Abstract
Purpose:
Since nanoparticles (NPs) are beginning to be introduced in medicine and industry, it is mendatory to evaluate their biological side-effects, among other things. The present study aimed to investigate the pathways by which nickel nanoparticles (NiNPs) enter nephrons and to evaluate their localization and effects on cellular ultrastructure.
Methods:
Rats were injected intraperitoneally with 20 nm NiNPs (20 mg/Kg/b.w./day) for 28 consecutive days. Transmission electron microscope technique was used to detect localization of NiNPs and their effects on cellular ultrastructure in rat kidneys. Additionally, measurements of certain biochemical parameters such as creatinine, urea, uric acid and phosphorus for investigating renal function following NiNPs treatment were taken.
Results:
The presence of NiNPs in the nephrons in treated rats was confirmed by transmission electron microscopy. NiNPs entered the renal tubules cells via various pathways. The results indicated that NiNPs administration induced ultrastructural changes in the proximal cells of renal tubules and certain glomerular cells (podocytes and mesangial cells). Additionally, NiNPs were found to be localized in the mitochondria, which led to a significant decrease in their density and morphology. Furthermore, cell death was induced in the glomerular cells as found with a Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) assay and through detection of p35 using immunohistochemical staining.
Conclusion:
Herein, NiNPs were found to induce various cellular ultrastructural changes in the kidneys of rats. NiNPs used diverse pathways to internalize into the cytoplasm of the proximal convoluted tubules (PT) cells across the basement membrane, and also through the plasma membrane of two adjacent PT cells. NiNPs internalization, accumulation and their alterations of the cellular ultrastructure affected rat renal function.
Introduction
Nanoparticles (NPs) are particles that have dimensions equivalent to 100 nm or less.Citation1 The increased use of NPs in the industry and in biomedical applications has led to increased public concern regarding the biological and medical effects of NPs.Citation2 Over the past several decades, nickel nanoparticles (NiNPs) have been widely used in hydrogen storages, as chemical catalysts, in ceramic capacitors, in sensors and conductive paint, and in nanomedicine.Citation3 Nanoparticles translocate across the plasma membrane via endocytosis using different direct permeation pathwaysCitation1 that depend on their physicochemical properties.Citation4 Translocation of NPs across the cell membrane is a requirement during drug delivery and nanotoxicological research, however, the fate of these particles following their internalization and the mechanisms of their eventual elimination require further investigation.Citation5
The main mechanism suggested for NiNPs-induced cell damage is via induction of increased levels of reactive oxygen species (ROS), which are markers of oxidative stress,Citation6–Citation10 this has also been found to be a key mechanism for the cytotoxicity of other nanomaterials.Citation11–Citation13 NiNPs were found to induce oxidative DNA damage,Citation14 inflammation, cell degeneration,Citation9 cell cycle arrest,Citation10 cytogenetic alterations and apoptosis.Citation15
Kidneys are one of the main organs where there is accumulation of nanoparticles, particularly NiNPs.Citation16 The kidneys of rats injected with nanoparticles were previously shown to display various histopathological changes such as vacuolation and pyknosis in renal tubular epithelial cells and glomerular damage.Citation17–Citation19 Nickel ions have also found to induce significant kidney damage, as well as lead to reduced activities of enzymatic and non-enzymatic antioxidants.Citation20
In the present study, we investigated the internalization and localization of NiNPs and their effects on the cellular ultrastructure in rat kidneys. NiNPs induced cellular ultrastructural alterations in the proximal cells of the renal tubules and certain glomerular cells. NiNPs led to a remarkable reduction in the density and morphology of mitochondria. Interestingly, NiNPs significantly enhanced apoptosis/cell death in the cells of the rat kidneys.
Material and methods
Material
Powdered 20 nm nickel nanoparticles (NiNPs) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other chemicals were of laboratory grade and were used as received.
Preparation of NINPs suspension
A stock suspension of 20 nm NiNPs was prepared in normal saline (10 mg\mL) by sonication for 30 seconds in an ultrasonic homogenizer (model 150 VT, manufactured by biologica, Inc., Manassas, VA, USA); the particle suspensions were kept on ice for 15 seconds and sonicated again on ice for a total of 3 minutes at a power of 400 W. The NiNPs were vibrated for 2 minutes, immediately prior to the eventual injection. A concentration of 20 mg/kg body weight was prepared from this stock suspension. The morphology of the NiNPs was examined in images taken by FEI Tecnai Spirit 10 Transmission Electron Microscope (TEM).
Animals
Twelve 12-week-old male Wistar rats weighing 200–220 g were obtained from the animal house of Salahaddin University-Erbil. Animals were maintained in stainless steel mesh cages with free access to tap water and pellets. Moreover, they were maintained at a mean temperature of 21±2°C and a mean relative humidity of 35%. The experiment was conducted following the protocols approved by the Animal Care Ethical Committee of College of Science, Salahaddin University-Erbil, under the number 4N/115 on April 5, 2017 according to the Institutional Animal Care and Use Committee (IACUC) guidelines for animal careCitation21 and UBC Animal Care Guidelines for intraperitoneal injections.Citation22
Experimental design
The 12 rats were equally and randomly divided into two groups (kept in two separate cages); each group contained six animals. In group 1 (control group); the rats received the vehicle, while in group 2 the rats received 20 nm NiNPs (20 mg/kg) for 28 consecutive days. A needle gauge that was 22 g was used for the intraperitoneal injection. The selected NiNPs dose was based on previous studies.Citation23,Citation24 Then, 24 hours after the last treatment, all rats were sacrificed and kidney samples were collected for the cellular ultrastructure analysis.
Electron microscopy
Kidney tissues (≤1 mm3) were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.2–7.4 for 24 hours, washed by cacodylate buffer 0.1 M, postfixed in 1% osmium tetroxide, dehydrated through a graded series of ethanol (50%, 70%, 95% and 100%), cleaned in propylene oxide and finally embedded in an Araldite mixture. The ultrathin sections were stained by uranyl acetate and lead citrate.Citation25 The NiNPs were photographed by FEI Tecnai Spirit 10 TEM, while the ultrathin sections of kidney were photographed by the JEOL JEM 1400 TEM. The number of mitochondria in the proximal convoluted tubules (PT) cells per 100 µm2 area of the electron micrographs were counted according to the method of Adachi 1967.Citation26
Immunohistochemical analysis
For detection of apoptotic cells, TUNEL assay was performed using the HRP-DAB (ab206386) kit. Immunohistochemical kits for the detection of p53 were used (manufactured by Leica Biosystems Newcastle Ltd, Newcastle upon Tyne, UK) and staining was carried out using an automated immunostainer(AutostainerLink48 DAKO, Agilent, Santa Clara, CA, USA).
Blood collection and biochemical analysis
Blood samples were collected from all anesthetized rats through cardiac puncture in which the collected blood samples were immediately placed into gel tubes for serum collection, then later centrifuged (Hettich D-78532/Germany) at 3000 rpm for 15 minutes. The sera were stored at −80°C (Sanyo – Ultra – Low Temperature, Moriguchi, Osaka, Japan) for the measurement of creatinine. Serum creatinine, urea and uric acid were determined spectrophotometrically (Cobas c311 Hitachi, Tokyo, Japan) using a kit from BIOLABO S.A.S. (Maizy, France) at 500 nm. Phosphorous was also estimated in serum using the ABNOVA calorimetric kits (Catalaog No. KA0815).
Statistical analysis
Statistical analyses were performed using Students t-test. Data were reported as mean±standard errors (SEs). P-values≤0.05 were considered significant.
Results
Throughout the study, the rats displayed no behavioral changes or unusual responses following treatment with NiNPs. Moreover, none of the rats died due to the treatment.
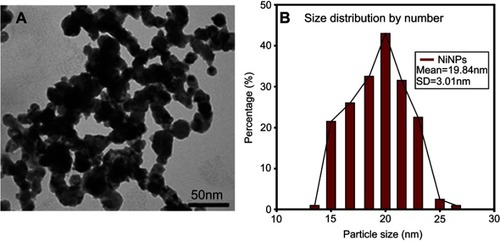
The shape and size of the 20 nm NiNPs are shown in . Following the examination of the electron micrographs, the mean size of the NiNPs was found to be 19.84 nm±3.01 (), which was approximately close to the size reported by the manufacturer.
Figure 1 NiNPs shape and size: (A) the shape of NiNPs as revealed by transmission electron micrographs of NiNPs. (B) Size distribution by number using a large number of TEM photos.
Abbreviations: NiNPs, nickel nanoparticles; SD, standard deviation; TEM, transmission electron microscope.
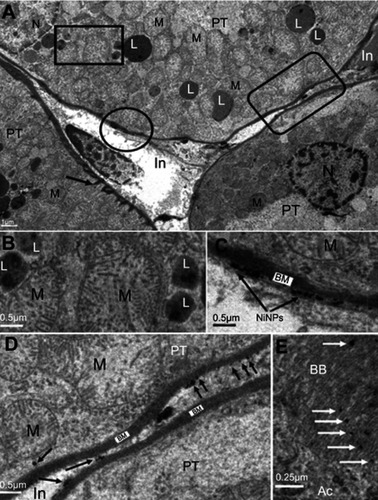
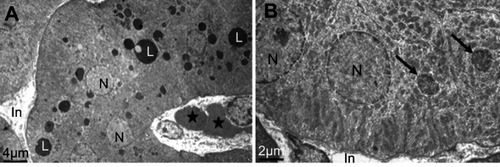
The electron microscope images confirmed the internalization of the NiNPs into the kidney tissues of the treated rats, and were localized within lysosome-vacuoles in the epithelial cells lining PT () or in the interstitial cells adherent to the basement membrane of the PT (). The NiNPs aggregated near the basement membrane appeared either as individual free particles or localized within vacuoles. The NiNPs were also found as individual particles in the cytoplasm of PT cells passed through the basement membrane (). Several numbers of NiNPs were also seen as individual particles within the brush border present in the lumen of the PT ().
Figure 2 Internalization of NiNPs in rat kidney. (A) PT cell with different NiNPs localizations, within lysosome-like vacuoles (rectangle) and adhered to the basement membrane (circle and round curved rectangle) as shown in (B–D) respectively. Arrow indicates the lateral ridge of the basement membrane. (B) Lysosome-like vacuoles containing dense particles. (C) NiNPs (arrows) adhered to the basement membrane (BM). (D) NiNPs (arrows) either as individual particles inside the PT cell or adhered to the basement membrane of both adjacent PT cells. (E) Apical cytoplasmic region of PT cells showing discharged NiNPs (arrows) through the brush border into the lumen.
Abbreviations: PT, proximal convoluted tubule; NiNPs, nickel nanoparticles; BM, basement membrane; L, lysosome-like vacuoles; In, interstitial region; Ac, apical cytoplasmic region; BB, brush border; M, mitochondria; N, nucleus.
As shown in , NiNPs also accumulated in the interstitial region of the cells and close to the basement membrane of the PT. The administration of NiNPs caused alterations in structure of the lateral ridges and disturbed the structure of the basal microvilli in the treated rats compared to the control group (). Subsequently, the damage that occurred in the kidneys following the exposure of the rats to NiNPs affected the level of serum creatinine, urea, uric acid and phosphorus compared to the control group ().
Table 1 Biochemical analysis in the serum of NiNP-treated rats
Figure 3 Internalization of NiNPs into the interstitial region of kidney cortex: (A) control group showing interstitial region between two proximal convoluted tubules. (B and C) the NiNP-treated group showing (B) deposition of NiNPs close to membranes (arrows), and (C) number of electron dense particles within vacuole close to the PT basement membrane (arrow).
Abbreviations: PT, proximal convoluted tubule; NiNPs, nickel nanoparticles; BM, basement membrane; In, interstitial region; M, mitochondria.
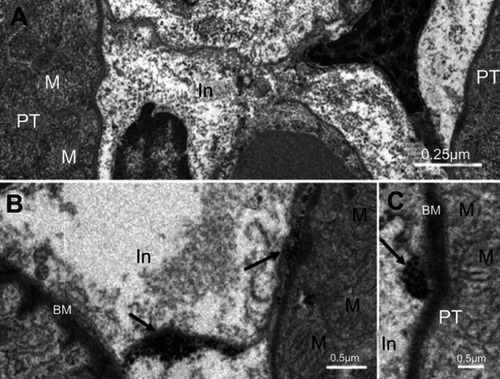
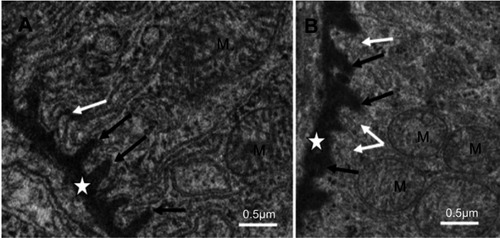
Figure 4 Basal region in proximal convoluted tubule cells of NiNP-treated rats. (A) Basal villi of control group (white arrow), lateral ridges (black arrows) extending from PT basement membrane (star). (B) Thickened basement membrane (star), disturbed basal villi (white arrows) and affected lateral ridges (black arrows).
Abbreviations: NiNPs, nickel nanoparticles; M, mitochondria; PT, proximal convoluted tubule.
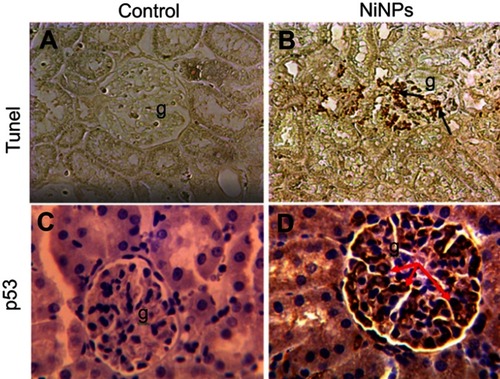
Within the glomerulus, NiNPs administration caused degeneration of podocytes and mesangial cells; the podocytes shrank and their nuclei condensed, while the mesangial cells displayed fragmented nuclei (). Furthermore, the foot processes of the podocytes, which normally form a slit in between, appeared fused in some sites forming a continuous layer (). The mode of cell death of mesangial cells appeared to be apoptotic, which was confirmed by a number of apoptotic bodies that were engulfed by adjacent neutrophil (). The mode of cell death in the glomerular cells was confirmed using the TUNEL assay and p53 immunohistochemical staining ().
Figure 5 Ultrastructure of glomerulus after NiNPs administration: (A) control group showing well developed podocytes with their foot processes (arrow), endothelial and mesangial cells. Red blood cells in the blood capillaries are seen; (B) the NiNP-treated group showing degenerated podocytes and mesangial cells. The nuclei of mesangial cells (1) are seen fragmented.
Abbreviations: P, podocytes; EN, endothelial cells; RBC, red blood cells; BC, blood capillary; Mc, mesangial cells; NiNPs, nickel nanoparticles.
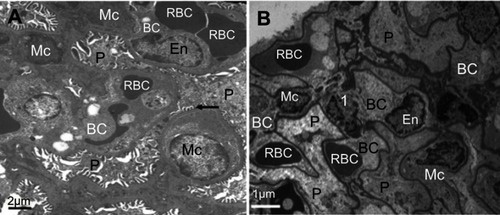
Figure 6 Ultrastructure changes in podocytes and mesangial cells in NiNP-treated rat kidney. (A) Degeneration of podocytes showing the fusion of foot processes (effacement) (arrows). Mesangial cells appeared degenerated having fragmented nucleus (black arrows). Neutrophil in the blood capillary showing phagocytosis of the degenerating mesangial cells. (B) Enlargement of (A) showing the phagocytosis process and engulfing of apoptotic bodies of mesangial cells and fusion of foot processes of podocytes.
Note: P1, primary podocyte foot processes; P2, secondary podocyte foot processes.
Abbreviations: NiNPs, nickel nanoparticles; P, podocytes; RBC, red blood cells; BC, blood capillary; MC, mesangial cells; Npl, neutrophil; ap, apoptotic bodies.
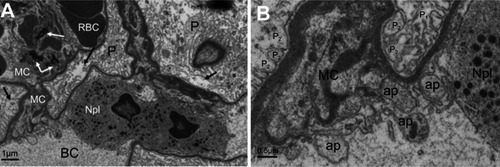
Figure 7 Immunohistochemical images of apoptotic cells in the glomerulus of NiNP-treated rat kidney. (A) Control group showing negative Tunel reaction. (B) NiNPs group showing positive Tunel reaction (arrows). (C) Control group showing no positive p53 reaction. (D) NiNPs group showing positive p53 reaction (arrows).
Abbreviations: NiNPs:, nickel nanoparticles; g, glomerulus.
As clearly shown in , exposure to NiNPs caused fusion or effacement of secondary foot processes of the podocytes. Both the proximal and distal renal tubules displayed degeneration of epithelial cells following NiNPs exposure, where some degenerated cells and cellular debris were shed into the lumen of the tubules ().
Figure 8 Ultrastructure changes in the podocytes foot processes. (A) Control group showing regular slit distances due to normal secondary foot processes structure (arrows; P2). (B) Fusion (effacement) of some of the secondary foot processes (P2). Arrow indicates a slit.
Note: P1, primary podocyte foot processes; P2, secondary podocyte foot processes.
Abbreviations: En, endothelial cells; RBC, red blood cells; BC, blood capillary; NiNPs, nickel nanoparticles; GBM, glomerular basement membrane.
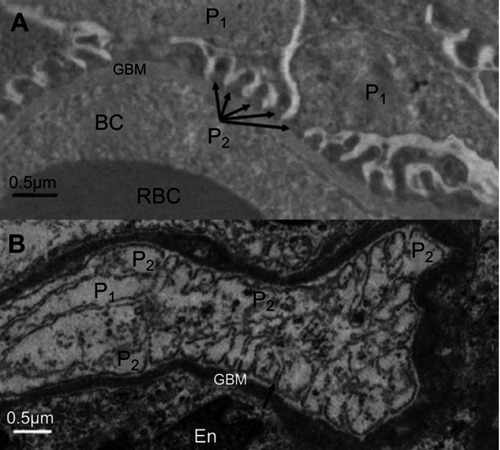
Figure 9 Ultrastructure of renal tubules of kidney of rats treated with NiNPs. (A) Proximal convoluted tubules of the NiNP-treated group showing variable sized lysosome-like vacuoles. Cellular debris is seen in the lumen of the duct shed from the renal tubules (stars). (B) Distal tubule of NiNP-treated rat showing pyknotic nuclei (arrows) against normal nuclei.
Abbreviations: NiNPs, nickel nanoparticles; L, lysosome-like vacuoles; N, nucleus; In, interstitial region.
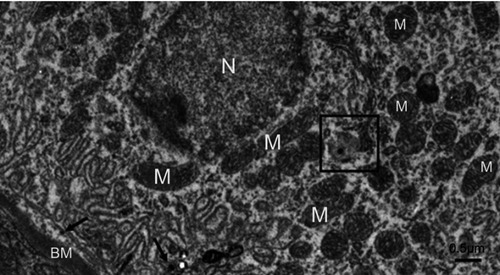
The most prominent effects of the NiNPs on the appearance of the renal tubules, as ultrastructurally revealed, were the lower density and the structural damage of the mitochondria compared to the control (). shows NiNPs uptake by mitochondria and their transmembrane transport between the two adjacent PT cells through endocytosis. A significant decrease (P≤0.05) occurred in the mitochondrial number in the PT cells of the NiNPs-treated rats compared to control (). In comparison to the normal ultrastructural architecture of rough endoplasmic reticulum (RER)-mitochondria close location system, a number of electron-dense particles of variable size, probably NiNPs, were also found to be adherent to the region of the RER that attached to the mitochondria (). NiNP-like particles were detected in the cells of the distal tubule, some were close to the plasma membrane, others were found as individual particles in the cytoplasm, and the rest of the particles were engulfed in vacuoles. Interestingly, a mitochondrion was found engulfing a particle-containing vacuole (see black box in ).
Figure 10 Electron microscope images of proximal convoluted tubule showing the status of mitochondria. (A) In the control group showing the normal density and ultrastructure. (B) In NiNP-treated rats showing the low density normal and damaged mitochondria. NiNPs are seen in different locations, free in the cytoplasm (black arrow), endocytosed by plasma membrane (white arrow), within lysosome-like vacuoles and uptaken by mitochondria (curved arrow connector).
Note: M2, damaged mitochondria.
Abbreviations: NiNPs, nickel nanoparticles; PT, proximal convoluted tubule; L, lysosome-like vacuoles; N, nucleus; M, mitochondria; bb, brush border.
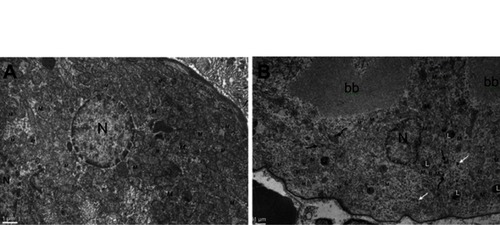
Figure 11 Mitochondria number counted by TEM photomicrographs per 100 µm2 area in PT cells of NiNPs treated rats which showed a significant decrease compared to control.
Note: *Significant decrease at P≤0.05.Abbreviation: NiNPs: nickel nanoparticles; TEM, transmission electron microscope; PT, proximal convoluted tubule.
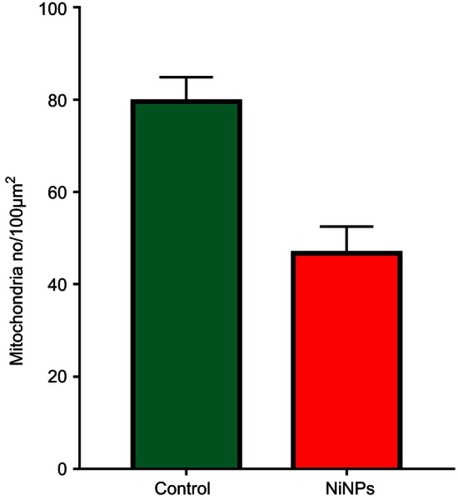
Figure 12 NiNPs intracellular localization in PT cells: (A) control group with normal ultrastructure of mitochondria, RER (arrow) and Golgi apparatus. (B) The NiNP-treated group showing electron dense particles, probably NiNPs, adhered to the RER which surround the mitochondria (arrows) and some are still free (arrow head).
Abbreviations: NiNPs, nickel nanoparticles; N, nucleus; RER, rough endoplasmic reticulum; M, mitochondria; PT, proximal convoluted tubule; G, Golgi apparatus.
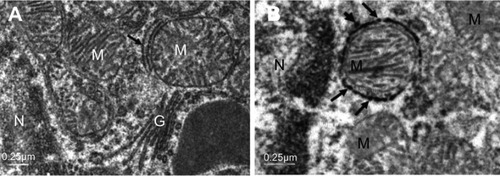
Figure 13 A distal tubule lining epithelial cell in the NiNP-treated group. Few numbers of mitochondria are seen. The box shows a vacuole containing an electron dense particle and whole vacuole is seen surrounded by an adjacent mitochondrion. Number of electron dense particles, probably NiNPs, near the basement membrane are seen (arrows).
Abbreviations: NiNPs, nickel nanoparticles; M, mitochondria; N, nucleus; BM, basement membrane.
Discussion
Manufactured NPs and their applications are expanding in the industry, commodities, biology and medicine. This has led to an increased number of studies to improve our understanding regarding the entry of these NPs into different cell types in vivo in order to examine their effects on cellular responses.Citation27–Citation29
In this study, the cellular ultrastructural localization of NiNPs was found to be within vacuoles or were found as free individual particles or aggregated/accumulated particles in renal tubule epithelial cells, and in the interstitium close to the basement membrane of the cells lining the renal tubules. The accumulation of NiNPs at the PT membrane may be due to many factors; firstly, difficulty in their indirect penetration rate leading to accumulation, secondly: due to their high extracellular concentration,Citation30,Citation31 the repetitive daily injections, thirdly: due to their large size. Moreover, physicochemical properties of the NPs could be another possible factor behind their aggregation, accumulation, and adhesion.Citation31–Citation33 Previously, nickel was found to accumulate in the lysosomes of proximal tubule cells and this was attributed to their strongly acidic, lipid-like properties that allow them to bind cationic and lipophilic substances.Citation34 Herein, NiNPs penetrated the basement membrane of the PT cells after their adhesion to the plasma membrane, but the penetration rate was slow although there was a high density of accumulated particles. Although lysosome-like vacuoles contained electron-dense particles, endocytosis of NiNPs close to the basement membrane couldn’t be observed in this study. Endocytosis, on the other hand, was detected at the plasma membrane of adjacent PT cells. Nanoparticles such as titanium dioxide (TiO2NPs), gold (AuNPs), silicon dioxide (SiO2NPs), iron oxide (Fe3O4NPs) and silver (AgNPs) have been found to penetrate directly into the plasma membrane.Citation1 Modes of direct penetration include diffusion, permeation, pore formationCitation35 and wrapping by membrane.Citation5 Although free individual NiNPs are detected close to the basement membrane, the mode of direct penetration couldn’t be confirmed, but the most probable pathway may be through translocation.Citation36 The size of the NiNPs facilitated their entrance into the target cells.Citation1 NP size, shape and core composition are strong determinants of cellular uptake and accumulation.Citation36,Citation37 Although it is still unclear whether translocation, distribution, and elimination are affected by NP size,Citation38 the size of NPs remains a critical factor in their internalization and accumulation. The size of NiNPs used in this study was 20 nm in diameter; this could facilitate NiNPs entrance into cells.Citation1 Nevertheless, NPs with a size ranged of 20–50 nm are considered appropriate application involving drug delivery.Citation39 NPs with a size less than 20 nm (eg 10 nm) have been shown to accumulate in the liver and spleen of rats,Citation40 while NPs of larger size (20–100 nm) have been found to accumulate in the kidney.Citation41 On the other hand, NPs with a size less than 20 nm could be rapidly cleared by the kidneys,Citation42,Citation43 while larger NiNPs may be difficult to be uptaken by cells.Citation44 The rapid clearance of NPs limits their use because they will take longer to reach target tissues.Citation45 Currently, no agreement has been reached on the effect of size on the cellular uptake and accumulation of NPs. For example, Cho et al46 observed maximum cellular uptake for AuNPs with a size less than 30 nm,Citation46 while Chithrani and Chan47 found maximum uptake for AuNPs with a size between 50 and70 nm.Citation47 These discrepancies may be due to the varied physicochemical properties of NPs.Citation37 The size and surface charge are found to interact in an interrelated fashion to modulate nanoparticle uptake into cells. For anionic AuNPs, cellular uptake is decreased with increasing size, whereas with cationic particles, cell uptake is increased with increasing particle size.Citation48 In addition to cellular uptake, particle size can also affect the mode of endocytosis.Citation49 With respect to size-dependent toxicity, small-sized NPs have larger surface area and particle concentrations than NPs of larger size which may increase the chance of interaction with surrounding macromolecules such as DNA and proteins, and as a consequence, trigger adverse responses.Citation50 Smaller-sized NPs have been shown to induce much higher production of ROS and interleukin-8 than NPs of larger size.Citation51,Citation52
On the other hand, the route of administration may play a significant role in NPs accumulation in different organs causing different levels of toxicity. In the present investigation, the intraperitoneal route of administration was selected since it is widely used in NPs research, particularly in nanomedicine.Citation53 It is well known that drug absorption via the intraperitoneal route is good and rapid due to the high intensity of blood vessels and lymph in the murine peritoneum.Citation54 Intraperitoneal injection has been demonstrated to improve the uptake of NP-labeled macromolecules compared with the intravenous route.Citation55 Intraperitoneal injection may cause a higher accumulation of AuNPs in kidneys compared with micromolecules.Citation56
As revealed by the TEM images, the NiNPs used here were spherical. Previously, when a comparison between cubic, spherical and rod-like gold nanoparticles was made, spherical particles showed the highest cellular uptake.Citation57 During renal excretion, the spherical NPs are cleared faster than rod NPs.Citation58 Differential toxicity due to the varying shapes of NPs has been reported. For example, the dendritic structure of NiNPs is found to result in higher toxicity in comparison to a spherical structure.Citation59 In a toxicity study using AgNPs with epithelial cells, wire-shaped silver was found to be more toxic than sphere-shaped silver.Citation60 Localization of clusters of NiNPs inside vacuoles in our images may be due to their agglomeration through curvature- mediated attraction.Citation61
Biochemically, sub-acute administration of NiNPs caused a significant increase of serum creatinine, urea and uric acid and a non significant increase of phosphorous level compared to the control. Recently, acute administration of nickel oxide nanoparticles (NiONPs) was found to induce an elevation of creatinine level.Citation62 The high levels of creatinine may reflect an abnormal glomerular filtration rate due to metals accumulation in kidneys.Citation63 Degeneration of renal tubular cells and mitochondrial damage due to NiNPs localization and accumulation, as revealed by this present work, may be related to these biochemical changes.Citation64
In this study, NiNPs caused damage to the mitochondria which also displayed a lower density. Several studies suggested the targeting of mitochondria by NPs due to their ability to bind with mitochondrial complex II of the electron transport chain to induce apoptosis.Citation33,Citation65 Mitochondria-targeting NPs have become a potential novel strategy in drug delivery for cancer therapy.Citation65,Citation66 The NiNPs-induced damage of the mitochondrial inner membrane, as revealed by the current investigation and consistently observed in previous work, may result in cytochrome c release leading to apoptosis.Citation66,Citation67 NiNPs uptake by mitochondria may be behind elevated oxidative stress, since mitochondria play a crucial role in cellular oxidative stress.Citation68,Citation69 Recently, other NPs such as silica NPs, were found to induce oxidative stress causing impaired mitochondrial function leading to apoptosis.Citation67 NiNPs-induced oxidative stress has been suggested to cause nanotoxicity, particularly induction of apoptosis.Citation7 The observation of NiNPs in the apical villi of PT cells in the present study indicates that excretion of NiNPs may occur via the urinary route, but further confirmation is required through further NP elimination studies.Citation70
Conclusion
NiNPs were found to induce various cellular ultrastructure changes in the kidneys of rats. The cellular ultrastructure alterations, which subsequently affected renal function may be due to the direct contact of NiNPs with the renal cells or due to the oxidative stress induced by NiNPs. The NiNPs used different ways to translocate into the cytoplasm of the PT cells across the basement membrane and through the plasma membrane of the two adjacent PT cells. Such pathways of internalization may be affected by the characteristic physicochemical properties of the NPs.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Prof. S. Polat in Cukurova University, Adana, Turkey; Dr Dara K. Mohammad, Karolinska Institutet, Solna, Sweden; Prof. Mojtaba Falahati, Azadi Islamic University, Tehran, Iran; and Dr Abbas Salihi, Salahaddin University-Erbil, Erbil, Iraq for their technical assistance. The authors also acknowledge Dr Fawaz Abumaray, Karolinska Institutet, Solna, Sweden for the linguistic revision.
References
- Nakamura H, Watano S. Direct permeation of nanoparticles across cell membrane: a review. KONA Powder Particle J. 2018;35:49–65.
- Savolainen K, Alenius H, Norppa H, Pylkkanen L, Tuomi T, Kasper G. Risk assessment of engineered nanomaterials and nanotechnologies–a review. Toxicology. 2010;269:92–104. doi:10.1016/j.tox.2010.01.01320105448
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2017. doi:10.1016/j.arabjc.2017.05.011
- Chen P, Zhang Z, Xing J, Gu N, Ji M. Physicochemical properties of nanoparticles affect translocation across pulmonary surfactant monolayer. Mol Phys. 2017;115:3143–3154. doi:10.1080/00268976.2017.1351005
- Nakamura H. Molecular dynamics simulation study on interaction of nanoparticle with cell membrane. Japanese Journal of Multiphase Flow 2018;32(3):321–328.
- Kong L, Hu W, Lu C, Cheng K, Tang M. Mechanisms underlying nickel nanoparticle induced reproductive toxicity and chemo-protective effects of vitamin C in male rats. Chemosphere. 2019;218:259–265. doi:10.1016/j.chemosphere.2018.11.12830472609
- Yu S, Liu F, Wang C, et al. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol Med Rep. 2018;17:3133–3139. doi:10.3892/mmr.2017.822629257258
- Latvala S, Hedberg J, Di Bucchianico S, et al. Nickel release, ROS generation and toxicity of Ni and NiO micro- and nanoparticles. PLoS One. 2016;11:e0159684. doi:10.1371/journal.pone.015968427434640
- Tahereh Razavipour S, Behnammorshedi M, Razavipour R, Ajdary M. The Toxic Effect of Nickel Nanoparticles on Oxidative Stress and Inflammatory Markers. Biomedical Research 2015;26(2):370–374.
- Ma C, Song M, Zhang Y, Yan M, Zhang M, Bi H. Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicol Rep. 2014;1:114–121. doi:10.1016/j.toxrep.2014.04.00828962232
- Meng H, Xia T, George S, Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3:1620–1627. doi:10.1021/nn900597321452863
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi:10.1126/science.111439716456071
- Zhang Q, Kusaka Y, Sato K, Nakakuki K, Kohyama N, Donaldson K. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: role of free radicals. J Toxicol Environ Health A. 1998;53:423–438.9537280
- Abudayyak M, Guzel E, Ozhan G. Nickel oxide nanoparticles induce oxidative DNA damage and apoptosis in kidney cell line (NRK-52E). Biol Trace Elem Res. 2017;178:98–104. doi:10.1007/s12011-016-0892-z27878512
- Saquib Q, Attia SM, Ansari SM, et al. p53, MAPKAPK-2 and caspases regulate nickel oxide nanoparticles induce cell death and cytogenetic anomalies in rats. Int J Biol Macromol. 2017;105:228–237. doi:10.1016/j.ijbiomac.2017.07.03228690165
- Amara S, Slama IB, Mrad I, et al. Effects of zinc oxide nanoparticles and/or zinc chloride on biochemical parameters and mineral levels in rat liver and kidney. Hum Exp Toxicol. 2014;33:1150–1157. doi:10.1177/096032711351032724501101
- Alidadi H, Khorsandi L, Shirani M. Effects of Quercetin on Tubular Cell Apoptosis and Kidney Damage in Rats Induced by Titanium Dioxide Nanoparticles. The Malaysian Journal of Medical Sciences 2018;25(2):72–81.
- Abdel-Wahhab MA, Aljawish A, El-Nekeety AA, Abdel-Aziem SH, Hassan NS. Chitosan nanoparticles plus quercetin suppress the oxidative stress, modulate DNA fragmentation and gene expression in the kidney of rats fed ochratoxin A-contaminated diet. Food Chem Toxicol. 2017;99:209–221. doi:10.1016/j.fct.2016.12.00227923682
- Mohammadi Fartkhooni F, Noori A, Mohammadi A. Effects of Titanium Dioxide Nanoparticles Toxicity on the Kidney of Male Rats. International Journal of Life Sciences 2016;10(1):65–69.
- Amudha K, Pari L. Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chem Biol Interact. 2011;193:57–64. doi:10.1016/j.cbi.2011.05.00321600195
- National Research Council Committee for the Update of the Guide for the CUoL, Animals. The National Academies Collection: Reports Funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press (US), National Academy of Sciences; 2011.
- Andrews K. UBC Animal Care Guidelines. Acc-2012-Tech10. Canada: University of British Columbia; 2014.
- Magaye RR, Yue X, Zou B, et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomedicine. 2014;9:1393–1402. doi:10.2147/IJN.S5621224648736
- Abdulqadir S, Aziz F. Nephrotoxicity of Nickel Nanoparticles in Rat: Effect of Different Doses. Presented at: 10th International Conference on Chemical, Biological, Environmental and Medical Sciences. March 21-22; 2018; Istanbul, Turkey.
- Kiernan J. Histological and Histochemical Methods: Theory and Practice. 5th ed. Banbury: Scion Publishing Ltd; 2015.
- Adachi F. Electron microscopic investigation of the regenerating hepatic cells of the senile rat. Nagoya J Med Sci. 1967;30:109–120.5624248
- Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018;13:339.30361809
- Panzarini E, Mariano S, Carata E, Mura F, Rossi M, Dini L. Intracellular transport of silver and gold nanoparticles and biological responses: an update. Int J Mol Sci. 2018;19(5):E1305.
- Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9(Suppl 1):51–63. doi:10.2147/IJN.S2659224872703
- Lawrence MG, Altenburg MK, Sanford R, et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci USA. 2017;114:2958–2963. doi:10.1073/pnas.161645711428246329
- Shin SW, Song IH, Um SH. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials. 2015;5:1351–1365. doi:10.3390/nano503135128347068
- Wu T, Tang M. Review of the effects of manufactured nanoparticles on mammalian target organs. J Appl Toxicol. 2018;38:25–40. doi:10.1002/jat.349928799656
- Mallick A, More P, Syed MM, Basu S. Nanoparticle-mediated mitochondrial damage induces apoptosis in cancer. ACS Appl Mater Interfaces. 2016;8:13218–13231. doi:10.1021/acsami.6b0026327160664
- Dingle JT, Barrett AJ. Uptake of biologically active substances by lysosomes. Proc R Soc Lond B Biol Sci. 1969;173:85–93. doi:10.1098/rspb.1969.00404389355
- Guarnieri D, Sabella S, Muscetti O, et al. Transport across the cell-membrane dictates nanoparticle fate and toxicity: a new paradigm in nanotoxicology. Nanoscale. 2014;6:10264–10273. doi:10.1039/c4nr02008a25061814
- Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi:10.1021/acsnano.5b0318426256227
- Wong AC, Wright DW. Size-dependent cellular uptake of DNA functionalized gold nanoparticles. Small. 2016;12:5592–5600. doi:10.1002/smll.20160169727562251
- Yang L, Kuang H, Zhang W, Aguilar ZP, Wei H, Xu H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci Rep. 2017;7:3303. doi:10.1038/s41598-017-03015-128607366
- Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23(8):E1848.
- Ruiz A, Mancebo A, Beola L, Sosa I, Gutiérrez L. Dose–response bioconversion and toxicity analysis of magnetite nanoparticles. IEEE Magn Lett. 2016;7:1–5. doi:10.1109/LMAG.2016.2535414
- Takeuchi I, Nobata S, Oiri N, Tomoda K, Makino K. Biodistribution and excretion of colloidal gold nanoparticles after intravenous injection: effects of particle size. Biomed Mater Eng. 2017;28:315–323. doi:10.3233/BME-17167728527194
- Sadat S, Jahan S, Haddadi A. Effects of Size and Surface Charge of Polymeric Nanoparticles on in Vitro and in Vivo Applications. Journal of Biomaterials and Nanobiotechnology 2016;7:91–108.
- Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11:673–692. doi:10.2217/nnm.16.527003448
- Mao BH, Tsai JC, Chen CW, Yan SJ, Wang YJ. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology. 2016;10:1021–1040. doi:10.1080/17435390.2016.118961427240148
- Sun YN, Wang CD, Zhang XM, Ren L, Tian XH. Shape dependence of gold nanoparticles on in vivo acute toxicological effects and biodistribution. J Nanosci Nanotechnol. 2011;11:1210–1216.21456161
- Cho EC, Au L, Zhang Q, Xia Y. The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells. Small. 2010;6:517–522. doi:10.1002/smll.20090162220029850
- Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi:10.1021/nl070363y17465586
- Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145. doi:10.1038/nnano.2008.3018654486
- Liu X, Huang N, Li H, Jin Q, Ji J. Surface and size effects on cell interaction of gold nanoparticles with both phagocytic and nonphagocytic cells. Langmuir. 2013;29:9138–9148. doi:10.1021/la401556k23815604
- Cho YM, Mizuta Y, Akagi JI, Toyoda T, Sone M, Ogawa K. Size-dependent acute toxicity of silver nanoparticles in mice. J Toxicol Pathol. 2018;31:73–80. doi:10.1293/tox.2017-004329479144
- Park J, Lim DH, Lim HJ, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb). 2011;47:4382–4384. doi:10.1039/c1cc10357a21390403
- Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine. 2018;14:1–12. doi:10.1016/j.nano.2017.08.011
- Almansour M, Alarifi S, Jarrar B. In vivo investigation on the chronic hepatotoxicity induced by intraperitoneal administration of 10-nm silicon dioxide nanoparticles. Int J Nanomedicine. 2018;13:2685–2696. doi:10.2147/IJN.S16284729765215
- Zhang XD, Wu HY, Wu D, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi:10.2147/IJN.S842821042423
- Jung C, Kaul MG, Bruns OT, et al. Intraperitoneal injection improves the uptake of nanoparticle-labeled high-density lipoprotein to atherosclerotic plaques compared with intravenous injection: a multimodal imaging study in ApoE knockout mice. Circ Cardiovasc Imaging. 2014;7:303–311. doi:10.1161/CIRCIMAGING.113.00060724357264
- Abdelhalim MA. Uptake of gold nanoparticles in several rat organs after intraperitoneal administration in vivo: a fluorescence study. Biomed Res Int. 2013;2013:353695. doi:10.1155/2013/35369523956977
- Niikura K, Matsunaga T, Suzuki T, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi:10.1021/nn305700523631767
- Zhao Y, Wang Y, Ran F, et al. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci Rep. 2017;7:4131. doi:10.1038/s41598-017-03834-228646143
- Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol. 2009;43:6349–6356.19746736
- Stoehr LC, Gonzalez E, Stampfl A, et al. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011;8:36. doi:10.1186/1743-8977-8-3622208550
- Reynwar BJ, Illya G, Harmandaris VA, Muller MM, Kremer K, Deserno M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature. 2007;447:461–464. doi:10.1038/nature0584017522680
- Dumala N, Mangalampalli B, Kalyan KSS, Grover P. Biochemical alterations induced by nickel oxide nanoparticles in female Wistar albino rats after acute oral exposure. Biomarkers. 2018;23:33–43. doi:10.1080/1354750X.2017.136094328748734
- Abdel Aziz I, Zabut B. Determination of blood indices of albino rats treated with aluminum chloride and investigation of antioxidant effects of vitamin E and C. Egypt J Biol. 2011;13:1–7.
- Sarhan OMM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine. 2014;9:1505–1517. doi:10.2147/IJN.S5672924711700
- Li WQ, Wang Z, Hao S, et al. Mitochondria-targeting polydopamine nanoparticles to deliver doxorubicin for overcoming drug resistance. ACS Appl Mater Interfaces. 2017;9:16793–16802. doi:10.1021/acsami.7b0154028481505
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843.12749418
- Kusaczuk M, Kretowski R, Naumowicz M, Stypulkowska A, Cechowska-Pasko M. Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int J Nanomedicine. 2018;13:2279–2294. doi:10.2147/IJN.S15839329695906
- Bustos PL, Volta BJ, Perrone AE, Milduberger N, Bua J. A homolog of cyclophilin D is expressed in Trypanosoma cruzi and is involved in the oxidative stress-damage response. Cell Death Discov. 2017;3:16092. doi:10.1038/cddiscovery.2016.9228179991
- Lee JE, Park JH, Shin IC, Koh HC. Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2012;263:148–162. doi:10.1016/j.taap.2012.06.00522714038
- Kanwal U, Bukhari NI, Rana NF, et al. Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: uptake by cells and organs. Int J Nanomedicine. 2019;14:1–15. doi:10.2147/IJN.S17686830587981
