Abstract
Cancer cells have been reported to exhibit an enhanced capacity for protoporphyrin IX (PpIX) synthesis facilitated by the administration of 5-aminolevulinic acid (ALA). We investigated the effect of ALA-based photodynamic therapy (PDT) on human cholangiocarcinoma cells (HuCC-T1). Since protoporphyrin IX (PpIX), a metabolite of ALA, can produce reactive oxygen species (ROS) under irradiation and then induce phototoxicity, ALA-based PDT is a promising candidate for the treatment of cholangiocarcinoma. When various concentrations of ALA (0.05–2 mM) were used to treat HuCC-T1 cells for 6 or 24 hours, the intracellular PpIX level increased according to the ALA concentration and treatment time. Furthermore, an increased amount of PpIX in HuCC-T1 cells induced increased production of ROS by irradiation, resulting in increased phototoxicity.
Introduction
Cholangiocarcinoma (CC) is the second most common primary hepatobiliary malignancy in adults, and has high mortality. It often arises from background conditions that cause longstanding inflammation, injury, and reparative biliary epithelial cell proliferation, such as primary sclerosing cholangitis, clonorchiasis, hepatolithiasis, or complicated fibropolycystic diseases.Citation1–Citation5 The most common curative therapy for CC is surgical resection. However, the majority of patients are diagnosed in a latent CC state and, unfortunately, they are not considered for surgery. For this reason, alternative therapies such as stent replacement, chemotherapeutic, radiation therapy, and photodynamic therapy (PDT) are the treatments of choice for the management of CC.Citation6–Citation9 PDT typically involves systemic administration of a tumor-localizing photosensitizer followed by its activation by light of an appropriate wavelength to create a photochemical reaction, thus causing photodamage to the tumor.Citation10,Citation11
5-aminolevulinic acid (ALA) is a precursor of PpIX, which is well known as a strong photosensitizer, and administered ALA is interconverted to PpIX via the heme biosynthetic pathway.Citation12–Citation15 PpIX accumulation in tumor cells is generally higher than that in normal cells, as the activity of key enzymes in tumor cells is significantly changed.Citation16–Citation18 Excitation of PpIX under irradiation in tumor cells can produce a significant amount of reactive oxygen species (ROS), including singlet oxygen, superoxide, hydroxyl radical, and hydrogen peroxide. ROS causes DNA and cell membrane damage through lipid peroxidation and/or alterations in membrane fluidity. This reaction results in obvious damage to cellular organelles, such as mitochondria and microsomes,Citation19–Citation22 and causes the death of tumor cells.
One of the main advantages of ALA-based PDT is that ALA-derived PpIX is cleared from the body within 24–48 hours after systemic ALA administration. This fact reduces or avoids the risk of prolonged phototoxicity to the human body.Citation14–Citation17 Recently, researchers reported the effects of ALA-based PDT in various tumor cells such as oral squamous cell carcinoma, melanoma, glioma, lymphoma, esophageal carcinoma, and basal cell carcinoma.Citation10,Citation23–Citation27 ALA and its derivatives are therefore considered a potential treatment option for hepatobiliary cholangiocarcinoma therapy.
In this study, the effect of ALA treatment on PpIX synthesis, ROS production, and cell death in cholangiocarcinoma cells was investigated. To the authors’ knowledge, there has been no in vitro study of the potential of ALA-based PDT in HuCC-T1 cells. Since ALA is regarded as safe for PDT compared with other photosensitizers, the application of ALA-based PDT will provide a new treatment option for CC.
Methods
Materials
5-aminolevulinic acid (ALA), 2′, 7′-dichlorofluorescein diacetate (DCFH-DA), 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromid (MTT), and propidium iodide (PI) were purchased from Sigma-Aldrich Co (St Louis, MI). FITC-Annexin V was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Cell culture materials were purchased from Invitrogen (New York, NY).
Cell culture
The human intrahepatic cholangiocarcinoma cell line (HuCC-T1) was used in this study. The cells were routinely propagated in Gibco® RPMI medium supplemented with 10% fetal bovine serum and 1% antibiotics at 37°C in a humidified atmosphere of 5% CO2:95% air according to the procedure described by Miyagiwa et al.Citation1 Every 3 days, trypsin-EDTA (0.05% trypsin and 0.02% EDTA) was used for subculture.
Dark toxicity
HuCC-T1 cells (1 × 104) were seeded into a 96-well plate and treated with various concentrations (0.05–2 mM) of ALA in serum-free medium for 6 or 24 hours. After that, the medium was changed to growth medium containing 10% fetal bovine serum and incubated for 24 hours. Cell viability was measured by MTT assay.Citation28
PpIX accumulation assay
HuCC-T1 cells were treated with various concentrations of ALA for 6 or 24 hours. After that, cells were lysed using cell lysis buffer (GenDEPOT, Barker, TX). PpIX accumulation in the cells was measured by fluorescence intensity using a microplate reader (Infinite M200 Pro, Tecan Group Ltd, Mannerdorf, Switzerland) at an excitation wavelength of 485 nm and emission wavelength of 635 nm.
Light sources for photodynamic therapy
Cultured cell plates were exposed to LED lamps at 635 nm for irradiation (SH System Co, Gwangju, Korea). Light intensity was 0.25 J/cm2, as measured by a photo radiometer (Delta Ohm, Padua, Italy).
Cell viability assay
HuCC-T1 cells were treated with various concentrations of ALA in serum-free medium for 6 or 24 hours. After incubation with ALA, the cells were irradiated at 635 nm with 0.25 J/cm2. The medium was changed to growth medium containing 10% fetal bovine serum and incubated for 24 hours. Cell viability was measured by MTT assay.Citation28
ROS measurement
After irradiation, ROS generation by PpIX-accumulated HuCC-T1 cells was measured by the DCFH-DA method.Citation29 In this method, fluorogenic substrate DCFH-DA, a cell-permeable dye, is oxidized to highly fluorescent DCF by ROS, and can be used to monitor intracellular generation of ROS. The cells were treated with various concentrations (0.05–2 mM) of ALA for 24 hours in phenol red free RPMI media. DCFH-DA was added to the media at a final concentration of 20 μM and incubated at 37°C for 30 minutes. After irradiation at 635 nm, ROS generation was measured by fluorescence intensity at an excitation wavelength of 485 nm and emission wavelength of 535 nm using a microplate reader.
Flow cytometry analysis
Two reagents, PI and FITC-annexin V, were used to identify apoptosis and necrosis of HuCC-T1 cells, respectively. Cells were treated with various concentrations of ALA for 24 hours. After that, cells were irradiated at 635 nm with 0.25 J/cm2, then collected cells were washed with phosphate-buffered saline (PBS). The pellets were resuspended with binding buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES] pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) containing FITC-annexin V (1 ug/mL) and further incubated for 30 minutes. Ten minutes prior to termination of incubation, PI (10 ug/mL) was added to stain necrotic cells under dark conditions. Following that, the cells were immediately analyzed using a FACScan flow cytometer (Becton Dicknson Biosciences, San Jose, CA).
Fluorescence microscopy
PpIX products were observed by fluorescence microscopy. The microscope (Eclipse 80i; Nikon, Tokyo, Japan) was equipped with a 460–480 mm excitation filter and a 610 nm filter for detection of PpIX fluorescence. 1 × 106 HuCC-T1 cells were seeded on a cover glass in a 6-well plate. The cells were then treated with 0.25 mM ALA for 24 hours in serum-free media, after which the medium was discarded and washed with PBS. The cells were fixed by 4% paraformaldehyde in PBS, mounted on a glass slide, and observed using a fluorescence microscope.
Phase contrast microscopy
Cell death by ALA-PDT was observed by phase contrast microscopy (MCXI 600; Micros, Vienna, Austria). 1 × 106 HuCC-T1 cells were seeded in a 6-well plate. The cells were then treated with 0.25 mM ALA for 24 hours in serum-free media.
Statistical analysis
All the experiments in this study were performed in triplicate, ie, average plus standard deviation value was obtained from three independent experiments. All data are shown as the mean ± SEM of the independent experiments. Statistical analysis was performed using Student’s t-test (SPSS Data Collection [IBM Corp, Somers, NY] software). Statistical significance was taken as P < 0.001.
Results
Dark toxicity of HuCC-T1 cells by ALA
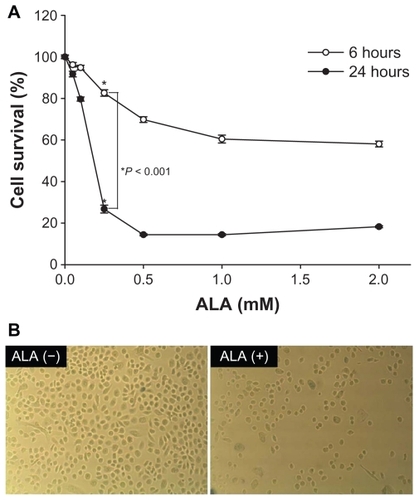
To identify dark toxicity, various concentrations of ALA were treated to HuCC-T1 cells for 6 or 24 hours without radiation (). When the HuCC-T1 cells were incubated with less than 0.5 mM ALA for 6 hours or 0.25 mM ALA for 24 hours, their survivability did not significantly change, ie, more than 90% of cells survived. Dark toxicity of cells appeared at greater than 0.5 mM at 6 hours treatment and 0.25 mM at 24 hours treatment.
Figure 1 Cell dark toxicity by ALA treatment in HuCC-T1 cells. HuCC-T1 cells were treated with ALA (0, 0.05, 0.1, 0.25, 0.5, 1, 2 mM) in serum-free culture medium. These cells were incubated for 24 hours in normal media containing 10% FBS. Cell survival was determined by measuring MTT. HuCC-T1 cells treated with ALA concentrations of 0.5 mM for 6 hours and 0.25-mM for 24 hours did not significantly influence cell survival (>90%).
Abbreviations: ALA, 5-aminolevulinic acid; HuCC-T1, human cholangiocarcinoma cells; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide.
![Figure 1 Cell dark toxicity by ALA treatment in HuCC-T1 cells. HuCC-T1 cells were treated with ALA (0, 0.05, 0.1, 0.25, 0.5, 1, 2 mM) in serum-free culture medium. These cells were incubated for 24 hours in normal media containing 10% FBS. Cell survival was determined by measuring MTT. HuCC-T1 cells treated with ALA concentrations of 0.5 mM for 6 hours and 0.25-mM for 24 hours did not significantly influence cell survival (>90%).Abbreviations: ALA, 5-aminolevulinic acid; HuCC-T1, human cholangiocarcinoma cells; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide.](/cms/asset/7e7ba75a-7bfe-4271-9801-69194fec6bbe/dijn_a_21395_f0001_b.jpg)
PpIX production by ALA
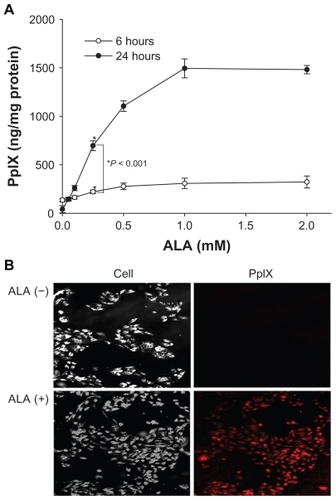
PpIX accumulation in HuCC-T1 cells incubated with ALA was assessed for 6 or 24 hours. Production of PpIX in HuCC-T1 cells was extremely high after 24 hours treatment with 1 mM ALA (). PpIX accumulation in HuCC-T1 cells induced by 0.25 mM ALA treatment for 24 hours was observed by phase contrast and fluorescence confocal microscopy. As shown in , red fluorescence was observed in the sample treated with 0.25 mM ALA, but no fluorescence signal was observed in the control group. These results indicate that administered ALA could be successfully interconverted to PpIX in HuCC-T1 cells.
Figure 2 PpIX accumulation graph induced by ALA treatment for 6 or 24 hours in HuCC-T1 cells (A) and photomicrographs of PpIX accumulation induced by ALA (0.25 mM) or non-treatment in HuCC-T1 cells (B).
Abbreviations: ALA, 5-aminolevulinic acid; HuCC-T1, human cholangiocarcinoma cells; PpIX, protoporphyrin IX.
Cell death induced by ALA-PDT
First, cell death by ALA-PDT was measured by MTT assay. HuCC-T1 cells were treated with ALA at various concentrations for 6 or 24 hours. The survivability of HuCC-T1 cells after irradiation was significantly decreased by 1 mM ALA treatment for 6 hours and 0.5 mM ALA for 24 hours, as shown in . When the exposure time of tumor cells to ALA was increased, photocytotoxicity increased in a similar range. Second, cell death induced by ALA-PDT in HuCC-T1 cells was identified by photomicrographs, as shown in . Whereas cell density was clearly decreased by treatment with 0.25 mM ALA, control treatment resulted in many cells. These results indicate that ALA-PDT induces death of HuCC-T1 cells.
Apoptosis and necrosis induced by ALA-PDT
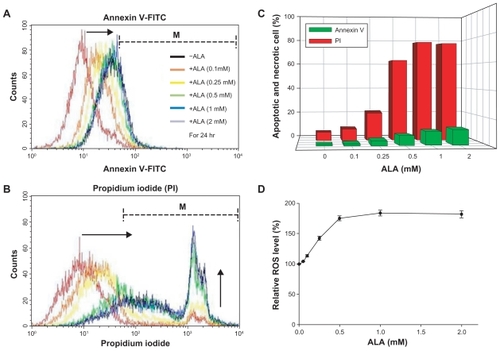
After ALA-based PDT, tumor cells were stained with FITC- annexin V for apoptotic cells () and PI for necrotic cells (). As shown in , the number of apoptotic cells gradually increased according to the increase in ALA concentration. Furthermore, the number of necrotic cells also dramatically increased according to ALA concentration (). These results indicate that ALA-based PDT induces death of HuCC-T1 cells through apoptosis and necrosis.
Figure 4 Apoptosis, necrosis, and ROS generation induced by ALA-PDT in HuCC -T1 cells. After ALA or nontreatment for 24 hours, HuCC -T1 cells were illuminated at 635 nm. Cells were stained with FITC-annexin V (A) and propidium iodide (B) and analyzed using a flow cytometer. Arrows indicate direction of graph movement. (C) This graph displays percentage (%) of cells in M (dotted line) area of total cells. After irradiation, ROS production in the cells was immediately measured by fluorescence intensity (D).
Abbreviations: ALA-PDT, 5-aminolevulinic acid-photodynamic therapy; HuCC-T1, human cholangiocarcinoma cells; ROS, reactive oxygen species.
ROS generation by ALA-PDT
As shown in , the ROS level gradually increased as the ALA concentration was increased to 0.5 mM after ALA-based PDT. These results could be explained by the fact that synthesis of PpIX after 24 hours incubation was saturated above 0.5 mM ALA. Therefore, ROS were produced by ALA- PDT, and the ROS level increased according to the increase in ALA concentration. In addition, ROS generation by ALA-based PDT was associated with cell death ().
Discussion
Administration of ALA both in vitro and in vivo is known to enhance PpIX synthesis in cancer cells. Frank et al reported that PpIX accumulation in cancer cells is significantly higher than that in normal human fibroblasts.Citation18 After irradiation, the PpIX-accumulated tumor cells produced ROS, which destroy tumor cells by apoptosis or necrosis.Citation20–Citation22,Citation30 The advantage of ALA-based PDT is its ability to target tumor cells. ROS generation in cancer cells can be specifically amplified by local irradiation even though ALA is distributed throughout the whole body.
Recently, researchers have reported that ALA-based PDT can effectively inhibit tumor cell growth and enhance patient survivability. Inoue et al reported massive apoptotic cell death in human glioma cells after ALA-based PDT.Citation27 Furthermore, the efficacy of ALA-based PDT was reported in melanoma cellsCitation10 and oral squamous cell carcinoma.Citation11
The main obstacle to the treatment of CC is the fact that CC develops deeper than the skin, making application of chemotherapy and radiotherapy difficult. ALA-based PDT has advantages in that ALA can be delivered locally by endoscopic operation, which allows for specific accumulation of PpIX in the tumor cells.Citation31 Furthermore, local application of ALA-based PDT by endoscopic operation enables avoidance of the undesirable side effects of photosensitizers compared with systemic application.
Before ALA-based PDT using HuCC-T1 cells was tested, it was confirmed whether the cell death effect was caused only by photoinduced cytotoxicity. The cytotoxicity of ALA in the absence of a light source (without irradiation and dark conditions) was investigated. When the ALA concentration was less than 0.5 mM at 6 hours incubation, the survivability of HuCC-T1 cells did not significantly change, ie, more than 90% of tumor cells survived as shown in . Furthermore, a similar result was observed at a greater incubation time and lower dose, ie, 24 hours incubation at 0.25 mM ALA also did not significantly affect cell survivability. The viability of tumor cells decreased at more than 0.5 mM ALA at 6 hours incubation. Moreover, when the incubation time was increased to 24 hours, the effective dose of ALA decreased; ie, viability of tumor cells decreased at 0.25 mM ALA. In the absence of irradiation, ALA itself did not affect cell survivability at any concentration. PpIX accumulation induced by ALA in HuCC-T1 cells reached its maximum at 1 mM ALA (). These results indicate that the ALA-based-PDT effect against human cholangiocarcinoma cells is dependent on the ALA concentration and incubation time. In addition, apoptosis and necrosis was identified in HuCC-T1 cells by ALA-PDT using FITC-annexin V and PI. The numbers of apoptotic and necrotic cells gradually increased on treatment with 1 mM ALA for 24 hours, indicating that the viability of HuCC-T1 cells decreased (). This result corresponds with the fact that PpIX accumulation was maximal at 1 mM ALA. Although the necrosis of tumor cells was dominant over apoptosis as shown in , secondary necrotic cells/late apoptotic cells might have been present in the necrotic population, since these secondary necrotic/late apoptotic cells represent cells that were initially apoptotic before losing membrane integrity due to a lack of timely phagocytosis. Even though apoptosis/necrosis dual staining did not strictly differentiate secondary necrotic/late apoptotic cells from necrosis of tumor cells, the results show a positive corelationship between apoptosis/necrosis of tumor cells and cell death. Furthermore, ROS generation in HuCC-T1 cells gradually increased upon treatment with 1 mM ALA for 24 hours (Figure 5). This result supports ALA-dependent death of HuCC-T1 cells after 24 hours incubation as shown in . After 6 hours incubation with various concentrations of ALA, the survivability of cells gradually decreased, and a significant decrease in cell survival was observed after 24 hours incubation with 0.5 mM ALA. These results indicate that ROS generation by PDT in HuCC-T1 cells is simultaneously dependent on incubation time and ALA concentration. Cao et al reported that hematoporphyrin, a photosensitizer similar to ALA, exhibits a PDT-mediated cytotoxic effect against human cholangiocarcinoma cells both in vitro and in vivo.Citation32 The results of the authors’ study show that ALA-based PDT is no less effective for treatment of human cholangiocarcinoma than hematoporphyrin.
In conclusion, a positive relationship between ALA concentration, PpIX accumulation, ROS production, and cell death by ALA-based PDT was observed. The results indicate that an appropriate concentration of ALA and treatment time is required to maximize the effects of ALA-based PDT and to minimize side effects. It is concluded that ALA-based PDT is a promising treatment option for CC.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health Welfare and Family Affairs, Republic of Korea (A091047).
Disclosure
The authors declare no conflicts of interest in relation to this paper.
References
- MiyagiwaMIchidaTTokiwaTSatoJSasakiHA new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free mediumIn Vitro Cell Dev Biol19892565035102544546
- WuGSZouSQLiuZRTangZHWangJHCelecoxib inhibits proliferation and induces apoptosis via prostaglandin E2 pathway in human cholangiocarcinoma cell linesWorld J Gastroenterol2003961302130612800245
- WuTLengJHanCDemetrisAJThe cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cellsMol Cancer Ther20043329930715026550
- AffordSCAhmed-ChoudhuryJRandhawaSCD40 activation- induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cellsFASEB J200115132345235411689460
- WiseCPilanthananondMPerryBFAlpiniGMcNealMGlaserSSMechanisms of biliary carcinogenesis and growthWorld J Gastroenterol200814192986298918494047
- KaramitopoulouETornilloLZlobecIClinical significance of cell cycle- and apoptosis-related markers in biliary tract cancer: a tissue microarray-based approach revealing a distinctive immunophenotype for intrahepatic and extrahepatic cholangiocarcinomasAm J Clin Pathol2008130578078618854271
- JoplinRIsolation and culture of biliary epithelial cellsGut19943578758788063212
- KangRSaitoHIharaYTranscriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1J Biol Chem19962714326706267128900148
- MuffMAMasyukTVStroopeAJDevelopment and characterization of a cholangiocyte cell line from the PCK rat, an animal model of autosomal recessive polycystic kidney diseaseLab Invest200686994095016783394
- GrimmSMvondoDGruneTBreusingNThe outcome of 5-ALA-mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1Biofactors2011371172421328623
- MoonYHParkJHKimSALeeJBAhnSGYoonJHAnticancer effect of photodynamic therapy with hexenyl ester of 5-aminolevulinic acid in oral squamous cell carcinomaHead Neck20103291136114219953630
- MalikZKostenichGRoitmanLEhrenbergBOrensteinATopical application of 5-aminolevulinic acid, DMSO and EDTA: protoporphyrin IX accumulation in skin and tumours of miceJ Photochem Photobiol B19952832132187623186
- PengQBergKMoanJKongshaugMNeslandJM5-Aminolevulinic acid-based photodynamic therapy: principles and experimental researchPhotochem Photobiol19976522352519066303
- PengQWarloeTBergK5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challengesCancer19977912228223089191516
- De RosaFSMarchettiJMThomaziniJATedescoACBentleyMVA vehicle for photodynamic therapy of skin cancer: influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopyJ Control Release200065335936610699294
- PengQWarloeTMoanJAntitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy can be enhanced by the use of a low dose of photofrin in human tumor xenograftsCancer Res200161155824583211479222
- PierreMBTedescoACMarchettiJMBentleyMVStratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: preparation and in vitro permeation studyBMC Dermatol20011511545679
- FrankJLornejad-SchäferMRSchöfflHFlaccusALambertCBiesalskiHKInhibition of heme oxygenase-1 increases responsiveness of melanoma cells to ALA-based photodynamic therapyInt J Oncol20073161539154517982681
- SharmaSJajooADubeA5-aminolevulinic acid-induced protoporphyrin-IX accumulation and associated phototoxicity in macrophages and oral cancer cell linesJ Photochem Photobiol B20078823156162
- ChenRHuangZChenGKinetics and subcellular localization of 5-ALA-induced PpIX in DHL cells via two-photon excitation fluorescence microscopyInt J Oncol200832486186718360713
- DonnellyRFMcCarronPAWoolfsonADDerivatives of 5-aminolevulinic acid for photodynamic therapyPerspect Med Chem20071114963
- FotinosNMikulicJConvertM5-ALA derivative-mediated photoinactivation of Propionibacterium acnesJ Dermatol Sci200956321421619709860
- Ickowicz SchwartzDGozlanYGreenbaumLBabushkinaTKatcoffDJMalikZDifferentiation-dependent photodynamic therapy regulated by porphobilinogen deaminase in B16 melanomaBr J Cancer20049091833184115150593
- MaierATomaselliFMatziVRehakPPinterHSmolle-JüttnerFMPhotosensitization with hematoporphyrin derivative compared to 5-aminolaevulinic acid for photodynamic therapy of esophageal carcinomaAnn Thorac Surg20017241136114011603425
- RedondoPMarquinaMPretelMAguadoLIglesiasMEMethyl- ALA-induced fluorescence in photodynamic diagnosis of basal cell carcinoma prior to Mohs micrographic surgeryArch Dermatol2008144111511718209183
- AmoTKawanishiNUchidaMMechanism of cell death by 5-aminolevulinic acid-based photodynamic action and its enhancement by ferrochelatase inhibitors in human histiocytic lymphoma cell line U937Cell Biochem Funct200927850351519735078
- InoueHKajimotoYShibataMAMassive apoptotic cell death of human glioma cells via a mitochondrial pathway following 5-aminolevulinic acid-mediated photodynamic therapyJ Neurooncol200783322323117245620
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651255636361138
- HeoHYParkJMKimCHHanBSKimKSSeolWLRRK2 enhances oxidative stress-induced neurotoxicity via its kinase activityExp Cell Res2010316464965619769964
- ItoSMiyoshiNDegraffWGNagashimaKKirschenbaumLJRieszPEnhancement of 5-aminolevulinic acid-induced oxidative stress on two cancer cell lines by gold nanoparticlesFree Radic Res200943121214122419905984
- BrancaleonLMoseleyHLaser and non-laser light sources for photodynamic therapyLasers Med Sci200217317318612181632
- CaoLQXuePLuHWZhengQWenZLShaoZJHematoporphyrin derivative-mediated photodynamic therapy inhibits tumor growth in human cholangiocarcinoma in vitro and in vivoHepatol Res200939121190119719788692