?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Recently there has been a remarkable surge of interest about natural products and their applications in the cosmetic industry. Topical delivery of antioxidants from natural sources is one of the approaches used to reverse signs of skin aging. The aim of this research was to develop a nanoemulsion cream for topical delivery of 30% ethanolic extract derived from local Phyllanthus urinaria (P. urinaria) for skin antiaging.
Methods
Palm kernel oil esters (PKOEs)-based nanoemulsions were loaded with P. urinaria extract using a spontaneous method and characterized with respect to particle size, zeta potential, and rheological properties. The release profile of the extract was evaluated using in vitro Franz diffusion cells from an artificial membrane and the antioxidant activity of the extract released was evaluated using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) method.
Results
Formulation F12 consisted of wt/wt, 0.05% P. urinaria extract, 1% cetyl alcohol, 0.5% glyceryl monostearate, 12% PKOEs, and 27% Tween® 80/Span® 80 (9/1) with a hydrophilic lipophilic balance of 13.9, and a 59.5% phosphate buffer system at pH 7.4. Formulation F36 was comprised of 0.05% P. urinaria extract, 1% cetyl alcohol, 1% glyceryl monostearate, 14% PKOEs, 28% Tween® 80/Span® 80 (9/1) with a hydrophilic lipophilic balance of 13.9, and 56% phosphate buffer system at pH 7.4 with shear thinning and thixotropy. The droplet size of F12 and F36 was 30.74 nm and 35.71 nm, respectively, and their nanosizes were confirmed by transmission electron microscopy images. Thereafter, 51.30% and 51.02% of the loaded extract was released from F12 and F36 through an artificial cellulose membrane, scavenging 29.89% and 30.05% of DPPH radical activity, respectively.
Conclusion
The P. urinaria extract was successfully incorporated into a PKOEs-based nanoemulsion delivery system. In vitro release of the extract from the formulations showed DPPH radical scavenging activity. These formulations can neutralize reactive oxygen species and counteract oxidative injury induced by ultraviolet radiation and thereby ameliorate skin aging.
Introduction
Solar ultraviolet radiation (280–400 nm) is a dangerous environmental health hazard for humans. Human skin is the biological tissue most susceptible to solar ultraviolet radiation, which is absorbed by various chromophores in the skin, such as melanin, DNA, RNA, proteins, lipids, water, and aromatic amino acids, such as tyrosine. Absorption of solar ultraviolet radiation by these chromophores leads to various photochemical reactions which induce reactive oxygen species and free radicals. Citation1 Glutathione and α-tocopherol are antioxidants located in the epidermis and dermis which protect the skin against the effects of reactive oxygen species and free radicals induced by solar ultraviolet radiation.Citation2 Induction of reactive oxygen species and free radicals above the physiological limits of the natural antioxidant defense mechanism in the skin lead to skin damage, including erythema, photoaging, and skin cancer.Citation3,Citation4 Prolonged exposure to solar ultraviolet radiation activates biochemical reactions in human skin that cause depletion of cellular antioxidants and antioxidant enzyme producers, leading to a state of oxidative stress. Oxidative stress is a state of fragmented cellular DNA and membrane damage that can lead to death of cells and tissues due to protein denaturation and lipid peroxidation caused by reactive oxygen species and free radicals.Citation5 The damage caused by oxidative stress contributes to skin photoaging.Citation6 Many approaches have been used to protect the skin against solar ultraviolet radiation. Novel photoprotective approaches are increasingly being used to prevent photodamage and to ameliorate sun-induced skin damage by prevention of penetration of ultraviolet radiation into the skin.Citation2–Citation4 Topically applied antioxidants can directly enrich the natural antioxidant defense system and prevent/reduce oxidative damage to the skin.Citation7
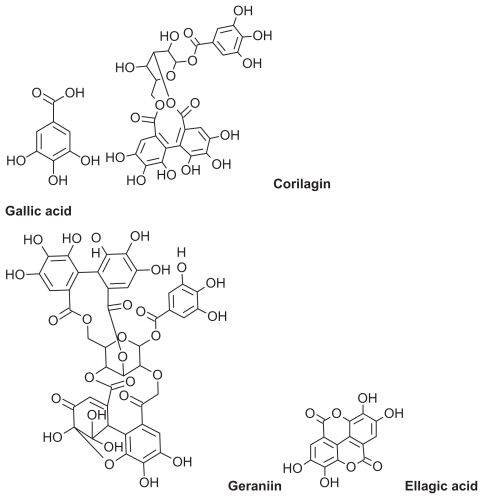
Phyllanthus urinaria is a herbal plant belonging to the genus Phyllanthus, family Euphorbiaceae. The genus Phyllanthus is widely distributed in tropical and subtropical countries, including Malaysia, Indonesia, China, Southern India, and South America.Citation8 Various antioxidant compounds, such as polyphenols, have been identified in P. urinaria ().Citation9,Citation10 Polyphenols are secondary plant metabolites with important commercial and biological roles due to their antioxidant activity.Citation11,Citation12 They are good electron donors with a good redox potential, so are able to scavenge free radicals and reactive oxygen species and reduce or prevent the harmful effects of reactive oxygen species.Citation13 Polyphenols are also relatively stable due to delocalization of resonance and a hydroxyl group attached to the aromatic ring system.Citation14 Polyphenols have been a major research interest for the last two decades,Citation15 and their commercial application as food supplements, food preservatives in nutraceuticals, skin antiaging agents, sunblock agents, and whiteners in cosmeceuticals has increased dramatically.Citation16
Figure 1 Two-dimensional chemical structure of polyphenolic compounds in Phyllanthus urinaria extract.
Topically applied antioxidant preparations protect against the harmful effects of the sun by scavenging free radicals and reactive oxygen species induced by solar ultraviolet radiation. The purpose of the present study was to prepare a nanoemulsion cream formulation for topical delivery of a 30% ethanolic extract of P. urinaria as a skin antiaging product. Permeation of the two formulations across an artificial cellulose membrane and their antioxidant activity in vitro was evaluated using 2, 2-diphenyl-1-picrylhydrazyl (DPPH).
Materials and methods
Materials
Nonionic surfactants, ie, Tween® 80, Span® 80, cetyl alcohol 99%, and DPPH were purchased from Sigma Chemical Co (St Louis, MO). Sodium benzoate, sodium hydroxide, and potassium dihydrogen phosphate were purchased from R and M Marketing (Essex, UK). Palm kernel oil esters (PKOEs) were gifted by the Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, Selangor, Malaysia. Ethanol 99.7% was purchased from Brightchem (Pulau Pinang, Malaysia), and P. urinaria extract was gifted by Nova Laboratories (Sepang, Malaysia). Glyceryl monostearate was purchased from Euro Chemo Pharma (Pinang, Malaysia). Cellulose acetate filter 0.2 μm pore diameter was purchased from Sterlitech™ (Kent, WA).
Methods
Prediction of permeability coefficient
The permeability coefficient of the active ingredients of the extract (gallic acid, corilagin, geraniin, and ellagic acid) were predicted using Advanced Chemistry Development Chemsketch drawing package (v 12.01; Advanced Chemistry Development, Toronto, Canada).Citation17
Phase diagram construction
A phase diagram was constructed using the water titration method as described in our previous report.Citation18 A blend of Tween® 80/Span® 80 (9/1) surfactants with a hydrophilic lipophilic balance (HLB) of 13.9 has a high solubilization capacity with water in PKOEs as well as being able to form larger conventional oil-in water emulsions.Citation18 The blend of hydrophilic and hydrophobic components in these surfactants is also very efficient for stabilizing oil-in-water (o/w) dispersions due to the formation of dense adsorption layers in this mixed system.Citation19 Therefore, a phase diagram for mixtures of PKOEs, Tween® 80/Span® 80 (9/1) at an HLB of 13.9, and water were selected to design a P. urinaria-loaded PKOEs nanoemulsion.
Preparation of nanoemulsion
Primary o/w nanoemulsions were prepared using PKOEs as the oil phase, with Tween® 80/Span® 80 (9/1) at HLB 13.9, and a phosphate buffer system at pH 7.4 as the aqueous phase (). The aqueous phase was added through an emulsification path crossing a micellar liquid crystalline phase.Citation20,Citation21 PKOEs and nonionic surfactants at various ratios were heated gently at 55°C and stirred with a magnetic bar at 350 rpm for 30 minutes. The aqueous phase (containing 0.1% sodium benzoate) was added dropwise from a burette at a rate of 1 mL/min. The nanoemulsion system was continuously mixed using a magnetic stirrer at 350 rpm with the aid of spatula in order to overcome the liquid crystalline phase. After complete addition of the aqueous phase, the mixture was stirred at a rate of 1500 rpm for 30 minutes in a water bath at 55°C. The final mixture was homogenized using a T25 Ultra-Turrax at 17,500 rpm for 1.5 minutes.
Table 1 Molecular weight and permeability coefficient (log P) predicted by the Advanced Chemistry Development Chemsketch drawing packageTable Footnote*
Table 2 Component percentages of primary nanoemulsion-unloaded and 0.05% extract-loaded formulations
Preparation of formulations
The rheological properties of the primary PKOEs-based nanoemulsion system were modified using cetyl alcohol and glyceryl monostearate. The unloaded and 0.05% extract-loaded formulations were obtained by the same method used to prepare the primary nanoemulsion base. The percentages of cetyl alcohol and glyceryl monostearate as rheology modifiers were derived from the PKOEs percentages in the primary nanoemulsions. Cetyl alcohol and glyceryl monostearate were first mixed with PKOEs using a magnetic bar at 350 rpm and 55°C for 30 minutes. The surfactant was then added to the mixture with stirring for another 30 minutes. P. urinaria 0.05% powder was added to the PKOEs-surfactant mixture with continuous stirring for a further 30 minutes. Phosphate buffer containing 0.1% sodium benzoate at pH 7.4 was used as the aqueous phase in the unloaded and extract-loaded formulations, which were stored at room temperature for 72 hours before investigation.
Evaluation of rheological properties
The rheological properties of the unloaded and extract-loaded formulations were studied using a stress-controlled cone and plate rheometer from Reologica Instruments AB (Lund, Sweden). The formulations were allowed to equilibrate for 72 hours prior to evaluation of their rheological properties.Citation22 We used a minimum stress of 0.05 Pa and a maximum stress of 550 Pa and 600 Pa for the unloaded and extract-loaded formulations, respectively. Rheograms were produced automatically in triplicate by gradually increasing the shear stress exerted by the instrument to the maximum stress specified by the method used. The relationship between the shear stress and the shear rate of each formulation was evaluated using the Power law equation:
Physical characteristics
The physical characteristics of the unloaded and extract-loaded nanoemulsions were evaluated by droplet size analysis, pH measurement, zeta potential determination, and transmission electron microscopy (TEM). The pH values of the primary nanoemulsions, unloaded and extract-loaded nanoemulsions were measured using a microprocessor pH meter (pH211, Hanna Instruments Inc, Woonsocket, RI) at 26°C ± 2°C.
Droplet size and zeta potential determination
Droplet size and zeta potential for the primary nanoemulsions, unloaded and extract-loaded nanoemulsions were measured by photon correlation spectroscopy using a Zetasizer Nano Series (Malvern Instruments, Worcestershire, UK). The samples were diluted to 1:100 with Milli-Q water.Citation23 The measurements were carried out in fully automatic mode, with each sample analyzed once and each analysis consisting of five replicates.
Transmission electron microscopy
The size and morphology of the extract-loaded formulations was studied using a FEI CM 12 high resolution TEM (Philips, Electron Optics, Eindhoven, Netherlands) equipped with a Docu Version 3.2 image analysis system (Soft Imaging System GmbH, Munster, Germany). The droplet size of the extract-loaded nanoemulsion sample was examined on collodion formvar carbon film-coated 400 mesh copper grid held with self-locking fine forceps. After 1–3 minutes, the droplet was wicked to dryness using a piece of filter paper. After 1 minute, a droplet of 2% methylamine tungstate as a negative stain solution was added to the surface of the grid. After another minute, the stained droplet was wicked to dryness using pieces of filter paper. The grid was placed on a Petri dish lined with filter paper for 10 minutes at room temperature before examination under the microscope.
In vitro formulation release
In vitro release of P. urinaria from the extract-loaded nano-emulsions was evaluated using a vertical Franz diffusion cell system composed of three cells with an effective surface diffusion area of 0.636 cm2 and a cell receptor volume of 5.0 mL. Artificial cellulose acetate membrane filters with a 0.2 μm pore diameter were mounted between the donor and receptor compartments. The receptor cells were filled with the phosphate buffer system and kept in contact with the cellulose membrane filters for the duration of the experiment. The receptor solution was mixed with a small magnetic bar at a speed of 500 rpm.Citation24,Citation25 The temperature of the receptor compartment was maintained at 37°C ± 0.2°C using a circulating water bath. A 0.5 g sample of each of the 13.9 F12U and 13.9 F36U formulations, each containing 0.25 mg of P. urinaria (0.05% wt/wt), was applied evenly on the surface of the cellulose membrane facing the donor compartment and kept there for 8 hours. Thereafter, the solutions in the receptor compartments were tested for antioxidant activity using DPPH. The experiment was performed in triplicate and the results are presented as the mean ± standard deviation.
In vitro radical scavenging activity of the extract released
The free radical scavenging activity of the extract released into the receptor compartment solution was evaluated using stable radical DPPHCitation26 with slight modification. A 200 μL sample of the receptor solution was added to 2.5 mL of distilled water with 100 μL of 0.04% w/v DPPH in 99.7 ethanol at 26°C ± 2°C. The mixture was vortexed for 2 to 3 minutes and stored in the dark at room temperature (26°C ± 2°C) for 30 minutes. Scavenging activity was measured at an ultraviolet wavelength of 517 nm using an Hitachi U-2000 Lambda spectrophotometer (Perkin Elmer, Plano, TX). The results are expressed as the percentage of DPPH reduced by the extract released from the formulations using formula (Equation2(2) ), where Sc. A is the scavenging activity of the active ingredients released by the extract, Ao is the absorbance of the blank solution (absorbance of reaction mixture without the solution released), and Armr is the absorbance of the reaction mixture with the released solution. The experiment was carried in triplicate and the results are shown as the mean ± standard deviation. The scavenging activity of the extract released was compared with the scavenging activity of the raw extract in the formulation using DPPH with a slight modification.Citation26 The reaction mixture was prepared by adding 200 μL from a 50 μg/mL 70:30 ethanol:water solution to DPPH 0.04% w/v diluted in 2.5 mL of ethanol 99.7%.
Results and discussion
The polyphenolic compounds in P. urinaria () have a high molecular weight and a high predicted permeability coefficient. The permeability coefficient of a topically applied dose formulation depends on its physicochemical characteristics of the loaded drug, particularly lipophilicity and molecular size.Citation27 On the basis of the predicted permeability coefficient, which is the Log of octanol/water partition coefficient of the antioxidant polyphenolic compounds in P. urinaria (), the extract components can be delivered to the epidermis and dermis using a nanoemulsion technique.
Components of formulations
Selection of an appropriate oil phase for a formulation is crucial because it influences selection of the other ingredients. The importance of this study arises from the use of novel PKOEs, ie, modified oil esters which are able to improve the delivery of pharmaceuticals and cosmetics.Citation28 PKOEs is similar to but less expensive than jojoba oil, which is used widely in the pharmaceutical and cosmetic industry. Use of PKOEs, which is cheaper than jojoba oil, can reduce the cost of cosmetics. Moreover, PKOEs has better physical characteristics, including good thermal stability, good fat-soluble properties, excellent wetting behavior, minimal greasiness, no irritating properties, no toxicity, and an ability to penetrate the stratum corneum easily.Citation28
The selection of surfactant is also a key factor when designing a formulation. Selection of the optimal surfactant or blend of surfactants reduces surface tension and stabilizes droplets against recoalescence.Citation29 Nonionic surfactants are generally recognized as safe and biocompatible, and are not affected by changes in pH or ionic strength. From our previous study of selection of surfactants according to solubilization capacity ability to mix with PKOEs,Citation18 Tween® 80/Span® 80 blend at a ratio of 9:1 and HLB 13.9 has a larger area of conventional o/w emulsion than other surfactant blends at various HLB values.Citation18 An o/w microemulsion obtained by dropwise addition of an aqueous phase passing a larger liquid crystalline area has been described.Citation18,Citation20,Citation21 To extend the region of the o/w microemulsion in our phase diagram study, we used gentle heating and mechanical energy from the magnetic stirrer and high pressure from a T25 Ultra-Turrax. Introduction of energy to obtain and extend the o/w microemulsion was performed successfully and produced primary nanoemulsions with a droplet size less than 100 nm using Tween® 80/Span® 80 blend at the ratio of 9:1 and a hydrophilic lipophilic balance of 13.9. The primary nanoemulsions were subjected to rheological investigation and modified using rheology modifiers. The primary nanoemulsions and formulations shown in were characterized using various techniques.
The P. urinaria 0.05% extract was selected as the loading dose for the formulation based on the solubility of the extract according to the PKOEs to surfactant ratio in the formulations. The solubility of the extract at PKOEs to surfactant ratios of F12 and F36 was 1.33 mg/g for PKOEs and 1.25 mg/g for the surfactant blend in the formulations, while the solubility of the extract in PKOEs was 0.59 mg/g. The slightly higher solubility of the extract in the formulations was due to polyoxethylene, a hydrophilic head group in the Tween® 80/Span® 80 surfactant blend at the ratio of 9:1 and HLB 13.9. Similarly, the phosphate buffer system was chosen as the aqueous phase for the formulations on the basis of the high solubility and apparent partition coefficient of the extract in the PKOEs/phosphate buffer system at pH 7.4.
Rheological properties
The rheological characteristics of semisolid preparations are important physical parameters in technical applications, including manufacturing, pumping, filling, and storage, as well as in the esthetic use of the final product.Citation30 The applications and acceptance of new pharmaceuticals and cosmetics depends on the flow properties of the finished product.Citation31 In this study, cetyl alcohol and glyceryl monostearate were used to modify the rheological properties of the primary nanoemulsions. Cetyl alcohol and glyceryl monostearate are used as stabilizers and thickening agents in a wide variety of cosmetics and pharmaceutical emulsions.Citation32,Citation33 The percentages of cetyl alcohol and glyceryl monostearate were calculated from the PKOEs content in the primary nanoemulsions. The rheological properties of the unloaded and extract-loaded formulations are shown by the rheograms in – and in . These rheograms clearly show two regions, ie, a yield stress region and a shear-thinning region, representing shear stress as a function of shear rate at an ambient temperature of 26°C ± 2°C. The yield stress region shows a slight increase in viscosity at a relatively low range of shear stress, while the shear-thinning region shows a decrease in viscosity with increasing shear rate on the upward curve. These two characteristics are common in semisolid products used in the pharmaceutical, cosmetic, and biomedical industries.Citation31
Table 3 Rheological parameters of unloaded formulation (n = 3)
Figure 2 Rheogram of extract-unloaded nanoemulsion formulation HLB13.9 F12.
Abbreviations: HLB, hydrophilic lipophilic balance; F, formulation.
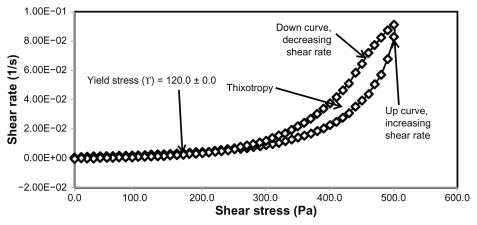
Figure 3 Rheogram of extract-loaded formulation HLB13.9F12.
Abbreviations: HLB, hydrophilic lipophilic balance; F, formulation.
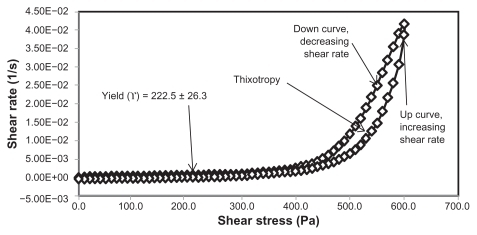
Yield stress is known as the minimum shear stress required to initiate flow or the stress below which a material does not exhibit fluid-like behavior. The yield stress of pharmaceutical and cosmetic materials should be high enough that they do not flow out of a container when the container is placed in an upside-down position. At the same time, it should not be so large that it offers resistance to flow during application on the human body. From and and it is clear that unloaded F36 has a high yield stress (215.0 ± 21.2 Pa) compared with F12 (120.0 ± 0.0 Pa). This could be due to the optimum surfactant/oil ratio in the unloaded F36 formulation, which forms more hydrogen bonds with the aqueous phase. A higher aqueous phase content in the formulation affects yield stress because the excess aqueous phase disrupts the hydrogen bonding network between the hydrophilic surfactant and the aqueous phase, as in F12. In the extract-loaded formulations, the yield stress of F12 was markedly increased (222.5 ± 26.3 Pa) due to the self-emulsification properties of the extract, leading to more hydrogen bonding with the aqueous phase. In contrast, unavailability of a free aqueous phase in F36 led to less hydrogen bonding with the extract and hence a slight increase in yield stress (220.0 ± 15.3 Pa).
Figure 4 Rheogram of extract-unloaded formulation HLB13.9F36.
Abbreviations: HLB, hydrophilic lipophilic balance; F, formulation.
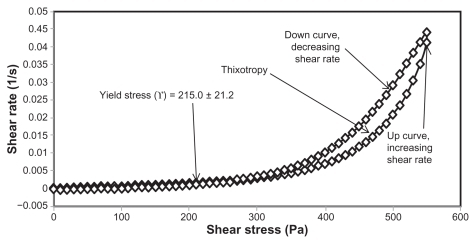
The rheograms in – also show a second region in the nanoemulsion indicating non-Newtonian, shear thinning, (pseudo) plastic flow behavior with a hysteresis loop. The hysteresis loop characterizes a thixotropic material, and the area within the loop is a measure of thixotropy. Shear thinning behavior with thixotropy is a desirable property in topical preparations because it facilitates even application on the skin.Citation34 The thixotropy of the unloaded and extract-loaded F12 formulations (–) was slightly larger compared with the unloaded and extract-loaded F36 formulation. This is due to a high surfactant to oil ratio which leads to molecular attraction between Tween® 80 and the continuous aqueous phase, as already mentioned. This also increases consistency () because the high surfactant to oil ratio leads to increased resistance to deformation, and there is more curvature when the load is released at maximum shear stress.
Figure 5 Rheogram of extract-loaded formulation HLB13.9F36.
Abbreviations: HLB, hydrophilic lipophilic balance; F, formulation.
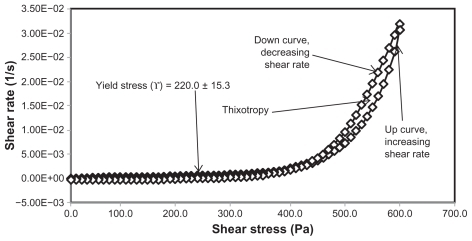
The flow index of each formulation was evaluated by linear regression of the logarithmic form of the Power law shown in Equationequation (1)(1) ,Citation34 τ is the shear stress, σ is the shear rate, k is the consistency index, and n is the flow index. Flow is Newtonian when n = 1, whereas n > 1 or n < 1 indicates shear thickening or shear thinning, respectively. The results shown in confirm that the studied formulations have shear thinning behavior. For non-Newtonian fluids, the lower the value of n, the more the shear thinning of the formulation. The flow index values for the unloaded F12 and F36 formulations were 0.386 and 0.291, respectively. The slight mobility of F12 was due to its slightly higher flow index compared with F36. Formulation F12 had a slightly lower consistency index (1306.691) than F36 (1403.579), and hence F36 has more thinning behavior than the unloaded F12 formulation.
The rheological characteristics of the extract-loaded F12 and F36 formulations were slightly different from those of the unloaded F12 and F36 formulations. There were marked increases in yield stress and consistency index for the extract-loaded F12 formulation. As a result, there was a decrease in the flow index (n) and hence a decrease in the mobility of the F12 formulation, whereas the extract-loaded F36 formulation showed a slight increase in consistency and yield stress and a decrease in flow index (n). It was also noted that the thixotropy decreased in both the extract-loaded formulations compared with the unloaded formulations (–). The slight change in rheological properties could be due to the self-emulsifying behavior of the extract.
Assessment of pH
The acidity of the skin ranges from pH 4–6, depending on the skin area and the age of the individual.Citation35 The low pH of the skin is because of the buffer system in the skin that is able to absorb small quantities of acid or alkali material applied to the skin in order to reduce irritation.Citation35 The pH of skin cosmetics is very important in order to avoid skin irritation if the product is highly acidic or alkaline. In this study, the pH of the trial nanoemulsions was close to the neutral skin pH of 7.0. The pH of the extract-loaded formulations was slightly below physiological pH, because the aqueous phase of these nanoemulsions was prepared using a phosphate buffer system at pH 7.4. This buffer system had a high partitioning coefficient in the extract-loaded nanoemulsion as well as high solubility of the extract to improve its release from the formulation. Cosmetics and skin care products in alkaline vehicles can cause skin irritation and make the skin susceptible to bacterial infection. Therefore, skin care products should have a pH close to that of the pH of skin at the area of application. Elderly individuals have a skin pH close to neutral, making this pH the most suitable for skin antiaging products. Here the pH of our formulations at 7.24 was close to that of skin pH, as shown in .
Table 4 pH values for primary nanoemulsions, and unloaded and extract-loaded formulations
Droplet size
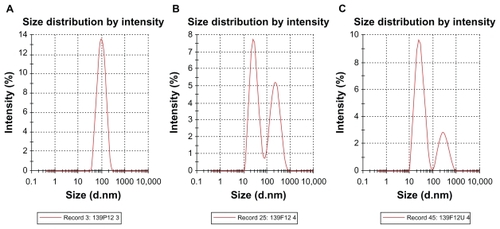
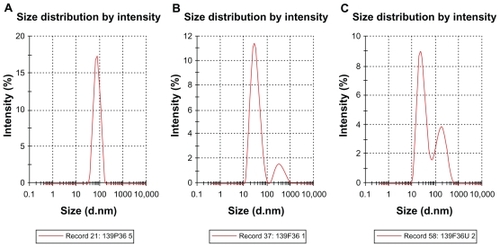
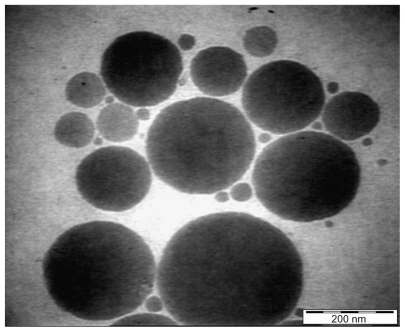
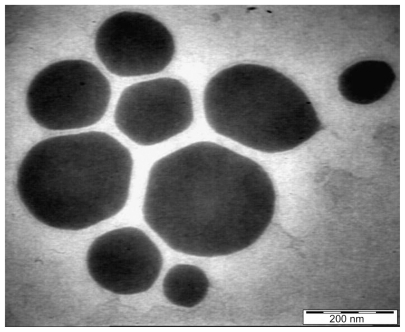
Droplet size distribution is one of the more important physical characteristics of a nanoemulsion used for dermal and transdermal delivery, and affects activity and bioavailability. Nanoemulsions have many advantages as drug delivery systems, including ease of manufacture, enhanced drug solubility, and hence increased drug release and improved bioavailability, as well as the opportunity to control the rate of release of the drug and improve patient compliance.Citation36 The small size of droplets in nanoemulsions for cutaneous use allows them to deposit uniformly on the skin and enhances their penetration.Citation37 The small droplet size of nanoemulsions stabilizes them against gravitational separation and flocculation. Citation29 Smaller droplet size also increases the total surface area for transfer, release, and absorption of the drug.Citation38 In this way, nanoemulsion systems improve the bioavailability of many topical preparations.Citation25,Citation26 Droplet size analysis in this study showed that the formulations had a small droplet size in the range desirable for a nanoemulsion.Citation37 Addition of a rheological modifier greatly affected the droplet size in our trial nanoemulsions via two mechanisms. Firstly, reduction in oil composition by rheological modifiers increased the surfactant to oil ratio and hence facilitated the emulsification process. Secondly, the rheological modifiers acted as an emulsifier and stabilizer, thereby improving the emulsification of oil in water and reducing particle size. The extract-loaded F12 nanoemulsion showed a greater decrease in droplet size (from 43.22 ± 0.21 nm to 30.74 ± 0.18 nm) whereas the extract-loaded F36 formulation showed a slight increase in droplet size (from 32.97 ± 0.31 nm to 35.71 ± 0.092 nm) as shown in and and . This could be due to the self-emulsifying activity of the extract as a result of its polyphenolic components. The second peak shown in is thought to be the peak size of the rheology modifiers because the second peak in was not present in the primary nanoemulsions (). Therefore, it was likely to be due to the rheology modifiers. The rheology modifiers (cetyl alcohol and glyceryl monostearate) are solid particles fused by gentle heating in the oil phase. They exert their effect by solidification when cooled to room temperature. When they are solidified, they form particles which act as a thickening and stabilizing agent. The size of these particles was identified, as shown by the second peaks in . It is also clear from that the shape and width of the first peak are similar to that in the primary nanoemulsions and differ only in intensity due to the presence of the second peak. This indicates that the first peak is for the extract-loaded nanoemulsion. The results showed a lower polydispersity index for the unloaded and extract-unloaded nanoemulsions despite the presence of the peak for the rheology modifier. In addition, the small standard deviations for the droplet size and polydispersity index of the nanoemulsions indicate consistency of these results.
Table 5 Droplet size of primary nanoemulsions, and unloaded and extract-loaded formulations
Zeta potential
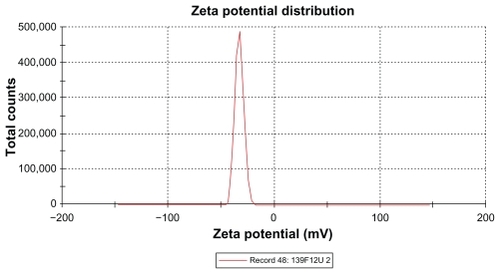
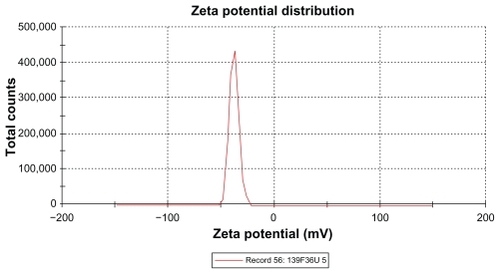
The zeta potential is the relative quantity of repulsive electrical potential difference at the emulsion interface and the electroneutral region of the solution.Citation24 It is estimated by measuring the ionic migration of particles dispersed in a charged field. The zeta potential results show that the potential difference in the unloaded and extract-loaded formulations increased, with a high potential difference in the extract-loaded formulations. This indicates relatively high stability in the extract-loaded formulation compared with the primary nanoemulsions. When the zeta potential is relatively high, the repulsive forces exceed the attracting van der Waals forces, hence the particles are dispersed and the system is deflocculated, whereas when the zeta potential is low, the attractive forces exceed the repulsive forces, and the particles aggregate, leading to flocculation.Citation24 The zeta potentials of both extract-loaded formulations carried a negative charge ( and and ). The adsorption of hydroxyl ions from water at the oil–water interface is the mechanism behind the negative interfacial charging of dispersed droplets of oil in water.Citation39 The presence of surfactant in the dispersion system at the interface decreases the inherent negative potential difference between oil and water at their interface, and the degree of suppression depends on the concentration of surfactant.Citation39 This could be due to depletion of the surface area for adsorption of hydroxyl ions in the presence of the surfactant at the interface.Citation39 From the above findings, this corresponds to adsorption of hydroxyl ions from the polyoxyethylene available at the interface, and the magnitude of the zeta potential also depends on the surfactant concentration. It is clear that the extract-loaded formulations had a higher zeta potential than the unloaded formulation. This might be due to the ionized forms of the phenolic compounds in the extract at the interface and would lead to formation of phenoxide ions.
Table 6 Zeta potential and conductivity of primary nanoemulsions, and unloaded and extract-loaded formulations
Transmission electron microscopy
Photon correlation spectroscopy is a common technique used to investigate smaller particles. The photon correlation spectroscopy method uses laser light at 90° to measure the dynamic fluctuations of scattered light intensity due to the Brownian motion of the scattered particles. The fluctuations are dynamic and high dilution is needed in order to avoid multiple scattering. Smaller particles can suspend very well in highly diluted media, therefore can be measured accurately by photon correlation spectroscopy. Sometimes, if the globules are large, they cannot be suspended well, especially in concentrated solution, so particle size measurements might be incorrect. TEM is used to confirm the droplet size obtained by photon correlation spectroscopy. Electron microscopy produces an image using a principle similar to that of a light microscope. A beam of electrons of much shorter wavelength passes through electrostatic or electromagnetic condenser lenses before passing through the object. When the electron beam passes through the object the beam scatters, and the pattern of scattering characterizes the internal view of the object. However, a higher resolution image is obtained by TEM because of the much shorter wavelength of the electron beam. TEM has better resolution in highly concentrated media than photon correlation spectroscopy, which requires high dilution. The inconsistency between photon correlation spectroscopy and TEM measurements might be due to the techniques themselves, ie, photon correlation spectroscopy measures particle size distribution whereas TEM estimates average droplet size.
Results obtained by high resolution TEM for the extract-loaded F12 and F36 formulations are shown in and , respectively. The F12 and F36 formulations clearly show droplet sizes in the nanoscale range. This might be due to a slightly high concentration of surfactant with respect to oil content which increases the emulsification of PKOEs in water. The droplets of nanoemulsion loaded with the extract appear as dark globules in the electron micrographic images shown in and .
In vitro release and radical scavenging activity
Release and transport of extract through an acetate cellular membrane is a useful indicator of how much of the active extract is available for absorption. It can also provide useful information on how well the absorption process transfers the extract through full-thickness rat skin, so might provide useful information on how well the extract can be absorbed through human skin. This will have an important bearing on how well the formulae will exert their predicted effects. The release of extract from the formulation is greatly affected by the partition coefficient of the extract in PKOEs and the solubility of the phosphate buffer system at pH 7.4. The high solubility of the drug in the external phase will enhance drug partition from the formulation (oil phase) and hence increase drug release.Citation25 In this study, the extract is highly soluble, and partitioning from the PKOEs in phosphate buffer at pH 7.4 (external phase) and the receptor solution will drive release of the extract.
We evaluated the antioxidant activity of the studied formulations released into the receptor solution compartment by in vitro assay toward stable radical DPPH. DPPH is a free radical that is stable at room temperature and produces a deep purple color in a solution containing ethanol. The purple color is reduced to yellow-colored diphenylpicryl hydrazine by antioxidant compounds.Citation40 The polyphenol compounds are responsible for the pharmacological effect of the extract, and have amphiphilic properties which facilitate their antioxidant properties in both the aqueous and lipid phase.Citation41 The presence of these compounds which could scavenge stable radical DPPH in the receptor solutions indicates good release of the extract from the formulations. Therefore, DPPH was useful as a marker to identify release of the extract and to evaluate the release in terms of antioxidant activity in the receptor solutions. The F12 and F36 formulations loaded with 0.05% P. urinaria extract inhibited 30.05% and 29.89%, respectively, of DPPH radical activity. The scavenging activity of the released extract was compared with that of the scavenging activity of 0.5 g of the raw material (equivalent to 0.25 mg of P. urinaria). The raw extract scavenged 58.58% of DPPH radical activity and the extract released from the F12 and F36 formulations scavenged 30.05% and 29.89%, respectively. Therefore, 51.30% and 51.02% of the extract material was released from formulations F12 and F36, respectively. The in vitro results of scavenging activity of the formulations released through the artificial membrane towards DPPH confirms that sufficient polyphenolic compounds were released. Hence, this product could result in good penetration when applied to the skin.
Conclusion
This study clearly shows that P. urinaria-loaded PKOEs nanoemulsions can be formulated successfully by a spontaneous method. The droplet sizes of the extract-loaded F12 and F36 nanoemulsions were 30.74 nm and 35.71 nm, respectively. This in vitro release study demonstrates that 51.30% and 51.02% of the extract-loaded formulations were released through the artificial membrane, scavenging 30.05% and 29.89% of DPPH activity, respectively. The release results are a good indicator that PKOEs-based nanoemulsions can penetrate the skin easily to deliver P. urinaria extract as a skin antiaging agent. The use of DPPH is a good method of studying the in vitro release of natural antioxidant compounds used in skin cosmetics.
Acknowledgments
The authors gratefully acknowledge the Biotechnology Directorate, Malaysia, for allocation of a research grant to undertake this work, and the financial support provided by the Universiti Sains Malaysia for ESM, Nova Laboratories Sdn Bhd Malaysia, as well as fellow researchers from the Chemistry Department, Faculty of Sciences, Universiti Putra Malaysia, for supplying the P. urinaria extract and palm kernel oil esters.
Disclosure
The authors report no conflicts of interest in this work.
References
- GonzálezSFernández-LorenteMGilaberte-CalzadaYThe latest on skin photoprotectionClin Dermatol200826661462618940542
- ChengYSLamKWNgKMKoRKMWibowoCAn integrative approach to product development – a skin-care creamComputers and Chemical Engineering200933510971113
- FonsecaYCatiniCVicentiniFCardosoJCavalcantiDeAlbuquerqueRJuniorVieira FonsecaMEfficacy of marigold extract-loaded formulations against UV induced oxidative stressJ Pharm Sci201110062182219321491442
- AlmeidaIValentãoPAndradePIn vivo skin irritation potential of a Castanea sativa (chestnut) leaf extract, a putative natural antioxidant for topical applicationBasic Clin Pharmacol Toxicol2008103546146718793273
- RatnamDAnkolaDBhardwajVSahanaDKumarMRole of antioxidants in prophylaxis and therapy: A pharmaceutical perspectiveJ Control Release2006113318920716790290
- PillaiSOresajoCHaywardJUltraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation induced matrix degradation – a reviewInt J Cosmet Sci2005271173418492178
- VicentiniFTCasagrandeRVerriWAJrGeorgettiSRBentleyMVFonsecaMJQuercetin in lyotropic liquid crystalline formulations: Physical, chemical and functional stabilityAAPS Pharm Sci Tech200892591596
- HuangSTYangRCYangLJLeePNPangJHPhyllanthus urinaria triggers the apoptosis and Bcl-2 down-regulation in Lewis lung carcinoma cellsLife Sci200372151705171612559392
- XuMZhaZJQinXLZhangXLYangCRZhangYJPhenolic antioxidants from the whole plant of Phyllanthus urinariaChem Biodivers2007492246225217886844
- MahdiESSakeenaMHAbdullahGZAbdulkarimMFSattarMANoorAMIdentification of phenolic compounds and assessment of in vitro antioxidants activity of 30% ethanolic extracts derived from two Phyllanthus species indigenous to MalaysiaAfrican Journal of Pharmacy and Pharmacology2011 In press
- Rice-EvansCMillerNPagangaGAntioxidant properties of phenolic compoundsTrends Plant Sci199724152159
- BendiniACerretaniLCarrasco-PancorboAPhenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decadeMolecules20071281679171917960082
- PiettaPSimonettiPMauriPAntioxidant activity of selected medicinal plantsJ Agric Food Chem1998461144874490
- SrinivasanMSudheerAMenonVFerulic acid: Therapeutic potential through its antioxidant propertyJ Clin Biochem Nutr20074029210018188410
- GourineNYousfiMBombardaINadjemiBStockerPGaydouEMAntioxidant activities and chemical composition of essential oil of Pistacia atlantica from AlgeriaInd Crops Prod2010312203208
- PeschelWSánchez-RabanedaFDiekmannWAn industrial approach in the search of natural antioxidants from vegetable and fruit wastesFood Chem2006971137150
- Nemeikaite-CenieneAImbrasaiteASergedieneECenasNQuantitative structure-activity relationships in prooxidant cytotoxicity of polyphenols: role of potential of phenoxyl radical/phenol redox coupleArch Biochem Biophys2005441218219016111645
- MahdiESSakeenaMHAbdulkarimMFAbdullahGZSattarMANoorAMEffect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil estersDrug Des Devel Ther20115311323
- GolemanovKTcholakovaSDenkovNGurkovTSelection of surfactants for stable paraffin-in-water dispersions, undergoing solid-liquid transition of the dispersed particlesLangmuir20062283560356916584227
- SolèIMaestroAPeyCMGonzálezCSolansCGutiérrezJMNano-emulsions preparation by low energy methods in an ionic surfactant systemColloids Surf A Physicochem Eng Asp20062881–3138143
- GutiérrezJMGonzálezCMaestroASolèIPeyCMNollaJNano-emulsions: New applications and optimization of their preparationCurr Opin Colloid Interface Sci2008134245251
- JiménezMMFresnoMJRamírezARheological study of binary gels with Carbopol® Ultrez TM 10 and hyaluronic acidChem Pharm Bull (Tokyo)20075581157116317666837
- RolandIPielGDelattreLEvrardBSystematic characterization of oil-in-water emulsions for formulation designInt J Pharm20032631–2859412954183
- AbdulkarimMFAbdullahGZChitneniMTopical piroxicam in vitro release and in vivo anti-inflammatory and analgesic effects from palm oil esters-based nanocreamInt J Nanomedicine2010591592421116332
- AbdullahGZAbdulkarimMFSalmanIMIn vitro permeation and in vivo anti-inflammatory and analgesic properties of nanoscaled emulsions containing ibuprofen for topical deliveryInt J Nanomedicine2011638739621499428
- HungCFFangCLLiaoMHFangJYThe effect of oil components on the physicochemical properties and drug delivery of emulsions: Tocol emulsion versus lipid emulsionInt J Pharm20073351–219320217129692
- CrossSEMagnussonBMWinckleGAnissimovYRobertsMSDetermination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layersJ Invest Dermatol2003120575976412713577
- KengPBasriMZakariaMAbdul RahmanMBAriffABAbdul RahmanRNZSallehABNewly synthesized palm esters for cosmetics industryInd Crops Prod20092913744
- WoosterTJGoldingMSanguansriPImpact of oil type on nano-emulsion formation and Ostwald ripening stabilityLangmuir20082422127581276518850732
- Jiménez SorianoMMFresno ContrerasMJSellés FloresEDevelopment of a cream from a self-emulsifying base and moisturizing activesFarmaco2001565–751352211482788
- ParkEKSongKWRheological evaluation of petroleum jelly as a base material in ointment and cream formulations: Steady shear flow behaviorArch Pharm Res201033114115020191355
- RibeiroHMMoraisJAEcclestonGMStructure and rheology of semisolid o/w creams containing cetyl alcohol/non-ionic surfactant mixed emulsifier and different polymersInternational Int J Cosmet Sci20042624759
- RibeiroHMMoraisJAEcclestonGMStructure and rheology of semisolid o/w creams containing cetyl alcohol/nonioinic surfactant mixed emulsifiers and different polymersInt J Cosmet Sci200426475918494913
- ShahinMAbdel HadySHammadMMortadaNNovel jojoba oil-based emulsion gel formulations for clotrimazole deliveryAAPS PharmSciTech201112123924721225383
- IssacharNGallYBorfllMPoelmanMpH measurements during lactic acid stinging test in normal and sensitive skinContact Dermatitis19973631521559145266
- HeWTanYTianZFood protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: Preparation, in vitro characterization, and pharmacokinetics in ratsInt J Nanomedicine2011652153321468355
- BouchemalKBriançonSPerrierEFessiHNano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisationInt J Pharm20042801–224125115265563
- SakeenaMHMuthannaFAGhassanZAFormulation and in vitro evaluation of ketoprofen in palm oil esters nanoemulsion for topical deliveryJ Oleo Sci201059422322820299769
- MarinovaKAlargovaRDenkovNCharging of oil–water interfaces due to spontaneous adsorption of hydroxyl ionsLangmuir199612820452051
- KumaranAJoel KarunakaranRIn vitro antioxidant activities of methanol extracts of five Phyllanthus species from IndiaLWT – Food Science and Technology2007402344352
- BajpaiVYoonJChul KangSAntioxidant and antidermatophytic activities of essential oil and extracts of Metasequoia glyptostroboides Miki ex HuFood Chem Toxicol20094761355136119303043