Abstract
Nanotechnology is an emerging field which has created great opportunities either through the creation of new materials or by improving the properties of existing ones. Nanoscale materials with a wide range of applications in areas ranging from engineering to biomedicine have been produced. Gold nanoparticles (AuNPs) have emerged as a therapeutic agent, and are useful for imaging, drug delivery, and photodynamic and photothermal therapy. AuNPs have the advantage of ease of functionalization with therapeutic agents through covalent and ionic binding. Combining AuNPs and other materials can result in nanoplatforms, which can be useful for biomedical applications. Biomaterials such as biomolecules, polymers and proteins can improve the therapeutic properties of nanoparticles, such as their biocompatibility, biodistribution, stability and half-life. Serum albumin is a versatile, non-toxic, stable, and biodegradable protein, in which structural domains and functional groups allow the binding and capping of inorganic nanoparticles. AuNPs coated with albumin have improved properties such as greater compatibility, bioavailability, longer circulation times, lower toxicity, and selective bioaccumulation. In the current article, we review the features of albumin, as well as its interaction with AuNPs, focusing on its biomedical applications.
Introduction
Nanotechnology is an emerging field which has provided a wide range of opportunities in applied sciences such as materials science and medicine.Citation1 Novel therapies for chronic diseases, including cancer, aim to overcome the adverse effects caused by the lack of specificity of conventional treatments, thereby improving drug administration.Citation2 In this context, a new and interesting branch of nanotechnology has recently emerged which involves the use of noble metal nanoparticles covered by albumin.Citation3
Gold nanoparticles (AuNPs), of different geometric shapes, can be used for spatially and temporally controlled drug release, thereby taking advantage of their biocompatibility, size and ease of functionalization, in addition to their optic, plasmonic and photothermal properties.Citation4 Optic properties in AuNPs can be modulated by controlling their shape, size, and surface modification through synthetic approaches. A wide variety of AuNP shapes have been reported, including gold nanospheres (AuNSs), nanorods (AuNRs), nanoprisms (AuNPrs), nanoclusters (AuNCs), nanoflowers and core-shells.Citation5–Citation7 Irradiation of AuNPs in their plasmonic band, allows them to absorb and dissipate the energy as local heat, which can be exploited in biomedical applications, including photothermal therapy and drug release.Citation8
Localized surface plasmon resonance (LSPR) on AuNPs is highly dependent on their shape, size, and the surrounding medium, and in this context they are useful for biosensing and detection.Citation9 However, their unique optical properties allow not only transport and release of molecules with therapeutic properties, but also theranostic applications.Citation10 The term “theranostic” was coined in 2002 and is defined as a material that combines the modalities of therapy and diagnostic imaging. Therefore, theranostics deliver therapeutic drugs and diagnostic imaging agents at the same time.Citation11 Small nanoparticles can easily scatter light and be detected through techniques as simple as dark field microscopy to more complex methods such as computerized tomography.Citation9
In a complementary way, AuNP functionalization and coating with endogenous proteins can increase the circulation time, biocompatibility, selective uptake and biodistribution of AuNP-based nanosystems.Citation12,Citation13 Serum albumin (SA) is the most abundant protein in plasma. SA includes a lot of amino acids with charged functional groups, such as carboxyl, amino and sulfhydryl,Citation14 which allow it to have several binding sites for attachment to therapeutic systems, including polyconjugated dyes, drugs, and nanoparticles.Citation2 In addition, SA can undergo cell uptake through specific receptors in tumor cellsCitation15,Citation16 as a nutrient and amino acid source, thereby increasing the bioavailability of loaded agents.Citation17–Citation20
AuNPs can be capped with SA molecules or encapsulated into SA nanocapsules, thereby giving rise to multifunctional nanoplatforms, which can transport drugs and deliver them to target sites.Citation21,Citation22 Furthermore, the AuNP-SA system can be used in other biomedical applications, as described in the current review, including drug delivery, photothermal therapy, diagnostics and theranostics.
Recently, numerous studies concerning AuNP-SA-based systems have been reported, which represent the beginning of a promising nanoplatform for biomedical applications. Albumin has been employed extensively for pharmaceutical applications and also in clinical trials. For a more extensive discussion of the applications of albumin the reader can consult illustrative literature.Citation2,Citation15,Citation16,Citation23–Citation28 With regard to AuNPs-SA, we believe that further developments are possible, and in the future these systems will be trialed clinically.
In the current review, we present a description of SA, a conventional but promising material in nanocarrier design, in addition to discussing AuNPs as a new and versatile material with fascinating properties, as well as forefront approaches for the creation of hybrid AuNP-SA systems.
AuNP properties for biomedical applications
Nanoparticles are defined as particles with sizes that lie between 1 and 100 nm, and which are formed in two or three dimensions. AuNPs exhibit a single structure, consisting of a highly symmetric face center cubic crystalline network.Citation29 AuNPs are characterized by specific geometrically dependent surface properties, as well as susceptibility to several environmental stimuli, such as light or heat.Citation30 Existing methodologies allow for AuNPs of diverse geometries, and the most common in biomedical applications are nanospheres, nanorods, nanoshells, nanocages and nanoprisms. shows a pictorial representation of the above-mentioned systems.
Figure 1 Different shapes of gold nanoparticles used in biomedical applications. Reprinted from Journal of Experimental & Clinical Medicine, 6, Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H, Chang J, Trends of gold nanoparticle-based drug delivery system in cancer therapy, pages 172-178, Copyright (2014) with permission from Elsevier.Citation47

For noble metal particles, where the particle size is smaller than the incident wavelength, the properties of the surface of the material influence interactions with electromagnetic radiation, and such interactions usually occur in visible (Vis) and near infrared (NIR) wavelengths.Citation31 Interestingly, AuNPs can absorb electromagnetic radiation and convert it to thermal energy through photoexcitation mechanisms, thereby releasing heat efficiently. The energy emission and absorption properties depend on the particle shape and size.
Optical properties of AuNPs
The unique properties of AuNPs are caused by the electromagnetic effect of the collective oscillation of the surface of the electrons (conduction electrons). When conduction electrons interact with light (), the restoring force, ie Coulombic attraction, from the electrons to the nuclei in the crystalline network, establishes a resonating condition in a relatively tight spectral band, the so-called LSPR, or surface plasmon.Citation32 In nanoparticles that possess this property, the excitation of the surface plasmon results in substantial electromagnetic fields at the surface of the nanoparticles, which strongly outweigh the intensity of the incident light field.Citation4
Figure 2 LSPR on gold nanoparticles, generated by the interaction between the conduction electrons on gold surface and the incident light on (A) non-anisotropic spherical AuNP, (B) longitudinal and (C) transversal anisotropic rod-shape AuNP.
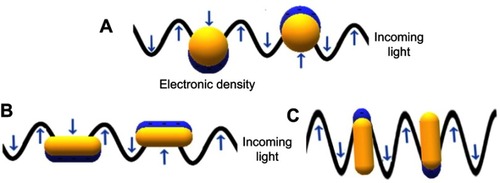
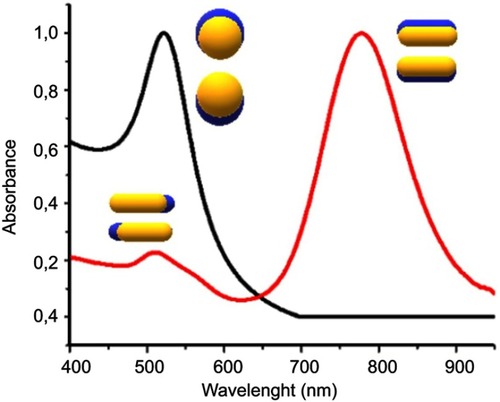
The electron density at the surface of the nanoparticle depends on its size, shape, metal structure and the surroundings, which directly affects the location of the surface plasmon in the electromagnetic spectrum (ES). In spherical AuNPs (AuNS), for example, a strong absorption band centered in the visible region (approximately 520 nm) occurs because the frequency of the electromagnetic wave of the incident light is resonant with the collective oscillation of the conduction electrons, creating the reddish tones observed in spherical AuNPs in solution. In contrast, for anisotropic AuNPs, such as rod and prism shaped (AuNRs and AuNPrs) nanoparticles, more than one band appears in the ES. AuNRs have two bands of surface plasmon resonance, which correspond to the resonant electrons in the transverse and longitudinal bands (less and more intense, respectively).Citation33–Citation35 shows a comparison between the spectra of AuNSs and AuNRs.
Figure 3 UV-Vis absorption spectrum AUNSs (black line), AUNRs (red line) and the oscillation modes on AuNPs.
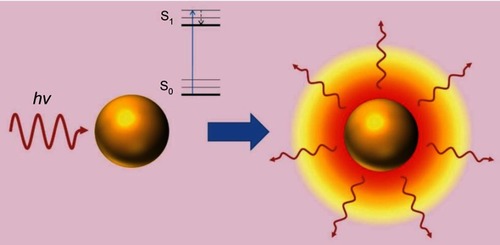
The LSPR produces unique effects in metallic nanoparticles, such as the photothermal effect through phonons, in which energy is transformed into the vibration of crystalline structures. Absorbed photons are converted into phonons, in a process that involves a rapid relaxation of electrons and phonons, followed by phonon-phonon relaxation, which results in an increase in the system temperature and conduction to its surroundings, thereby producing an increase in local temperature ().Citation36–Citation39 The temperature increase produced at the AuNP surface can be used in diverse therapeutic applications, and in particular, photothermal therapy.
Figure 4 Photothermal effect on gold nanoparticles. After irradiation AuNPs absorbs light, which leads to an electronic transition of the surface electrons from a ground state (S0) to an excited state (S1). The energy is released in the environment of the nanostructure as local heat. Republished with permission of Future Medicine Ltd, from Gold nanoparticles for photothermally controlled drug release, Guerrero AR, Hassan N, Escobar CA, Albericio F, Kogan MJ, Araya E, 9, 2014; permission conveyed through Copyright Clearance Center, Inc.Citation32
It is important to mention that for biomedical applications the system should have the least possible toxicity, which requires that the absorption of the plasmon be in the so-called biological window (700–1,100 nm). In this region of the spectrum, none of the radiation is absorbed by the molecules present in human tissue, allowing the irradiation to penetrate tissues and generate a photothermal effect, without causing secondary damage.Citation40
The development of multimodal functional nanoparticles has gained attention as a way to produce promising nanomaterials for biomedical applications.Citation41,Citation42 AuNPs have been one of the most used building blocks, not only for their optical properties, but also because of their load capacity, low toxicity and modifiable surface.Citation43 AuNPs in combination with biomaterials have shown significant efficacy in the treatment of various diseases, such as Alzheimer’s diseaseCitation13,Citation44,Citation45 and several types of cancer.Citation43,Citation46,Citation47
An important issue that should be considered for the pharmaceutical use of nanoparticles is that when nanoparticles are intravenously administered, several plasma proteins bind to their surface, forming the so-called protein corona.Citation48 These capped nanoparticles can then be recognized by the macrophage cell surface and be internalized,Citation49 leading to a significant loss of the nanoparticles from circulation. A portion of the serum proteins that bind and cap the nanoparticles are termed opsonins. Nanoparticles capped with these proteins can be recognized and captured by macrophages, contributing to the loss of the majority of the administrated dose because of retention by the reticuloendothelial system (RES).Citation50
It is important to mention that the protein corona can change over time to create different protein profiles.Citation51,Citation52 Modification of colloidal particles with polymers such as polyethyleneglycol (PEG) can increase their half-life in the bloodCitation47 and also enhance the selective delivery of nanoparticles. For example, capping nanoparticles with endogenous serum proteins, such as apolipoprotein-B and/or ApoE, could support blood brain barrier penetration through transcytosis via a low-density lipoprotein receptor.Citation53,Citation54 Considering the importance of the protein corona for targeting purposes, it is important to reduce non-specific protein adsorption and favor the recruiting of specific proteins.
Serum albumin
SA is of great interest because it is the most abundant protein in plasma, and it has many important functions, including the regulation of blood pH and osmotic pressure.Citation55 Albumin is a globular protein, and is non-toxic, water soluble, stable from pH 4–9 and in organic solvents,Citation24 and exhibits several binding sites.Citation14 Albumin acts as a solubilizing agent for fatty acids and detoxifies blood plasma, thereby reducing the levels of harmful substances such as heavy metals, reactive oxygen species and ions.Citation25 Furthermore, SA can transport and release many compounds, such as drugs, through the plasma.Citation55
Albumin sources
Albumin can be obtained from diverse sources, such as egg (ovalbumin, OVA), bovine serum (bovine serum albumin, BSA), human serum (human serum albumin, HSA), rat serum, soy, milk and grains.Citation24,Citation56 OVA, BSA and HSA are most commonly used for biophysical and biochemical studies, and all are commercially available. They have the advantages of great stability, biocompatibility, and pH and low temperature sensitivity.Citation57 Albumin is chemically attractive because of its disulfide bonds and sulfhydryl groups, which allow interaction with organic and inorganic ligands. presents some properties and advantages of OVA, BSA and HSA.
Table 1 Properties of OVA, BSA and HSA
Structural considerations
OVA, BSA and HSA demonstrate similarities, whereby all three are formed mainly of an α-helix with a globular formation, contain hydrophilic and hydrophobic sites, and demonstrate an acid nature. There are, however, numerous differences. Structurally, OVA consists of a single polypeptide chain of 385 amino acids, with a serpin-like structureCitation58 and a helical reactive loop arrangement.Citation24 OVA is widely used in the food industry because of its ability to foam and form gels.Citation59
Figure 5 Crystal structure of BSA. Reprinted from Elsevier, 52(3-4) , Majorek KA, Porebski PJ, Dayal A, et al. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins, 174-182, Copyright 2012, with permission from Elsevier.Citation55
BSA () has 583 amino acids,Citation60 and this protein is made up of three homologous domains, which themselves are the product of two subdomains, and their structure is 67% α-helical. BSA is attractive because of its availability, binding affinity in the formation of ligand-protein complexes, intrinsic fluorescence through its two tryptophan residues (Trp-134 in the first domain and Trp-212 in the second domain),Citation61 and structural and functional similarities to HSA.Citation62
Similar to BSA, HSA consists of 585 amino acids in a globular conformation, which contains three homologous domains in an α-helical ellipsoid shape.Citation15,Citation24,Citation55 The amino acids in HSA can be dissimilated and used as a source of nutrition for peripheral tissues and preferentially undergo uptake by tumors and inflamed tissue.Citation15,Citation16 Moreover, HSA possesses different binding sites, which gives it the ability to transport drugs, hormones, fatty acids and ions through the body.Citation24,Citation63 These characteristics make it a good candidate for a drug delivery vehicle,Citation57 and for use in pharmaceutical preparations.
BSA and HSA
BSA and HSA are homologous in structure, conformation and properties. In fact, BSA and HSA share 76% sequential identity,Citation27 and the major difference lies in tryptophan (Trp) residue location. In HSA there is just one Trp, located at position 214, whilst BSA has a Trp-212 at subdomain IIA, and additionally a Trp-134 exposed in solvent at subdomain IB.Citation64
The important differences between OVA and the other two albumins are shown in and . The molecular weight, size, structure, S-S bridges and SH groups differ significantly between OVA and BSA/HSA. These properties are related to binding abilities, surface modificationsCitation2 and the formation of nanostructures, which will be the core issue in the current review. For this reason, we will focus only on BSA and HSA for biomedical applications.
Compared with other albumins, BSA and HSA are less immunogenic and they are considered to be well tolerated by humansCitation15 and are used as a protein carrier in different delivery systems.Citation64 BSA and HSA are currently used in drug delivery approaches in which drugs are conjugated to the binding sites of the protein,Citation65 thereby improving water solubility, therapeutic efficiency, bioavailability, biocompatibility,Citation66 biodistributionCitation67 and reducing drug side effects,Citation68–Citation70 including for anti-inflammatory, chemotherapeuticCitation19 and hypoglycemic drugs.Citation18,Citation28
Capping AuNPs with albumin to increase their colloidal stability and to reduce their interaction with plasma proteins
Surface modification of nanoparticles generally provides better water solubility, increases colloidal stability, increases biocompatibility, modifies cell uptake and intracellular trafickingCitation71 and improves blood circulation time compared with bare nanoparticles. Nanoparticles can be stabilized using a wide variety of molecules, such as peptides, proteins, DNA and polymers.Citation72–Citation74 Functionalization of AuNPs using PEG helps to diminish interactions with plasma proteins, reduces nanoparticle phagocytosis by the reticuloendothelial system with consequent accumulation in the liver and spleen, increases the half-life in circulation and allows selective delivery to tumors.Citation74,Citation75 Stabilization can also be achieved by capping AuNPs with albumin.Citation75 Capping is produced through electrostatic or hydrophobic interactions between nanoparticles and albumin or through chemisorption of the thiols to the gold surface.Citation76 For capping, it is possible to incubate AuNPs in a solution of the protein, taking into account various parameters, such as concentration, ionic strength and pH. Controlling the pH is necessary to regulate the degree of ionization of the species, considering the isoelectric point of albumin (approximately 5) and the zeta potential of the nanoparticles to allow electrostatic interactions. Albumin-capped nanoparticles can be stabilized through steric stabilization. BSA-encapsulated AuNPs (Au-BSA) have been used to load large amounts of methotrexate, which was useful for the delivery of this drug to breast cancer cells.Citation77
Albumin to favor the selective delivery of AuNPs
One of the exploitable features of cancer cells, in terms of increasing the selectivity of anticancer treatments, is their accelerated rate of proliferation, resulting in a higher demand for energy and nutrients.Citation17 This demand can be supplied by increasing the protein consumption and metabolism rate. Since albumin is the predominant plasmatic protein, it is the primary nutrition source for tumor cells.Citation18–Citation20 This can be seen by the fact that advanced cancer patients have a low level of serum albumin (hypoalbuminemia), as a result of the widespread need for tumors to be supplied with amino acids.Citation25
For drug delivery, there are two predominant ways in which nanosystems can reach a tumor site; these are active targeting and passive targeting. The first involves the interaction of a nanosystem with overexpressed or specific receptors in a tumor, thereby resulting in capture and further internalization at the tumor site or inside cancer cells. In this manner, albumin interacts with several specific receptors, including glycoproteins (Gp18, Gp30 and Gp60) and Secreted Protein Acidic Rich in Cysteine (SPARC), which is responsible for its recycling and transcytosis.Citation78
Because of the active targeting demonstrated by albumin, in addition to its excellent biocompatibility, several albumin-based formulations have been proposed to overcome the drawbacks of unmodified drugs, such as rapid clearance from the body, poor solubility, and a short-half life. As a result, the FDA has approved several albumin-based drugs, including Abraxane, Levimir and Victoza, which are currently used in medical treatments for cancer and diabetes. It has been shown that these albumin-conjugated drugs can accumulate in inflamed tumors and tissues modulated by GP60 and SPARC receptors, as shown in .Citation15,Citation16 Moreover, through BSA conjugation, drug biodistribution can be increased,Citation67 and release can be promoted through protein digestion.Citation66 In the case of albumin nanoparticles conjugated to paclitaxel, as is the case with Abraxane (clinically probed), the selective effect on tumor cells is achieved by using the endogenous mechanism for delivering proteins to cells. Albumin binding to the glycoprotein receptor gp60 on endothelial cells results in activation of caveolin-1 and the transcytosis of intact nanoparticles across the cell membrane. In addition to the active albumin-mediated transport of drugs into tumor cells, a degree of tumor-selective targeting is provided by SPARC, a protein which modulates the interaction of cells with the extracellular matrix and is over-expressed in many cancer types.Citation79,Citation80 Although associated with tumors, SPARC is not tumor-specific since it is found in healthy tissues, especially during embryonic development.Citation81 Increased levels of SPARC are associated with tissue and bone remodeling and hyperproliferation, but a pronounced increase in expression is seen with malignant transformation. SPARC is present on the surface of MX-1 human mammary carcinoma cells.Citation81 It is thought that the SPARC-mediated concentration of paclitaxel-carrying albumin molecules in the vicinity of tumor cells could lead to locally high levels of drug release.
Figure 6 Albumin uptake in tumor interstitial mediated by transcytosis with GP60 and subsequent binding to SPARC in the extracellular tumor matrix. Reprinted from Journal of Controlled Release, 157, Elsadek B, Kratz F, Impact of albumin on drug delivery - New applications on the horizon, Pages 4-28, Copyright (2012), with permission from Elsevier. Citation99
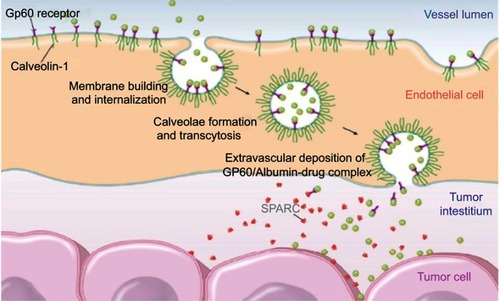
In contrast, passive targeting refers to the accumulation of macromolecules through the enhanced permeability and retention effect (EPR), which in summary, refers to angiogenesis-generated anatomic defects in tumor blood vessels. Tumors demonstrate increased permeability, because of intercellular gaps in blood vessels, which can reach up to 4 μm (in healthy tissues such gaps are typically less than 5 nm),Citation82–Citation84 and which allow for the extravasation of macromolecules and nanoparticles of more than 10 nm, including viruses and bacteria.
The EPR effect depends on the size of drugs.Citation83,Citation85 Small drugs can freely diffuse in/out of the tumor blood vessels without accumulating, while objects between 20 and 200 nmCitation1 can enter the tumor interstitial space without returning to the bloodstream, because of their large size.
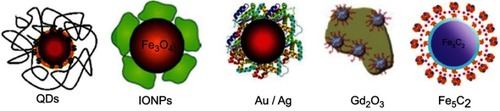
Albumin, in addition to being used to load therapeutic and imaging agents, is also useful for surface modification of numerous types of nanoparticle, as shown in . Surface modification of nanoparticles generally provides greater water solubility, and increased biocompatibility and blood circulation time, compared with uncoated nanoparticles.Citation2
Figure 7 Nanoparticles surface modification with albumin. Copyright © 2016. John Wiley and Sons. Reproduced from Chen Q, Liu Z. Albumin carriers for cancer theranostics: a conventionalplatform with new promise. Adv Mater. 2016;28:10557–10566.Citation2
Interesting functional drug delivery systems can be obtained from AuNPs, which possess numerous useful properties such as a high surface area, protection of cargo molecules from degradation, and energy absorption and emission dependent on their size, shape and surface modification, which will be subsequently described.Citation32,Citation70,Citation86 However, nanoparticles with a suitable size to allow for accumulation through passive targeting, if combined with SA, may demonstrate a dual ability to be captured through passive and active targeting, and therefore represent useful nanoplatforms for drug delivery with significant physiological stability and enhanced half-life compared with albumin or AuNPs alone.Citation87
AuNPs and albumin
Nanotransporters have provided a wide range of opportunities in the area of drug administration.Citation88 AuNPs offer the possibility of trapping and transporting poorly soluble or minimally biocompatible drugs, thereby improving circulation time, biocompatibility and, most importantly, preferential accumulation at a disease site.Citation89 Metallic nanoparticles, such as magnetic nanoparticles, plasmonics and quantum dots, have various properties that make them useful for biomedical applications.
SA interaction with AuNPs induces structural changes in SA, which are dependent on the size, shape and surface modification of the AuNPs. AuNP size is an important aspect to consider in AuNP-SA interactions, and small nanoparticles have a more efficient interaction with SA compared with larger nanoparticles.Citation90–Citation93 However, nanoparticle shape also has a strong influence on protein adsorption in AuNPs, as demonstrated by Moustaoui et al,Citation94 who assessed protein interaction in AuNSs and branched-shaped nanoparticles (AuNU). AuNSs showed an increase in the hydrodynamic radius, indicating that each type of protein binds on the gold nanoparticle in a specific orientation, and AuNUs showed different protein orientations because of their multi-oriented surfaces (tips) with a higher surface to volume area.
In another study, ChakrabortyCitation6 et al showed that AuNRs and AuNSs induce variations in the stability, structure and function of SA. In this work, a spectroscopic analysis comparing the interaction between SA and two nanoparticle geometries, namely that of AuNRs and AuNS with either Cetyltrimethylammonium bromide (CTAB) or PEG, was performed. Variation in the HSA structure was found to be higher in the order HSA-CTAB-AuNRs > HSA-CTAB-AuNSs > HSA-PEG-AuNRs > HSA-PEG-AuNSs. Accordingly, a rod shaped structure showed greater levels of denaturation than a spherical counterpart. However, the presence of CTAB on the surface resulted in a higher aggregation level, and was correlated with a positive charge, whereas PEGylated AuNPs have a near zero charge because of the neutral charge of PEG.Citation39,Citation95
Interestingly, shapes with planar surfaces have reduced accessibility and therefore less interaction with SA, as demonstrated by Carnovale et alCitation96. In this novel study, the interaction between SA and AuNPs with different geometries was compared, namely AuNSs, AuNRs, AuNPrs and AuNCs. It was found that AuNRs have higher adsorption constant values, while geometric shapes with large flat planar surfaces, such as prisms and cubes, presented lower adsorption values.
Strategies for AuNP conjugation with albumin
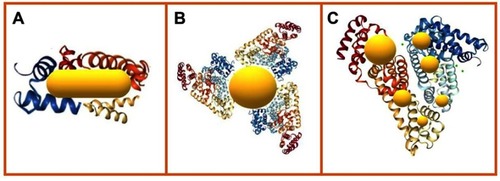
The characteristics of its structural domains allow SA to bind to different photothermal agents, including AuNPs. The resulting structures can be observed as AuNPs with a protein “halo” or take the form of nanocapsules in which AuNPs are confined in a cavity surrounded by albumin.Citation67
The first case, in which AuNPs present a halo, is attributable to SA attaching to AuNPs through physical or chemical adsorption, as summarized in . This can be achieved in several ways:
Passive adsorption: in which SA charged functional groups, or specific atoms are attached to the gold surface through covalent or non-covalent interactions. An example of this is the interaction between SH groups in the SA cysteine residues and Au atoms on the AuNP surface, giving rise to Au-S covalent bonds.Citation68 As a result of the binding abilities of SA, direct adsorption is usually achieved through simple incubation of an AuNP solution in the presence of SA. This adsorption strategy is remarkable because of its methodological simplicity and economy, avoiding the use of additional reagents and drastic conditions.
Active adsorption: this process is a bit more complicated because it involves the use of modified SA, with the aim to increase the amount or quality of interactions between SA-Au or SA-surface molecules on the AuNPs. Modification of SA can be achieved through functionalization, giving rise to carboxylated or thiolated SA or changes in the total charge, resulting in cationic or anionic SA.Citation27,Citation97 This is a convenient strategy when the AuNP surface is functionalized to other molecules or polymers, and an SA coating is required; for example, for an AuNP-PEG coating, cationic BSA can improve the covering.Citation67
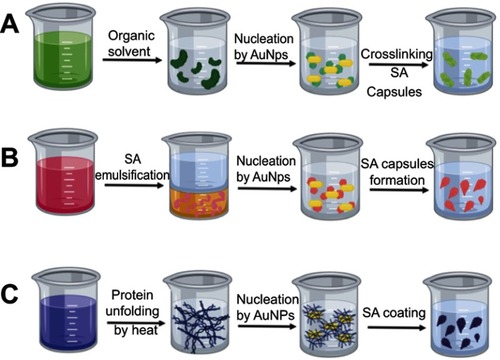
In situ: this strategy refers to the use of albumin, either as a synthesis reagent (eg as a reducing agent) or as a building block for AuNP synthesis, where SA is added during the synthesis procedure resulting in AuNPs with an SA coating.Citation2,Citation98 Several studies report the use of SA as a foaming and stabilizing agent or even as a template for AuNC synthesis. As a result, AuNC-SA hybrids can be obtained through one-pot synthesis.Citation2,Citation98 However, SA nanocapsules are also useful as a drug delivery platform. The use of SA encapsulation methods offers some benefits, including the loading of poorly soluble agents and the protection of cargo from natural degradation.Citation2,Citation23,Citation69,Citation99 Some of the most common procedures for SA nanocapsule creation are shown in and are detailed below:
Desolvation cross-linking: This method, also known as a coacervation process, is mainly used to produce core-shell SA-AuNPs, as shown in . In practice, AuNPs are added to an SA solution as nuclei for capsule formation, after organic solvent (usually methanol, ethanol or acetone) is added dropwise until a turbid solution is observed, in which SA is desolvated. Therefore, water solubility decreases considerably, giving rise to phase separation, and resulting in albumin aggregates. The desolvated albumin has exposed amine groups, which can be cross-linked by crosslinking agents, such as glutaraldehyde. Advantageously, this strategy allows chemical agents (including nanoparticles, drugs and diagnostic agents) to be physically entrapped inside SA capsules. SA nanocapsules are highly stable and protect entrapped agents against degradation.Citation24,Citation63,Citation100
Emulsification: Albumin has a wide variety of amino acids, some of which are hydrophobic. In a conventional emulsification procedure, a SA solution is mixed with a non-aqueous phase (oil), for shearing and high-pressure homogenization, giving rise to an emulsion. AuNPs functionalized with hydrophobic molecules can be used as seeds for interactions with hydrophobic amino acids in albumin in this procedure, generating a core-shell. For nanocapsule stabilization, either crosslinking agents or stirring at high temperatures can be used. Finally, the solvent is removed through low pressure evaporation.Citation23,Citation27 This is a methodology mainly used for the encapsulation of lipophilic drugs, and increases biocompatibility and water solubility.Citation101
Thermal gelation: Protein structure is susceptible to temperature changes. In thermal gelation, a SA aqueous solution is heated to produce protein unfolding, which induces protein-protein interactions through hydrophobic, electrostatic, disulfide and hydrogen bonding. Unfolding also gives rise to SA interactions with AuNPs, resulting in self-assembly on the AuNP surface and protein coating.Citation101
Figure 8 Absorption of SA on AuNP surface can be achieved by: (A) passive absorption of SA constituent functional groups attached to gold surface; (B) adsorption of activated SA modified with functional groups improving the surface binding properties; (C) growing of AuNPs embedded on SA structure after in situ synthesis.
Biomedical applications of albumin-AuNPs
Nanotransporters have provided a wide range of opportunities in the area of drug administration.Citation88 Metallic nanoparticles, such as magnetic nanoparticles, plasmonics and quantum dots, have interesting optic properties that make them useful for biomedical applications.Citation102 AuNPs offer the possibility of trapping and transporting poorly soluble or minimally biocompatible drugs, thereby improving circulation time, biocompatibility and, most importantly, preferential accumulation at a disease site.Citation89 An SA coating on the surface of AuNPs increases stability, targeting and biocompatibility.
The biomedical applications discussed in the current review are summarized in and are detailed in the following sections.
Table 2 Biomedical applications of gold nanoparticles
Albumin-AuNPs for photothermal therapy
Photothermal therapy involves the use of photoactive metals, which can convert light into heat through a previously described photothermal effect. The released heat kills malignant cells, resulting in weakening or removal of a treated tumor. Several studies have been conducted on photothermal therapy, and include the use of spherical AuNPs,Citation76,Citation103 nanorods, nanoshellsCitation104 and nanostarsCitation105 bound to SA. These nanobioconjugates achieved high stability, selective accumulation in malignant tissue, and selective photothermal ablation through laser irradiation.
An example of an AuNP photothermal system was reported by AL-Jawad et al,Citation6 where AuNPs (size <5 nm) covered with BSA were synthesized and administered to rhabdomyosarcoma murine fibroblasts (L20B) and RAW 264.7 monocyte-macrophage cells. A drastic concentration-dependent photothermal ablation was achieved after a 532 nm and 800 nm irradiation, reaching more than 74% cytotoxicity for all tested cell lines. In another study, Mocan et alCitation76 developed an original model of liver hepatocellular carcinoma, and tested the effect of AuNPs-BSA. The study showed selective accumulation of the nanosystem in tumor cells, as well as apoptosis induced by laser irradiation at 808 nm. Zhang et alCitation106 synthesized highly stable AuNRs functionalized with BSA and administered them to fibroblast L929 and gastric adenocarcinoma YCC-2 cells in vitro, resulting in significant anticancer efficacy through NIR hyperthermia.
The inclusion of targeting agents in nanosystems results in improved properties, and increased selectivity and uptake by malignant cells. Folic acid (FA) is a widely used cancer targeting agent, since cancer cells overexpress FA receptors. This strategy was demonstrated in a study by Li et alCitation100 in which AuNSs were synthesized and conjugated with BSA-FA, resulting in a highly stable BSA–FA–AuNS system with low toxicity. The system underwent cell uptake in HeLa cells because of overexpressed FA receptors, and cell ablation was possible after laser irradiation, reaching 70% and 75% toxicity after NIR and green laser irradiation, respectively.
Aside from FA, other molecular systems can be used to promote the cell uptake of nanocapsules, including AuNR-BSA encapsulated in ssPalmM:DOPE:cholesterol lipid, as described by Paraiso et al.Citation107 In vitro assays with this nanosystem were performed in 4T1 breast cancer cells, in which cell viability decreased from 100% in the absence of irradiation to 11% after NIR irradiation, and an increase in dosage killed all the cells. Cell uptake played a key role in these results, since without lipid encapsulation, the AuNR-BSA system showed a plateau in killing efficiency (30% cell viability after irradiation), showing the suitability of the system for hyperthermia.
However, nanoparticle-based photothermal therapy must be carefully assessed before conducting pre-clinical trials, since it is necessary to consider the impact and harm caused by laser irradiation; this is particularly relevant for systems in which irradiation is performed at wavelengths outside the biological window.Citation6,Citation108,Citation109 In such cases, the risk of radiation-induced damage makes it difficult to discern if cell death is caused by hyperthermia through the photothermal effect or by the laser itself. However, even though photothermal therapy works by killing a high percentage of malignant cells by itself, combined approaches including drug delivery and photodynamic therapy controlled by external stimuli provide added value to photothermal systems.Citation36,Citation110
Albumin-AuNPs for drug delivery
One of the issues that nanotechnology addresses is the lack of selectivity of existing drugs, since this problem is one of the major causes of poor efficacy, besides the undesirable secondary effects associated with current treatments.
Biomedical applications involving AuNPs are mainly based on nanoparticles with spherical,Citation22,Citation39,Citation44,Citation111–Citation113 nanorod,Citation12,Citation68,Citation107,Citation114–Citation118 nanoprismCitation5,Citation119,Citation120 and nanocluster geometry;Citation6,Citation121–Citation125 however, current synthetic methods allow for the development of nanoparticles with different morphologies. Morphological changes can also modulate the physicochemical properties of AuNPs, which in combination with SA may be improved and exploited for therapeutic applications.
Because of its bioaccumulation, targeting, biocompatibility and surface properties, albumin can transport diverse therapeutic systems in a localized way, which is denoted “spatially controlled release”. The interesting optical properties of AuNPs allow them to be monitored using imaging tools, and the photothermal effect can release adsorbed molecules on the AuNP surface, thereby temporally controlling drug release. Therefore, albumin-AuNPs are a promising system for drug delivery.
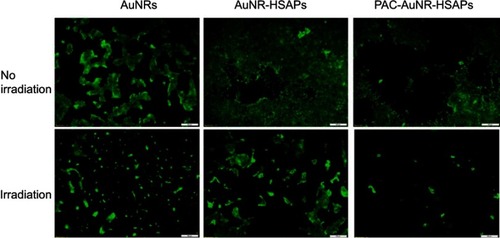
The photothermal effect is frequently used for controlled drug release, as in Peralta et al,Citation10 where AuNRs were synthesized, conjugated to the commercial anticancer drug paclitaxel (PAC) and encapsulated in HSA, and 3 μg of PAC/mg could be loaded into the system. The nanoplatform accumulated in 4T1 mouse breast cells through the EPR effect. After irradiation, the system generated approximately 94% cell death, through a combined photothermal effect and PAC release, as shown in , which demonstrates that the system reached its highest toxicity only after irradiation, as an external stimulus, and is therefore temporally controlled.
Figure 10 Fluorescence microscopy images of the cytotoxic effects on 4T1 breast cancer cells when treated with free AuNRs, AuNR-HSAPs or PAC-AuNR-HSAPs. (White scale bar denotes 100 μm). Reprinted with permission from Peralta DV, Heidari Z, Dash S, Tarr MA. Hybrid paclitaxel and 1030 gold nanorod-loaded human serum albumin nanoparticles for simultaneous chemotherapeutic and photothermal therapy on 4T1 breast cancer cells. ACS Appl Mater Interfaces. 2015;7:7101–7111.Citation10 Copyright 2015 American Chemical Society.
Callaghan et alCitation126 reported a controlled drug delivery system based on HSAP-AuNRs, for the tyrosine kinase inhibitor sorafenib (SRF). In cytotoxicity and uptake trials in renal carcinoma cells (RCCs), this system demonstrated a dual therapeutic action, with drug release and induction of photothermal ablation by hyperthermia. HSAP-AuNR-SRFs have potential for the treatment of advanced RCC. In an additional study by Liu et al,Citation127 AuNRs-HSA were used to encapsulate SRF and deliver it in a human metastatic cell carcinoma mouse model. The HSA-AuNR-SRF system produced 11.1% necrosis, while SRF alone produced only 4.2%; in contrast, after irradiation HSA-AuNR produced 62% necrosis and HSA-AuNR-SRF killed 100% of the cells, showing that AuNRs-HSA improved drug delivery compared with SRF alone. In addition to the ablation created by the photothermal effect, the drug load produced complementary effects, causing enhanced cell toxicity.
In another study, Wang et alCitation128 developed a delivery system for gemcitabine (Gem) based on AuNP@BSA. The system was synthesized in a facile one-pot reaction via the reduction of HAuCl4 with BSA as a stabilizer, which was subsequently conjugated to Gem through electrostatic interaction to give rise to the AuNP@BSA-Gem system. In vitro assays in pulmonary carcinoma cells showed that the AuNP@BSA system demonstrated high biocompatibility and, as expected, a high cell death rate was produced by Au@BSA-Gem, even in comparison with free Gem.
Precise control of drug release is required to achieve optimal delivery of therapeutic agents; with this aim, Chiu et alCitation129 developed a one-step synthesis for a doxorubicin (DOX) loaded gold nanorod/BSA core-shell (NR@DOX:BSA), which acted as an improved macrophage-mediated delivery system, with photothermal/chemotherapeutic drug distribution and retention abilities. The nanoconstruct showed low toxicity, but after irradiation, DOX was released, causing a drastic increase in cell death. In vivo studies showed that DOX exhibited limited antitumor effects because of its rapid clearance; in contrast, the synthesized nanosystem was delivered through tumor-tropic migration to tumor tissues.
Gold nanoflowers (AuNFs) represent an interesting and novel nanoparticle shape; in a recent study by Uppal and Bose,Citation7 AuNFs were successfully capped with HSA through either electrostatic or covalent attachment, resulting in a stable system that was non-toxic in a cancer cell line (human squamous carcinoma, Nt8e). This study demonstrates the promise of AuNF-BSA for biomedical applications, such as drug delivery or theranostics.
In another study, non-conventional gold nanocube-protein hybrids (PGHNs) were constructed by Ding et al,Citation123 using AuNCs, BSA and Trp as building blocks. The PGHNs demonstrated blue emission, high biocompatibility and cell internalization in S. ceresiviae cells. The PGHNs were loaded with rhodamine 6G and DOX separately and administered to the cells, resulting in internalization and release in the nucleus, demonstrating that they are a promising vehicle for drug delivery.
Scientific advances in nanotechnology have resulted in new nanoplatforms to treat diseases other than cancer; for example, the systems described in the current review may have applications ranging from the treatment of dermatological diseases to bacterial infections. Examples of this are included in the works of Lademann et alCitation130 and Rastogi et al.Citation112
Lademann et alCitation130 focused on dermatotherapy, resulting in photoactivated fluorescein isothiocyanate release from an AuNP-doped BSA system. In an ex vivo porcine skin model, the system selectively targeted follicular ducts, because of the suitable size and biocompatibility properties of the particles. The plasmonic release of the encapsulated drug after irradiation demonstrates that this system is feasible as a plasmonic nanocarrier for the treatment of dermatological diseases.
AuNPs have also been used as antibiotic delivery systems, as demonstrated in a study by Rastogi et al,Citation112 in which highly stable albumin-capped AuNSs were synthesized using sodium borohydride as a reducing agent. The system was functionalized for amino-glycosidic antibiotic delivery (streptomycin, neomycin, gentamicin and kanamycin), and the resulting nanoconstruct showed increased antibacterial activity against Gram-negative and Gram-positive bacterial strains, compared with free antibiotic; therefore, this system has considerable potential for infection prevention and therapeutics.
Albumin AuNPs for diagnostic devices
One of the objectives of nanomedicine is to improve the capabilities of current medicine and make it more selective and sensitive. In this context, it is important to develop diagnostic devices to identify diseased tissue in a non-invasive way.
AuNPs have emerged as a promising diagnostic system, because of their optic properties. Moreover, they can be functionalized; for example, they can be selectively delivered to a malignant site and therefore this site can be detected by the presence of the AuNPs. One of the techniques used to detect AuNPs is photoacoustic (PA) imaging, as it is a highly sensitive nonionizing imaging modality, in which a pulsed laser can penetrate deep tissue (up to 5 cm), thereby causing rapid heating and thermal expansion, which generates an acoustic pressure wave.Citation105 PA combined with computed tomography (CT) provides 3D visual images with high spatial resolution. For example, in Zu et al,Citation105 BSA-capped gold nanostars (AuNS-BSA) were synthesized, and demonstrated an average size of 85 nm, excellent biocompatibility in HepG2 cells and PA contrast enhancement, which improves CT accuracy, thereby giving rise to a dual-modal CT/PA imaging contrast agent.
In addition to PA/CT, there are other techniques to detect AuNPs and their derivates. Chen et alCitation131 synthesized AuNSs conjugated to indocyanine green (ICG) and stabilized using albumin (AuNS-ICG-BSA), which demonstrated good biocompatibility and low cytotoxicity, together with a superior photothermal ablation effect in U87 glioma cells. This represents a spatially controlled antitumor agent, and it caused cell damage only after light irradiation. However, this nanoconstruct is useful for surface enhanced Raman scattering (SERS) imaging-based real-time sensitive monitoring of intracellular temperature in phototh photothermal therapy (PTT). Interestingly, this system allows for the detection of small temperature variations within a cell during PTT, and this can be monitored through SERS, as a nanothermometer. A simpler system was demonstrated in a study by Florina et alCitation132 in which highly stable and biocompatible PEG-AuNP and BSA-PEG-AuNP systems, with good internalization in A549 cells and SERS activity, were demonstrated to be promising candidates for intracellular sensing applications.
Albumin AuNP theranostic devices
In recent years, a new strategy called theranostics has become increasingly important in nanomedicine. The acronym “theranostics” derives from the words therapy and diagnosis, and a theranostic agent can detect the signs of a pathology (including tumors, infections, damaged tissue, and overexpressed receptors) and generate treatment.Citation133 This field involves the use of systems with therapeutic action for the treatment of diseases, and which in turn, allow for non-invasive diagnosis and monitoring in real time,Citation27 providing an efficient treatment, and early (non-invasive) detection of the disease, in addition to personalized therapy.Citation133
To date, a great variety of theranostic platforms have been reported, including polymers, liposomes, micelles, dendrimers,Citation134 organic materials,Citation135,Citation136 inorganic nanoparticles,Citation137–Citation139 and proteins,Citation24,Citation140 of which, the latter have been used as nanotransporters. Surface modifications, changes in particle size and functionalization of nanomaterials can improve biocompatibility, retention and therapeutic efficacy.
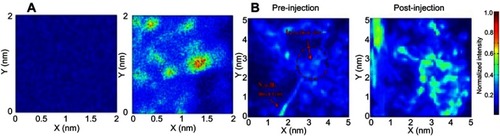
An AuNP-BSA system has shown therapeutic effectiveness in chronic diseases such as cancer, as demonstrated in a recent study by Chiu et al.Citation138 The study demonstrated a simple one-step fabrication of a biomimetic nanomaterial (AuNR@SA) from a synthetic source. AuNRs were synthesized and encapsulated with SA, giving rise to core-shell gold nanorods-albumin, AuNR@SA. The SA capping was achieved through either glutaraldehyde crosslinking (GTA), or a denaturing method using methanol/ethanol (EM). GTA showed less interaction with endogenous proteins (SA, transferrin and fibrinogen), thereby leading to reduced protein corona formation, compared to the EM system. Moreover, GTA crosslinking resulted in a higher photothermal efficiency and temperature increase. The system was tested for DOX delivery, and produced in vivo tumor growth inhibition through NIR laser exposure, through a combination of photothermal therapy and chemotherapy. Moreover, the GTA system has proven to be useful for diagnosis with PA, showing a 3.5-fold higher signal than AuNR-CTAB in tramp C1 cells and in vivo mouse models (), as well as accumulation in tumor cells.
Figure 11 Capability of NR@SA nanoplatform for PA imaging. (A) 2 × 2 mm area of projected C-scan PA images of nontreated and NR@SA treated tramp C1 tumor cells. (B) in vivo photoacoustic imaging from tumor-bearing mice during and after local delivery of NR@SA. Reprinted with permission from Chiu HT, Chen CH, Li ML, et al. Bioprosthesis of core-shell gold nanorod/serum albumin nanoimitation: a half-native and half-artificial nanohybrid for cancer theranostics. Chem Mater. 2018;30:729–747.Citation138 Copyright (2018) American Chemical Society.
Another interesting system is that described by Sashidaran et al,Citation110 where gold nanostars (AuNSs) were synthesized through a simple wet chemistry route and subsequently BSA-functionalized, yielding a stable monodispersed solution, with an average particle size of 120 nm. AuNS-BSA did not exhibit any inherent cytotoxic activity towards healthy (L929) and cancerous (KB) cells, but it showed significant photothermal ablation properties after laser exposure, thereby working as a therapeutic agent. Interestingly, the system also showed good CT imaging properties. AuNS-BSAs were loaded with rhodamine 6G, allowing the system to exhibit SERS and it is considered to be promising for Raman imaging.
AuNCs are prominent nanosystems because of their ease of functionalization and small size, which allows them to be easily cleared by the kidneys.Citation141 Cui et alCitation121 synthesized BSA stabilized AuNCs, which were subsequently cross-linked to create a well-defined gold cluster nanoassembly (AuCNA). The AuCNA showed high biocompatibility and precise tumor targeting to 4T1 breast cancer cells, as seen through NIR fluorescence images. However, laser irradiation (660 nm) generated reactive oxygen species (ROS), which caused significant cell death in vitro, and this effect resulted in tumor death in an in vivo assay.
In a study by Fu et al,Citation137 curcumin, a hydrophobic, but promising therapeutic agent, was encapsulated in BSA functionalized gold nanoclusters (BSA-AuNCs), and showed enhanced internalization in human neuroblastoma (SH-SY5Y) cells, followed by strong fluorescence within the cells and the inhibition of proliferation via the induction of apoptosis. This research is ongoing, but highlights that the system has significant potential as a candidate for theranostic applications, by taking advantage of such fluorescence signals.
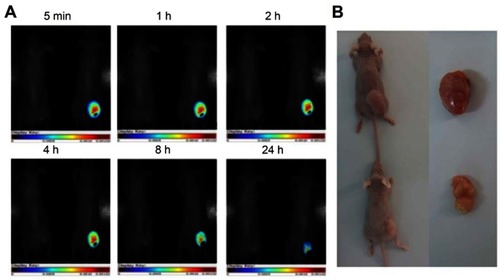
The AuNCs-BSA system has also been used for the delivery of approved anticancer drugs, such as in Ding et al,Citation125 where constructed BSA@AuNCs were modified using cyclic arginine-glycine-aspartate (cRGD) and loaded with DOX (DOX/RGD-BSA@AuNCs). This nanosystem produced growth inhibition in cancer cells, including Hela, MCF-7, U251 and CAL-27 cells. Remarkably, the DOX/RGD-BSA@AuNCs system proved to be useful not only for drug delivery, but also for localized in vivo imaging, as shown in , where a decrease in tumor size and specific detection of the tumor site is notable after treatment using the nanosystem and laser irradiation.
Figure 12 (A) In vivo targeted cancer fluorescence images of Hela tumor-bearing mice exposed to the laser (488 nm, 425 mW cm2, 25 min) after injection of the DOX/RGD-BSA@AuNCs system. (B) The images of tumor excised from mice after injection of PBS solution or DOX/RGD-BSA@AuNCs solutions at 19 day. Reprinted with permission from Ding C, Xu Y, Zhao Y, Zhong H, Luo X. Fabrication of BSA@AuNC-based nanostructures for cell fluoresce imaging 1435 and target drug delivery. ACS Appl Mater Interfaces. 2018;10:8947-8954.Citation125 Copyright (2018) American Chemical Society.
Nanosystems offer a wide variety of possibilities because of their versatility and functionalization potential, and they can present enhanced and special characteristics, such as sensitivity to external stimuli (such as light, laser radiation, heat and pH). By means of pH sensitivity, Huang et alCitation98 used BSA as a template for the creation of an Au-BSA core/shell, which was subsequently functionalized with DOX and FA (Au–BSA–DOX–FA). The nanocomposite showed in vitro non-toxicity in MGC-803 and GES-1 cells, pH-sensitive drug release properties, and superior antitumor activity compared with free DOX. In vivo CT imaging showed that the nanocomposite had selective antitumor activity by targeting overexpressed folate receptors in gastric cancer.
AuNPs can be combined with other metallic nanoparticles, such as Gd2O3 nanoparticles, which can improve the properties of the system for theranostic applications. An example is reported in a study by Han et al,Citation142 in which gadolinium oxide-gold nanocluster hybrids (Gd2O3−AuNCs), stabilized in BSA were synthesized, showing high biocompatibility, in vivo and in vitro uptake, as well as signal enhancement for magnetic resonance and X-ray CT imaging. This system, besides enhancing imaging, can be used to kill tumor cells through the production of singlet oxygen species (1O2) after NIR irradiation (808 nm). However, this nanosystem also proved to be useful for loading and delivering the fluorescent dye ICG, improving photodynamic properties. Similarly, You et alCitation143 designed a multifunctional delivery platform for Gd based on hollow gold nanoshells coated with BSA (Au@BSA-Gd), in which gold conferred photothermal properties, and the construct exhibited properties that are useful for CT and PA. The incorporation of ICG into the system provided photodynamic/photothermal properties and near-infrared fluorescence (NIRF)/PA imaging capabilities, thereby achieving an ideal theranostic agent for NIRF/PA/CT/MR quadmodal imaging.
Other non-conventional AuNP geometries also offer interesting properties, which in addition to SA enhanced biocompatibility, can be useful for theranostic applications. An example is branched gold nanoshells (BAuNSHs), as synthesized by Topete et alCitation144 through a seeded-growth, and which were loaded with DOX. The system was also coated with a BSA-ICG-FA complex. This is a multifunctional system useful for fluorescence imaging because of the inherent fluorescence of the internalized dye and the presence of FA. The system also has the potential to deliver chemotherapy through its loaded drug, and photothermal and photodynamic therapy upon NIR irradiation. The synergistic effect of the nanoconstruct was observed in terms of an increase in internalization and toxicity in HeLa cells, and bioaccumulation at tumor sites in a tumor-bearing mouse model, observed through fluorescence imaging.
Conclusion
Albumin is a promising biomaterial for new approaches, and its biocompatibility and transport properties allow for the creation of new materials and the improvement of existing materials. The special characteristics of malignant tissues, such as an accelerated rate of proliferation and increased cellular stress and cell growth, resulting in a higher demand for energy and nutrients, can be exploited for controlled drug delivery.
The synergy between SA and AuNPs results in interesting properties, such as an increase in stability, a reduction in the interaction with plasma proteins, passive and active targeting of malignant cells, and an increase in selectivity. The photothermal properties and the NIR absorption of light of AuNPs are suitable for local hyperthermia, photothermal ablation and photothermal release. The system demonstrates significant potential in terms of spatial and temporally controlled release, in addition to its applications in theranostics, using simple detection techniques such as PA, CT or a combination of both, as previously described. Coating AuNPs with SA decreases the interaction of the nanosytem with endogenous proteins in the body, protecting the cargo against degradation.
Future perspectives
Although, a limited number of clinical trials are now in progress, with promising resultsCitation145–Citation147 the use of AuNPs for clinical applications has been delayed due to the inherent potential toxicity of metal nanoparticles. As mentioned previously, the capping of AuNPs with SA allows for an improvement in their biological properties, which may encourage the scientific community to investigate AuNP-SA-based nanosystems for clinical trials, as well as other types of SA-coated NIR-plasmonic nanoparticles for biomedical applications.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by FONDAP 15130011, FONDECYT 1170929 and 1190623, and CONICYT 21180258. The abstract of this paper was presented at the as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the 11th Workshop on Computational Chemistry and Molecular Spectroscopy in October 2018 (11thWCCMS): http://www.wccms.cl/downloads/wccms_2018_proceedings.pdf.
References
- Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–121. doi:10.1016/j.jconrel.2016.11.01527871992
- Chen Q, Liu Z. Albumin carriers for cancer theranostics: a conventional platform with new promise. Adv Mater. 2016;28:10557–10566. doi:10.1002/adma.v28.4727111654
- Mariam J, Sivakami S, Dongre PM. Elucidation of structural and functional properties of albumin bound to gold nanoparticles. J Biomol Struct Dyn. 2017;35:368–379. doi:10.1080/07391102.2016.114422326821333
- Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35:209–217. doi:10.1039/B514191E16505915
- Alfranca, G,Artiga A, Stepien G, et al. Gold nanoprism – nanorod face off : comparing the heating efficiency, cellular internalization and thermoablation capacity. Nanomedicine (Lond.). 2016;11:2903–2916. doi:10.2217/nnm-2016-023327785974
- AL-Jawad SMH, Taha AA, Al-Halbosiy MMF, AL-Barram LFA. Synthesis and characterization of small-sized gold nanoparticles coated by bovine serum albumin (BSA) for cancer photothermal therapy. Photodiagnosis Photodyn Ther. 2018;21:201–210. doi:10.1016/j.pdpdt.2017.12.00429223737
- Uppal A, Bose B. Synthesis, stability, and in vitro oral cancer cell toxicity of human serum albumin stabilised gold nanoflowers. IEE. 2018. doi:10.1049/iet-nbt.2017.0002
- Kuo W-S, Chang Y-T, Cho K-C, et al. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials. 2012;33:3270–3278. doi:10.1016/j.biomaterials.2012.01.03522289264
- Santos NC, Domingues MM, Felício MR, Gonçalves S, Carvalho PM. Application of light scattering techniques to nanoparticle characterization and development. Front Chem. 2018;6:1–17.29441345
- Peralta DV, Heidari Z, Dash S, Tarr MA. Hybrid paclitaxel and gold nanorod-loaded human serum albumin nanoparticles for simultaneous chemotherapeutic and photothermal therapy on 4T1 breast cancer cells. ACS Appl Mater Interfaces. 2015;7:7101–7111. doi:10.1021/acsami.5b0085825768122
- Kelkar SS, Reineke TM. Theranostics : combining imaging and therapy. Bioconjug Chem. 2011;22:1879–1903. doi:10.1021/bc200151q21830812
- Adura C, Guerrero S, Salas E, et al. Stable conjugates of peptides with gold nanorods for biomedical applications with reduced effects on cell viability. ACS Appl Mater Interfaces. 2013;5:4076–4085. doi:10.1021/am401486h23597259
- Olmedo I, Araya E, Sanz F, et al. How changes in the sequence of the peptide CLPFFD-NH 2 can modify the conjugation and stability of gold nanoparticles and their affinity for -amyloid fibrils. Bioconjug Chem. 2008;19:1154–1163. doi:10.1021/bc800016y18510352
- Cheema MA, Taboada P, Barbosa S, et al. Human serum albumin unfolding pathway upon drug binding: A thermodynamic and spectroscopic description. J Chem Thermodyn. 2009;41:439–447. doi:10.1016/j.jct.2008.11.011
- Lee ES, Youn YS. Albumin-based potential drugs: focus on half-life extension and nanoparticle preparation. J Pharm Investig. 2016;46:305–315. doi:10.1007/s40005-016-0250-3
- Bairagi U, Mittal P, Albumin MB. A versatile drug carrier. Austin Ther. 2015;2:1–6.
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2013;2:881–898. doi:10.1158/2159-8290.CD-12-0345
- Swiercz R, Mo M, Khare P, et al. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget. 2017;8:3528–3541. doi:10.18632/oncotarget.1386927974681
- Commisso C,Davison S, Soydaner-Azeloglu R, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi:10.1038/nature1213823665962
- Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:1–7. doi:10.3389/fphys.2014.0026824478714
- Nguyen VH, Lee BJ. Protein corona: A new approach for nanomedicine design. Int J Nanomed. 2017;12:3137–3151. doi:10.2147/IJN.S129300
- Yu Z, Yu M, Zhang Z, Hong G, Xiong Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res Lett. 2014;9:1–7. doi:10.1186/1556-276X-9-34324380376
- Bhushan B, Khanadeev V, Khlebtsov B, Khlebtsov N, Gopinath P. Impact of albumin based approaches in nanomedicine: imaging, targeting and drug delivery. Adv Colloid Interface Sci. 2017;246:13–39. doi:10.1016/j.cis.2017.06.01228716187
- Karimi M, Bahrami S, Ravari SB, et al. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Deliv. 2016;13:1609–1623. doi:10.1080/17425247.2016.119314927216915
- Kratz F. A clinical update of using albumin as a drug vehicle - a commentary. J Control Release. 2014;190:331–336. doi:10.1016/j.jconrel.2014.03.01324637463
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi:10.1016/j.jconrel.2008.05.01018582981
- Kudarha RR, Sawant KK. Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater Sci Eng C. 2017;81:607–626. doi:10.1016/j.msec.2017.08.004
- Min Y, Caster JM, Eblan MJ, Wang AZ. Clinical translation of nanomedicine. Chem Rev. 2015;115:11147−11190. doi:10.1021/cr500441926088284
- Saverot S-E, Reese LM, Cimini D, Vikesland PJ, Bickford LR. Characterization of conventional one-step sodium thiosulfate facilitated gold nanoparticle synthesis. Nanoscale Res Lett. 2015;10:241. doi:10.1186/s11671-015-0940-1
- Jeremic B, Aguerri AR, Filipovic N. Radiosensitization by gold nanoparticles. Clin Transl Oncol. 2013;15:593–601. doi:10.1007/s12094-013-1034-023359187
- Pastoriza-Santos I, Liz-Marzan LM. Colloidal silver nanoplates. State of the art and future challenges. J Mater Chem. 2008;18:1724–1737. doi:10.1039/b716538b
- Guerrero AR, Hassan N, Escobar CA, Albericio F, Kogan MJ, Araya E. Gold nanoparticles for photothermally controlled drug release. Nanomedicine (Lond). 2014;9:2023–2039. doi:10.2217/nnm.14.12625343351
- Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem. 2003;B668–B677. doi:10.1021/jp026731y
- Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem. 2000;19:409–453. doi:10.1080/01442350050034180
- Link S, El-Sayed MA. Optical properties and ultrafast d ynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331–366. doi:10.1146/annurev.physchem.54.011002.10375912626731
- Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. doi:10.1016/j.jare.2010.02.002
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy : challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi:10.1016/j.jconrel.2014.12.03025545217
- Vetterlein C, Vasquez R, Bolaños K, et al. Exploring the influence of Diels-Alder linker length on photothermal molecule release from gold nanorods. Colloids Surf B Biointerfaces 2018;166:323–329. doi:10.1016/j.colsurfb.2018.03.021
- Alex SA, Chakraborty D, Chandrasekaran N, Mukherjee A. A comprehensive investigation of the differential interaction of human serum albumin with gold nanoparticles based on the variation in morphology and surface functionalization. RSC Adv. 2016;6:52683–52694. doi:10.1039/C6RA10506H
- Bayazitoglu Y, Kheradmand S, Tullius TK. An overview of nanoparticle assisted laser therapy. Int J Heat Mass Transf. 2013;67:469–486. doi:10.1016/j.ijheatmasstransfer.2013.08.018
- Schleich N, Danhier F, Préat V. Iron oxide-loaded nanotheranostics: major obstacles to in vivo studies and clinical translation. J Control Release. 2015;198:35–54. doi:10.1016/j.jconrel.2014.11.02425481448
- Sahu A, Lee JH, Lee HG, Jeong YY, Tae G. Prussian blue/serum albumin/indocyanine green as a multifunctional nanotheranostic agent for bimodal imaging guided laser mediated combinatorial phototherapy. J Control Release. 2016;236:90–99. doi:10.1016/j.jconrel.2016.06.03127349352
- Qiu Y, Liu Y, Wang L, et al. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010;31:7606–7619. doi:10.1016/j.biomaterials.2010.01.04220656344
- Prades R, Guerrero S, Araya E, et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33:7194–7205. doi:10.1016/j.biomaterials.2012.06.06322795856
- Kogan MJ, Bastus NG, Amigo R, et al. Nanoparticle-mediated local and remote manipulation of protein aggregation. Nano Lett. 2006;6:110–115. doi:10.1021/nl052110f16402797
- Stone J, Jackson S, Wright D. Biological applications of gold nanorods. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:100–109. doi:10.1002/wnan.12020967876
- Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H, Chang J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Clin Med. 2014;6:172–178. doi:10.1016/j.jecm.2014.10.015
- Velasco-Aguirre C, Morales F, Gallardo-Toledo E, et al. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: preclinical approaches. Int J Nanomed. 2015;10:4919–4936.
- Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi:10.1021/ja208433822191645
- Dobrovolskaia MA, Aggarwal P, Hall JB, Mcneil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi:10.1021/mp800032f18510338
- Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552:328–339. doi:10.1016/j.ijpharm.2018.10.01130308270
- Charbgoo F, Nejabat M, Abnous K, et al. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J Control Release. 2018;272:39–53. doi:10.1016/j.jconrel.2018.01.00229305922
- Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J Nanosci Nanotechnol. 2004;4:484–488. doi:10.1166/jnn.2003.07715503433
- Reddy JS, Vobalaboina V. novel delivery systems for drug targeting to the brain. Drugs Future. 2004;29:63–83. doi:10.1358/dof.2004.029.01.872585
- Majorek KA, Porebski PJ, Dayal A, et al. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol Immunol 2012;52(3-4):174-82. doi:10.1016/j.molimm.2012.05.011
- Bhatty RS. Albumin proteins of eight edible grain legume species: electrophoretic patterns and amino acid composition. J Agric Food Chem. 1982;30:620–622. doi:10.1021/jf00111a0577096818
- Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–182. doi:10.1016/j.jconrel.2011.07.03121839127
- Majorek KA, Porebski PJ, Dayal A, Zimmerman MD, Jablonska K, Stewart AJ, Chruszcz M, Minor W. PDB ID: 3V03. Mol Immunol 52, 174–182 (2012).
- Lv L, Chi Y, Chen C, Xu W. Structural and functional properties of ovalbumin glycated by dry-heating in the presence of maltodextrin. Int J Food Prop. 2015;18:1326–1333. doi:10.1080/10942912.2011.620204
- Wang Y, Yu H, Shi X, Luo Z, Lin D, Huang M. PDB ID: 4K2C. J Biol Chem 288, 15980–15987 (2013).
- Binaymotlagh R, Hadadzadeh H, Farrokhpour H, et al. In situ generation of the gold nanoparticles-bovine serum albumin (AuNPs-BSA) bioconjugated system using pulsed-laser ablation (PLA). Mater Chem Phys. 2016;177:360–370.
- Apadopoulou ATP, Reen REJG, Razier RIAF. Interaction of flavonoids with bovine serum albumin : a fluorescence quenching study. Agric Food Chem. 2005;53:158–163. doi:10.1021/jf048693g
- Nosrati H, Salehiabar M, Manjili HK, Danafar H, Davaran S. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int J Biol Macromol. 2018;108:909–915. doi:10.1016/j.ijbiomac.2017.10.18029101048
- Peters TJ. All About Albumin. San Diego: Academic Press; 1995.
- Pragna Lakshmi T, Mondal M, Ramadas K, Natarajan S. Molecular interaction of 2,4-diacetylphloroglucinol (DAPG) with human serum albumin (HSA): the spectroscopic, calorimetric and computational investigation. Spectrochim Acta A Mol Biomol Spectrosc. 2017;183:90–102. doi:10.1016/j.saa.2017.04.01228441541
- Li C, Xing L, Che S. Coordination bonding based pH-responsive albumin nanoparticles for anticancer drug delivery. Dalt Trans. 2012;41:3714. doi:10.1039/c2dt30226h
- Mariam J, Sivakami S, Dongre PM. Albumin corona on nanoparticles – a strategic approach in drug delivery. Drug Deliv. 2015;1–9. doi:10.3109/10717544.2015.1048488
- Zhiya MA, Xia H, Liu Y, Liu B, Chen W, Zhao Y. Applications of gold nanorods in biomedical imaging and related fields. Chin Sci Bull. 2013;58:2530–2536. doi:10.1007/s11434-013-5720-7
- Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int J Pharm. 2017;522:172–197. doi:10.1016/j.ijpharm.2017.01.06728188876
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi:10.1038/nbt.333026348965
- Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7(10):1322–1337. doi:10.1002/smll.20110000121520409
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Toxicity of nanoparticles gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;3:325–327. doi:10.1002/smll.200400093
- Hussain I, Nichols RJ, Schiffrin DJ, Brust M, Fernig DG. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Nanosci Nanotechnol. 2004;126:10076–10084.
- Liu Y, Shipton MK, Ryan J, Kaufman ED, Franzen S, Feldheim DL. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide - poly (ethylene glycol) monolayers. Anal Chem. 2007;79:2221–2229. doi:10.1021/ac061578f17288407
- Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB. Gold nanosphere-antibody conjugates for hyperthermal therapeutic applications. Gold Bull. 2007;40:121–129. doi:10.1007/BF03215568
- Mocan L, Matea C, Tabaran FA, et al. Selective ex vivo photothermal nano-therapy of solid liver tumors mediated by albumin conjugated gold nanoparticles. Biomaterials. 2017;119:33–42. doi:10.1016/j.biomaterials.2016.12.00927992805
- Murawala P, Tirmale A, Shiras A, Prasad BLV. In situ synthesized BSA capped gold nanoparticles : effective carrier of anticancer drug Methotrexate to MCF-7 breast cancer cells. Mater Sci Eng C. 2014;34:158–167. doi:10.1016/j.msec.2013.09.004
- Larsen MT, Kuhlmann M, Hvam ML, Howard KA. Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther. 2016;4:3. doi:10.1186/s40591-016-0048-826925240
- Brekken RA, Sage EH, Brekken RA. Mini review SPARC, a matricellular protein: at the crossroads of cell matrix SPARC, a matricellular protein : at the crossroads of cell matrix communication. Matrix Biol. 2001;19:815–827.
- Kouros M. SPARC (osteonectin/BM-40). Int J Biochem Cell Biol. 1999;31:1363–1366. doi:10.1016/S1357-2725(99)00090-410641790
- Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, Llera AS. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:53. doi:10.1007/s10555-008-9146-7
- Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013;104:779–789. doi:10.1111/cas.1215223495730
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi:10.1016/j.drudis.2006.07.00516935749
- Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft : molecular size dependence and cutoff size advances in brief vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756.7641188
- Grobmyer SR, Moudgil B. What is cancer nanotechnology? Methods Mol Biol. 2010;624:1–9. doi:10.1007/978-1-60761-609-2_120217585
- Bhamidipati M, Fabris L. Multiparametric assessment of gold nanoparticle cytotoxicity in cancerous and healthy cells: the role of size, shape, and surface chemistry. Bioconjug Chem. 2016;28:449–460. doi:10.1021/acs.bioconjchem.6b00627
- Sanchez-Iglesias A, Grzelczak M, Altantzis T, et al. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano. 2012;6:11059–11065. doi:10.1021/nn304760523186074
- Ahmad R, Fu J, He N, Li S. Advanced gold nanomaterials for photothermal therapy of cancer. J Nanosci Nanotechnol. 2016;16:67–80. doi:10.1166/jnn.2016.1077027398434
- Elzoghby AO, Hemasa AL, Freag MS. Hybrid protein-inorganic nanoparticles: from tumor-targeted drug delivery to cancer imaging. J Control Release. 2016;243:303–322. doi:10.1016/j.jconrel.2016.10.02327794493
- Pramanik S, Banerjee P, Sarkar A, Bhattacharya SC. Size-dependent interaction of gold nanoparticles with transport protein: a spectroscopic study. J Lumin. 2008;128:1969–1974. doi:10.1016/j.jlumin.2008.06.008
- Boulos SP, Davis TA, Yang JA, et al. Nanoparticle − protein interactions: a thermodynamic and kinetic study of the adsorption of bovine serum albumin to gold nanoparticle surfaces. Langmuir. 2013;29(48):14984–14996. doi:10.1021/la402920f24215427
- Rafaela G-Á, Marilena H, Ana S-I, Luis -ML-M, Kostas K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale. 2018;10:1256–1264. doi:10.1039/C7NR08322J29292433
- Chakraborty S, Joshi P, Shanker V, et al. Contrasting effect of gold nanoparticles and nanorods with different surface modifications on the structure and activity of bovine serum albumin. Langmuir. 2011;27(12):7722–7731. doi:10.1021/la200787t21591651
- Moustaoui H, Saber J, Djeddi I, et al. A protein corona study by scattering correlation spectroscopy: a comparative study between spherical and urchin-shaped gold nanoparticles. Nanoscale. 2019;11:3665–3673. doi:10.1039/C8NR09891C30741295
- Online VA, Chandrasekaran N, Mukherjee A. A comprehensive investigation of the differential interaction of Human Serum Albumin with Gold nanoparticles based on the variation in morphology and surface functionalization. RSC Adv. 2016;58:52683–52694.
- Carnovale C, Bryant G. Impact of nanogold morphology on interactions with human serum. Phys Chem Phys. 2018;30:29558–29565. doi:10.1039/C8CP05938A
- Maleki MS, Moradi O, Tahmasebi S. Adsorption of albumin by gold nanoparticles: equilibrium and thermodynamics studies. Arab J Chem. 2017;10:S491–S502. doi:10.1016/j.arabjc.2012.10.009
- Liu M. pH-sensitive Au – BSA – DOX – FA nanocomposites for combined CT imaging and targeted drug delivery. Int J Nanomedicine. 2017;12:2829–2843. doi:10.2147/IJN.S12827028435261
- Elsadek B, Kratz F. Impact of albumin on drug delivery - New applications on the horizon. J Control Release. 2012;157:4–28. doi:10.1016/j.jconrel.2011.09.06921959118
- Li J, Cai R, Kawazoe N, Chen G. Facile preparation of albumin-stabilized gold nanostars for the targeted photothermal ablation of cancer cells. J Mater Chem B. 2015;3:5806–5814. doi:10.1039/C4TB02051K
- Loureiro A, Azoia NG, Gomes AC, Cavaco-Paulo A. Albumin-based nanodevices as drug carriers. Curr Pharm Des. 2016;22:1371–1390. doi:10.2174/138161282266616012511490026806342
- Chen L, Feng W, Zhou X, et al. Facile synthesis of novel albumin-functionalized flower-like MoS 2 nanoparticles for in vitro chemo-photothermal synergistic therapy. RSC Adv. 2016;6:13040–13049. doi:10.1039/C5RA27822H
- Lademann J, Richter H, Knorr F, et al. Acta Biomaterialia Triggered release of model drug from AuNP-doped BSA nanocarriers in hair follicles using IRA radiation. Acta Biomater. 2016;30:388–396. doi:10.1016/j.actbio.2015.11.05226621698
- Peralta DV, He J, Wheeler DA, Zhang JZ, Tarr MA. Encapsulating gold nanomaterials into size-controlled human serum albumin nanoparticles for cancer therapy platforms. J Microencapsul. 2014;31:824–831. doi:10.3109/02652048.2014.94001225090588
- Zu L, Liu L, Qin Y, Liu H, Yang H. Multifunctional BSA-Au nanostars for photoacoustic imaging and X-ray computed tomography. Nanomed Nanotechnol Biol Med. 2016;12:1805–1813. doi:10.1016/j.nano.2016.05.003
- Zhang L, Xia K, Bai YY, et al. Synthesis of gold nanorods and their functionalization with bovine serum albumin for optical hyperthermia. J Biomed Nanotechnol. 2014;10:1440–1449.25016644
- Paraiso WKD, Tanaka H, Sato Y, et al. Preparation of envelope-type lipid nanoparticles containing gold nanorods for photothermal cancer therapy. Colloids Surf B Biointerfaces. 2017;160:715–723. doi:10.1016/j.colsurfb.2017.10.00929035819
- Sun J, Guo Y, Xing R, et al. Synergistic in vivo photodynamic and photothermal antitumor therapy based on collagen-gold hybrid hydrogels with inclusion of photosensitive drugs. Colloids Surf A Physicochem Eng Asp. 2017;514:155–160. doi:10.1016/j.colsurfa.2016.11.062
- Liu P, Zheng H, Yang Z, et al. Facile preparation of versatile gadolinium-chelated protein nanocomposite for T1magnetic resonance imaging-guided photodynamic and photothermal synergetic therapy. J Mater Chem. 2018;B 6:1688–1698. doi:10.1039/C8TB00148K
- Sasidharan S, Bahadur D, Srivastava R. Albumin stabilized gold nanostars: A biocompatible nanoplatform for SERS, CT imaging and photothermal therapy of cancer. RSC Adv. 2016;6:84025–84034. doi:10.1039/C6RA11405A
- Liu J, Peng Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017;55:13–27. doi:10.1016/j.actbio.2017.03.05528377307
- Rastogi L, Kora AJ, Arunachalam J. Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater Sci Eng C. 2012;32:1571–1577. doi:10.1016/j.msec.2012.04.044
- Klebowski B, Depciuch J, Parlinska-Wojtan M, Baran J. Applications of noble metal-based nanoparticles in medicine. Int J Mol Sci. 2018;19. doi:10.3390/ijms19124031
- Li D, Zhang M, Xu F, et al. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm Sin B. 2018;8:74–84. doi:10.1016/j.apsb.2018.04.00629872624
- Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via raman imaging in living mice. ACS Nano. 2012;6:10366–10377. doi:10.1021/nn304347g23101432
- Almada M, Leal-Martínez BH, Hassan N, et al. Photothermal conversion ef fi ciency and cytotoxic effect of gold nanorods stabilized with chitosan, alginate and poly (vinyl alcohol). Mater Sci Eng C. 2017;77:583–593. doi:10.1016/j.msec.2017.03.218
- Zhang Z, Wang J, Nie X, et al. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J Am Chem Soc. 2014;136:7317–7326. doi:10.1021/ja412735p24773323
- Encinas-Basurto D, Ibarra J, Juarez J, et al. Hybrid folic acid-conjugated gold nanorods-loaded human serum albumin nanoparticles for simultaneous photothermal and chemotherapeutic therapy. Mater Sci Eng C. 2018;91:669–678. doi:10.1016/j.msec.2018.06.002
- Pelaz B, Grazu V, Ibarra A, et al. Tailoring the synthesis and heating ability of gold nanoprisms for bioapplications. Langmuir. 2012;28:8965–8970. doi:10.1021/la204712u22260484
- Bao C, Beziere N, del Pino P, et al. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small. 2013;9:68–74. doi:10.1002/smll.20120177923001862
- Cui H-D, Hu D-H, Zhang J-N, et al. Theranostic gold cluster nanoassembly for simultaneous enhanced cancer imaging and photodynamic therapy. Chin Chem Lett. 2017;28:1391–1398. doi:10.1016/j.cclet.2016.12.038
- Santhosh M, Chinnadayyala SR, Singh NK, Goswami P. Human serum albumin-stabilized gold nanoclusters act as an electron transfer bridge supporting specific electrocatalysis of bilirubin useful for biosensing applications. Bioelectrochemistry. 2016;111:7–14. doi:10.1016/j.bioelechem.2016.04.00327126550
- Ding H, Yang D, Zhao C, et al. Protein-gold hybrid nanocubes for cell imaging and drug delivery. ACS Appl Mater Interfaces. 2015;7:4713–4719. doi:10.1021/am508373325669930
- Yu X, Liu W, Deng X, Yan S, Su Z. Gold nanocluster embedded bovine serum albumin nanofibers-graphene hybrid membranes for the efficient detection and separation of mercury ion. Chem Eng J. 2018;335:176–184. doi:10.1016/j.cej.2017.10.148
- Ding C, Xu Y, Zhao Y, Zhong H, Luo X. Fabrication of BSA@AuNC-based nanostructures for cell fluoresce imaging and target drug delivery. ACS Appl Mater Interfaces. 2018;10:8947–8954. doi:10.1021/acsami.7b1849329457719
- Callaghan C, Peralta D, Liu J, et al. Combined treatment of tyrosine kinase inhibitor-labeled gold nanorod encapsulated albumin with laser thermal ablation in a renal cell carcinoma model. J Pharm Sci. 2016;105:284–292. doi:10.1016/j.xphs.2015.11.01726852860
- Liu J, Abshire C, Carry C, et al. Nanotechnology combined therapy: tyrosine kinase-bound gold nanorod and laser thermal ablation produce a synergistic higher treatment response of renal cell carcinoma in a murine model. BJU Int. 2017;119:342–348. doi:10.1111/bju.2017.119.issue-227431021
- Wang Z, Chen L, Chu Z, Huang C, Huang Y, Jia N. Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging. Mater Sci Eng C. 2018;89:106–118. doi:10.1016/j.msec.2018.03.025
- Chiu HT, Su CK, Sun YC, Chiang CS, Huang YF. Albumin-gold nanorod nanoplatform for cell-mediated tumoritropic delivery with homogenous chemodrug distribution and enhanced retention ability. Theranostics. 2017;7:3034–3052. doi:10.7150/thno.1927928839462
- Lademann J, Richter H, Knorr F, et al. Triggered release of model drug from AuNP-doped BSA nanocarriers in hair follicles using IRA radiation. Acta Biomater. 2016;30:388–396. doi:10.1016/j.actbio.2015.11.05226621698
- Chen J, Sheng Z, Li P, et al. Indocyanine green-loaded gold nanostars for sensitive SERS imaging and subcellular monitoring of photothermal therapy. Nanoscale. 2017;9:11888–11901. doi:10.1039/C7NR02798B28561825
- Leopold LF, Tódor IS, Diaconeasa Z, et al. Assessment of PEG and BSA-PEG gold nanoparticles cellular interaction. Colloids Surfaces A Physicochem. Eng Asp. 2017;532:70–76. doi:10.1016/j.colsurfa.2017.06.061
- Baum RP, Kulkarni HR. Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy - the bad berka experience. Theranostics. 2012;2:437–447. doi:10.7150/thno.364522768024
- Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi:10.1016/j.jconrel.2007.12.01018261822
- Fernandez-Fernandez A, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Appl Biochem Biotechnol. 2011;165:1628–1651. doi:10.1007/s12010-011-9383-z21947761
- Fiegel V, Harlepp S, Begin-Colin S, Begin D, Mertz D. Design of protein-coated carbon nanotubes loaded with hydrophobic drugs through sacrificial templating of mesoporous silica shells. Chem Eur J. 2018. doi:10.1002/chem.201705845
- Fu C, Ding C, Sun X, Fu A. Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater Sci Eng C. 2018. doi:10.1016/j.msec.2017.12.028
- Chiu HT, Chen CH, Li ML, et al. Bioprosthesis of core-shell gold nanorod/serum albumin nanoimitation: a half-native and half-artificial nanohybrid for cancer theranostics. Chem Mater. 2018;30:729–747. doi:10.1021/acs.chemmater.7b04127
- Quan Q, Xie J, Gao H, et al. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Mol Pharm. 2011;8:1669–1676. doi:10.1021/mp200125j21838321
- Abbas M, Zou Q, Li S, Yan X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv Mater. 2017;29. doi:10.1002/adma.201700681
- Khlebtsov B, Prilepskii A, Lomova M, Khlebtsov N. Au-nanocluster-loaded human serum albumin nanoparticles with enhanced cellular uptake for fluorescent imaging. J Innov Opt Health Sci. 2015;9:1650004. doi:10.1142/S1793545816500048
- Han L, Xia J-M, Hai X, Shu Y, Chen X-W, Wang J-H. Protein-stabilized gadolinium oxide-gold nanoclusters hybrid for multimodal imaging and drug delivery. ACS Appl Mater Interfaces. 2017;9:6941–6949. doi:10.1021/acsami.7b0024628177224
- You Q, Sun Q, Yu M, et al. BSA-bioinspired gadolinium hybrid-functionalized hollow gold nanoshells for NIRF/PA/CT/MR quadmodal diagnostic imaging-guided photothermal/photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9:40017–40030. doi:10.1021/acsami.7b1192629087183
- Topete A, Alatorre-Meda M, Iglesias P, et al. Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells. ACS Nano. 2014;8:2725–2738. doi:10.1021/nn406425h24571629
- Aaron C, Mitragotri AS. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1:10–29. doi:10.1002/btm2.1000329313004
- Ali MRK, Rahman MA, Wu Y, et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc National Acad Sci. 2017;114:E3110–E3118. doi:10.1073/pnas.1619302114
- Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7:8003–8015. doi:10.1039/C5NR01050K25864858