Abstract
Purpose
Multiwalled carbon nanotubes (MWCNTs) have been known to enter the circulatory system via the lungs from inhalation exposure; however, its carcinogenicity and subsequent accumulation in other organs have not been adequately reported in the literature. Moreover, the safety of MWCNTs as a biomaterial has remained a matter of debate, particularly when the material enters the circulatory system. To address these problems, we used carcinogenic rasH2 transgenic mice to intravenously administer highly dispersed MWCNTs and to evaluate their carcinogenicity and accumulation in the organs.
Methods
Two types of MWCNTs (thin- and thick-MWCNTs) were intravenously administered at a high dose (approximately 0.7 mg per kg body weight) and low dose (approximately 0.07 mg per kg body weight).
Results
MWCNTs showed pancreatic accumulation in 3.2% of mice administered with MWCNTs, but there was no accumulation in other organs. In addition, there was no significant difference in the incidence of tumor among the four MWCNTs-administered groups compared to the vehicle group without MWCNTs administration. Blood tests revealed elevated levels in mean red blood cell volume and mean red blood cell hemoglobin level for the MWCNTs-administered group, in addition to an increase in eotaxin.
Conclusion
The present study demonstrated that the use of current technology to sufficiently disperse MWCNTs resulted in minimal organ accumulation with no evidence of carcinogenicity.
Introduction
Although multiwalled carbon nanotubes (MWCNTs) are used in various products for their mechanical and electrical properties,Citation1,Citation2 these materials have been problematized for their fibrous nanoparticle structure.Citation3 As a result, numerous safety assessments have since been conducted internationally.Citation4 Safety concerns have been placed on some of the thickest and longest MWCNTs in recent years;Citation5,Citation6 however, MWCNTs have been increasingly reported as safe biomaterials, provided that the extent of inhalation exposure does not reach critical levels.Citation7–Citation10 The results of these safety assessments have mainly reported on the lung carcinogenicity of the biomaterial and were intended to prevent lung cancer in those exposed to asbestos. It is clear from reports in the literature that inhaled MWCNTs can translocate to the circulatory system.Citation11,Citation12 However, the in vivo kinetics of MWCNTs remains largely unknown, and the safety assessment for the translocation of inhaled MWCNTs from the circulatory system to other organs is an important and understudied subject that would benefit from further research.Citation13
The application of MWCNTs as a biomaterial has become increasingly popular and internationally competitive as a field of research.Citation14 Various studies on drug delivery systems (DDS), imaging, scaffolds for regenerative medicine, and composite reinforcement materials have been conducted, wherein the delivery is performed via localized implantation or intravenous administration.Citation15–Citation20 Although the application of MWCNTs as a biomaterial has not yet reached clinical use due to the lack of available data to support its safety, the most important issue at hand is whether or not the organ accumulation of MWCNTs within the circulatory system can induce cancer.Citation21 Because studies that utilize DDS and imaging for cancer treatment are common, MWCNTs tend to be intravenously injected by which the amount entering the circulatory system is much greater than that of inhalation exposure or implantation.Citation22–Citation24
There have been numerous reports that have investigated the in vivo kinetics of MWCNTs in the circulatory system of normal mice, and various reports have shown that MWCNTs accumulate in organs such as the liver, spleen, pancreas, lung, and kidney.Citation25,Citation26 According to these reports, no carcinogenicity was found in any organs, regardless of organ accumulation.Citation27 However, MWCNTs are consistently under considerable social scrutiny for their carcinogenicity, as one animal study has demonstrated tumor formation under intraperitoneal administration;Citation28 thus, further assessments under strict conditions are recommended. We intravenously administered MWCNTs in carcinogenic rasH2 transgenic mouse models and performed weekly assessments on their debilitation/death and body weight. Furthermore, the mice were euthanized at 26 weeks after final evaluation, and tissue evaluations were performed for all prescribed organs for the presence of MWCNTs and evidence of tumors.Citation29 Thin-MWCNTs and thick-MWCNTs under evaluation were intravenously administered using the most current and advanced technology for dispersion, including the use of dispersion liquid and ultrasonic dispersion machine.Citation30 This study is the first to report the organ accumulation of highly-dispersed MWCNTs in the circulatory system and the use of a transgenic mouse model to assess carcinogenicity under the severest of conditions.
Materials and methods
MWCNTs
Flotube 9110 (CNano Technology, Santa Clara, USA) and MWNT7 (Hodogaya Chemical, Tokyo, Japan) were used as thin-MWCNTs and thick-MWCNTs, respectively. Thin-MWCNTs had a mean diameter of 10–15 nm, length of 10 μm, and carbon purity of over 99.8%. The residual metallic impurities reported by the manufacturer were as follows: Fe, <50 ppm; Cr, <10 ppm; Co, <10 ppm; Ni, <10 ppm; Cu, <10 ppm; Zn, <10 ppm. Thick-MWCNTs had a mean diameter of 60 nm, length of 10 μm, and carbon purity of over 99.5%. The residual metallic impurities reported by Takaya et alCitation31 were found to contain 4400 ppm Fe, 48 ppm Cr, and 17 ppm Ni. The thin-MWCNTs and thick-MWCNTs were both observed under a transmission electron microscope (TEM) (JEM-2100; JEOL, Tokyo, Japan).
High dispersion liquids for MWCNTs
For dispersion, polysorbate 80 (Nichiyu, Tokyo, Japan) was diluted to 0.1% with Dulbecco’s phosphate-buffered saline (DPBS) without Ca and Mg (Nakalai Tesque, Kyoto, Japan). We reported in 2018 that this dispersion liquid is an optimal solution for the dispersion of MWCNTs.Citation30 MWCNTs were sterilized in an autoclave at 121 °C for 15 mins and subsequently suspended in a dispersion liquid with a Nano Premixer PR-1 ultrasonic dispersion machine (Thinky, Tokyo, Japan). The dispersion characteristics of the thin-MWCNTs and thick-MWCNTs were evaluated using the Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK).
Animal experimentation
Seven-week old male transgenic CBYB6F1-Tg(HRAS)2Jic mouse models (rasH2 mouse) (CLEA Japan, Tokyo, Japan) were used for animal experiments. All animal experimentation procedures were approved and carried out in compliance with the guidelines of the institutional animal care committee of Shinshu University.
MWCNTs groups and their negative control
The volume of the intravenously injected solution was 50 μl. The mass (concentration) of thin-MWCNTs and thick-MWCNTs were 0.02 mg (0.4 mg/ml) in the high-dose group and 0.002 mg (0.04 mg/ml) in the low-dose group. This concentration corresponds to approximately 0.7 mg and 0.07 mg per kg body weight, respectively. Four groups consisting of low- and high-doses in each of the two MWCNTs groups were administered in rasH2 mice, in addition to a negative control that was administered with polysorbate 80 alone (vehicle group) for a total of five groups. There were 16 animals in the thick-MWCNTs (low-dose) and thick-MWCNTs (high-dose) groups, while the other three groups included 15 animals for a total of 77 animals.
Method of intravenous administration in rasH2 mouse models
The rasH2 mice were sedated with inhaled anesthetics at 2.0 ml/min with 3.0% isoflurane (Abbott Japan, Tokyo, Japan). A 5 mm skin incision was made in the right cervix of rasH2 mice, and the sternocephalic muscle was identified to be subsequently displaced to the median side. The right external jugular vein between the sternocephalic and cleidbrachial muscles was identified to apply tension with traction to the sternocephalic muscle towards the cranial side. Under this condition, 50 μl of solution was injected with a syringe using a 32 G needle (). After the injection, pressure was applied with a gauze and the skin was then sutured with a 5–0 nylon thread.
Follow-up of the rasH2 mouse models
Death and/or debilitation of the rasH2 mice were followed up every week, and the body weight was measured for all mice. Debilitated rasH2 mice were euthanized by inhalation of isoflurane anesthesia. Mice were dissected at the time of death or euthanization as described below.
Dissection of rasH2 mouse models
Mice that survived 26 weeks after injection were euthanized by inhalation of isoflurane anesthesia. The brain, tongue, esophagus, trachea, thyroid, thymus, heart, lung, liver, kidney, spleen, stomach, small intestine, large intestine, testis, and epididymis were prepared according to the provision of the Central Institute for Experimental Animals (CIEA) (Kanagawa, Japan), an independent organization that promotes the development of quality humanized animals. Organs with macroscopically observed abnormalities were removed and fixed with 20% neutral buffered formalin solution (Wako Pure Chemical Industries, Osaka, Japan). Tissue specimens were also prepared under the CIEA guidelines. Each organ was cleaved according to the CIEA tissue assessment guidelines for rasH2 model mouse, and one hematoxylin and eosin stained tissue specimen was prepared per organ. Similarly, according to the provisions of CIEA, histological evaluation was performed with an optical microscope BX-50F (Olympus, Tokyo, Japan) to assess the presence of tumors. Moreover, the presence or absence of CNTs was confirmed with optical, fluorescence, and polarization microscopy using the same tissue sample.
Blood examination
Blood was collected from the heart of all rasH2 mice at the time of dissection, and no blood was collected from mice that were found dead. The collected blood was measured for blood count with pocH100iV (Sysmex, Hyogo, Japan). Measurement of cytokines in plasma was performed with a Bio-Plex MAGPIX system (Bio Rad, Hercules, USA) using a Bio-Plex Pro mouse cytokine GI 23-Plex panel kit (Bio Rad, Hercules, USA) and analyzed with Bio-Plex Manager™ 6.1 software (Bio Rad, Hercules, USA).
The blood count for white blood cell (WBC), red blood cell (RBC), hemoglobin (Hb), hematocrit (HCT), mean red blood cell volume (MCV), mean red blood cell hemoglobin level (MCH), mean red blood cell hemoglobin concentration (MCHC), and platelet (PLT) were measured.
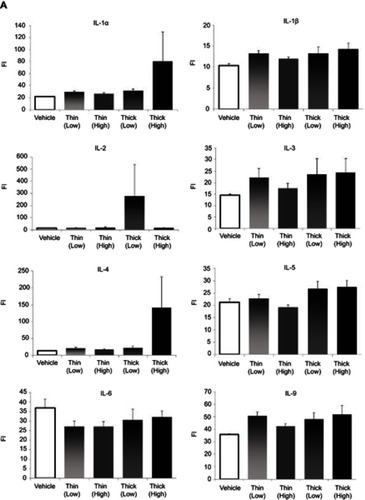
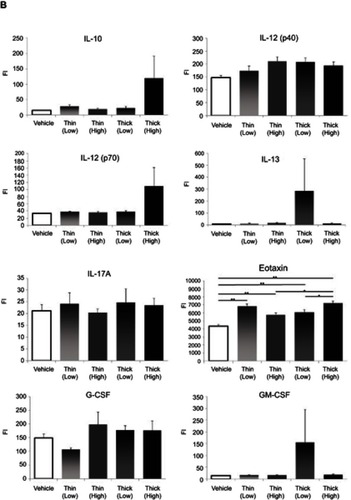
Cytokine measurements included IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1a, MIP-1b, RANTES, and TNF-α.
Statistical analysis
For statistical analysis of tumorigenesis, a significance test was conducted with the Fisher’s exact test. Statistical analyses of blood examinations were performed by a one-way analysis of variance and multiple comparison test (Tukey-Kramer). A P-value less than 0.05 was considered statistically significant.
Ethics
All animal experiments were performed after receiving approval by the ethics committee of Shinshu University in accordance with the Shinshu University Animal Use and Care Rules.
Results
Morphology and dispersibility of MWCNTs
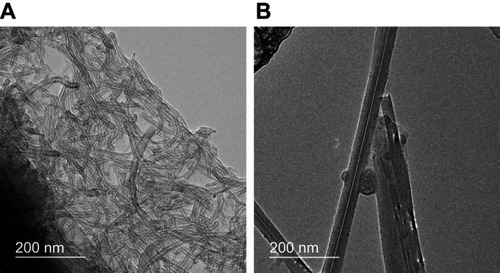
SEM imaging revealed that thin MWCNTs were curly and thick MWCNTs were straight (). shows the dispersibility of the thin-MWCNTs and thick-MWCNTs solutions. We found that both show particle sizes that are similar to that of a single MWCNT and are highly dispersed.
Figure 1 Morphology and dispersibility of MWCNTs. (A) The mean diameter, length, and morphology of the thin-MWCNTs were 10–15 nm, 10 µm, and curly, respectively. For thick-MWCNTs, the mean diameter, length, and morphology were 60 nm, 10 µm, and straight, respectively. (B) The particle diameter of the thin-MWCNTs and thick-MWCNTs solutions were both similar to a single MWCNTs and were highly dispersed.
Abbreviation: MWCNTs, multiwalled carbon nanotubes.
Oncogenesis of rasH2 mice
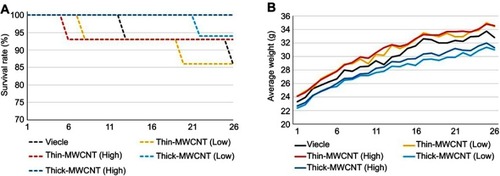
Six mice were euthanized due to death or debilitation prior to 26 weeks postoperatively (). At 5 weeks postoperatively, one animal in the thin-MWCNTs group (high-dose) died, and a tumor was confirmed in the spleen. At 7 weeks, one animal in the thin-MWCNTs group (low-dose) died, and a tumor was found in the spleen. At 12 weeks, one animal in the vehicle group was debilitated, and a stomach tumor was confirmed. At 19 weeks, an animal in the thin-MWCNTs group (low-dose) was debilitated, and a tumor of the thymus was observed. One debilitated animal in the thick-MWCNTs group (low-dose) exhibited a stomach tumor at 21 weeks. Finally, one animal in the vehicle group died at 25 weeks, and a lung tumor was observed. Other mice survived up to 26 weeks. Changes in the mean body weight for all groups are shown in , but there was no significant difference between groups.
Figure 2 The survival rate and mean body weight of rasH2 mouse models. (A) Survival rate. Two animals in the vehicle group, two in the thin-MWCNTs (low-dose) group, one in the thin-MWCNTs (high-dose) group, and one in the thick-MWCNTs (low-dose) were euthanized within 26 weeks due to debilitation. The other 71 mice survived up to week 26. (B) Average weight. The vehicle group, thin-MWCNTs (low-dose) group, thin-MWCNTs (high- dose) group, thick-MWCNTs (low-dose) group, and thick-MWCNTs (high-dose) group showed similar changes in weight.
Abbreviation: MWCNTs, multiwalled carbon nanotubes.
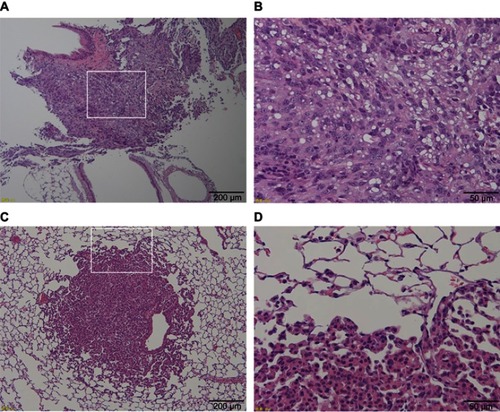
The presence or absence of tumor occurrence at final observation is shown in . Tumor occurrence was most frequently observed in the lungs for all groups at 15 of 77 (19.5%) animals, but there was no significant difference between groups. One case in the vehicle group developed mesothelioma that was thought to originate from the pleura (), but all other cases were adenomas (). Although we checked for the presence of thin- and thick-MWCNTs in cases with tumors that occurred in the lungs, we found no evidence of MWCNTs. The lung is the most common site of tumor occurrence in rasH2 mice, and we believe that these cases were spontaneous in onset, including those in the MWCNTs groups.Citation32,Citation33 The next common site of tumor occurrence was the spleen, and a tumor was found in a group other than the thick-MWCNTs group (high-dose). Like the lungs, there were no significant differences between the groups in the spleen. A tumor was also found in the stomach of an animal in the vehicle and thick-MWCNTs (low-dose) groups, respectively, and in the perineum of an animal in the thin-MWCNTs (low-dose) and thick-MWCNTs (high-dose) groups, respectively. There were no significant differences between groups in the stomach and perineum. Although we observed whether MWCNTs were present at the site where these tumors occurred, none were found at any of the sites. In terms of statistical analysis, there were no significant differences in the number of tumor occurrence in each organ of all groups that were administered under two different dosages for two types of MWCNTs as compared with the vehicle group.
Table 1 Histopathological findings in rasH2 mice treated with vehicle (control), thin-MWCNTs (low-dose), thin-MWCNTs (high-dose), thick-MWCNTs (low-dose), and thick-MWCNTs (high-dose) by jugular vein administration
Figure 3 Histological images of two tumor types occurring in the lungs of rasH2 mice. (A) Low power field image of vehicle group in which mesothelioma occurred. A large number of tumor cells with diffused large nuclei were present in the lungs. (B) An enlarged image of (A) surrounded by a white frame. The enlargement of nucleolus and irregular cells were observed, and numerous multinucleated cells were also found that were diagnosed as mesothelioma derived from the pleura. (C) Low power field image of the thin-MWCNTs (high-dose) group in which adenomas occurred. A large nuclear cell was found in a part of the lungs. (D) An enlarged image of (C) surrounded by a white frame. Irregular cells forming gland-like structures were proliferating. MWCNTs were not found in and around the tumor.
Organ accumulation of MWCNTs
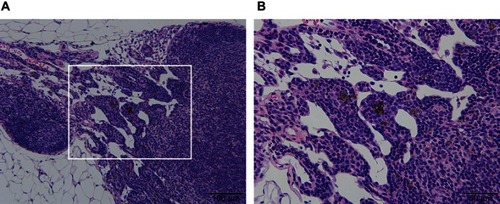
For the pancreas, there were two animals in the thin-MWCNTs group (high-dose), and MWCNTs deposition was observed from their appearance at the time of dissection. Thus, although the pancreas is an organ that is not included in the CIEA guideline, a tissue specimen was prepared and evaluated. However, there was no tumor formation despite the deposition of MWCNTs that was observed in both animals (). Because MWCNTs had accumulated in the pancreas of both animals, a pancreatic tissue specimen was prepared and observed for all of the other rasH2 mice, but no deposition of MWCNTs was observed. No deposition of thin- or thick-MWCNTs were observed in the tissue specimens of other organs for all mice.
Figure 4 Histological image of the thin-MWCNTs (high-dose) group in which MWCNTs. (A) Black MWCNTs were deposited in part of the pancreas. (B) An enlarged image of (A) surrounded by a white frame. No occurrence of tumors were found surrounding the MWCNTs.
Abbreviation: MWCNTs, multiwalled carbon nanotubes.
Blood count measurements
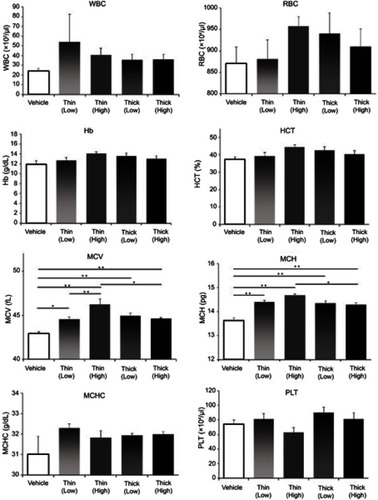
Blood count measurements are shown in . There were no significant differences between the WBC, RBC, Hb, HCT, MCHC, and PLT groups. However, in comparing the MCV with the vehicle group, the low-dose thin-MWCNTs (42.9357 vs 44.5500, P=0.0145), high-dose thin-MWCNTs (42.9357 vs 46.2286, P<0.001), low-dose thick-MWCNTs (42.9357 vs 44.9500, P<0.001), and high-dose thick-MWCNTs (42.9357 vs 44.6333, P=0.0071) groups all showed significantly higher volumes. In addition, there was a significant difference between the low- and high-dose thin-MWCNTs groups (44.5500 vs 46.2286, P=0.0097), as well as between the high-dose thin-MWCNTs and thick-MWCNTs groups (46.2286 vs 44.6333, P=0.0138). In comparing the MCH with the vehicle group, the low-dose thin-MWCNTs (13.6214 vs 14.3929, P<0.001), high dose thin-MWCNTs (13.6214 vs 14.6714, P<0.001), low-dose thick-MWCNTs (13.6214 vs 14.3500, P<0.001), and high-dose thick-MWCNTs (13.6214 vs 14.2867, P<0.001) groups exhibited significantly higher values. Moreover, there was also a significant difference between the high-dose thin-MWCNTs and thick-MWCNTs groups (14.6714 vs 14.2867, P=0.0236).
Figure 5 Blood count measurements. At the time of euthanization, blood was collected from the heart. In MCV and MCH, there were significant differences in the four MWCNTs groups and the vehicle group. There was no significant difference in WBC, RBC, Hb, HCT, MCHC and PLT. *P<0.05, **P<0.01.
Abbreviations: MWCNTs, multiwalled carbon nanotubes; MCV, mean red blood cell volume ; MCH, mean red blood cell hemoglobin level; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; HCT, hematocrit; MCHC, mean red blood cell hemoglobin concentration; PLT, platelet.
Cytokine measurements
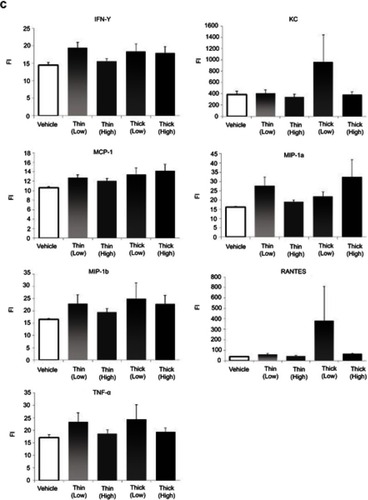
In terms of cytokine measurements, a significant difference was only found in the eotaxin levels (). In comparing the eotaxin level with the vehicle group, the low-dose thin-MWCNTs (4331.6923vs 6821.5769, P<0.001), high-dose thin-MWCNTs (4331.6923 vs 5768.2857, P=0.0064), low-dose thick-MWCNTs (4331.6923 vs 6075.5313, P<0.001), and high-dose thick-MWCNTs (4331.6923 vs 7215.4667, P<0.001) groups all exhibited a significantly higher value. A significant difference was found between the high-dose thin-MWCNTs and thick-MWCNTs groups (5768.2857 vs 7215.4667, P=0.0039), as well as between the low-dose thick-MWCNTs and high-dose thick-MWCNTs groups (6075.5313 vs 7215.4667, P=0.0321). There was no significant difference in cytokine measurements in other groups.
Figure 6 Cytokine measurements (A–C). An anticoagulant was applied to the blood that was collected from the heart at the time of euthanization. After centrifugation, the plasma was extracted to measure the cytokine levels. Eotaxin levels were significantly higher in all four MWCNTs groups compared to that of the vehicle group. None of the other cytokines showed any significant difference. *P<0.05, **P<0.01.
Abbreviations: FI, fluorescence intensity; MWCNTs, multiwalled carbon nanotubes.
Discussion
Ras is a G protein that is isolated from rat sarcoma and binds GTP to continually sustain cell division in its activated form.Citation34–Citation37 The rasH2 mouse models used in this study were introduced to human c-Ha-ras proto-oncogene and spontaneously developed tumors in multiple organs, eventually dying at approximately 35 weeks. However, it is unlikely that tumors will occur before 33 weeks, and carcinogenicity tests can be conducted during this time frame. As conducted in this study, testing substances are usually administered to six-week old mice and observed for 26 weeks. Carcinogenic substances develop tumors during this period, and mice die as a result of its severity.Citation38,Citation39 Therefore, compared to common mice that require two years of follow-up for their carcinogenicity assessment, these mouse models may be assessed at approximately a quarter of the normal duration. Since this method of evaluation is highly sensitive and can reduce time and labor, it is mainly used for predicting human carcinogenicity for chemical substances and has already been approved by ISO.Citation40,Citation41 Moreover, rasH2 mouse models have also been recently used in carcinogenesis tests for biomaterials of solid implants. For example, the models were used in assessing the carcinogenicity of IC tags for human implantation.Citation42 In terms of MWCNTs, we have previously reported the use of subcutaneously implanted rasH2 mouse models.Citation43 This study is the first to report the use of rasH2 mouse to assess the carcinogenicity of MWCNTs that enter the circulatory system.
In evaluating the carcinogenicity of chemical substances, the positive control is an intraperitoneal administration of N-methyl-N-nitrosourea (MNU). Because its carcinogenic potential has already been established, the positive control group is not indicated. Moreover, even if MNU is administered intravenously, it does not show carcinogenicity and cannot be used as a positive control (). On the other hand, there is no internationally approved positive control of nanoparticles such as MWCNTs. Currently, the safety assessment of nanoparticles is often performed according to chemical substances.Citation44–Citation47 Therefore, we did not prepare a positive control group for the intraperitoneal administration of MNU in this study.
Organ accumulation by the intravenous administration of MWCNTs showed clear macroscopic deposits in the pancreas of two animals in the high-dose group of thin-MWCNTs. This corresponds to 3.2% of the 62 mice that were administered with MWCNTs. However, MWCNTs were not observed in the pancreas of other mice and other organs of all mice. Because these results were obtained from detailed observations of a single tissue specimen for each organ with an optical microscope, there is a possibility that MWCNTs could have accumulated in the pancreas and other organs of other mice; thus, this was the limitation of our research method in terms of assessing specimens. Regardless of this limitation, we observed that MWCNTs accumulate in the pancreas by intravenous administration, which is consistent with other recent reports.Citation48,Citation49 MWCNTs accumulation was only found in two organs in total, and an intravenous injection of 0.02 mg in the high-dose group and 0.002 mg in the low-dose group corresponded to approximately 0.7 mg and 0.07 mg per kg of body weight, respectively. This is a considerable amount compared to what has been reported in previous studies.Citation20,Citation21 The high dose corresponds to approximately 42 mg when converted to a 60 kg human, and such a high concentration is never introduced into the blood stream when used as a DDS. The high dose was determined in order to evaluate the maximum intravenously injectable volume of MWCNTs, and the low dose was set at 1/10 of that volume. In this study, sufficient dispersion procedures were carried out using technology that is considered to be the most current and advanced; thus, we conducted the experiment with minimal dispersion and aggregation, and we believe that there was less organ accumulation and damage than that of previously described experiments with intravenous administration.Citation50–Citation52 Conversely, it can be said that MWCNTs can be used for DDS and imaging without accumulation in unintended organs, even if MWCNTs are intravenously administered using present-day technologies.
In the carcinogenicity assessment, there were no mice that developed tumors in the lung, thymus, spleen, stomach, and perineum in both the MWCNTs group and vehicle group administered with a solvent alone, and no statistically significant differences were found between groups. No occurrences of tumor caused by MWCNTs were observed in any of the organs. Tissue specimens showing tumors were examined in detail, but MWCNTs were not found in any specimens. In addition, tissue specimens were evaluated in detail in the pancreas of two mice that exhibited the presence of MWCNTs, but there were no tumors that were observed in either cases. Moreover, despite assessing the tissue specimens in detail for the two mice with MWCNTs in the pancreas, both showed no evidence of tumor occurrence. The carcinogenicity of straight thick-MWCNTs has been problematized in previous reports.Citation53 Although curly thin-MWCNTs and straight thick-MWCNTs were compared, no tumors developed after intravenous administration in our study. With the technologically advanced dispersion techniques that are available today, the fact that no occurrence of tumor was observed in the pancreas under the most stringent assessment using transgenic mice is an important finding, considering that the greatest accumulation is known to be found in the pancreas.Citation54–Citation56 We found that the possibility of developing tumor in the body is exceptionally low, provided that intravenous injections of MWCNTs are kept under 0.7 mg per kg body weight and are sufficiently dispersed as conducted in this study.
In the blood examination, the MCV and MCH of red blood cells were significantly higher in the MWCNTs-administered group in terms of blood count. These changes have not been previous described in the literature and is reported for the first time in this research.Citation57 In addition, eotaxin, which is a chemotactic cytokine (chemokine), showed significant increase in the MWCNTs-administered groups. In addition to potent eosinotaxis, eotaxin has a wide range of effects that include the promotion of myeloid progenitor cell differentiation, eosinophil recruitment from the bone marrow, enhancement of adhesion between the eosinophils and vascular endothelial cells, degranulation, and active oxygen production, as well as serving as a “key player” in allergic inflammation.Citation58–Citation60 This study was unable to clarify the mechanism that resulted in high MCV and MCH levels and increased eotaxin, in addition to their mutual relationship. Since these may be reactions that are particular to rasH2 mice, further studies are recommended for future research. On the other hand, there was no increase in white blood cell count and inflammatory cytokine, and persistent inflammation was not observed. In addition, there were no significant differences in other blood tests. We believe these results demonstrate the safety of highly dispersed MWCNTs entering the circulatory system.
Conclusion
As the most stringent carcinogenicity assessment for highly dispersed MWCNTs entering the circulatory system, an intravenous administration test was performed with transgenic oncogenic rasH2 mice for the first time. Although some mice exhibited an accumulation of MWCNTs in the pancreas, the incidence of tumors did not increase in all organs. We believe that it is unlikely that carcinogenesis will occur due to an intravenous administration of highly dispersed MWCNTs, even if the amount of administration is relatively large (0.7 mg/kg). The results of this study provide important information on the organ accumulation and carcinogenicity assessment in using MWCNTs as a biomaterial, such as when the material is translocated to the circulatory system by inhalation, when clarifying the DDS of the cancer treatment, and obtaining the imaging of the lesion.
Disclosure
Naoto Saito reports grants from the Japan Agency for Medical Research and Development, during the conduct of the study. The authors report no other conflicts of interest in this work.
Acknowledgments
We thank Kiyokazu Kametani (Research Center for Human and Environmental Sciences, Shinshu University) for his technical assistance in TEM analysis, Kayo Suzuki (Research Center for Human and Environmental Sciences, Shinshu University), Misako Yamada (Research Center for Human and Environmental Sciences, Shinshu University) for their technical assistance in the preparation of tissue sections. We would like to thank Sho Sugita (OrthoTranslations) for English language editing. This work was supported in part by the Japan Agency for Medical Research and Development (study numbers: 15hk0102021h0001, 16hk0102021h0002, and 17hk0102021h0003).
Supplementary materials
Figure S1 Method of intravenous administration of rasH2 mice. After sufficient sedation with inhaled anesthesia, a skin incision of approximately 5 mm was performed on the right cervical skin of rasH2 mouse to identify the sternocephalic muscle, which was displaced to the median side. The right external jugular vein between the sternocephalic and cleidbrachial muscle was verified, and tension was applied with traction to the sternocephalic muscle towards the cranial side. Under this condition, 50 μl of solution was injected with a 32G syringe while being cautious to minimize leakages. The figure shows the intravenous administration of thin-MWCNTs (high-dose).Abbreviation: MWCNTs, multiwalled carbon nanotubes

Table S1 Histopathological findings in rasH2 mice treated with vehicle and MNU by jugular vein administration
References
- Park HS, Kim JS, Choi BG, et al. Sonochemical hybridization of carbon nanotubes with gold nanoparticles for the production of flexible transparent conducing films. Carbon. 2010;48(5):1325–1330. doi:10.1016/j.carbon.2009.11.054
- He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C. Carbon nanotubes: applications in pharmacy and medicine. Biomed Res Int. 2013;2013:578290. doi:10.1155/2013/57829024195076
- Silva RM, Doudrick K, Franzi LM, et al. Instillation versus inhalation of multiwalled carbon nanotubes: exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano. 2014;8(9):8911–8931. doi:10.1021/nn503887r25144856
- Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi:10.1016/j.taap.2005.02.02116129115
- Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–116. doi:10.2131/jts.33.10518303189
- Yamashita K, Yoshioka Y, Higashisaka K, et al. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation. 2010;33(4):276–280. doi:10.1007/s10753-010-9182-720174859
- Nagai H, Okazaki Y, Hwu S, Misawa N, Yamashita Y, Akatsuka S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108(49):E1330–E1338. doi:10.1073/pnas.111001310822084097
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium : a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi:10.1186/1743-8977-7-520307263
- Matsumoto M, Serizawa H, Sunaga M, et al. No toxicological effects on acute and repeated oral gavage doses of single-wall or multi-wall carbon nanotube in rats. J Toxicol Sci. 2012;37(3):463–474.22687986
- El-Gazzar AM, Abdelgied M, Alexander DB, et al. Comparative pulmonary toxicity of a DWCNT and MWCNT-7 in rats. Arch Toxicol. 2019;93(1):49–59. doi:10.1007/s00204-018-2336-330341734
- Mercer RR, Scabilloni JF, Hubbs AF, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxico. 2013;10:38. doi:10.1186/1743-8977-10-56
- Thompson LC, Holland NA, Snyder RJ, et al. Pulmonary instillation of MWCNT increases lung permeability, decreases gp130 expression in the lungs, and initiates cardiovascular IL-6 transsignaling. Am J Physiol Lung Cell Mol Physiol. 2016;310(2):L142–L154. doi:10.1152/ajplung.00384.201426589480
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T. Lung carcinogenicity of inhaled multi- walled carbon nanotube in rats. Part Fibre Toxicol. 2016;13(1):53. doi:10.1186/s12989-016-0164-227737701
- Hara K, Aoki K, Usui Y, et al. Evaluation of CNT toxicity by comparison to tattoo ink. Mater Today. 2011;14(9):434–440. doi:10.1016/S1369-7021(11)70188-2
- Eletskii AV. Carbon nanotube-based electron field emitters. Phys Usp. 2010;53:863–889. doi:10.3367/UFNe.0180.201009a.0897
- Rosen Y, Elman NM. Carbon nanotubes in drug delivery: focus on infectious diseases. Expert Opin Drug Deliv. 2009;6(5):517–530. doi:10.1517/1742524090286557919413459
- Usui Y, Aoki K, Narita N, et al. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small. 2008;4(2):240–242. doi:10.1002/smll.20070067018205152
- Shimizu M, Kobayashi Y, Mizoguchi T, et al. Carbon nanotubes induce bone calcification by bidirectional interaction with osteoblasts. Adv Mater. 2012;24(16):2176–2185. doi:10.1002/adma.20110383222447724
- Tanaka M, Sato Y, Zhang M, et al. In vitro and in vivo evaluation of a three-dimensional porous multi-walled carbon nanotube scaffold for bone regeneration. Nanomaterials (Basel). 2017;7(2):46. doi:10.3390/nano7120458
- Tanaka M, Sato Y, Haniu H, et al. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS One. 2017;12(2):e0172601. doi:10.1371/journal.pone.017260128235026
- Jain S, Thakare VS, Das M, et al. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24(11):2028–2039. doi:10.1021/tx200372821978239
- Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci. 2010;113(1):226–242. doi:10.1093/toxsci/kfp24719822600
- U.S. Department of Health, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. Current intelligence bulletin 65, occupational exposure to carbon nanotubes and nanofibers. DHHS (NIOSH) Publication No. 2013-145. 4 2013 Available from: https://www.cdc.gov/niosh/docs/2013-145/pdfs/2013-145.pdf. Accessed 211, 2019.
- Hosseinpour M, Azimirad V, Alimohammadi M, Shahabi P, Sadighi M. The cardiac effects of carbon nanotubes in rat. Bioimpacts. 2016;6(2):79–84. doi:10.15171/bi.2016.1127525224
- Rittinghausen S, Hackbarth A, Creutzenberg O, et al. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol. 2014;11:59. doi:10.1186/s12989-014-0059-z25410479
- Mercer RR, Scabilloni JF, Hubbs AF, et al. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2013;10:33. doi:10.1186/1743-8977-10-5623895460
- Yamaguchi A, Fujitani T, Ohyama K, et al. Effects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in mice. J Toxicol Sci. 2012;37(1):177–189.22293422
- Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110(2):442–448. doi:10.1093/toxsci/kfp10019429663
- Urano K, Suzuki S, Machida K, et al. Examination of percutaneous application in a 26-week carcinogenicity test in CB6F1-TG rasH2 mice. J Toxicol Sci. 2007;32(4):367–375. doi:10.2131/jts.32.36717965551
- Kuroda C, Ueda K, Haniu H, et al. Different aggregation and shape characteristics of carbon materials affect biological responses in RAW264 cells. Int J Nanomedicine. 2018;13:6079–6088. doi:10.2147/IJN.S17762730323595
- Takaya M, Serita F, Yamazaki K, et al. Characteristics of multiwall carbon nanotubes for an intratracheal instillation study with rats. Ind Health. 2010;48:452–459. doi:10.2486/indhealth.MS112820720337
- Paranjpe MG, Elbekaei RH, Shah SA, Hickman M, Wenk ML, Zahalka EA. Historical control data of spontaneous tumors in transgenic CByB6F1-Tg(HRAS)2Jic (Tg.rasH2) mice. Int J Toxicol. 2013;32(1):48–57. doi:10.1177/109158181247156523324159
- Suguro M, Numano T, Kawabe M, et al. Tumor induction by 26-week dermal application of 1, 2-Dichloroethane in CB6F1-Tg rasH2 mice. Toxicol Pathol. 2017;45(3):427–434. doi:10.1177/019262331770100328421967
- Morton D, Alden CL, Roth AJ, Usui T. The Tg rasH2 mouse in cancer hazard identification. Toxicol Pathol. 2002;30(1):139–146. doi:10.1080/0192623025282485111890467
- Macdonald J, French J, Gerson RJ, et al. The utility of genetically modified mouse assays for identifying human carcinogens : a basic understanding and path forward. Toxicol Sci. 2004;77(2):188–194. doi:10.1093/toxsci/kfh03714657512
- Long GG, Morton D, Peters T, Short B, Skydgaard M. Alternative mouse models for carcinogenicity assessment : industry use and issues with pathology interpretation. Toxicol Pathol. 2010;38(1):43–50. doi:10.1177/019262330935410719915137
- Nambiar PR, Morton D. The rasH2 mouse model for assessing carcinogenic potential of pharmaceuticals. Toxicol Pathol. 2013;41(8):1058–1067. doi:10.1177/019262331347725723423820
- Tamaoki N. The rasH2 transgenic mouse: nature of the model and mechanistic studies on tumorigenesis. Toxicol Pathol. 2001;29(Suppl):81–89. doi:10.1080/01926230175338593311695564
- Tsuji S, Kuwahara Y, Takagi H, Sugiura M. Gene expression analysis in the lung of the rasH2 transgenic mouse at week 4 prior to induction of malignant tumor formation by urethane and N-methylolacrylamide. J Toxicol Sci. 2015;40(6):685–700. doi:10.2131/jts.40.88726558449
- ISO 10993-1: 2018. Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a Risk Management Process.
- ISO 10993-3:2014. Biological Evaluation of Medical Devices - Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity.
- Urano K, Suzuki S, Machida K, Sawa N, Eguchi N. Use of IC tags in short-term carcinogenicity study on CB6F1 TGrasH2 mice. J Toxicol Sci. 2006;31:407–418. doi:10.2131/jts.31.40717202757
- Takanashi S, Hara K, Aoki K, et al. Carcinogenicity evaluation for the application of carbon nanotubes as biomaterials in rasH2 mice. Sci Rep. 2012;2:498. doi:10.1038/srep0038622787556
- Castranova V, Schulte PA, Zumwalde RD. Occupational nanosafety considerations for carbon nanotubes and carbon nanofibers. Acc Chem Res. 2013;46(3):642–649. doi:10.1021/ar300004a23210709
- ISO/TS 13278: 2017. Nanotechnologies - Determination of Elemental Impurities in Samples of Carbon Nanotubes Using Inductively Coupled Plasma Mass Spectrometry.
- ISO/TS 11888: 2017. Nanotechnologies - Characterization of Multiwall Carbon Nanotubes - Mesoscopic Shape Factors.
- ISO/TS 12805: 2011. Nanotechnologies - Materials Specifications - Guidance on Specifying Nano-Objects.
- Badie BH, Rezaee R, Valokala GM, et al. Cardiotoxicity of nano-particles. Life Sci. 2016;165:91–99. doi:10.1016/j.lfs.2016.09.01727686832
- Wu T, Tang M. Review of the effects of manufactured nanoparticles on mammalian target organs. J Appl Toxicol. 2018;38(1):25–40. doi:10.1002/jat.v38.128799656
- Qu G, Bai Y, Zhang Y, Jia Q, Zhang W, Yan B. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon. 2009;47(8):2060–2069. doi:10.1016/j.carbon.2009.03.056
- Zhang T, Tang M, Zhang S, et al. Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study. Int J Nanomedicine. 2017;12:1539–1554. doi:10.2147/IJN.S12334528280324
- Ji Z, Zhang D, Li L, et al. The hepatotoxicity of multi-walled carbon nanotubes in mice. Nanotechnology. 2009;20:44. doi:10.1088/0957-4484/20/44/445101
- Toyokuni S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev. 2013;65:2098–2110. doi:10.1016/j.addr.2013.05.01123751780
- Firme CP, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine (Basel). 2010;6(2):245–256. doi:10.1016/j.nano.2009.07.003
- Iancu C, Mocan L. Advances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermia. Int J Nanomedicine. 2011;6:1675–1684. doi:10.2147/IJN.S2564621904457
- Sakamoto Y, Nakae D, Fukumori N, et al. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34(1):65–76.19182436
- Kavosi A, Hosseini S, Noei G, Madani S, Khalighfard S. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep. 2018;8(1):8375. doi:10.1038/s41598-018-26790-x29849103
- Crouzier D, Follot S, Gentilhomme E, et al. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology. 2010;272(1–3):39–45. doi:10.1016/j.tox.2010.04.00120381574
- Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Løvik M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol Sci. 2009;109(1):113–123. doi:10.1093/toxsci/kfp05719293371
- Sato Y, Yokoyama A, Shibata K, et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol Biosyst. 2005;1(2):176–182. doi:10.1039/b502429c16880981
- Yoshizawa K, Yuki M, Kinoshita Y, et al. N-methyl-N-nitrosourea-induced schwannomas in male Sprague-Dawley rats with a literature review of inducible and spontaneous lesions. Exp Toxicol Pathol. 2016;68(7):371–379. doi:10.1016/j.etp.2016.05.00527233116
- Teixeira-Guedes CI, Faustino-Rocha AI, Talhada D, et al. A liver schwannoma observed in a female Sprague-Dawley rat treated with MNU. Exp Toxicol Pathol. 2014;66(2–3):125–128. doi:10.1016/j.etp.2013.11.00324315155
References
- Yoshizawa K, Yuki M, Kinoshita Y, et al. N-methyl-N-nitrosourea- induced schwannomas in male Sprague-Dawley rats with a literature review of inducible and spontaneous lesions. Exp Toxicol Pathol. 2016;68(7):371–379. doi:10.1016/j.etp.2016.05.00527233116
- Teixeira-Guedes CI, Faustino-Rocha AI, Talhada D, et al. A liver schwannoma observed in a female Sprague-Dawley rat treated with MNU. Exp Toxicol Pathol. 2014;66(2–3):125–128. doi:10.1016/j.etp.2013.11.00324315155