati. Department of Biosciences and Bioengineering; 2018.
- Jebakumar Immanuel Edison TN, Sethuraman MG. Electrocatalytic reduction of benzyl chloride by green synthesized silver nanoparticles using pod extract of Acacia nilotica. ACS Sustainable Chem Eng. 2013;1(10):1326–1332. doi:10.1021/sc4001725
- Mallikarjuna K, Narasimha G, Dillip G, et al. Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Dig J Nanomater Biostruct. 2011;6(1):181–186.
- Satyavani K, Ramanathan T, Gurudeeban S. Plant mediated synthesis of biomedical silver nanoparticles by using leaf extract of Citrullus colocynthis. Res J Nanosci Nanotechnol. 2011;1(2):95–101. doi:10.3923/rjnn.2011.95.101
- Arunachalam R, Dhanasingh S, Kalimuthu B, Uthirappan M, Rose C, Mandal AB. Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf B. 2012;94:226–230. doi:10.1016/j.colsurfb.2012.01.040
- Gopinath K, Gowri S, Arumugam A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostruct Chem. 2013;3(1):68. doi:10.1186/2193-8865-3-68
- Vanaja M, Annadurai G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci. 2013;3(3):217–223. doi:10.1007/s13204-012-0121-9
- Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A. 2009;348(1):212–216. doi:10.1016/j.colsurfa.2009.07.021
- Kathiravan V, Ravi S, Ashokkumar S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta A. 2014;130:116–121. doi:10.1016/j.saa.2014.03.107
- Li S, Shen Y, Xie A, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9(8):852–858. doi:10.1039/b615357g
- Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47(12):2405–2410. doi:10.1016/j.procbio.2012.09.025
- Loo YY, Chieng BW, Nishibuchi M, Radu S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int J Nanomedicine. 2012;7:4263. doi:10.2147/IJN.S3063122904632
- Ajitha B, Reddy YAK, Reddy PS. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater Sci Eng C. 2015;49:373–381. doi:10.1016/j.msec.2015.01.035
- Sathyavathi R, Krishna MB, Rao SV, Saritha R, Rao DN. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett. 2010;3(2):138–143. doi:10.1166/asl.2010.1099
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol Prog. 2006;22(2):577–583. doi:10.1021/bp050142316599579
- Elavazhagan T, Arunachalam KD. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed. 2011;6:1265–1278. doi:10.2147/IJN.S18347
- Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys E. 2010;42(5):1417–1424. doi:10.1016/j.physe.2009.11.081
- Reddy NJ, Vali DN, Rani M, Rani SS. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater Sci Eng C. 2014;34:115–122. doi:10.1016/j.msec.2013.08.039
- Mallikarjuna K, Balasubramanyam K, Narasimha G, Kim H. Phyto-synthesis and antibacterial studies of bio-based silver nanoparticles using Sesbania grandiflora (Avisa) leaf tea extract. Mater Res Express. 2018;5(1):015054. doi:10.1088/2053-1591/aaa67d
- Vasanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B. 2014;117:354–359. doi:10.1016/j.colsurfb.2014.02.052
- Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B. 2013;108:80–84. doi:10.1016/j.colsurfb.2013.02.033
- Prabhu D, Arulvasu C, Babu G, Manikandan R, Srinivasan P. Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochem. 2013;48(2):317–324. doi:10.1016/j.procbio.2012.12.013
- Rajaram K, Aiswarya D, Sureshkumar P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater Lett. 2015;138:251–254. doi:10.1016/j.matlet.2014.10.017
- Kumar HAK, Mandal BK, Kumar KM, et al. Antimicrobial and antioxidant activities of Mimusops elengi seed extract mediated isotropic silver nanoparticles. Spectrochim Acta A. 2014;130:13–18. doi:10.1016/j.saa.2014.03.024
- Kumar DA, Palanichamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A. 2014;127:168–171. doi:10.1016/j.saa.2014.02.058
- Nabikhan A, Kandasamy K, Raj A, Alikunhi NM. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B. 2010;79(2):488–493. doi:10.1016/j.colsurfb.2010.05.018
- Muniyappan N, Nagarajan N. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 2014;49(6):1054–1061. doi:10.1016/j.procbio.2014.03.015
- David L, Moldovan B, Vulcu A, et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf B. 2014;122:767–777. doi:10.1016/j.colsurfb.2014.08.018
- Savitha R, Saraswathi U. A study on the preventive effect of silver nano particles synthesized from millingtonia hortensis in isoproterenol induced cardio toxicity in male wistar rats. World J Pharm Pharm Sci. 2016;5(8):1442–1450.
- Atale N, Saxena S, Nirmala JG, Narendhirakannan R, Mohanty S, Rani V. Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: a green approach. Appl Biochem Biotechnol. 2017;181(3):1140–1154. doi:10.1007/s12010-016-2274-627734287
- Chitra G, Balasubramani G, Ramkumar R, Sowmiya R, Perumal P. Mukia maderaspatana (Cucurbitaceae) extract-mediated synthesis of silver nanoparticles to control Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2015;114(4):1407–1415. doi:10.1007/s00436-015-4320-725601441
- Santhoshkumar T, Rahuman AA, Rajakumar G, et al. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108(3):693–702. doi:10.1007/s00436-010-2115-420978795
- Gnanadesigan M, Anand M, Ravikumar S, et al. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med. 2011;4(10):799–803. doi:10.1016/S1995-7645(11)60197-122014736
- Li X, Xu H, Chen Z-S, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:16. doi:10.1155/2011/270974
- Otari S, Patil R, Ghosh S, Thorat N, Pawar S. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim Acta A. 2015;136:1175–1180. doi:10.1016/j.saa.2014.10.003
- Kannan N, Subbalaxmi S. Green synthesis of silver nanoparticles using Bacillus subtillus IA751 and its antimicrobial activity. Res J Nanosci Nanotechnol. 2011;1(2):87–94. doi:10.3923/rjnn.2011.87.94
- Malarkodi C, Rajeshkumar S, Paulkumar K, Gnanajobitha G, Vanaja M, Annadurai G. Bacterial synthesis of silver nanoparticles by using optimized biomass growth of Bacillus sp. J Nanosci Nanotechnol. 2013;3:26–32.
- Hallol M. Studies on Bacterial Synthesis of Silver Nanoparticles Using Gamma Radiation and Their Activity against Some Pathogenic Microbes. Cairo (Egypt): Department of Microbiology and Immunology, Cairo University; 2013.
- Das VL, Thomas R, Varghese RT, Soniya E, Mathew J, Radhakrishnan E. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. 2014;4(2):121–126. doi:10.1007/s13205-013-0130-8
- Saravanan C, Rajesh R, Kaviarasan T, Muthukumar K, Kavitake D, Shetty PH. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol Rep. 2017;15:33–40. doi:10.1016/j.btre.2017.02.006
- Gandhi H, Khan S. Biological synthesis of silver nanoparticles and its antibacterial activity. J Nanomed Nanotechnol. 2016;7(2):366. doi:10.4172/2157-7439.1000366
- Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B. 2010;81(1):358–362. doi:10.1016/j.colsurfb.2010.07.036
- Sintubin L, De Windt W, Dick J, et al. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol. 2009;84(4):741–749. doi:10.1007/s00253-009-2032-619488750
- Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi -A-A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. 2007;42(5):919–923. doi:10.1016/j.procbio.2007.02.005
- Parikh RY, Ramanathan R, Coloe PJ, et al. Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One. 2011;6(6):e21401. doi:10.1371/journal.pone.002140121713008
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B. 2009;74(1):328–335. doi:10.1016/j.colsurfb.2009.07.048
- Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011;46(9):1800–1807. doi:10.1016/j.procbio.2011.06.008
- Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009;5(4):452–456. doi:10.1016/j.nano.2009.01.01219523420
- Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi:10.1016/j.micpath.2018.01.03829407231
- Merin DD, Prakash S, Bhimba BV. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac J Trop Med. 2010;3(10):797–799. doi:10.1016/S1995-7645(10)60191-5
- Prasad TN, Kambala VSR, Naidu R. Phyconanotechnology: synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J Appl Phycol. 2013;25(1):177–182. doi:10.1007/s10811-012-9851-z
- Govindaraju K, Kiruthiga V, Kumar VG, Singaravelu G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J Nanosci Nanotechnol. 2009;9(9):5497–5501.19928252
- Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci. 2015;5(4):499–504. doi:10.1007/s13204-014-0341-2
- El-Rafie H, El-Rafie M, Zahran M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym. 2013;96(2):403–410. doi:10.1016/j.carbpol.2013.03.07123768580
- Kannan RRR, Arumugam R, Ramya D, Manivannan K, Anantharaman P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl Nanosci. 2013;3(3):229–233. doi:10.1007/s13204-012-0125-5
- Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi:10.1016/j.micpath.2017.11.01329146498
- Ahmad A, Mukherjee P, Senapati S, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B. 2003;28(4):313–318. doi:10.1016/S0927-7765(02)00174-1
- Mukherjee P, Ahmad A, Mandal D, et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1(10):515–519. doi:10.1021/nl0155274
- Bhainsa KC, D‘Souza S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B. 2006;47(2):160–164. doi:10.1016/j.colsurfb.2005.11.026
- Kathiresan K, Manivannan S, Nabeel M, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B. 2009;71(1):133–137. doi:10.1016/j.colsurfb.2009.01.016
- Vigneshwaran N, Ashtaputre N, Varadarajan P, Nachane R, Paralikar K, Balasubramanya R. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61(6):1413–1418. doi:10.1016/j.matlet.2006.07.042
- Basavaraja S, Balaji S, Lagashetty A, Rajasab A, Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull. 2008;43(5):1164–1170. doi:10.1016/j.materresbull.2007.06.020
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5(4):382–386. doi:10.1016/j.nano.2009.06.00519616127
- Banu A, Rathod V, Ranganath E. Silver nanoparticle production by Rhizopus stolonifer and its antibacterial activity against extended spectrum β-lactamase producing (ESBL) strains of enterobacteriaceae. Mater Res Bull. 2011;46(9):1417–1423. doi:10.1016/j.materresbull.2011.05.008
- Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog. 2018;117:68–72. doi:10.1016/j.micpath.2018.02.00829427709
- Wei G, Zhou H, Liu Z, et al. One-step synthesis of silver nanoparticles, nanorods, and nanowires on the surface of DNA network. J Phys Chem B. 2005;109(18):8738–8743. doi:10.1021/jp044314a16852035
- Nithyaja B, Misha H, Nampoori V. Synthesis of silver nanoparticles in DNA template and its influence on nonlinear optical properties. Nanosci Nanotechnol. 2012;2(4):99–103. doi:10.5923/j.nn.20120204.02
- Kasyanenko N, Varshavskii M, Ikonnikov E, et al. DNA modified with metal nanoparticles: preparation and characterization of ordered metal-DNA nanostructures in a solution and on a substrate. J Nanomater. 2016;2016:12. doi:10.1155/2016/3237250
- Dai S, Zhang X, Li T, Du Z, Dang H. Preparation of silver nanopatterns on DNA templates. Appl Surf Sci. 2005;249(1–4):346–353. doi:10.1016/j.apsusc.2004.12.026
- Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Biomimetic synthesis and patterning of silver nanoparticles. Nat Mater. 2002;1(3):169. doi:10.1038/nmat75812618805
- Patil MP, Singh RD, Koli PB, et al. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb Pathog. 2018;121:184–189. doi:10.1016/j.micpath.2018.05.04029807133
- Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5(1):33–40. doi:10.2217/nnm.09.7720025462
- Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18(4):356–363. doi:10.1016/j.jscs.2013.09.011
- Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B. 2012;98:112–119. doi:10.1016/j.colsurfb.2012.04.006
- Bindhu M, Umadevi M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim Acta A. 2015;135:373–378. doi:10.1016/j.saa.2014.07.045
- Ghosh S, Patil S, Ahire M, et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomed. 2012;7:483. doi:10.2147/IJN.S30631
- Manikandan R, Manikandan B, Raman T, et al. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim Acta A. 2015;138:120–129. doi:10.1016/j.saa.2014.10.043
- Patil RS, Kokate MR, Kolekar SS. Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim Acta A. 2012;91:234–238. doi:10.1016/j.saa.2012.02.009
- Wongpreecha J, Polpanich D, Suteewong T, Kaewsaneha C, Tangboriboonrat P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr Polym. 2018;199:641–648. doi:10.1016/j.carbpol.2018.07.03930143172
- Lok C-N, Ho C-M, Chen R, et al. Silver nanoparticles: partial oxidation and antibacterial activities. JBIC. 2007;12(4):527–534. doi:10.1007/s00775-007-0208-z17353996
- Martinez-Castanon G, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza J, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res. 2008;10(8):1343–1348. doi:10.1007/s11051-008-9428-6
- Asghar MA, Zahir E, Shahid SM, et al. Iron, copper and silver nanoparticles: green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B 1 adsorption activity. LWT. 2018;90:98–107. doi:10.1016/j.lwt.2017.12.009
- Khatami M, Sharifi I, Nobre MA, Zafarnia N, Aflatoonian MR. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem Lett Rev. 2018;11(2):125–134. doi:10.1080/17518253.2018.1444797
- Muthamil S, Devi VA, Balasubramaniam B, Balamurugan K, Pandian SK. Green synthesized silver nanoparticles demonstrating enhanced in vitro and in vivo antibiofilm activity against Candida spp. J Basic Microbiol. 2018;58(4):343–357. doi:10.1002/jobm.20170052929411881
- Bonilla JJA, Guerrero DJP, Rgt S, et al. Green synthesis of silver nanoparticles using maltose and cysteine and their effect on cell wall envelope shapes and microbial growth of candida spp. J Nanosci Nanotechnol. 2017;17(3):1729–1739. doi:10.1166/jnn.2017.12822
- Marulasiddeshwara M, Dakshayani S, Kumar MS, Chethana R, Kumar PR, Devaraja S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: a promising therapeutic agent. Mater Sci Eng C. 2017;81:182–190. doi:10.1016/j.msec.2017.07.054
- Sonker AS, Pathak J, Kannaujiya V, Sinha R, Pathak J, Kannaujiya V. Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can J Biotechnol. 2017;1(1):26–37. doi:10.24870/cjb.2017-000103
- Phull A-R, Abbas Q, Ali A, Raza H, Zia M, Haq I-U. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J Pharm Sci. 2016;2(1):31–36. doi:10.1016/j.fjps.2016.03.001
- Khatami M, Nejad MS, Salari S, Almani PGN. Plant-mediated green synthesis of silver nanoparticles usingTrifolium resupinatumseed exudate and their antifungal efficacy onNeofusicoccum parvumandRhizoctonia solani. IET Nanobiotechnol. 2016;10(4):237–243. doi:10.1049/iet-nbt.2015.007827463795
- Sánchez-Navarro M, Ruiz-Torres CA, Niño-Martínez N, et al. Cytotoxic and bactericidal effect of silver nanoparticles obtained by green synthesis method using annona muricata aqueous extract and functionalized with 5-fluorouracil. Bioinorg Chem Appl. 2018;2018:8. doi:10.1155/2018/6506381
- Lakshmanan G, Sathiyaseelan A, Kalaichelvan P, Murugesan K. Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Mod Sci. 2018;4(1):61–68.
- Elella MHA, Mohamed RR, Abdel-Aziz MM, Sabaa MW. Green synthesis of antimicrobial and antitumor N, N, N-trimethyl chitosan chloride/poly (acrylic acid)/silver nanocomposites. Int J Biol Macromol. 2018;111:706–716.29339279
- Palem RR, Ganesh SD, Kroneková Z, Sláviková M, Saha N, Sáha P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: a comparative study on physico-chemical, antimicrobial and anticancer activity. Bull Mate Sci. 2018;41(2):55.
- Dadashpour M, Firouzi-Amandi A, Pourhassan-Moghaddam M, et al. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater Sci Eng C. 2018;92:902–912.
- Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng. 2018;89:429–443.
- Sun RW-Y, Chen R, Chung NP-Y, Ho C-M, Lin C-LS, Che C-M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Cheml Commun. 2005;40:5059–5061.
- Zodrow K, Brunet L, Mahendra S, et al. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43(3):715–723.19046755
- Hebeish A, El-Rafie M, El-Sheikh M, Seleem AA, El-Naggar ME. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol. 2014;65:509–515.24530328
- Maneerung T, Tokura S, Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym. 2008;72(1):43–51.
- Chen J-P, Chiang Y. Bioactive electrospun silver nanoparticles-containing polyurethane nanofibers as wound dressings. J Nanosci Nanotechnol. 2010;10(11):7560–7564.21137982
- Jacob JM, John MS, Jacob A, et al. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater Res Express. 2018;6(4):45401.