?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel design of an optical trapping tool for tangle protein (tau tangles, β-amyloid plaques) and molecular motor storage and delivery using a PANDA ring resonator is proposed. The optical vortices can be generated and controlled to form the trapping tools in the same way as the optical tweezers. In theory, the trapping force is formed by the combination between the gradient field and scattering photons, and is reviewed. By using the intense optical vortices generated within the PANDA ring resonator, the required molecular volumes can be trapped and moved dynamically within the molecular buffer and bus network. The tangle protein and molecular motor can transport and connect to the required destinations, enabling availability for Alzheimer’s diagnosis.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the aging population in which the loss of neural cells associated with AD is believed to be caused by the accumulation of β-amyloidal plaques.Citation1,Citation2 The tangles inside neuronsCitation3 and genesCitation1 are caused by abnormal axons in the neuronal networks – an important area of the brain. A study of axonal transport begins with the observation of nerve cell bodies.Citation4 The axon consists of many microtubules. Each microtubule is a hollow cylindrical tube with an external diameter about 25 nm and a wall thickness of approximately 4 nm. The cross section of each microtubule consists of 13 proto-filaments with the fastest axonal transport occurring at a velocity of 5 μm per second.Citation5 The main mechanism is to deliver cellular components to their action site, which is long-range microtubule-based transport. The major components of the transport machinery are the “engines”, or molecular motors.
In AD, the link protein is affected by disease disturbing the axonal transportation.Citation6 The microtubules in the axon are more resistant to the severing protein katanin than microtubules in other parts of the neuron. The sustained loss of tau from axonal microtubules over time renders them more sensitive to endogenous severing proteins. Thus, it causes the microtubule array to gradually disintegrate in tauopathies such as AD.Citation7–Citation9 The optical trapping tool was first invented by Ashkin et al.Citation10 It has emerged as a powerful tool with broad-reaching applications in biology, physics, engineering, and medicine. The ability of optical trapping and manipulation of viruses, living cells, bacteria, and organelles by laser radiation pressure without damageCitation11,Citation12 has been demonstrated,Citation13,Citation14 and is of particular interest in the fields of medicine and nanotechnology. The possibility of liquids transportation and delivery at the nanoscale has been rapidly developed within the capillary or microchannel.Citation15 Micronanofluidics is a multi-dimensional field of science functioning in the 1–100 nm range.Citation15 It is a burgeoning field with important applications in areas such as medical devices, biotechnology, chemical synthesis, and analytical chemistry.Citation16
Researchers have used this promising technique in the study of optical trapping applications such as the control of kinesin movement on the microtubule surfaceCitation17–Citation19 to the axon terminal. In the study of micronanofluidics combined with optics, Erickson laboratory researchers’Citation20–Citation24 interests include advancing flows, delivery, and implantable devices in living organs.Citation25–Citation27 New advances in optics strategy using light to drive and halt neuronal activity with molecular specificity have been investigated. Moreover, the optical methods that have been developed to date encompass a broad array of strategies, including photorelease of caged neurotransmitters, engineered light-gated receptors and channels, naturally light-sensitive ion channels and pumps,Citation28 and artificial neural networks.Citation29 Recently, the use of optical trapping tool microscopic volume transportation within an add/drop multiplexer has been reported both in theoryCitation30 and experimentally.Citation31 Here the transporter is known as an optical tweezer. The optical tweezer generation technique is used as a powerful tool for the manipulation of micrometer-sized particles. To date, the useful static tweezers are well recognized and realized. The use of dynamic tweezers is now also realized in practical work.Citation32–Citation34 Schulz et alCitation35 have shown the possibility of trapped atoms being transferred between two optical potentials. In principle, an optical tweezer uses the forces exerted by intensity gradients in the strongly focused beams of light to trap and move the microscopic volumes of matter via a combination of forces induced by the interaction between photons, due to the photon scattering effects. In application, the field intensity can be adjusted and tuned to the desired gradient field. The scattering force can then form the suitable trapping force. Hence, the appropriate force can be configured as the transmitter/receiver for performing a long distance microscopic transportation.
In this paper, the dynamic optical tweezers/vortices are generated using the optical pulse propagating within an add/drop optical multiplexer incorporating two nanoring resonators (PANDA ring resonator), and using light pulse control within an add/drop optical filter.Citation36 By using the proposed system, the tangle protein and molecular motor can be trapped, transported, and received into the optical waveguide by optical tweezers. By utilizing the reasonable optical pulse input power, the dynamic tweezers can be controlled and stored within the system before reaching the desired destinations via the molecular buffer and bus network. In application, the neuron network can be connected by the link protein that can be available for AD and recovery, which will be discussed in detail.
Theoretical background
In theory, the trapping forces are exerted by the intensity gradients in the strongly focused beams of light to trap and move the microscopic volumes of matter, in which the optical forces are customarily defined by the following relationship:Citation37
In the Rayleigh regime, trapping forces decompose naturally into two components. Since, at this limit, the electromagnetic field is uniform across the dielectric, particles can be treated as induced point dipoles. The scattering force is given by:Citation37
Here σ is the scattering cross section of a Rayleigh sphere with radius r. 〈S〉 is the time averaged Poynting vector, n is the index of refraction of the particle, m = n/nm is the relative index, and k = 2πnm/λ is the wave number of the light. The scattering force is proportional to the energy flux and points along the direction of propagation of the incident light. The gradient field (Fgrad) is the Lorentz force acting on the dipole induced by the light field. It is given by:Citation37
Alzheimer’s diagnosis using molecular network
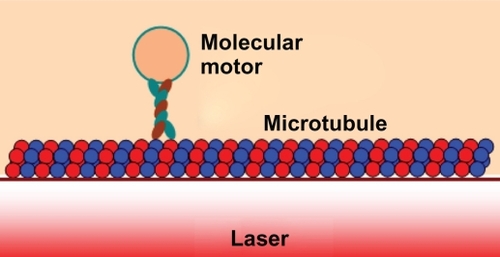
In operation, the optical tweezers can be trapped, transported, and stored within the PANDA ring resonator and wavelength router, which can be used to form the microscopic volume (molecular motor, tau tangles, and β-amyloids plaques) transportation, and drug delivery via the waveguide.Citation39 The manipulation of trapped and removed tangle proteins within the optical tweezers has been reported. Optical trapping is one of the most powerful single-molecule techniques with wide reaching applications in medicine. Living cells and important biological applications can be investigated using optical tweezers.Citation40 Hosokawa et alCitation41 have demonstrated the optical trapping of synaptic vesicles in a hippocampal neuron and found that the intracellular synaptic vesicles can be trapped at the focal spot within the laser irradiation time. This occurs because the vesicles form clusters in neurons, and are effectively trapped at the focal spot due to its high polarizability.
In this paper, we propose the use of the optical trapping tools for removing tangle protein and transportation out of neuronal cells, which induces the neurofibrillary degeneration caused by AD. Amyloid and tau proteins are both implicated in memory impairment, mild cognitive impairment (MCI), and early AD, however their interaction is unknown.Citation42 In operation, the optical tweezer can be trapped, transported, and stored within the PANDA ring resonator,Citation30 incorporating a wavelength router in the same drug-delivery network system.Citation7 We used the theory of optical trapping and transportation techniqueCitation43–Citation45 to trap kinesins for the manipulation of synaptic vesicles in critical areas of the neuronal network; processing and removing the amyloid plaques to prevent the accumulation of them between nerve cells in the brain. The spherical kinesin motor molecules are directly moved on to the microtubules where they could be activated by ATP,Citation40 which is activated by the interaction of nerve cells to each other.
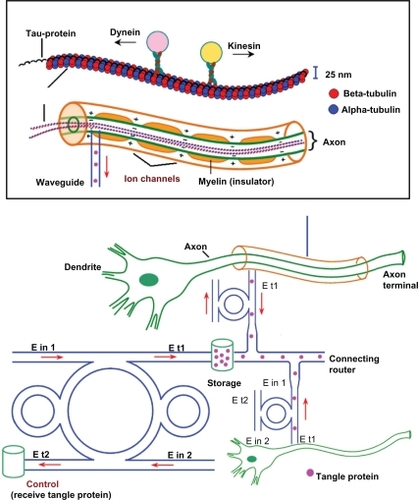
The proposed AD system is as shown in . By using the molecular buffer and bus network, the required trapped volumes can be transported within the network to the required destinations. The trapped tangle protein can be filtered via the add/drop filter before reaching the desired destinations. The throughput port (Et1) output of the add/drop filter is connected to the axon, in which the effective area of the waveguide is 2.01 μm2 (r = 800 nm) and the outside diameter of the microtubule is 25 nm.Citation5 The diameter of axons at birth is 1 μm, increasing through childhood (7 years) to 12 μm, and later to 24 μm in adulthood.Citation46 The optical tool is connected to the axon and between the nerve cells to trap the tangle protein into the removal storage by an add/drop filter (control port), otherwise, the bus network is designed to trap the molecular motor to activate the information of the neuronal cell at the same time. shows a schematic of the waveguide and microtubule position in the axon. The proposed design system is used to trap kinesinCitation47 and moves/stores the tangle protein via the molecular buffer and bus network. The light waveguide is inserted into the axon to trap the kinesin and traps/stores/receives the tangle protein. The ungrouped form of these proteins causes Alzehimer’s disease.Citation48 The optical tweezer can induce the mechanical unfolding and refolding of a single protein molecule in the absence and the presence of molecular chaperones.Citation49 Moreover, this noninvasive optical trapping technique can be used to unfold the poly-proteinCitation50 in adult neurons.Citation51
Figure 1 Schematic diagram of an Alzheimer’s diagnosis system using a molecular buffer and bus network.
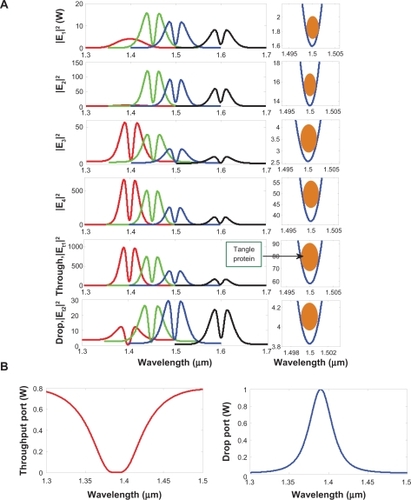
In simulation, the bright soliton with center wavelength at 1.50 μm, peak power 2 W, pulse 35fs is input into the system via the input port. The coupling coefficients are given as κ0 = 0.5, κ1 = 0.35, κ2 = 0.1, and κ3 = 0.35, respectively. The ring radii are Rad = 20 and 1 μm, RR = 5 μm and 0.8 μm, and RL = 5 and 0.8 μm, respectively. To date, the evidence of a practical device with radius of approximately 0.8 μm has been reported by the authorsCitation52 in which Aeff is 2.01 μm2 (r =800 nm). In this case, the dynamic tweezers (gradient fields) can be in the forms of bright solitons, Gaussian pulses, and dark solitons, which can be used to trap the required tangle protein. In , there are four different center wavelengths of tweezers generated, where the dynamical movements are (a) |E1|2, (b) |E2|2, (c) |E3|2, (d) |E4|2, (e) through port, and (f) drop port signals, where in this case all microscopic volumes are received by the drop port. In practice, the fabrication parameters that can be easily controlled are the ring resonator radii instead of coupling constants. The important aspect of the result is that the tunable tweezers can be obtained by tuning (controlling) the add (control) port input signal, in which the required number of single protein (tau-protein/beta myeloid, plaque) can be obtained and seen at the drop/through ports. Otherwise, they propagate within a PANDA ring before collapsing/decaying into the waveguide.
Figure 3 Result of the dynamic tweezers within the buffer with different (A) wavelengths and (B) coupling constants, where Rad = 20 μm, RR = RL = 5 μm.
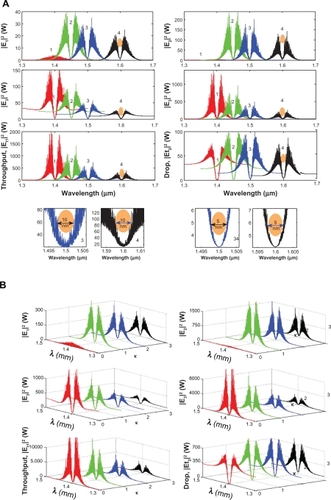
More results of the optical tweezers generated within the PANDA ring are shown in , where in this case the bright soliton is used as the control port signal to obtain the tunable results. The output optical tweezers of the through and drop ports with different coupling constants are shown in , while the different wavelength results are shown in , which is allowed to form the selected targets. In application, the trapped microscopic volumes (molecules) can transport into the wavelength router via the through port, while the retrieved microscopic volumes are received via the drop port (connecting target). The advantage of the proposed system is that the transmitter and receiver can be fabricated on-chip and alternatively operated by a single device.
Conclusion
Tau protein and β-amyloid deposits are known as the targets in Alzheimer’s disease. Recent studies show tau as a potential diagnostic marker and a candidate for change and maintenance via drug application.Citation53–Citation55 In this work, we propose the future design of Alzheimer’s therapy in that the tangle protein and molecular motor can be trapped, transported, and received into the optical waveguide by optical tweezers. By utilizing the reasonable optical pulse input power, the dynamic tweezers can be controlled and stored within the system before reaching the final destination via the molecular buffer and bus network. The tweezer can also be amplified by using the nanoring resonators and modulated signals via the control port. In conclusion, we have shown that the use of the molecular buffer and bus network for long distance protein trapping and transportation can be realized by using the proposed system. The trapped volumes or molecules are then transported via the wavelength router and bus network to the required (connecting) targets, and are applicable to AD. However, the large microscopic volumes and networks are potential problems, and the search for a new guide pipe medium,Citation40 such as nano tubes and crosstalk effects will be the next topic of investigation.
Acknowledgements
We would like to thank the Institute of Advanced Photonics Science, Nanotechnology Research Alliance, Universiti Teknologi, Malaysia (UTM), and King Mongkut’s Institute of Technology (KMITL), Thailand, for providing the research facilities. This research work has been supported by UTM’s Tier 1/Flagship Research Grant, MyBrain15 Fellowship/MOHE SLAB Fellowship and the Ministry of Higher Education (MOHE) research grant. N Suwanpayak would like to acknowledge King Mongkut’s Institute of Technology Ladkrabang, Bangkok (KMITL), Thailand, for the partial support in higher education at KMITL, Thailand.
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- SelkoeDJAlzheimer’s disease: genes, proteins, and therapyPhysiol Rev20018174176611274343
- JeffreyLCummingsJLAlzheimer’s diseaseN Engl J Med2004351566715229308
- MayeuxREarlyMDAlzheimer’s diseaseN Engl J Med2010232194220120558370
- MorganJECirculation and axonal transport in the optic nerveEye2004181089109515534594
- KarpGCell and Molecular Biology6th edHoboken, NJJohn Wiley & Sons, Inc2010
- StokinGBGoldsteinLSBAxonal transport and Alzheimer’s diseaseAnnu Rev Biochem20067560762716756504
- de VosKJGriersonAJAckerleySMillerCCJRole of axonal transport in neurodegenerative diseasesAnnu Rev Neurosci20083115117318558852
- FlahertyDBSoriaJPTomasiewiczHGWoodJGPhosphorylation of human tau protein by microtubule-associated kinases: GSK3b and cdk5 are key participantsJ Neurosci Res20006246347211054815
- YuWBaasPWChanges in microtubule number and length during axon differentiationJ Neuroscience19941428182829
- AshkinADziedzicJMYamaneTObservation of a single-beam gradient force optical trap for dielectricOpt Lett19861128829019730608
- LuSJQiangFParkJSBiological properties and enucleation of red blood cells from human embryonic stem cellsBlood20081124362436319029448
- ChenHDGeKLiYApplication of optical tweezers in the research of molecular interaction between lymphocyte function associated antigen-1 and its monoclonal antibodyCell Mol Immunol2007422122517601377
- AshkinADziedzicJMOptical trapping and manipulation of viruses and bacteriaScience1987235151715203547653
- ZhaoXSunYBuJZhuSYuanXCMicrolens array enabled on-chip optical trapping and sortingApplied Opt201150318322
- HarrisonRVHarelNPanesarJMountRJBlood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortexCerebral Cortex20021222523311839597
- PsaltisDQuakeSRYangCDeveloping optofluidic technology through the fusion of microfluidics and opticsNature200644238138616871205
- SchnitzerMJBlockSMKinesin hydrolyses one ATP per 8-nm stepNature19973883863909237757
- BlockSMAsburyCLShaevitzJWLangMJProbing the kinesin reaction cycle with a 2D optical force clampPNAS20031002351235612591957
- BlockSMKinesin motor mechanics: binding, stepping, tracking, gating, and limpingBiophys20079229862995
- YangAJHEricksonDOptofluidic ring resonator switch for optical particle transportLab Chip20101076977420221566
- ChungAJHuhYSEricksonDA robust, electrochemically driven microwell drug delivery system for controlled vasopressin releaseBiomed Microdevices20091186186719353273
- KrishnanMTolleyMLipsonHEricksonDHydrodynamically tunable affinities for fluidic assemblyLangmuir2009253769374419708253
- ChungAJEricksonDEngineering insect flight metabolics using immature stage implanted microfluidicsLab Chip2009966967619224016
- SchmidtBSYangAHJEricksonDLipsonMOptofluidic trapping and transport on solid core waveguides within a microfluidic deviceOptics Express20071143221433419550709
- SegevMChristodoulidesDNRotschildCMethod and system for manipulating fluid medium US 2011/0023973 A (patent).2011
- BuggeMPalmersGImplantable device for utiliztion of the hydraulic enerty of the heart US RE41,394 E (patent).2010
- ChenSYHuSHLiuDMKuoKTDrug delivery nanodevice, its preparation method and used there of US 2011/0014296 A1 (patent).2011
- SzobotaSIsacoffEYOptical control of neuronal activityAnnu Rev Biophys20103932934820192766
- SuwanpayakNJalilMATeekaTAliJYupapinPPOptical vortices generated by a PANDA ring resonator for drug trapping and delivery applicationsBio Med Opt Express20112159168
- PiyatamrongBKulsiriratKTechithdeeraWMitathaSYupapinPPDynamic potential well generation and control using double resonators incorporating in an add/drop filterMod Phys Lett B20102430713082
- CaiHPoonAOptical manipulation and transport of microparticle on silicon nitride microring resonator – based add-drop devicesOpt Lett2010352855285720808347
- AshkinADziedzicJMYamaneTOptical trapping and manipulation of single cells using infrared laser beamsNature19873307697713320757
- EgashiraKTerasakiAKondowTPhoton-trap spectroscopy applied to molecules adsorbed on a solid surface: probing with a standing wave versus a propagating waveApp Opt19988051135115
- KachynskiAVKuzminANPudavarHEKaputaDSCartwrightANPrasadPNMeasurement of optical trapping forces by use of the two-photon-excited fluorescence of microspheresOpt Lett2003282288229014680158
- SchulzMCrepazHSchmidt-KalerFEschnerJBlattRTransfer of trapped atoms between two optical tweezer potentialsJ Mod Opt20075416191626
- TasakornMTeekaCJomtarakRYupapinPPMultitweezers generation control within a nanoring resonator systemOpt Eng201049075002
- SvobodaKBlockSMBiological applications of optical forcesAnnu Rev Biophys Biomol Struct1994232472837919782
- ZhuJOzdemirSKXiaoYFLiLHeLChenDROn-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonatorNat Photonics201044649
- SuwanpayakNJalilMAAzizMSAliJYupapinPPMolecular buffer using a PANDA ring resonator for drug delivery useInt J Nanomed20116575580
- AshkinAOptical trapping and manipulation of neutral particles using lasersProc Natl Acad Sci199794485348589144154
- HosokawaCKudohSNKiyoharaATaguchiTOptical trapping of synaptic vesicles in neuronsAppl Phys Lett201198163705
- ShiptonOALeitzJRDworzakJTau protein is required for amyloid induced impairment of hippocampal long-term potentiationJ Neurosci2011311688169221289177
- SvobodaKSchmidtCFSchnappBJBlockSMDirect observation of kinesin stepping by optical trapping interferometryNature19933657217278413650
- BlockSMGoldsteinLSSchnappBJBead movement by single kinesin molecules studied with optical tweezersNature19903483483522174512
- KolomeiskyABMichaelEFisherMEMolecular motors: a theorist’s perspectiveAnnu Rev Phys Chem20075867569517163836
- PausTToroRCould sex differences in white matter be explained by g ratio?Front Neuroanat200931719169410
- BechtluftPvan LeeuwenRGHTyremanMDirect observation of chaperone-induced changes in a protein folding pathwayScience20073181458146118048690
- Ou-YandHDWeiMTComplex fluids: probing mechanical properties of biological system with optical tweezersAnnu Rev Phys Chem201042144020055681
- XiaXHuZMarquezMPhysically bonded nanoparticle network: a novel drug delivery systemJ Controlled Release20051032130
- YingJLingYWestfieldLASadlerJEShaoJYUnfolding the A2 domain of von Willebrand factor with the optical trapBiophys J2010981685169320409490
- ChoiJHWolfMToronovVNoninvasive determination of the optical properties of adult brain: near-infrared spectroscopy approachJ Biomed Opt2004922122914715077
- PrabhuAMTsayAHanZVanVExtreme miniaturization of silicon add-drop microring filters for VLSI photonics applicationsJ IEEE Photon20102436444
- IqbalKLiuFGongCXGrundke-EqbalITau in Alzheimer’s disease and related tauopathiesCurr Alzheimer Res2010765365521214513
- TakashimaATau aggregation us a therapeutic target for Alzheimer’s diseaseCurr Alzheimer Res2010766566920678070
- GozesITau pathology and future therapeuticsCurr Alzheimer Res2010768569820678069