Abstract
Inflammatory bowel disease (IBD), which mainly consists of Crohn’s disease and ulcerative colitis, is a chronic and relapsing inflammatory condition of the gastrointestinal tract. The traditional treatment strategies relied on frequent administration of high dosages of medications, including antibiotics, non-steroidal anti-inflammatory drugs, biologics, and immunomodulators, with the goal of reducing inflammation. Some of these medications were effective in alleviating the early-stage inflammatory symptoms, but their long-term efficacies were compromised by the accumulation of toxicities. Recently, nanoparticle (NP)-based drugs have been widely studied for their potential to solve such problems. Various mechanisms/strategies, including size-, charge-, pH-, pressure-, degradation-, ligand-receptor-, and microbiome- dependent drug delivery systems, have been exploited in preclinical studies. A certain number of NP delivery systems have sought to target drugs to the inflamed intestine. Although several NP-based drugs have entered clinical trials for the treatment of IBD, most have failed due to premature drug release, weak targeting ability, and the high immune toxicity of some of the synthetic nanomaterials that have been used to fabricate the NPs. Therefore, there is still a need for rationally designed and stable NP drug delivery system that can specifically target drugs to the disease site, prolong the drug’s residence time, and minimize systemic side effects. This review will analyze the current state of the art in NP-mediated drug delivery for IBD treatment. We will focus on topics such as deliverable targets (at the tissue or cellular level) for treating inflammation; the target-homing NP materials that can interact with such targets; and the major administration routes for treating IBD. These discussions will integrate notable trends in the research and development of IBD medications, including multi-responsive NP-mediated delivery and naturally-derived targeting NPs. Finally, current challenges and future directions will be presented in the hopes of advancing the study of NP-mediated strategies for treating IBD.
Introduction
More than 1.8 million patients in the United States and approximately 3.5 million worldwide are suffering from inflammatory bowel disease (IBD), which is a set of chronic and idiopathic inflammatory conditions that may affect the entire gastrointestinal (GI) tract.Citation1,Citation2 IBD primary comprises two clinically defined forms: ulcerative colitis (UC) and Crohn’s disease (CD). UC, which is confined to the colon, can spread from the rectum to the cecum in a non-interrupted fashion and show extensive mucosal ulceration. CD, in contrast, mostly affects the ileum and the colon but can also be found discontinuously in other regions of the GI tract.Citation3 Both UC and CD are associated with increased colorectal cancer risk, high morbidity, and a decreased quality of life.Citation4–Citation6 The rise of global industrialization has been paralleled by a dramatic increase in the incidence of IBD worldwide, putting a substantial burden on public healthcare.Citation7
The etiology of IBD has been extensively studied, but the causative factors are not yet fully understood. The current knowledge of complex diseases suggests that their causes are often multifactorial.Citation8 Endogenous triggers (such as genetic predisposition and immunoregulatory dysfunction), external environmental triggers (including diet, chemicals, and psychological stress), and microbial exposure are thought to contribute to the development of IBD.Citation3,Citation9,Citation10 Recent studies of the human microbiome revealed that dysbiosis (an alteration of the regular composition of the microbiota) also plays a pivotal role in the development of IBD,Citation3,Citation11–Citation13 adding another layer of complexity of the etiology of this important disease.
Colon-targeted drug delivery system (DDS) has received significant attention for their potential to treat IBD, which predominantly affects the colon.Citation14 Conventional formulations, such as capsules, tablets, and solutions, are still used in the clinic to deliver anti-inflammatory drugs (e.g., 5-aminosalicylic acid, corticosteroids) or immunosuppressive agents (e.g., azathioprine, 6-mercaptopurine) for the treatment of IBD. In contrast, IBD-targeting biologics (e.g., anti-tumor necrosis factor [TNF]-monoclonal antibodies) are mainly delivered via intravenous (IV) or subcutaneous (SC) injection.Citation15 These approaches do not yield colon-targeted drug delivery and often trigger severe systemic side effects. Therefore, scientists have developed several advanced targeting DDSs, which employ different mechanisms for the controlled and colon-targeted release of a loaded drug. Such strategies include the use of time-dependent erodible hydrogel capsules, pH-dependent coating formulations derived from methacrylate derivatives or poly-(lactic-co-glycolic acid) (PLGA),Citation16 pressure-sensitive osmotic agents with capsulated semi-permeable membranes, and microbiota-triggered ethylcellulose/amylose particles.Citation17 Although researchers have expended tremendous effort in creating colon-targeted DDSs, several of which have been approved for clinical studies, their potential seems limited for clinical use in treating IBD. The available single factor-triggered NP therapies have failed to control the disease in many patients and carry risks for severe systemic side effects.Citation18 Clearly, there is no easy cure for IBD.
Recent design language conveyed strategies for explicitly targeting the loaded drug to a subtype of cells, instead of the entire colon tissue.Citation19 The use of a cell-targeting DDS in IBD is expected to provide a high local drug concentration at the site of inflamed cells or tissues for a prolonged period. Given that higher drug concentrations in inflamed cells are associated with a lower histological index of inflammation,Citation20 inflammation-targeting DDS can maximize the anti-inflammatory efficacy of the loaded drug. Moreover, targeted deliveries have been shown to prevent or reduce the premature drug delivery that occurs when the delivery system is degraded before it reaches the site of action.Citation21 For example, biologics, such as antibodies or small interfering RNAs (siRNAs), generally undergo rapid degradation and exhibit a short half-life in circulation, and thus benefit from the protection of a DDS.Citation22 A targeted DDS yields an elevated drug concentration and enables prolonged drug-target interactions, and thus should be able to be given at a lower dosing frequency. Since the targeted DDS-loaded drug is directly released at the inflamed cells, this strategy should minimize systemic drug exposure and the associated side effects.
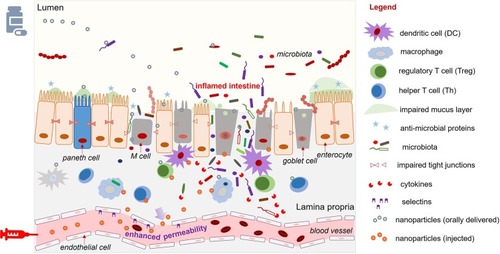
Among the cell-specific targeting approaches, NP-based DDSs have gained considerable attention due to their favorable small size, strong drug-loading ability, and versatile surface structure. Smaller-sized NPs (<200 nm) were found to penetrate the mucus layer more deeply than larger submicron particles and were shown to reach the intestinal tissues.Citation23 There is an overall increase in the colon permeability of IBD patients, who lose tight junctions and cellular integrity upon the activation of pro-inflammatory cytokines. NPs passively target the drug to the site of inflammation through the enhanced permeability effect (EPR), which increases their uptake by activated immune cells.Citation24 NP-based delivery modifies the pharmacokinetics of the encapsulated drug, potentially improving its stability and reducing its immunogenicity compared to the free drug. Furthermore, surface modification of the NP can facilitate its specific cell-targeting functions, enabling nano-drugs to exhibit controlled release at the site of inflamed cells and minimizing systemic toxicity.Citation25
The published studies have largely focused on treating IBD with mechanism-driven DDSs (including size-, charge-, pH-, pressure-, enzymatic hydrolysis-, degradation-, ligand-receptor-, and microbiome-dependent) and synthetic NP-mediated DDSs (including amphiphilic prodrugs, hydrogels, mesoporous silica, and solid lipid NPs), and such applications have been extensively reviewed.Citation14,Citation21,Citation24,Citation25 This review will touch only lightly on these topics, instead focusing on deliverable targets in IBD treatment, including the intestinal epithelium and mucus, immune cells, the lamina propria, the extracellular matrix (ECM), and the microbiota (a newly emerged target). In each section, we will describe the deliverable targets and present the corresponding NP materials that show activity toward each target. We will then discuss the main administration routes of different NPs for IBD treatment. Finally, we will conclude by summarizing the challenges and predicting future directions for NP-mediated IBD treatment.
Deliverable Targets In IBD And Corresponding NP Materials
Intestinal Epithelium And Mucus
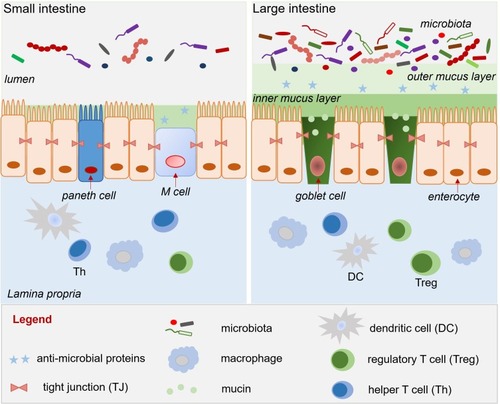
The intestinal epithelium is lined by a monolayer of columnar intestinal epithelial cells (IECs), which comprise several specialized cell types, such as enterocytes, goblet cells, Paneth cells, M cells, and stem cells.Citation26 These different IECs are connected by intercellular junctions, such as adherens and tight junctions (TJs). TJs act as intestinal barriers and regulate the permeability of water, ions, and nutrients. The goblet cells generate secreted mucins (mainly mucin 2), which form a “loose” layer of gel-like mucus. There are two mucus layers in the colon: a loose outer layer of secreted mucins and a “firm” inner layer of cell membrane-anchored mucins []; in contrast, the mucus layer of the small intestine is a loose, discontinuous, unattached single layer that sometimes reveals the tips of the villi.Citation27
Logically, mucoadhesive NPs can locally deliver drugs to the mucus of the small or large intestine.Citation28 Mucin, which forms the building blocks of the mucus layer, is a glycoprotein that is composed of single-chain amino acid backbones (mainly containing proline, serine, and threonine) with branched oligosaccharide side chains, including fructose, galactose, N-acetyl galactosamine, and N-acetyl glucosamine.Citation29 Given the hydrophilicity of mucin, NP materials that seek to target the mucous membrane should contain hydrophilic functional groups, such as carboxyl or hydroxyl, which will allow hydrogen bonds to form between the mucins and the NPs. Many NP materials, including the synthetic polymers (acrylic acid derivative/polyacrylate) and many natural polymers (hyaluronic acid, cellulose derivative, chitosan, alginates, and pectin), can nonspecifically adhere to mucin, as they lack ligand molecules that can recognize and bind to particular receptors on mucus. However, some natural materials reportedly exhibit specific mucin-binding functions. For example, tomato lectins can recognize and bind to N-acetyl glucosamine-containing complexes on cell surfaces, and these lectins show a strong association with mucus gel.Citation30 Some strain-specific bacterial adhesins were also found to selectively attach to the glycoarray presented by mucin.Citation31 Therefore, tomato lectins and bacterial adhesins might be useful for constructing specific mucin-binding NPs.
In addition to its unique chemical properties, mucin is also characterized by its continuous degradation and formation. Many studies have shown that the reduced mucus thickness seen in IBD patients compared with non-IBD controls may result from depletion of goblet cells in the affected colorectal mucosa.Citation32 Patients with UC also exhibit altered glycosylation and reduced sulfation of mucin, indicating that there is enhanced microbiota-mediated degradation of the outer mucus layer.Citation33,Citation34 Although this scenario makes mucus-penetrating delivery much more accessible in IBD patient than in healthy people, fast deterioration of the thin mucus layer in IBD patient can limit the application of mucoadhesion-based DDS. Therefore, although many published reports have supported the delivery of IBD-treating therapeutics to the mucus, this may not be the best choice
As an alternative to delivering the drug to the mucus layer, many other systems have sought to deliver NPs to the intestinal epithelium.Citation35 These NPs penetrate the mucus layer following oral delivery or, if administrated by intravenous injection, they exit the leaky vasculature of the inflamed intestine to reach IECs via the EPR effect [].Citation36 In the context of IEC-targeted drug delivery, rational DDS design focuses primarily on the NP size and charge, and the specific ligand/receptor on the IECs.Citation18,Citation37 Several studies have indicated that the best tissue-penetrating NPs for IBD treatment are less than 200 nm in diameter and have a negative surface charge (such as anionic liposomes), as these characteristics allow the NPs to benefit from the EPR effect of inflammation and the accumulation of positively charged proteins at the damaged epithelium of IBD patients, respectively.Citation38 Many edible plant-derived NPs, including broccoli-, ginger-, grape-, and grapefruit-derived NPs, have demonstrated excellent IEC-targeting functions, partly due to their suitable sizes and negatively charged surfaces.Citation39,Citation40 In the context of ligand/receptor-dominated IEC targeting, some of the membrane proteins that are upregulated in IBD can be used as anchors for the attachment of nano-drugs. For example, the glycoprotein, CD98, which is a heterodimer that forms the large neutral amino acid transporter, is abnormally up-regulated on both epithelial and immune cells in IBD.Citation41 NPs decorated on their surfaces with a CD98 antibody demonstrated an IEC-targeting function and successfully delivered CD98 Fab’-bearing quantum dot (QD)-loaded NPs and CD98 siRNA to reduce inflammation in a mouse model of IBD.Citation42 Peptide transporter 1 (PepT1), an oligopeptide transporter, was also found to be overexpressed in the colon epithelium of IBD patients.Citation43–Citation45 Numerous pro-drug strategies have been examined for their ability to target PepT1 as a means to enhance drug absorption. Some have used dipeptides (e.g., valine-glycine and tyrosine-valine) chemically linked to poly-lactic acid-poly-ethylene glycol (PLA-PEG) to deliver drugs.Citation46,Citation47 The results from these studies have suggested that upregulated membrane proteins, especially transporters, are excellent anchors for IEC-targeted NP delivery.
Immune Cells, The Lamina Propria, And The Extracellular Matrix
The intestinal immune system, which is separated from the GI lumen by the intestinal epithelium, prevents the invasion of pathogens and maintains the tolerance of commensal microorganisms and ingested foods.Citation48,Citation49 The innate immune system comprises a variety of cells, including dendritic cells (DCs), granulocytes (such as neutrophils), macrophages, and monocytes. In active IBD, there is a noticeable infiltration of innate immune cells, including DCs, macrophages, natural killer cells, neutrophils, and some adaptive immune cells, such as B cells and T cells, into the lamina propria.Citation50,Citation51 Notably, the infiltration of macrophages and neutrophils is recognized as a hallmark of IBD.Citation52,Citation53 Recently, the ECM has been proven to play an essential role in interacting with the immune response.Citation54 The ECM contains numerous macromolecules (e.g., collagen, elastin, proteoglycan, and non-collagenous proteins) that control critical cellular events, such as adhesion, differentiation, migration, proliferation, and survival.Citation55 In the inflamed tissues, the ECM is affected by the infiltrating immune cells and the activated tissue-resident cells; this results in a remodeled ECM microenvironment with high concentrations of proinflammatory cytokines (e.g., interferons [IFNs], TNF-α, and transforming growth factor-β [TGF-β]), esterases, matrix metalloproteinases (MMPs), myeloperoxidase (MPO), and reactive oxygen species/reactive nitrogen species (ROS/RNS).Citation56 These released inflammatory regulators can modulate a wide range of ECM macromolecules. The proteases, especially MMPs (e.g., MMP8 or MMP9), selectively cleave ECM macromolecules (such as type I collagen) into small bioactive peptides that may reversely alter immune cell activities.Citation57
In active IBD, altered immune cells and macromolecules of the lamina propria and ECM offer opportunities for targeted drug delivery. Notably, some specific ligands/receptors are overexpressed at the leukocyte-endothelial interface to selectively recruit leukocytes to the site of inflammation.Citation58 Endothelial cells, especially those from capillary blood vessels, are activated by inflammatory cytokines to upregulate several adhesion molecules, including E-selectin, intercellular adhesion molecule (ICAM)-1, P-selectin, and vascular cell adhesion molecule (VCAM)-1, on their luminal surfaces.Citation59 Meanwhile, ligands that participate in anchoring these leukocyte receptors are increasingly expressed by the immune cells. Targeting the molecules that modulate leukocyte traffic has recently become a popular approach for drug delivery.Citation60 NPs that mimic the structures of these ligands have been designed to attach to endothelial cells and release drugs to the adjacent immune cells. For example, recombinant P-selectin glycoprotein ligand-1 (PSGL-1) was conjugated to PEGylated PLA particles, and the synthesized NPs demonstrated a significantly stronger ability to adhere to the inflamed endothelium in an in vivo model, compared to unconjugated PEG-PLA particles.Citation61,Citation62 Another example is the use of P- and E-selectins (which bind carbohydrate) to attach to the surface of PLGA particles and mimic the leukocyte adhesion on selectins.Citation63,Citation64 Although these innovative approaches have shown excellent delivery efficiency both in vitro and in vivo, additional work is needed to evaluate their toxicity and therapeutic potential in IBD and further improve their targeting specificity.
The ultimate goal of NP-mediated drug delivery for IBD is to target the NPs to a specific subtype of inflammation-associated cells. In a breakthrough study, surface-modified nontoxic liposomal nanomaterials illustrated the specific-cell targeting ability of NPs. In this work, neutral phospholipids were conjugated with hyaluronan to form liposomal NPs, whose surfaces were further decorated with integrin-targeted antibodies against β7 integrin (β7 I). This enabled them to target a specific subset of leukocytes: the gut mononuclear leukocytes. Through the cell-specific targeting and precise delivery of siRNAs against the cell cycle-regulating molecule, cyclin D1 (CyD1), the authors verified that CyD1 is a potent anti-inflammatory therapeutic target.Citation65 The targeting of activated macrophages via recognition of their overexpressed surface receptors has also been applied in NP-based cell-specific therapies for IBD. The relevant studies used mannosylated poly-(amidoamine)-based NPs to target mannose,Citation66 galactosylated chitosan NPs to target the macrophage galactose/N-acetyl galactosamine-specific lectins (MGLs),Citation67–Citation69 and PLA-PEG NPs grafted with the Fab’ portion of F4/80 antibodies to target the F4/80 membrane proteins.Citation70 These studies showcased the power of NPs to target specific cells through ligand/receptor interactions.
Another emerging direction in the cell-specific targeting field is to employ naturally occurring nanoscale extracellular vesicles for delivery purposes, such as by using edible plant- or mammalian cell-derived exosomes. The lipid bilayer of edible plant-derived exosomes has a unique chemical composition characterized by the noticeable enrichment of phosphatidylethanolamine (PE) and phosphatidylcholine (PC) on the outer layer.Citation40 Two interesting studies showed that the PE- and PC-enriched outer layer could guide grapefruit-derived exosomes to target macrophages via the clathrin-dependent pathway and macropinocytosis.Citation71,Citation72 Another set of studies showed that exosomes derived from TGF-β1 gene-modified DCs could specifically interact with T cell subsets, induce the regulatory T cells (CD4+Foxp3+ Tregs) from lymph nodes of the inflammatory site, and decrease the proportion of helper T cells (Th17) at inflammatory sites, thereby inhibiting the development of DSS-induced colitis in mice.Citation73,Citation74 Given their lipid bilayer membranes and specific surface proteins, naturally occurring exosomes exhibit a highly selective homing ability and specific targeting capability, and thus might be an ideal NP DDS for IBD.
Microbiota
It is estimated that more than 1013 commensal microorganisms reside in the adult human’s intestine, over 90% of which live in the colon.Citation75,Citation76 These commensal microorganisms are commonly known as the human microbiota and include bacteria, fungi, and viruses. In this review, our discussion will focus on the bacteria. Commensal bacteria in a healthy gut were found to comprise at least 400–500 different bacterial species and many different strains within each species, indicating the enormous complexity of this ecosystem.Citation77 It is commonly observed that the members of the human microbiota mainly belong to four phyla; the majority are Bacteroidetes and Firmicutes (represented mostly by Clostridia), with minor representations of Actinobacteria and Proteobacteria. Intestinal inflammation is typically associated with significant decreases in the population of Bacteroidetes and Firmicutes, especially in species of Clostridium coccoides and Clostridium leptum, and substantial increases in the community of Actinobacteria and Proteobacteria, mainly Enterobacteriaceae.Citation75,Citation77 In general, IBD patients show an overall decrease in microbial diversity and a reduction of about 25% in microbial genes.Citation78
Most of the commensal microbiota is located in the intestine; this site has a significantly higher pH (5.5–7.5) than the stomach (pH 1.5–2.0), and the delivery of antibiotics to the intestine can alter the structure of the microbiota. Thus, many nano-delivery systems use pH-sensitive polymeric materials to target the microbiota with antibiotic drugs.Citation79,Citation80 Synthetic polymers, including poly (acrylic acid), poly (acrylamide), poly (diethyl-aminoethyl methacrylate), poly (dimethyl-aminoethyl methacrylate), and poly (methacrylic acid), have been widely studied for the construction of pH-sensitive antibiotic nano-drugs that yield enhanced antimicrobial effects through sustained drug release.Citation81 Some natural polymers, including albumin and gelatin, have also been tested for the production of efficient pH-susceptible nanosystems that can improve the antimicrobial efficiency of antibiotics (i.e., ciprofloxacin, aminoglycosides) by ensuring a targeted and controlled release.Citation82
Beyond the polymer-based NPs, some novel strategies have harnessed genetically engineered probiotic bacteria as the NP delivery system and “nano-factory”. In the first such effort, the probiotic strain, Lactococcus lactis, was genetically modified to express the anti-inflammatory cytokine, interleukin-10 (IL-10), to treat colitis in a mouse model of IBD. When an engineered Lactococcus lactis was orally delivered to the GI tract, it was found to restore intestinal homeostasis.Citation83 Subsequently, Lactococcus lactis was genetically engineered to produce the low calcium response V protein, an immunomodulatory pathogenic protein, and orally delivered to mice. The distributed bacteria released a low dosage of the pathogenic protein, which triggered IL-10 secretion by the host immune cells and reduce colitis.Citation84,Citation85 In similar studies, engineered bacteria, such as Lactobacillus casei,Citation86,Citation87 Lactococcus plantarum,Citation88,Citation89 and Streptococcus gordonii,Citation90 have been used to deliver various molecules to treat colitis in animal models of IBD. Strictly speaking, these probiotics-based NPs are not explicitly designed to tailor the microbiota structure, as their secreted proteins are intended to target immune cells. However, the administration of the probiotics themselves will naturally modify the microbial ecosystem, and the immune changes triggered by their secreted proteins will also reshape the microbiota structure.
Recent studies have shown that edible plant-derived NPs are potent weapons for targeting specific strains of the microbiota. Orally administered ginger-derived lipid nanoparticles (GDLPs) were found to specifically target the Lactobacillaceae in a lipid-dependent manner. The delivered GDLPs contained microRNAs that were shown to affect various genes in Lactobacillus rhamnosus (LGG). In particular, the GDLPs microRNA, mdo-miR7267-3p, mediated targeting of an LGG monooxygenase (ycnE), increased indole-3-carboxaldehyde (I3A), and subsequently induced the production of IL-22, which can improve barrier function and ameliorate mouse colitis.Citation91 These findings indicated that edible plant-derived NPs might be used to target specific components of the microbiome to alleviate inflammation in IBD.
Delivery Routes Of NPs In IBD
Because of their unique size and size-dependent physical properties, NPs are able to pass through mucus layer and deliver loaded drugs to intestinal cells.Citation23 NPs can also be engulfed by macrophage cells through phagocytosis, and thereby modulate the immune environment of the gut.Citation92 Surface-modified NPs can attach to the target tissue for an extended period, and thus can be used for intestinal imaging or other therapeutic purposes.Citation93 Therefore, NPs are mainly used for targeted delivery of drugs, immune-modulating, and medical imaging. In general, the routes of NP delivery include oral administration and non-oral drug delivery (injection and rectal administration).
Oral Administration
The major challenge for oral NP systems targeting the inflamed intestine lies in the environmental extremes in the GI tract. Multiple factors (e.g., digestive enzyme, pH variation, transit time, and microbiota composition) affect the stability and delivery efficiency of NPs. This scenario becomes even more complicated in a chronic inflammatory condition.Citation94 The pH value in the colon can vary widely under inflammation, and studies have shown that the colonic pH can be significantly more acidic in IBD patients (pH 2.3–5.5) than under normal conditions (pH 7.0 ± 0.7).Citation18 The transit time in the GI tract can also vary remarkably in different IBD patients and healthy individuals.Citation95 The physiological characteristics of the respective GI tract segments have been well exploited for designing traditional oral DDS. Ligand-receptor, enzyme-, pH-, time-, microbiota-, and pressure-mediated mechanisms have all been considered for the design of colonic-targeting NPs for IBD treatment.Citation14,Citation18
NP drug delivery strategies that involve only a single drug release mechanism have not succeeded well in clinical studies due to their lack of flexibility and/or the loss of selectivity upon encountering the complex and harsh gut microenvironment. Recently, the combination of multi-responsive mechanisms has gained traction in efforts to design NP-based delivery systems. A multi-responsive DDS would be expected to overcome the limitations of single mechanism-guided delivery and largely enhance the drug delivery efficiency. For example, CODES® (for 5-aminosalicylic acid) and TARGIT® (for budesonide) employed both pH-responsive and microbiota-mediated degradation strategies to target the drug to the colon, and demonstrated more efficient targeting ability than NPs that exhibited only pH- or microbiota-mediated degradation ().Citation96,Citation97 Going forward, multi-responsive drug delivery is expected to become more and more popular in the treatment of IBD.
Table 1 US Food And Drug Administration Approved Multi-Responsive Oral Drug Delivery Systems For IBD Treatment
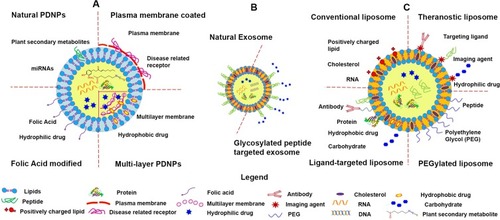
An emerging branch in the multi-responsive DDS field is the therapeutic application of naturally derived nanoparticles, such as NPs isolated from edible plants and mammalian cells. The plant-derived NPs have shown excellent targeting ability with negligible toxicity. Owning to their biocompatible lipid bilayer structure [], edible plant-derived NPs do not cause an immune response. If properly manufactured, these NPs often maintain their surface structures, which are typically characterized by a few transmembrane proteins, ligands, abundant encapsulated genetic materials, and enriched bioactive metabolites. For example, GDNPs have been shown to carry proteins, ginger miRNAs, and ginger active compounds. Orally administrated GDNPs were found to efficiently target the colon and were mainly absorbed by cells in the lining of the intestine, which is the site of IBD-associated inflammation.Citation98 The colon-targeting function of GDNPs is multi-factorial and is likely to involve size-, ligand-, and receptor-mediated actions. The capacity of other plant-derived NPs, including grape-, grapefruit-, and broccoli-derived NPs, to target different tissues is also likely to be mediated via multiple factors.Citation71,Citation72,Citation99–Citation101 These mechanisms warrant further study. Unlike the edible plant-derived NPs, most mammalian cell-derived NPs are not suitable for oral administration (see the injectable DDS section for more information on these NPs).
Figure 3 Structures of plant-derived nanoparticles (PDNPs), exosome, and synthetic nanoparticles (artificial liposomes). (A) Major forms of PDNPs, (B) mammalian exosome, and (C) major forms of the artificial liposomes. Reprinted with permission from The Royal Society of Chemistry. Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6(9):1312–1321.Citation40
In addition to considering the targeting mechanisms for oral-deliverable NPs, the drugs’ properties need to be considered during NP design efforts. For example, antibodies or small molecules that target G-protein coupled receptor (GPCR) signaling can be delivered to the ECM, siRNA drugs require a DDS for high cellular uptake, and DNA drugs need to be escorted into the nucleus. For biologics, the traditional delivery route is the intravenous injection; however, recent advances in plant-derived NPs have demonstrated that GDLPs could orally deliver CD98 siRNAs to the inflamed colon, offering a new approach for orally delivering biologics to treat IBD.Citation102 Although many small molecules may be delivered to the colon by NPs, efforts should be made to prevent the premature drug release caused by acidic erosion in the stomach and enzymatic degradation along the GI tract. Enteric coatings or degradation-resistant NP delivery systems can potentially be used to protect encapsulated drugs.
Injectable NPs For IBD
Injectable administration, including IV, intramuscular (IM), and SC injection, is less favored by patients compared to oral administration, as injections require specialized personnel and devices, and are often associated with pain. Injectable administration offers benefits over oral administration in providing instant and high systemic bioavailability and largely reducing the liver’s first-pass metabolism of drugs. However, the consequent high systemic bioavailability inevitably increases the possibility of systemic side effects. For example, IV infusion of anti-TNF-α biologics may suppress the whole immune system,Citation103 and patients receiving SC delivery of Leukine® (sargramostim), a human granulocyte-macrophage colony-stimulating factor (GM-CSF) that activates innate immunity, reported more musculoskeletal pain than those that received a placebo.Citation104,Citation105
Currently, most biologics (anti-TNF-antibodies, antibodies against integrins) and some corticosteroids are administered through IV or SC injections. However, injectable NP-mediated drug delivery could enable such agents to reach the inflammation site and/or target it through the EPR effect and then undergo local cargo delivery via both size- and ligand/receptor interaction-dependent mechanisms. Other microenvironment-dependent release mechanisms, such as enzyme-, pH-, and ROS-driven degradation of NPs, can also be harnessed when designing injectable NPs. As seen with oral administration, multi-responsive degradation is becoming more and more popular in the development of injectable NPs.
Various polymer-based injectable NPs have been assessed in preclinical studies of IBD treatment. To ensure safety, however, each component of a synthetic polymer-based NP should be investigated for long-term toxicity. This could be an issue for further development of injectable polymer-based NPs, as relatively few polymers (e.g., polyethylene glycol [PEG], PLGA, and chitosan) have been verified as safe materials,Citation106,Citation107 and most synthetic polymers have not been sufficiently studied in terms of their long-term toxicity. Some synthetic NP materials have even been reported to promote tissue inflammation and sclerosis.Citation108–Citation111
In contrasts to synthetic polymers, mammalian cell-derived exosomes are much safer NP system for injectable drug delivery. In their role as part of the cell-cell communication system, mammalian cell-derived exosomes continuously shuttle between the host and receptor cells under the supervision of the immune system. Therefore, these exosomes are extraordinary biocompatible and can be used to generate highly specific NP systems.Citation112 Some exosomes can even have their own innate therapeutic effects. For example, intravenous injection of intestinal epithelial cell (IEC) exosomes into mice with dextran sulfate sodium (DSS)-induced colitis has been shown to inhibit the progress of colitis. In one study, IEC exosomes carrying transforming growth factor-beta 1 (TGF-β1) exhibited immunosuppressive activity by inducing Tregs and immunosuppressive DCs,Citation74 other studies found that exosomes released by granulocytic myeloid-derived suppressor cells (G-MDSCs) could attenuate DSS-induced acute colitis in mice, decreasing the proportion of Th1 cells and increasing the proportion of Tregs in mesenteric lymph nodes.Citation113,Citation114 Thus, it seems that exosomes could be active natural nanocarriers for delivering innately anti-inflammatory biological components for the treatment of IBD.
Other Delivery Routes
Although rectal delivery is recommended as the first-line treatment for patients with mild to moderate distal colitis, rectal administration of drugs to treat IBD is less favorable than oral administration or IV injection.Citation115–Citation117 Despite this, many antisense oligonucleotide (ASO) medications have been successfully delivered by rectal administration in preclinical and clinical IBD studies. For example, researchers used rectal administration of galactosylated low molecular-weight chitosan (gal-LMWC) and TNF-α ASO to deliver the latter into activated colonic macrophages, significantly reducing colonic TNF-α in mice with 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis.Citation118 Moreover, administration of signal transducer and activator of transcription 3 (STAT3) ASO by rectal enema effectively inhibited STAT3 expression and phosphorylation in the inflamed colonic mucosa of colitis models and significantly attenuated the intestinal inflammation.Citation119 Despite the recognized advantages of rectal administration, such as low systemic drug exposure, however, rectal therapies are generally unpopular and remain underused. This may reflect issues with poor drug retention, limited medication adherence to enema treatments, and problems with leakage and bloating.Citation18,Citation120
In general, the selection of the delivery route used to treat IBD depends on the individual disease stage. Injectable administration of drugs is particularly useful for IBD treatment when the inflammation has spread to extra-intestinal locations, such as the eyes, joints, and skin. When inflammation is only present in the GI tract, oral and/or rectal drug delivery would be a better solution, as they may reduce the systemic bio-distribution of the delivered drug.
Conclusion And Prospects
NP-mediated DDSs have been widely developed for the treatment of chronic inflammatory diseases, such as IBD. The delivery of an NP-encapsulated drug to the inflamed intestine has two prominent benefits: 1) an NP-loaded drug may offer similar or better efficacy at a lower concentration than the same drug delivered in a conventional formulation (e.g., tablets, capsules, or emulsions);Citation28 2) NPs may improve the pharmacokinetics of the loaded drug, reducing its systemic bio-distribution, toxicity, and/or dosing frequency. For NP-based drug delivery in the treatment of IBD, the utilized targeting strategies are typically based on the physiological changes exhibited by the inflamed tissues.Citation116 Compared to healthy intestine, inflamed intestine demonstrates alterations in pH, the colon-transit time, receptor expression, angiogenesis, and other microenvironmental factors. These differences between infected and healthy intestine can be exploited to improve the targeting function of NP-based drug delivery. Many single-factor (enzyme-, pH-, time-, pressure-, receptor-, ultrasound-, etc.) triggered NP delivery systems have been developed for the treatment of IBD.Citation16,Citation21 However, none of these single factor-triggered NP systems has proven able to easily cure the disease.
Recently, the study of IBD nanomedicine has turned to use multi-responsive NP systems to target the disease. Multi-responsive NP systems may be comprised of multi-responsive materials with complex structures, or they may take advantage of known NP systems that naturally have multi-responsive functions. Construction of multi-responsive materials and NPs with complex structures require extensive chemical modification and particle structure optimization, and the toxicity of each component must be carefully evaluated. At present, we know very little about the dispositions and metabolisms of these synthetic materials after drug delivery. The existing pharmacokinetic (PK) studies of NP-based DDSs have mainly focused on the carried drug; the PKs of the NP materials have been largely ignored.Citation121,Citation122 A hot topic in the field of complex NP design is the development of dual-function (i.e., having both diagnostic and therapeutic abilities) NP-based delivery systems. Some complex NP-based delivery systems have been fabricated to deliver multiple drugs, with the goal of producing synergic efficacy or performing multimodal imaging.Citation123,Citation124 These new and more sophisticated nanosystems might be the future of NP-mediated IBD treatment and diagnosis. However, toxicity should be carefully evaluated for these multi-functional NPs: the therapeutic NPs warrant long-term safety evaluations while the diagnostic NPs need to be tested for the safety of their acute administration.
The biomedical field has witnessed an exponential increase in the number of studies focused on exosomes and their diagnosis and treatment of diseases.Citation73,Citation125 Such progress has also extended to the area of NP-mediated IBD therapy. Exosomes are nano-sized spherical vesicles that are secreted by plant or animal cells. The lipid compositions differ drastically between plant- and animal cell-derived exosomes, but both exhibit equally powerful cell-specific targeting functions. For instance, ginger-derived lipid vesicles can specifically target IECs and deliver CD98 siRNA to reduce colonic CD98 gene expression,Citation102 while exosomes from antigen-presenting DC cells could activate both regulatory and helper T cells.Citation126 It has been suggested that these naturally occurring NPs could shift the current paradigm of NP drug delivery from using synthetic polymeric NPs to using natural NPs.
The development of 16s ribosomal RNA sequencing has allowed researchers to increasingly clarify the structures of various gut microbiota. Efforts to identify the antigenic variation of the microbiota in IBD, specifically the major bacterial species at the site of inflammation, could help researchers design microbiome-targeted NP delivery systems.Citation75 For instance, the surface properties of NPs could be tuned to target pathogenic bacteria or probiotics to improve the microbiota structure of the patient, thereby helping increase the efficacy of IBD treatment by altering the microbiota metabolome.Citation127 Several commensal enteric bacteria (Clostridia and Bacteroides) convert undigested fibers to short-chain fatty acids (SCFAs), including acetate and butyrate, which are energy substrates for colonic epithelial cells and regulatory factors of the adaptive immune system. These SCFAs were found to stimulate the proliferation of Foxp3+IL-10-producing colonic Tregs, which help to protect the colon against colitis.Citation128,Citation129 Therefore, microbiome-targeted NPs could be designed to elevate the population of these probiotics. Given the complex etiology of IBD, gaining a better understanding of the gut microbiota network, the microbiota metabolome, and their immunology will help us understand IBD pathophysiology and facilitate the design of novel drug delivery systems to target IBD.
Abbreviations
IBD, inflammatory bowel disease; ICAM, intercellular adhesion molecule; NP, nanoparticle; VCAM, vascular cell adhesion molecule; DDS, drug delivery system; PSGL-1, P-selectin glycoprotein ligand-1; GI, gastrointestinal; PLA, poly lactic acid; UC, ulcerative colitis; PLGA, poly lactic-co-glycolic acid; CD, Crohn’s disease; β7 I, β7 integrin; TNF, tumor necrosis factor; CyD1, Cyclin D 1; IV, intravenous; PE, phosphatidylethanolamine; SC, subcutaneous; PC, phosphatidylcholine; siRNA, small interfering RNA; Tregs, regulatory T cells; EPR, enhanced permeability effect; Ths, helper T cells; IECs, intestinal epithelial cells; IL-10, interleukin-10; TJs, tight junctions; GDLP, ginger derived lipid nanoparticle; QDs, quantum dots; LGG, lactobacillus rhamnosus; PepT1, peptide transporter 1; I3A, indole-3-carboxaldehyde; PEG, poly ethylene glycol; GDNPs, ginger derived nanoparticles; DCs, dendritic cells; GPCR, G-protein coupled receptor; ECM, extracellular matrix; IM, intramuscular; IFNs, interferons; ASO, antisense oligonucleotide; TGF-β, transforming growth factor- β; LMWC, low molecular weight chitosan; MMP, matrix metalloproteinases; TNBS, 2,4,6-trinitrobenzene sulfonic acid; MPO, myeloperoxidase; PK, pharmacokinetics; ROS, reactive oxygen species; SCFAs, short-chain fatty acids; RNS, reactive nitrogen species; MGL, macrophage galactose/N-acetylgalactosamine-specific lectins; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-MDSC, granulocytic myeloid-derived suppressor cells; STAT3, signal transducer and activator of transcription 3.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Institutes of Health of Diabetes and Digestive and Kidney (RO1-DK-116306 and RO1-DK-107739 to D.M.), the Department of Veterans Affairs (Merit Award BX002526 to D.M.). DM is a recipient of a Senior Research Career Scientist Award (BX004476) from the Department of Veterans Affairs. Authors appreciate the support from the Royal Society of Chemistry for reusing of Figure 3, the structure of edible-plant derived nanoparticles, from the Journal of Materials Chemistry B, 2018; 6(9): 1312-1321.
References
- Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2014;8(4):288–295. doi:10.1016/j.crohns.2013.09.00124074875
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.15026323879
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217. doi:10.1038/nrgastro.2015.3425732745
- Francescone R, Hou V, Grivennikov SI, Cytokines IBD. colitis-associated cancer. Inflamm Bowel Dis. 2015;21(2):409–418. doi:10.1097/MIB.000000000000023625563695
- Beaugerie L. [IBD and increased risk of cancer: what is the reality?]. Rev Infirm. 2014;63(199):28. doi:10.1016/j.revinf.2013.12.011
- Stormont JM, Shah AN, Sharma AK, Saubermann LJ, Farmer RG. Colorectal cancer in IBD patients. Am J Gastroenterol. 2013;108(9):1535. doi:10.1038/ajg.2013.203
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-029050646
- Venkataraman GR, Rivas MA. Rare and common variant discovery in complex disease: the IBD case study. Hum Mol Genet. 2019. doi:10.1093/hmg/ddz189
- Hammer T, Lophaven SN, Nielsen KR, et al. Dietary risk factors for inflammatory bowel diseases in a high-risk population: results from the Faroese IBD study. United Eur Gastroenterol J. 2019;7(7):924–932. doi:10.1177/2050640619852244
- Kaplan GG. IBD: global variations in environmental risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2014;11(12):708–709. doi:10.1038/nrgastro.2014.18225348851
- van der Sloot KWJ, Weersma RK, Dijkstra G, Alizadeh BZ. Development and validation of a web-based questionnaire to identify environmental risk factors for inflammatory bowel disease: the Groningen IBD Environmental Questionnaire (GIEQ). J Gastroenterol. 2019;54(3):238–248. doi:10.1007/s00535-018-1501-z30109418
- Lim JS, Lim MY, Choi Y, Ko G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol Brain. 2017;10(1):14. doi:10.1186/s13041-017-0292-028427452
- Putignani L, Del Chierico F, Vernocchi P, et al. Gut microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm Bowel Dis. 2016;22(2):487–504. doi:10.1097/MIB.000000000000060226588090
- Zhang M, Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm Bowel Dis. 2018;24(7):1401–1415. doi:10.1093/ibd/izy12329788186
- Sabino J, Verstockt B, Vermeire S, Ferrante M. New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol. 2019;12:1756284819853208. doi:10.1177/1756284819853208
- Zeeshan M, Ali H, Khan S, Mukhtar M, Khan MI, Arshad M. Glycyrrhizic acid-loaded pH-sensitive poly-(lactic-co-glycolic acid) nanoparticles for the amelioration of inflammatory bowel disease. Nanomedicine (Lond). 2019;14(15):1945–1969. doi:10.2217/nnm-2018-041531355705
- Samaan M, Campbell S, Cunningham G, Tamilarasan AG, Irving PM, McCartney S. Biologic therapies for Crohn’s disease: optimising the old and maximising the new. F1000Research. 2019;8. doi:10.12688/f1000research.18902.1
- Zhang S, Langer R, Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2017;16:82–96. doi:10.1016/j.nantod.2017.08.00631186671
- Mokhtarzadeh A, Hassanpour S, Vahid ZF, et al. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J Control Release. 2017;266:166–186. doi:10.1016/j.jconrel.2017.09.02828941992
- D’Inca R, Paccagnella M, Cardin R, et al. 5-ASA colonic mucosal concentrations resulting from different pharmaceutical formulations in ulcerative colitis. World J Gastroenterol. 2013;19(34):5665–5670. doi:10.3748/wjg.v19.i34.566524039359
- Viscido A, Capannolo A, Latella G, Caprilli R, Frieri G. Nanotechnology in the treatment of inflammatory bowel diseases. J Crohns Colitis. 2014;8(9):903–918. doi:10.1016/j.crohns.2014.02.02424686095
- Huang Z, Gan J, Jia L, et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi:10.1016/j.biomaterials.2015.01.01325701029
- Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158–171. doi:10.1016/j.addr.2008.11.00219133304
- Takedatsu H, Mitsuyama K, Torimura T. Nanomedicine and drug delivery strategies for treatment of inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11343–11352. doi:10.3748/wjg.v21.i40.1134326525603
- Lu L, Chen G, Qiu Y, et al. Nanoparticle-based oral delivery systems for colon targeting: principles and design strategies. Sci Bull. 2016;61(9):670–681. doi:10.1007/s11434-016-1056-4
- Xia F, Ding F, Lv Y, Di W, Sheng Y, Ding G. A high efficient method to isolate exosomes from small intestinal epithelium. Mol Biotechnol. 2019;61(5):325–331. doi:10.1007/s12033-019-00163-930796724
- Regueiro M, Swoger J. Clinical Challenges and Complications of IBD. Thorofare, NJ: SLACK Inc.; 2013.
- Melero A, Draheim C, Hansen S, et al. Targeted delivery of Cyclosporine A by polymeric nanocarriers improves the therapy of inflammatory bowel disease in a relevant mouse model. Eur J Pharma Biopharm. 2017;119:361–371. doi:10.1016/j.ejpb.2017.07.004
- Chassaing B, Gewirtz AT. Identification of inner mucus-associated bacteria by laser capture microdissection. Cell Mol Gastroenterol Hepatol. 2019;7(1):157–160. doi:10.1016/j.jcmgh.2018.09.00930510996
- Irache JM, Durrer C, Duchene D, Ponchel G. Bioadhesion of lectin-latex conjugates to rat intestinal mucosa. Pharm Res. 1996;13(11):1716–1719. doi:10.1023/A:10164051266568956340
- Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol. 2012;20(1):30–39. doi:10.1016/j.tim.2011.10.00122088901
- Swidsinski A, Loening-Baucke V, Theissig F, et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56(3):343–350. doi:10.1136/gut.2006.09816016908512
- Qin X. Damage of the mucus layer: the possible shared critical common cause for Both Inflammatory Bowel Disease (IBD) and Irritable Bowel Syndrome (IBS). Inflamm Bowel Dis. 2017;23(2):E11–E12. doi:10.1097/MIB.000000000000101028079624
- Scaldaferri F, Lopetuso LR, Petito V, et al. Gelatin tannate ameliorates acute colitis in mice by reinforcing mucus layer and modulating gut microbiota composition: emerging role for ‘gut barrier protectors’ in IBD? United Eur Gastroenterol J. 2014;2(2):113–122. doi:10.1177/2050640614520867
- Des Rieux A, Fievez V, Theate I, Mast J, Preat V, YJ S. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30(5):380–391. doi:10.1016/j.ejps.2006.12.00617291730
- Greish K, Taha S, Jasim A, et al. Styrene maleic acid encapsulated raloxifene micelles for management of inflammatory bowel disease. Clin Transl Med. 2017;6(1):28. doi:10.1186/s40169-017-0157-228770521
- Iglesias N, Galbis E, Diaz-Blanco MJ, Lucas R, Benito E, de-Paz MV. Nanostructured chitosan-based biomaterials for sustained and colon-specific resveratrol release. Int J Mol Sci. 2019;20(2):398. doi:10.3390/ijms20020398
- Xiao B, Xu Z, Viennois E, et al. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther. 2017;25(7):1628–1640. doi:10.1016/j.ymthe.2016.11.02028143741
- Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi:10.7717/peerj.518630083436
- Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6(9):1312–1321. doi:10.1039/C7TB03207B30034807
- Charania MA, Laroui H, Liu H, et al. Intestinal epithelial CD98 directly modulates the innate host response to enteric bacterial pathogens. Infect Immun. 2013;81(3):923–934. doi:10.1128/IAI.01388-1223297381
- Xiao B, Laroui H, Viennois E, et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146(5):1289–1300e, 1281–1219. doi:10.1053/j.gastro.2014.01.056
- Zucchelli M, Torkvist L, Bresso F, et al. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(10):1562–1569. doi:10.1002/ibd.2096319462432
- Wang Y, Hu Y, Li P, et al. Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice. Biochem Pharmacol. 2018;148:163–173. doi:10.1016/j.bcp.2017.12.02529305856
- Wang CY, Liu S, Xie XN, Tan ZR. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des Devel Ther. 2017;11:3511–3517. doi:10.2147/DDDT.S151725
- Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134(1):166–178. doi:10.1053/j.gastro.2007.10.02618061177
- Wu Y, Sun M, Wang D, et al. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater Sci. 2019;7:4299–4309. doi:10.1039/C9BM00925F31408067
- Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136(3):345–361.29797112
- Haag LM, Siegmund B. Intestinal microbiota and the innate immune system - a crosstalk in Crohn’s disease pathogenesis. Front Immunol. 2015;6:489. doi:10.3389/fimmu.2015.0048926441993
- Santiago L, Castro M, Pardo J, Arias M. Mouse model of Colitis-Associated Colorectal Cancer (CAC): isolation and characterization of mucosal-associated lymphoid cells. Methods Mol Biol. 2019;1884:189–202.30465204
- Matsui F, Inaba M, Uchida K, et al. Induction of PIR-A/B(+) DCs in the in vitro inflammatory condition and their immunoregulatory function. J Gastroenterol. 2018;53(10):1131–1141. doi:10.1007/s00535-018-1447-129508072
- Schoultz I, Keita AV. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells. 2019;8(2):193. doi:10.3390/cells8020193
- Butin-Israeli V, Bui TM, Wiesolek HL, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129(2):712–726.30640176
- Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2015;64(3):367–372. doi:10.1136/gutjnl-2014-30804825416065
- Lindholm M, Manon-Jensen T, Madsen GI, et al. Extracellular matrix fragments of the basement membrane and the interstitial matrix are serological markers of intestinal tissue remodeling and disease activity in dextran sulfate sodium colitis. Dig Dis Sci. 2019. doi:10.1007/s10620-019-05676-6
- Stronati L, Palone F, Negroni A, et al. Dipotassium glycyrrhizate improves intestinal mucosal healing by modulating extracellular matrix remodeling genes and restoring epithelial barrier functions. Front Immunol. 2019;10:939. doi:10.3389/fimmu.2019.0093931105713
- Gaggar A, Jackson PL, Noerager BD, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–5669. doi:10.4049/jimmunol.180.8.566218390751
- Ajdukovic J, Salamunic I, Hozo I, et al. Soluble P-selectin glycoprotein ligand - a possible new target in ulcerative colitis. Bratisl Lek Listy. 2015;116(3):147–149.25869560
- Weishaupt C, Steinert M, Brunner G, et al. Activation of human vascular endothelium in melanoma metastases induces ICAM-1 and E-selectin expression and results in increased infiltration with effector lymphocytes. Exp Dermatol. 2019. doi:10.1111/exd.14023
- Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharma Sin B. 2018;8(1):23–33. doi:10.1016/j.apsb.2017.12.002
- Farzi B, Young D, Scrimgeour J, Cetinkaya C. Mechanical properties of P-selectin PSGL-1 bonds. Colloids Surf B Biointerfaces. 2019;173:529–538. doi:10.1016/j.colsurfb.2018.10.01730342396
- Sakhalkar HS, Dalal MK, Salem AK, et al. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15895–15900. doi:10.1073/pnas.263143310014668435
- Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–670. doi:10.1016/j.biomaterials.2004.03.00315282144
- Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–2177. doi:10.1016/S0142-9612(01)00349-011962658
- Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319(5863):627–630. doi:10.1126/science.114985918239128
- Vieira AC, Chaves LL, Pinheiro M, Ferreira D, Sarmento B, Reis S. Design and statistical modeling of mannose-decorated dapsone-containing nanoparticles as a strategy of targeting intestinal M-cells. Int J Nanomedicine. 2016;11:2601–2617. doi:10.2147/IJN.S10490827354792
- Huang Y, Guo J, Gui S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-a siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur J Pharm Sci. 2018;125:232–243. doi:10.1016/j.ejps.2018.10.00930315858
- Wang C, Zhang Z, Chen B, Gu L, Li Y, Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J Colloid Interface Sci. 2018;516:332–341. doi:10.1016/j.jcis.2018.01.07329408121
- Zhang J, Tang C, Yin C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials. 2013;34(14):3667–3677. doi:10.1016/j.biomaterials.2013.01.07923419643
- Laroui H, Viennois E, Xiao B, et al. Fab’-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release. 2014;186:41–53. doi:10.1016/j.jconrel.2014.04.04624810114
- Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.19023939022
- Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867–1877. doi:10.1038/ncomms288623695661
- Ma ZJ, Wang YH, Li ZG, et al. Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int J Stem Cells. 2019. doi:10.15283/ijsc18139
- Cai Z, Zhang W, Yang F, et al. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012;22(3):607–610. doi:10.1038/cr.2011.19622157651
- Khan I, Ullah N, Zha L, et al. Alteration of gut microbiota in Inflammatory Bowel Disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019;8(3):126. doi:10.3390/pathogens8030126
- Vieira-Silva S, Sabino J, Valles-Colomer M, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbio. 2019. doi:10.1038/s41564-019-0483-9
- Sun Y, Li L, Xia Y, et al. The gut microbiota heterogeneity and assembly changes associated with the IBD. Sci Rep. 2019;9(1):440. doi:10.1038/s41598-018-37143-z30679676
- Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2019;1–14. doi:10.1080/15548627.2019.1635384
- Holota Y, Dovbynchuk T, Kaji I, et al. The long-term consequences of antibiotic therapy: role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS One. 2019;14(8):e0220642. doi:10.1371/journal.pone.022064231437166
- Liu M, Nazzal L. Enteric hyperoxaluria: role of microbiota and antibiotics. Curr Opin Nephrol Hypertens. 2019;28(4):352–359. doi:10.1097/MNH.000000000000051831145706
- Anderson EM, Noble ML, Garty S, et al. Sustained release of antibiotic from poly(2-hydroxyethyl methacrylate) to prevent blinding infections after cataract surgery. Biomaterials. 2009;30(29):5675–5681. doi:10.1016/j.biomaterials.2009.06.04719631376
- Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo). 2011;64(9):625–634. doi:10.1038/ja.2011.5821811264
- Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289(5483):1352–1355. doi:10.1126/science.289.5483.135210958782
- Foligne B, Dessein R, Marceau M, et al. Prevention and treatment of colitis with lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology. 2007;133(3):862–874. doi:10.1053/j.gastro.2007.06.01817678918
- Souza BM, Preisser TM, Pereira VB, et al. Lactococcus lactis carrying the pValac eukaryotic expression vector coding for IL-4 reduces chemically-induced intestinal inflammation by increasing the levels of IL-10-producing regulatory cells. Microb Cell Fact. 2016;15(1):150. doi:10.1186/s12934-016-0548-x27576902
- Bellavia M, Rappa F, Lo Bello M, et al. Lactobacillus casei and bifidobacterium lactis supplementation reduces tissue damage of intestinal mucosa and liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice. J Biol Regul Homeost Agents. 2014;28(2):251–261.25001657
- Llopis M, Antolin M, Carol M, et al. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15(2):275–283. doi:10.1002/ibd.2073618839424
- Kim MS, Byun JS, Yoon YS, Yum DY, Chung MJ, Lee JC. A probiotic combination attenuates experimental colitis through inhibition of innate cytokine production. Benef Microbes. 2017;8(2):231–241. doi:10.3920/BM2016.003128008786
- Kawahara M, Nemoto M, Nakata T, et al. Anti-inflammatory properties of fermented soy milk with lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int Immunopharmacol. 2015;26(2):295–303. doi:10.1016/j.intimp.2015.04.00425887264
- Ricci S, Macchia G, Ruggiero P, et al. In vivo mucosal delivery of bioactive human interleukin 1 receptor antagonist produced by Streptococcus gordonii. BMC Biotechnol. 2003;3:15. doi:10.1186/1472-6750-3-1513129437
- Teng Y, Ren Y, Sayed M, et al. Plant-Derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652 e638. doi:10.1016/j.chom.2018.10.00130449315
- Nguyen MA, Wyatt H, Susser L, et al. Delivery of MicroRNAs by Chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano. 2019;13(6):6491–6505. doi:10.1021/acsnano.8b0967931125197
- Petkau K, Kaeser A, Fischer I, Brunsveld L, Schenning AP. Pre- and postfunctionalized self-assembled pi-conjugated fluorescent organic nanoparticles for dual targeting. J Am Chem Soc. 2011;133(42):17063–17071. doi:10.1021/ja207534521913650
- Gao C, Liu L, Zhou Y, Bian Z, Wang S, Wang Y. Novel drug delivery systems of Chinese medicine for the treatment of inflammatory bowel disease. Chin Med. 2019;14:23. doi:10.1186/s13020-019-0245-x31236131
- Gazzaniga A, Maroni A, Foppoli A, Palugan L. Oral colon delivery: rationale and time-based drug design strategy. Discov Med. 2006;6(36):223–228.17250787
- Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4(3):160–170. doi:10.1038/ncpgasthep069617339853
- Ali H, Weigmann B, Neurath MF, Collnot EM, Windbergs M, Lehr CM. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167–177. doi:10.1016/j.jconrel.2014.03.03924685705
- Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.15927491931
- Deng Z, Rong Y, Teng Y, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25(7):1641–1654. doi:10.1016/j.ymthe.2017.01.02528274798
- Li W, Zhang T, Ye Y, Zhang X, Wu B. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int J Pharm. 2015;495(2):948–955. doi:10.1016/j.ijpharm.2015.10.01126453780
- Wang QL, Zhuang X, Sriwastva MK, et al. Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways. Theranostics. 2018;8(18):4912–4924.30429877
- Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (Lond). 2017;12(16):1927–1943. doi:10.2217/nnm-2017-019628665164
- Trinchard-Lugan I, Ho-Nguyen Q, Bilham WM, Buraglio M, Ythier A, Munafo A. Safety, pharmacokinetics and pharmacodynamics of recombinant human tumour necrosis factor-binding protein-1 (Onercept) injected by intravenous, intramuscular and subcutaneous routes into healthy volunteers. Eur Cytokine Netw. 2001;12(3):391–398.11566619
- Gendelman HE, Zhang Y, Santamaria P, et al. Evaluation of the safety and immunomodulatory effects of sargramostim in a randomized, double-blind phase 1 clinical Parkinson’s disease trial. NPJ Parkinsons Dis. 2017;3:10. doi:10.1038/s41531-017-0013-528649610
- Spitler LE, Cao H, Piironen T, Whiteside TL, Weber RW, Cruickshank S. Biological effects of anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) antibody formation in patients treated with GM-CSF (Sargramostim) as adjuvant therapy of melanoma. Am J Clin Oncol. 2017;40(2):207–213. doi:10.1097/COC.000000000000012425286079
- Hu X, Li D, Gao Y, Mu L, Zhou Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ Int. 2016;94:8–23. doi:10.1016/j.envint.2016.05.00127203780
- Boverhof DR, Bramante CM, Butala JH, et al. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul Toxicol Pharmacol. 2015;73(1):137–150. doi:10.1016/j.yrtph.2015.06.00126111608
- Can Demirdogen B. Potential role of calcifying nanoparticles in the etiology of multiple sclerosis. Med Hypotheses. 2019;128:25–27. doi:10.1016/j.mehy.2019.05.00531203904
- Roach KA, Stefaniak AB, Roberts JR. Metal nanomaterials: immune effects and implications of physicochemical properties on sensitization, elicitation, and exacerbation of allergic disease. J Immunotoxicol. 2019;16(1):87–124. doi:10.1080/1547691X.2019.160555331195861
- Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine. 2015;40(6):399–404. doi:10.1097/BRS.000000000000077825584952
- van den Brule S, Beckers E, Chaurand P, et al. Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats. Part Fibre Toxicol. 2014;11:67. doi:10.1186/s12989-014-0067-z25497478
- Domenis R, Cif A, Fabris M, Curcio F. JOINT MEETING OF PATHOLOGY AND LABORATORY MEDICINE SIPMET. Clinical applications of microenvironment-controlled immunosuppressive properties of mesenchymal stem cells-derived exosomes: a review. Journal of biological regulators and homeostatic agents. Jul-Aug 2018;32 (4 Suppl. 1):15–20.
- Wang Y, Tian J, Tang X, et al. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget. 2016;7(13):15356–15368. doi:10.18632/oncotarget.732426885611
- Su H, Cong X, Liu YL. Transplantation of granulocytic myeloid-derived suppressor cells (G-MDSCs) could reduce colitis in experimental murine models. J Dig Dis. 2013;14(5):251–258. doi:10.1111/1751-2980.1202923279711
- Wolk O, Epstein S, Ioffe-Dahan V, Ben-Shabat S, Dahan A. New targeting strategies in drug therapy of inflammatory bowel disease: mechanistic approaches and opportunities. Expert Opin Drug Deliv. 2013;10(9):1275–1286. doi:10.1517/17425247.2013.80048023721560
- Dahan A, Amidon GL, Zimmermann EM. Drug targeting strategies for the treatment of inflammatory bowel disease: a mechanistic update. Expert Rev Clin Immunol. 2010;6(4):543–550. doi:10.1586/eci.10.3020594127
- Klotz U, Schwab M. Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57(2):267–279. doi:10.1016/j.addr.2004.08.00715555742
- Zuo L, Huang Z, Dong L, et al. Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010;59(4):470–479. doi:10.1136/gut.2009.18455619951904
- Bai A, Hu P, Chen J, et al. Blockade of STAT3 by antisense oligonucleotide in TNBS-induced murine colitis. Int J Colorectal Dis. 2007;22(6):625–635. doi:10.1007/s00384-006-0229-z17089128
- Kesharwani SS, Ahmad R, Bakkari MA, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–179. doi:10.1016/j.jconrel.2018.08.00430142410
- Dogra P, Adolphi NL, Wang Z, et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat Commun. 2018;9(1):4551. doi:10.1038/s41467-018-06730-z30382084
- Mohammad AK, Reineke J. Quantitative nanoparticle organ disposition by gel permeation chromatography. Methods Mol Biol. 2012;926:361–367.22975975
- Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv. 2017;14(1):65–73. doi:10.1080/17425247.2016.120558327337289
- Tng DJ, Song P, Lin G, et al. Synthesis and characterization of multifunctional hybrid-polymeric nanoparticles for drug delivery and multimodal imaging of cancer. Int J Nanomedicine. 2015;10:5771–5786. doi:10.2147/IJN.S8646826396511
- Zhang W, Jiang X, Bao J, Wang Y, Liu H, Tang L. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front Immunol. 2018;9:90. doi:10.3389/fimmu.2018.0009029483904
- Kim SH, Bianco N, Menon R, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13(2):289–300. doi:10.1016/j.ymthe.2005.09.01516275099
- Lewis JD, Ruemmele FM, Wu GD. Nutrition, Gut Microbiota and Immunity: Therapeutic Targets for IBD. Nestlé Nutrition Institute; 2013.
- Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi:10.3390/nu704283925875123
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi:10.1126/science.124116523828891
- Katsuma M, Watanabe S, Kawai H, Takemura S, Masuda Y, Fukui M. Studies on lactulose formulations for colon-specific drug delivery. Int J Pharm. 2002;249(1–2):33–43. doi:10.1016/S0378-5173(02)00429-512433432
- Abinusawa A, Tenjarla S. Release of 5-Aminosalicylic Acid (5-ASA) from mesalamine formulations at various pH levels. Adv Ther. 2015;32(5):477–484. doi:10.1007/s12325-015-0206-425951927
- Yu A, Baker JR, Fioritto AF, et al. Measurement of in vivo gastrointestinal release and dissolution of three locally acting mesalamine formulations in regions of the human gastrointestinal tract. Mol Pharm. 2017;14(2):345–358. doi:10.1021/acs.molpharmaceut.6b0064128009518
- Nanda K, Moss AC. Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX((R)) mesalamine. Clin Pharmacol. 2012;4:41–50. doi:10.2147/CPAA.S2655622888278
- Goyanes A, Hatton GB, Merchant HA, Basit AW. Gastrointestinal release behaviour of modified-release drug products: dynamic dissolution testing of mesalazine formulations. Int J Pharm. 2015;484(1–2):103–108. doi:10.1016/j.ijpharm.2015.02.05125721685
- Nicholls A, Harris-Collazo R, Huang M, Hardiman Y, Jones R, Moro L. Bioavailability profile of Uceris MMX extended-release tablets compared with entocort EC capsules in healthy volunteers. J Int Med Res. 2013;41(2):386–394. doi:10.1177/030006051347658823569029
- Sinha SR, Nguyen LP, Inayathullah M, et al. A thermo-sensitive delivery platform for topical administration of inflammatory bowel disease therapies. Gastroenterology. 2015;149(1):52–55 e52. doi:10.1053/j.gastro.2015.04.00225863215
- Watts P, Smith A. TARGIT technology: coated starch capsules for site-specific drug delivery into the lower gastrointestinal tract. Expert Opin Drug Deliv. 2005;2(1):159–167. doi:10.1517/17425247.2.1.15916296742