Abstract
Angiogenesis is the formation of new blood vessels from pre-existing vessels. It is a highly regulated process as determined by the interplay between pro-angiogenic and anti-angiogenic factors. Under certain conditions the balance between angiogenesis stimulators and inhibitors is altered, which results in a shift from physiological to pathological angiogenesis. Therefore, the goal of therapeutic targeting of angiogenic process is to normalize vasculature in target tissues by enhancing angiogenesis in disease conditions of reduced vascularity and blood flow, such as tissue ischemia, or alternatively to inhibit excessive and abnormal angiogenesis in disorders like cancer. Gold nanoparticles (AuNPs) are special particles that are generated by nanotechnology and composed of an inorganic core containing gold which is encircled by an organic monolayer. The ability of AuNPs to alter vasculature has captured recent attention in medical literature as potential therapeutic agents for the management of pathologic angiogenesis. This review provides an overview of the effects of AuNPs on angiogenesis and the molecular mechanisms and biomedical applications associated with their effects. In addition, the main synthesis methods, physical properties, uptake mechanisms, and toxicity of AuNPs are briefly summarized.
Angiogenesis: overview
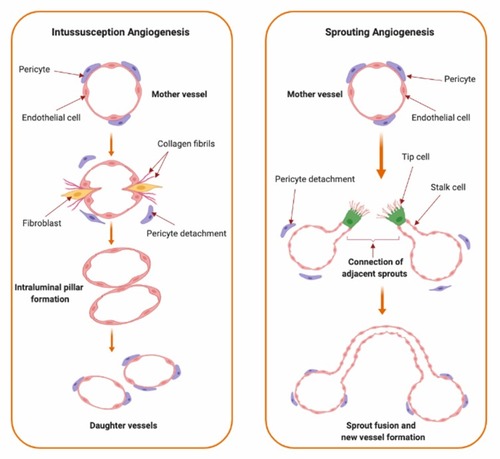
Angiogenesis is the formation of new blood vessels from pre-existing vessels.Citation1–Citation4 It is a highly regulated process in which the interplay between pro-angiogenic and anti-angiogenic factors mediates the transition of vascular endothelium through different stages of vascular growth. Angiogenesis is divided into multiple sequential phases of vascular development which are activation, progression, migration, differentiation, and maturation of endothelial cells.Citation5,Citation6 Under conditions of hypoxia, inflammation, or vascular injury; angiogenic signals stimulate quiescent endothelial cells to take an activated state.Citation5–Citation7 During the activation phase, vascular permeability is increased and pericytes detach away from endothelial cells through the effect of different angiogenic mediators. Activation of endothelial cells is followed by degradation of the extracellular matrix and subsequent migration of endothelial cells during the progression phase.Citation2,Citation8,Citation9 Endothelial cell destabilization and migration is regularly mediated by angiopoietins (Angs), matrix metalloproteinases (MMPs), chymases, and heparanases which collectively enhance degradation of matrix molecules and facilitate formation of new vascular structures by the activated endothelial cells.Citation5,Citation6,Citation10–Citation12 Upon development of new blood vessels, maturation and stabilization of the new vessels take place through pericyte attachment and the resumption of the quiescent state of the endothelial cells.Citation5,Citation6 Angiogenesis is mediated by two major mechanisms; sprouting and intussusception ().
Figure 1 Types of angiogenesis. Types of angiogenic growth include intussusceptive (left panel) and sprouting angiogenesis (right panel). Intussusceptive angiogenesis, also known as splitting angiogenesis, represents the splitting of an existing blood vessel to form new blood vessels by a splitting process involving series of changes of interstitial tissues which invade the existing blood vessel in the presence of pericytes and fibroblasts which ends up with the core splitting by intraluminal pillars development of periendothelial cells and the formation of new smaller vessels or capillaries. Sprouting angiogenesis, as indicated from the name, involves the sprouting of endothelial cells from pre-existing blood vessel by the interruption of endothelial cells basement membrane, migration of endothelial cells along the projection’s tip via stalk cells’ formation into the surrounding connective tissue with further sprouting and new capillary network formation.
Angiogenesis is controlled by multiple pro-angiogenic and anti-angiogenic factors. Angiogenic factors include vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), epidermal growth factor (EGF), placental growth factor (PlGF), cytokines (e.g. tumor necrosis factor-alpha (TNF-α), interleukin (IL)-8), Ang-1, Ang-2, and angiogenin.Citation13,Citation14 Among the pro-angiogenic factors, VEGF-A is a major regulator of vascular growth. Anti-angiogenic factors include thrombospondin-1, angiostatin, endostatin, vasostatin, tumstatin, interferon-γ (IF-γ), glycosaminoglycan (heparins), αv integrin antagonists, β1 integrin antagonists, anti-tissue factor/anti-factor VIIa, and tissue inhibitors of matrix metalloproteinases (TIMPs).Citation14–Citation18
Formation of new blood vessels can take place under physiologic or pathologic conditions. Physiological, normal, angiogenesis is regularly activated in the developing fetus and continues to form blood vessels of the adult tissues.Citation19 Physiological angiogenesis is activated in response to different physiological stimuli, such as tissue hypoxia, inflammation, and/or injury, and plays a vital role in physiological processes such as wound-healing and embryonic development.Citation7 However, under certain circumstances, the balance between angiogenesis stimulators and inhibitors is altered in favor of excessive angiogenic signaling, which ultimately drives pathological angiogenesis.Citation9,Citation20,Citation21 Pathological angiogenesis involves the formation of vessels which are hyperpermeable to plasma and its proteins and possess heterogeneous functions with irregular sprouting and non-uniformed vessel distribution.Citation22–Citation26 Pathologic angiogenesis contributes to multiple disease conditions such as ocular neovascularization, cancer, and inflammatory disorders.Citation13 Dysregulation of angiogenesis has been also shown to contribute to the pathogenesis of other disorders such as asthma, obesity, atherosclerosis, infectious diseases, heart and brain ischemia, pre-eclampsia, respiratory distress, hypertension, and osteoporosis.Citation7 Targeting pathologic angiogenesis has widespread biomedical applications with the ultimate goals to control the rate of angiogenic growth and normalize vasculature in target tissues.
Targeting angiogenesis for therapeutic indications
The goal of therapeutic angiogenesis is to improve perfusion to ischemic tissues or organs by generating new blood vessels and stimulating neovascularization to restore tissue functions, or to inhibit abnormal angiogenesis in disorders like cancer.Citation9,Citation13 Although promising results were shown in experimental animal studies for pro-angiogenic factors, such results were not attained in clinical studies.Citation27,Citation28 Despite the fact that the challenges associated with clinical translation are specific for each therapeutic approach, many hurdles were attributed to drug delivery, such as failure to deliver the treatment to the designated target with the required amounts or for the required duration of time.Citation29,Citation30 Regranex gel, which contains becaplermin; a recombinant angiogenic growth factor, is the first and only US-FDA approved pro-angiogenesis product. Becaplermin attaches to PDGF receptors in the targeted wound and initiates angiogenesis in diabetic neuropathic ulcers that extend to subcutaneous tissues.Citation31,Citation32 Regranex gel showed a significant increase in the incidence of complete ulcer healing in patients with full-thickness diabetic neurotrophic foot ulcers when compared to placebo.Citation33 Malignancies distant from the gel application site however have been reported in both clinical and post-marketing studies, in addition to an increase in the rate of death in patients using three tubes or more of Regranex gel.Citation34 Together, these adverse effects limited the clinical usefulness of this product and further warranted the need for more tolerable angiogenic modulators.
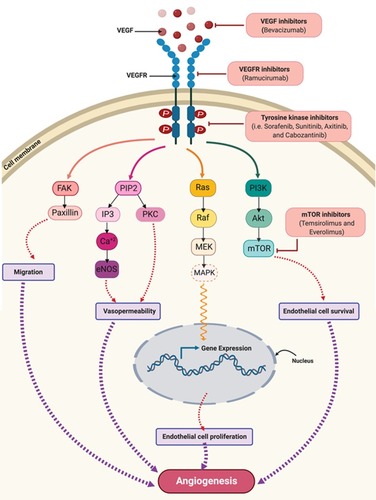
Several anti-angiogenic agents have been approved by the US-FDA for cancer therapy. Angiogenesis inhibitors include monoclonal antibodies that target specific pro-angiogenic growth factors and/or their receptors (bevacizumab and ramucirumab); tyrosine kinase inhibitors (TKIs) of growth factor receptors (sorafenib, sunitinib, axitinib, cabozantinib, pazopanib, regorafenib, and vandetanib); and inhibitors of mammalian target of rapamycin (mTOR) (temsirolimus and everolimus) ().Citation32 Despite the availability of anti-angiogenic drugs, many challenges limit their use in clinical practice. Among them is the development of more aggressive tumors once treatment is withdrawn.Citation35,Citation36 In addition, angiogenesis inhibitors have a wide range of serious adverse effects, such as bleeding, hypertension, leukopenia, and lymphopenia, which could further hinder their use in clinical settings. Angiogenesis inhibitors have been also shown to reduce delivery of chemotherapeutic drugs to target tumors and, in some cases, to affect the distribution and pharmacokinetic profiles of co-administered anti-cancer treatments.Citation14,Citation37–Citation40 Furthermore, the acquired resistance or “escape” of cancer cells against these agents is considered one of the most important challenges that limit their efficacy.Citation14,Citation41–Citation43 Moreover, angiogenesis inhibitors lack valid and standardized biomarkers for assessing their efficacy and/or tolerability, which further add to therapy limitations.Citation14,Citation41 Taken together, efforts to effectively target pathologic angiogenesis while overcoming current limitations to angiogenesis inhibitors are ongoing, especially in the area of cancer management. Targeting angiogenesis has been, without a doubt, impacted by technological advances in nanotechnology. The administration of angiogenesis modulators using nanoparticles, especially metal nanoparticles, has been increasingly utilized. This was driven by many advantages of nanoparticles; such as longer half-life, improved efficacy, and selective targeting of tumor-associated vasculature.Citation39,Citation44 Properties of nanoparticles that facilitate their utilization for biomedical applications include the enhanced permeability and retention effect (EPR), which allows the internalization of nanoparticles to tumor tissues, the ability to avoid the immune system, which prolongs the drug half-life and decreases its effective dose, and their high surface density, which results in selective targeting.Citation39
Figure 2 VEGF/VEGFR downstream signaling pathways and sites of actions of approved anti-angiogenic drugs in cancer therapy. Binding of VEGF to VEGFR leads to activation of multiple downstream cell signaling pathways including FAK/paxillins, PIP2, RAS/MAPK, and PI3K/Akt/mTOR-pathways. Monoclonal antibodies such as bevacizumab and ramucirumab bind to VEGF and VEGFR, respectively, preventing receptor activation and subsequent downstream signaling. Cabozantinib, pazopanib, regorafenib, sorafenib, sunitinib, and vandetanib are tyrosine kinase inhibitors which target the kinase domain of VEGFR. Temsirolimus and everolimus are inhibitors of mTOR which is a downstream effector for the VEGF/VEGFR axis. Collectively, these drugs will abolish angiogenic signaling in endothelial cells. These anti-angiogenic effects occur via inhibiting endothelial cells migration, vasopermeability, proliferation and survival.
Abbreviations: Akt, protein kinase B; eNOS, endothelial nitric oxide synthase; FAK, focal adhesion kinase; IP3, inositol trisphosphate; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; PKC, protein kinase C; PIP2, phosphatidylinositol 4,5-bisphosphate; PI3K, phosphoinositide-3-kinase; RAF, rapidly accelerated fibrosarcoma; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Among metal nanoparticles, gold nanoparticles (AuNPs) have captured recent attention for their potential use as angiogenic modulators. In the following subsections, the current evidence for utilizing AuNPs to modulate angiogenic growth along with the proposed molecular mechanisms and therapeutic applications for their effects are summarized. In addition, the physical characteristics and methods for the synthesis of AuNPs, as well as their uptake mechanisms, and toxicity are briefly outlined.
Gold nanoparticles (AuNPs)
AuNPs synthesis and properties
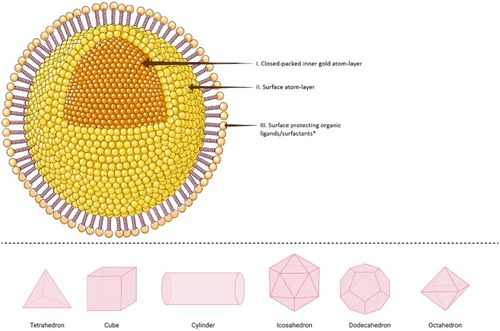
Gold nanoparticles (AuNPs) are composed of an inorganic core containing gold which is typically encircled by an organic monolayer.Citation45 A representative structural model of a single AuNP (, upper panel), is called “monolayer protected cluster; MPC”. MPC usually consists of central inner gold atoms “core” that determine the crystallinity of the structure; gold atoms on the outer surface of AuNPs structure, which usually have a different geometry than the core atoms, that create the surface’s facets and edges that control the catalytic activity of the particle, and a protecting outer layer of organic ligands or surfactants.Citation45,Citation46 The latter structures stabilize AuNPs and provide them with surface functionality.Citation45,Citation46 Depending on the size ranges of AuNPs, they could form clusters, catalytic particles or plasmonic crystals with different nanostructures (, lower panel).Citation46,Citation47 These include tetrahedrons,Citation48,Citation49 cubes,Citation50–Citation52 cylinders,Citation53 icosahedron,Citation49 dodecahedrons,Citation54 octahedrons,Citation49 in addition to a variety of branched structures.Citation55–Citation58
Figure 3 Schematic illustration of different shapes of a single crystalline gold nanoparticle (AuNP). Upper panel: In the simplest structure, AuNPs are commonly composed of central inner gold atoms with a closed-packed crystallographic face-centered cubic structure (domain I), surrounded by surface atoms on the outer surface of the gold atoms (domain II), which could be functionalized with an outmost layer of surface organic ligand or surfactants (domain III). The structure above is shown with a spherical shape, however, it could be also synthesized in various shapes as examples shown in 3D illustrations in the lower panel. *In the upper panel, the surface protecting ligands/surfactants were added to the posterior part of the spherical nanoparticle only for a clearer presentation of domains I and II.
Depending on the composition of AuNPs, a wide variety of organic ligands could be introduced to functionalize the surface of AuNPs and enhance their properties. Such modifications in drug delivery are crucial for adequate drug loading, proper drug-releasing, cellular uptake, and targeting.Citation59 Surface functionalization is used to introduce numerous biomolecules to the surface of AuNPs, such as peptides, proteins, and oligonucleotides.Citation60,Citation61 For example, gold nanoclusters coated with a mixed monolayer of n-alkanethiol and tetraalkylammonium followed by DNA assembly on the surface of the AuNPs were used by McIntosh et al to inhibit transcription by T7 RNA polymerase in vitro.Citation62 In addition, multifunctional AuNPs with polyethylene glycol (PEG), cell penetration and cell adhesion peptide, and small interfering RNA (siRNA) were tested for silencing c-Myc protooncogene, an important gene for cell cycle and homeostasis, both in vitro and in vivo.Citation63 Wu et al synthesized DNA gold nanoassembly composed of a gold core, a cationic peptide interlayer, and an outer layer of fluorophore-labeled hairpin DNA probes, which demonstrated highly efficient cellular delivery of DNA probes and overcome entrapment by endosomes.Citation64 Functionalization of AuNPs with a mixture of ammonium- and carboxylate- groups attached to the alkyl-thiol ligand terminals are also used to enhance cellular penetration and uptake.Citation65 Relevant functionalized AuNPs are discussed below while addressing the effects of AuNPs on angiogenesis.
Several methods, such as the Turkevich and Brust-Schiffrin methods, have been utilized for AuNP synthesis. The Turkevich method involves nucleation of gold ions through the reduction of gold salts by a reducing agent.Citation66 In this method, sodium citrate is added as a gold-reducing agent and capping agent to stabilize and prevent the aggregation of AuNPs (). Turkevich method results in monodispersed spherical AuNPs, in aqueous solution, that are 10–20 nm in diameter with a relatively narrow size distribution.Citation46 Turkevich method however is limited by the narrow size range of the synthesized AuNPs. It was later refined by Frens et al by varying the ratio of the reducing agent to the stabilizing agent to allow for the production of AuNPs with a wider size range.Citation67,Citation68
Figure 4 A schematic illustration of different AuNPs synthesis methods. (A) Turkevich method. This method uses sodium citrate as a gold-reducing agent and capping agent, (B) Brust-Schiffrin method includes the use of phase transfer agent (TOAB) and thiol-mediated capping of AuNPs, and (C) Murray method or place-exchange method, represented in organo-thiol (-SH) system, which can be used with different ligands and biomolecules.
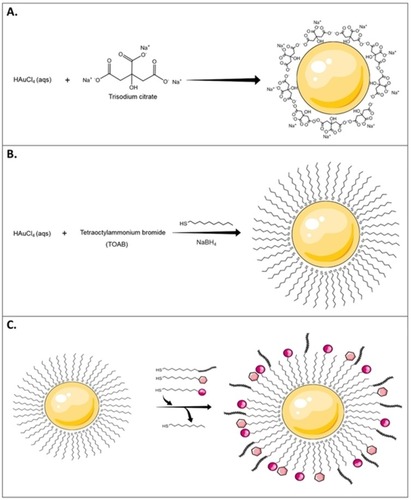
The Brust-Schiffrin method was inspired by Faraday’s two-phase system and results in large quantities of MPCs. The advantage of the Brust-Schiffrin method is that it results in more stable AuNPs that are smaller in size and monodispersed in terms of shape and size. This method involves the transfer of gold ions from a gold salt aqueous solution into an organic solvent (e.g. toluene) using a phase-transfer agent such as tetraoctylammonium bromide (TOAB).Citation69 This step is followed by a thiol-mediated capping of the gold clusters usually by using a thiol-terminated long-chain alkane, which is followed by the addition of a reducing agent such as sodium borohydride in the presence of alkanethiols (). The thiol-mediated capping step prevents aggregation of the reduced gold atoms and results in the production of stable organic monolayers on the AuNPs.Citation45,Citation46,Citation69–Citation71 The synthesized AuNPs can be further modified by ligand place-exchange reactions with different biomolecules to add a variety of specific functionalities to AuNPs in a process called the Murray method ().Citation45,Citation46,Citation72
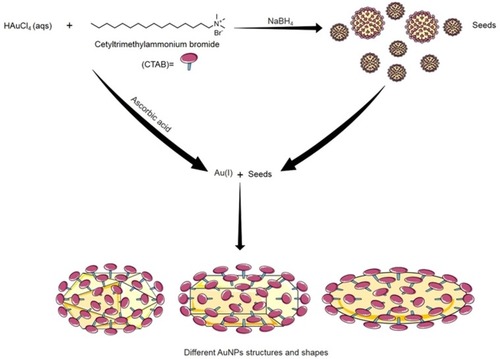
Turkevich and Brust-Schiffrin methods are usually used to produce spherical AuNPs. AuNPs can also be synthesized into different nanostructures, such as rods,Citation73 triangles,Citation74 wires,Citation73 cubes,Citation52 tubes,Citation75 and starsCitation76 via alternate procedures. The most commonly used procedure for the synthesis of non-spherical AuNPs is the seed-mediated growth, known as Murphy method.Citation77,Citation78 The basic idea of this method is to produce seed particles by the reduction of gold salts using a small amount of a strong reducing agent (e.g. sodium borohydride). These seeds are then added to a metal salt solution in the presence of a weak reducing agent (e.g. ascorbic acid) and surfactant (e.g. cetyltrimethylammonium bromide, CTAB), which is used as surface protecting agent ().Citation46,Citation77,Citation78 The geometry of the targeted gold nanostructure can be determined by altering the concentration of seed particles, reducing agents and/or the surface protecting agents.Citation70,Citation77,Citation78 Other methods for the synthesis of AuNPs include electrochemical-, photochemical-,Citation79–Citation81 sonochemical-,Citation82 and microwave-assisted methods.Citation83 These methods use different reducing energy sources, such as electron-, ultraviolet-, ultrasonic-, and microwave-sources, respectively.Citation46
Figure 5 The seed-mediated growth synthesis method of different shapes of AuNPs. In Seed-mediated growth method, seed particles are produced by the reduction of gold salts using a small amount of a strong reducing agent (e.g. Sodium borohydride; NaBH4). Then these seeds are added to a metal salt solution in the presence of weak reducing agent (e.g. ascorbic acid) and surfactant (e.g. cetyltrimethylammonium bromide; CTAB). Changing the concentration of seed particles, reducing agents and/or the surface-protecting agents control the geometry of the resulted gold nanostructure.
Despite the efficient synthesis of AuNPs using chemical methods, the generation of toxic byproducts along with the use of toxic materials and solvents are major concerns that could impact their large scale production and biological applications.Citation70,Citation84,Citation85 Thus, synthetic procedures for AuNPs production have been devised using biological components that lack toxicity. Green biosynthesis of AuNPs have many advantages when compared to chemical synthesis; such as reduced toxicity, lower cost, and safety to the environment.Citation70 Several biological resources were utilized in the synthesis of AuNPs such as plants or plant extracts,Citation86–Citation88 microorganisms,Citation89–Citation92 and algae.Citation93,Citation94 In addition, biomolecules have been used including amino acids,Citation95–Citation97 enzymes,Citation98 lipids,Citation99–Citation101 nucleic acids,Citation102,Citation103 fatty acids,Citation104 carbohydrates,Citation105 and polysaccharides.Citation106,Citation107
Cellular uptake and internalization of AuNPs
Several internalization mechanisms were suggested and studied for nanoparticles. In phagocytic cells, phagocytosis is a common mechanism for internalization of large clusters or aggregates of nanoparticles. Alternatively, nanoparticles are internalized by pinocytosis or passive uptake through the cell membrane in non-phagocytic cells.Citation108,Citation109 Depending on different nanoparticles properties, functionalization, and surface modifications; they could be internalized by either receptor-mediated endocytosis, or non-specific receptor-independent endocytosis.Citation108–Citation110
Cellular uptake and internalization pathways of AuNPs () are affected by many factors such as particle size, shape, surface charge, and functionalization.Citation108,Citation109 The effect of the size of AuNPs on their cellular internalization was widely studied. Chithrani et al showed that 50 nm AuNPs had the highest uptake by HeLa cells among a range of tested sizes; 10–100 nm.Citation111 Similarly, Ko et al demonstrated that the uptake of 50 nm AuNPs by human adipose-derived stem cells was more than other examined sizes; 15–100 nm.Citation112 However, other studies showed that cellular uptake of smaller gold nanoclusters (1–2 nm) was greater than larger gold nanoclusters (12 nm).Citation113,Citation114
Figure 6 Internalization and cellular uptake pathways of AuNPs. Different properties of AuNPs, such as particle size, shape, surface charge, and functionalization affect internalization and cellular uptake mechanisms in different cells. These pathways include: (a) passive uptake for small AuNPs, (b) phagocytosis and (c) micropinocytosis for large size nanoparticle or aggregates of AuNPs, different pinocytosis pathways; (d) clathrin-dependent endocytosis, (e) caveolin-dependent endocytosis, and (f) clathrin/caveolin-independent endocytosis which are usually the uptake pathways for AuNPs with functionalized-, charged- or neutral-surfaces. Spherical AuNPs are used for presentation purposes only, different shapes of AuNPs are internalized by a variety of cells and affect the internalization pathways as well.
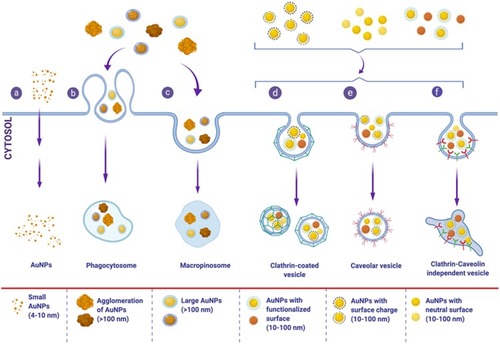
Surface coating and functionalization play a vital role in determining cellular uptake mechanism. Saha et al showed that the uptake of cationic AuNPs involves different endocytic pathways (caveolin- and dynamin-dependent pathways) and specific cell surface receptors, in HeLa cells and MCF10A cells.Citation115 On the other hand, coated AuNPs with PEG, polyvinyl alcohol (PVA) or a mixture of both demonstrated different uptake behaviors by monocyte-derived dendritic cells (MDDCs), as (PEG+PVA)-AuNPs and PVA-AuNPs showed higher uptake, compared to PEG-AuNPs.Citation116
In regards to AuNPs shape, Xie et al showed that AuNPs with triangular shape resulted in the highest uptake by RAW 264.7 macrophages, compared to rods and stars.Citation117 In addition, each shape has different endocytosis pathway; triangles are internalized via clathrin-mediated endocytosis and dynamin-dependent pathway, rods through both clathrin- and caveolin-mediated endocytosis, and stars through clathrin-mediated process.Citation117 Overall, the heterogeneity of AuNPs’ properties and synthesis methods determine their cellular internalization and their intracellular fate.
AuNPs and angiogenesis
The anti-angiogenic effects of AuNPs have been demonstrated in multiple studies. AuNPs reduced viability, migration, and tube formation of human umbilical vein endothelial cells (HUVECs).Citation118–Citation126 The anti-angiogenic effects of AuNPs on HUVECs were found to be dose-dependent.Citation118,Citation119,Citation122 AuNPs were also shown to inhibit VEGF-A-induced migration of human retinal microvascular endothelial cells (HRMECs) in vitro.Citation127 The anti-angiogenic activity of AuNPs has been further demonstrated utilizing ex vivo models such as the chick chorioallantoic membrane model (CAM).Citation128,Citation129 In the CAM model, AuNPs inhibited formation of new blood vessels, reduced total tubule complex, total vessel length, and number of vessel junctions.Citation124,Citation128
Administration of AuNPs has also been shown to result in anti-angiogenic activity in multiple models in vivo. AuNPs reduced vascular density and permeability while maintained vessel integrity in different tumor models. AuNPs treatment normalized tumor vasculature in nude mice inoculated with SW620 human colorectal cancer.Citation130 AuNPs reduced vascular density as indicated by staining tumor tissues with CD31 vessel marker. In addition, AuNPs improved vascular stability by increasing pericyte coverage as determined by detection of α-smooth muscle actin on tumor vasculature within 6–9 days of treatment.Citation130 In an animal model of melanoma, the anti-angiogenic activity of AuNPs was demonstrated in terms of reduced vascular density, reduced permeability, and improved vascular morphology and perfusion.Citation131 Normalization of tumor vasculature was associated with reduced lung metastasis in AuNPs-treated animals when compared to vehicle-treated controls.Citation131 In an animal model of liver cancer, the anti-angiogenic activity of AuNPs revealed a remarkable decrease in vascular density in treated animals as indicated by staining for the marker CD34.Citation121 Furthermore, AuNPs reduced vessel permeability in a mouse ovarian tumor model as indicated by reduced fluid accumulation in the peritoneal cavity of nanoparticle-treated animals.Citation132 A reduction in angiogenesis was also observed in a nude mouse ear model treated with AuNPs.Citation132
Molecular mechanisms associated with anti-angiogenic effects of AuNPs
Multiple molecular pathways have been shown to contribute to the anti-angiogenic effects of AuNPs (). The VEGF-A/VEGFR pathway has been a major molecular target mediating the anti-angiogenic activity of AuNPs. Accumulating evidence revealed that treatment with AuNPs suppressed the activity of VEGF-A/VEGFR2 axis and its downstream signaling in different endothelial cells both in vitro and in vivo. AuNPs were shown to decrease expression of VEGF-A and suppress VEGFR2 phosphorylation in HUVECs.Citation123,Citation125,Citation126,Citation133–Citation135 Inhibition of the VEGF-A/VEGFR2 axis leads to subsequent inhibition of AKT and ERK1/2 phosphorylation in endothelial cells.Citation121,Citation124,Citation134 In addition, AuNPs were shown to suppress intracellular calcium release and reduce RhoA activity mediated by VEGF-165 in HUVECs.Citation121,Citation133 AuNPs have been shown to downregulate the levels of pro-angiogenic factors, Ang-1 and Ang-2, in tumor tissues.Citation135 In addition, Vimalraj et al indicated that AuNPs exerted anti-angiogenic effects in CAM model by downregulating Ang-1/Tie2R pathway.Citation129
Figure 7 Molecular pathways affected by the anti-angiogenic effects of AuNPs. Anti-angiogenic effects of AuNPs are mediated by suppressing activation of VEGFR2, Tie2R, FGFR, and their downstream signaling pathways. AuNPs suppress intracellular calcium release mediated by VEGF-165. AuNPs upregulate E-cadherin and downregulate vimentin reducing epithelial-to-mesenchymal transition (EMT). AuNPs reduce ILs, MMPs, and TNF-α expression and inhibit neovascularization via induction of autophagy.
Abbreviations: Ang-1, angiopoietin 1; Ang-2, angiopoietin 2; Ca+2, calcium; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; ILs, interleukins; MMPs, matrix metalloproteases; Tie2R, angiopoietin receptor; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
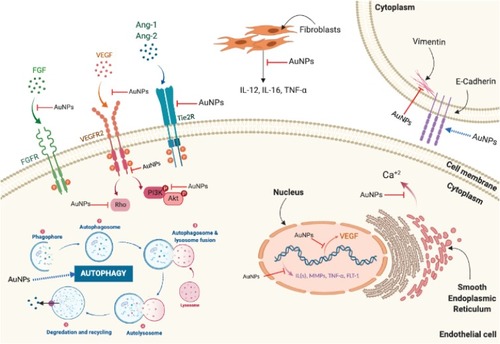
Animal studies showed that AuNPs suppressed inflammatory cytokines and release of MMPs. AuNPs depleted MMP-2 in H-357-PEMT cells in a p53- and p21-dependent manner.Citation135 They altered the level of inflammatory cytokines IL-6, IL-1β, TNF-α, and metastasis-associated markers (CD-44, CD-133) in H-357-PEMT cells and conditioned media-treated HUVECs in a p53/p21-dependent manner.Citation135 AuNPs decrease the production of IL-12, IL-6, MMP-1, and TNF-α from dermal fibroblasts suppressing angiogenesis.Citation136 AuNPs reduced epithelial-to-mesenchymal transition (EMT) as indicated by upregulation of the epithelial marker E-cadherin and downregulation of the mesenchymal protein, vimentin.Citation131 AuNPs also inhibited angiogenesis by reducing MMP-2 expression and increasing the levels of the junctional adaptor protein ZO-1 in endothelial cells.Citation131 AuNPs further inhibited basic FGF-2-induced angiogenesis.Citation126,Citation136 Recently, Shen et al showed that AuNPs inhibited retinal neovascularization through induction of autophagy, which was confirmed by upregulation and detection of autophagic markers LC3-II, ATG5, Beclin1, and p62.Citation122 The molecular pathways associated with the anti-angiogenic activity of AuNPs are illustrated in .
Biomedical applications of AuNPs
The unique physical and chemical properties of AuNPs make them the perfect choice for a variety of biomedical applications. AuNPs represent an appealing choice as angiogenic modulators due to their compatibility, along with their ease of synthesis and characterization owing to the presence of surface plasmon resonance band.Citation132,Citation137–Citation140 AuNPs can bind to amine- and/or thiol-groups in proteins, which represent unique ligands for AuNPs.Citation23,Citation133 In addition, AuNPs can be produced in a wide range of particle size and shapes to meet the indicated application. The vascular activity of AuNPs has been particularly important in mediating the medical applications for these nanoparticles. The medical applications of AuNPs that are associated with modifying the angiogenic process and vasculature are summarized below.
AuNPs as therapeutic agents in pathologic angiogenesis
Administration of AuNPs has been shown to block pathological angiogenesis in the retina, a leading cause of blindness.Citation127,Citation134 Intravitreal administration of AuNPs significantly inhibited retinal neovascularization in a mouse model of oxygen-induced retinopathy of prematurity when compared to control animals.Citation134 Histological examination showed no retinal changes in terms of thickness, inflammation, or cytotoxic effects to retinal cells in AuNPs treated-animals compared to vehicle-treated groups.Citation134 Reva et al examined the effect of subcutaneous injections of AuNPs on dermal structures in male CBA mice.Citation125 Injected nanoparticles were phagocytosed by macrophages and the anti-angiogenic activity of AuNPs was mediated by the toxic activity of macrophages loaded with nanoparticles. Cytotoxic activity of AuNPs on vascular endothelium in subcutaneous tissues was mediated by the deactivation of macrophages that produce VEGF-A, or through direct death of the endothelium as a result of the migration of macrophages through the vascular wall.Citation125 AuNPs treatment normalized tumor vasculature in a xenograft nude mice model of human colorectal carcinoma (CRC).Citation130 Treatment reduced expression of VEGFR2 and HIF-1α, which was accompanied by reduced vessel hyperpermeability and hypoxia, respectively. In addition plasma level of anterior gradient 2 (AGR2), a biomarker associated with cancer progression and angiogenesis, was reduced as a result of AuNPs treatment. Interestingly, effects on tumor vasculature have been found to abolish by day 14 of AuNPs treatment suggesting a time frame for the anti-angiogenic effects of AuNPs.Citation130
Other studies have demonstrated that AuNPs could enhance angiogenesis in animal models. The application of AuNPs in photo-bio-modulation therapy (PBMT) accelerated cutaneous wound healing in Sprague Dawley rats. Histological results indicated that AuNPs and PBMT is effective in stimulating angiogenesis and triggering inflammatory response at an early stage due to enhanced epithelialization, collagen deposition, and fast vascularization.Citation141 In addition, AuNPs increased the expression of CD31 endothelial marker and enhanced angiogenesis in an orthotopic co-implantation model of pancreatic cancer.Citation142 Roma-Rodrigues et al demonstrated modulation of angiogenesis using AuNP-peptide conjugates in CAM assay.Citation143 Specific peptide conjugates on the surface of AuNPs were observed to promote or inhibit angiogenesis in CAM assay by interacting with endothelial cell angiogenic receptors or by altering the balance between pro- and anti-angiogenic factors.Citation143
AuNPs for targeted drug delivery
Targeted AuNPs have emerged as potential delivery systems due to the ease by which they can be modified and the possibility of loading them with drug therapies. Arginine-glycine-aspartic acid peptide (RGD) has been widely used as a targeting ligand because of its selective binding to the ανβ3 and ανβ5 integrins.Citation144 These integrins are overexpressed in endothelial cells during tumor angiogenesis.Citation144 Multiple studies used RGD-modified AuNPs for detection of cancer angiogenesis and for tumor-targeted delivery in vivo. Peptides based on the cyclic RGD sequence have been designed to antagonize the function of αvβ3 integrin, thereby inhibiting angiogenesis in αvβ3-integrin-positive C6 gliomas.Citation145 In another study, radiolabeled 177Lu-AuNP-RGD were found to deliver the highest tumor radiation absorbed dose and to induce less tumor progression and metabolic activity than 177Lu-AuNP or 177Lu-RGD radiopharmaceuticals in glioma cells of athymic mice. 177Lu-AuNP-RGD decreased tumor vascularity and VEGF-A gene expression.Citation145
Li et al investigated the anti-tumor effects for the co-delivery of the anti-angiogenesis drug captopril with siRNA loaded into AuNPs in an animal model of breast cancer.Citation146 The complexes started to accumulate at the tumor site by passive targeting through enhanced permeability and retention effect within 1 h of administration. In vivo investigation on nude mice bearing MDA-MB-435 tumor xenografts revealed that captopril/siRNA/AuNP complexes possessed satisfying tumor homing ability and strong anti-tumor activity, which was further associated with downregulating VEGF-A mRNA and protein levels in tumor tissue.Citation146 A hybrid-nanoparticle using quinacrine and gold (QAuNP) induced anti-angiogenic and anti-metastatic effect on oral squamous cell carcinoma (OSCC). QAuNP significantly suppressed angiogenesis and induced tumor regression in xenograft mice model of OSCC in vivo.Citation135 Saber et al prepared and tested the effect of c(RGDfK) labeled chitosan-capped AuNPs [cRGD(CS-Au)NPs] as a carrier for selective intracellular delivery of sunitinib malate (STB) to the tumor vasculature. cRGD(CS-STB-Au)NPs were more toxic to cells than the free STB with the same drug dose indicating that the prepared nanoparticles improved delivery and uptake by cells.Citation120
Recently, recombinant human endostatin conjugated AuNPs (rhES-AuNPs) have shown significant activity in inhibiting AGR2-induced angiogenesis in animal model of metastatic colorectal cancer (mCRC).Citation118 AuNPs normalized vasculature by promoting vessel stability, indicated by increasing pericyte expression, and reducing VEGFR2 in mCRC xenografts. There was however no significant difference in vascularity between the control group and the rhES-AuNPs-treated group as determined by the endothelial marker CD31.Citation118 In agreement, Li et al reported on the targeted delivery of rhES using AuNPs as a drug delivery system in mice bearing H22 xenografted tumors.Citation147 rhES-AuNPs-PEG improved pericyte coverage and enhanced maturity and stability of tumor vessels.Citation147 AuNPs have been successfully used for the dual delivery of the chemotherapeutic drug doxorubicin and the anti-angiogenic drug sorafenib in tumor-bearing mice.Citation148 Intravenous administration of AuNPs carrying therapeutic drugs was found to accumulate at the tumor site.Citation148 Balakrishnan et al demonstrated a powerful anti-tumor activity for the bioflavonoid quercetin formulated into AuNPs-based drug delivery system in 7,12-dimethylbenz(a)anthracene induced mammary carcinoma in Sprague-Dawley rats.Citation119 The quercetin-AuNPs delivery system suppressed mammary tumor growth in vivo as a result of tumor EMT suppression and angiogenesis.Citation119 The conjugation of AuNPs and snake venom protein toxin NKCT1 showed anti-cancer effects, both in vivo and in vitro, in Ehrlich ascites carcinoma (EAC) cells and EAC induced mice. NKCT1-AuNP reduced proliferation and induced apoptosis, which resulted in reduced volume and weight of EAC tumors in male albino mice. These effects were mediated by decreased expression of VEGF-A, IL-10, and Bcl2, along with upregulation of Bax and caspase 3/9 in NKCT1-AuNP-treated animals.Citation149
AuNPs have also been modified to enhance angiogenesis in animal models. Kim et al examined the therapeutic effect of phytochemically stabilized AuNPs (pAuNPs) coated on a hydrocolloid membrane (HCM) for curing cutaneous wounds in Sprague Dawley rats.Citation150 Topical application of pAuNP-HCM enhanced skin regeneration and wound closure in injured animals. These effects were achieved through increasing collagen expression, enhanced expression of VEGF-A and angiopoietins, decreased MMP-1 and TGF-β1 expression, along with enhanced antioxidant effect in AuNPs-treated animals.Citation150 Conjugating AuNPs with VEGF-A for the efficient transdermal delivery of VEGF-A in the treatment of wound injuries in BALB/c mice was shown to improve vascular density and increase the number of subcutaneous vessels in the experimental group that was treated with VEGF-A-AuNP when compared to control group.Citation151 Topical application of anti-oxidant agents with AuNPs enhanced wound healing of diabetic mouse skin, an effect which was mediated by reduced expression of the receptor for advanced glycation end-products (RAGE) and increased VEGF-A levels.Citation152
Other examples of AuNPs conjugates were reported that combined the advantages of AuNPs with added components, such as hybrids of polyethylenimine/RNA-based AuNPs that combined the self-assembly and anti-VEGF-A characteristics of nucleic acid that inhibit tumor angiogenesis at its initial progression, and the capability of AuNPs to generate heat which resulted in photothermal treatment of cancer at its later stages.Citation153 Likewise, Nethi et al synthesized bio-conjugates of AuNPs using Hamelia patens (HP) leaf extract as a reducing and stabilizing agent,Citation154 which is also used as a wound-healing agent.Citation155 These AuNPs-HP conjugates introduced an eco-friendly green and cost-effective synthesis of AuNPs in addition to showing pro-angiogenic effects in both in vitro and ex vivo models.Citation154 Moreover, peptide conjugates with AuNPs were assessed by Wang et al. A multivalent ligand of a peptide sequence (REDV)-modified alginate (ALG) conjugated with AuNPs (cREDV-ALG) was prepared to promote angiogenesis.Citation156 REDV-peptide sequence was chosen as an adhesion peptide that adheres to α4 β1 integrin, which is expressed on the membrane of endothelial cells, to enhance the binding between endothelial cells and ligand modified scaffolds in tissue engineering.Citation156,Citation157 cREDV-ALG showed a selective adhesion to HUVECs and promoted their proliferation.Citation156,Citation157 In addition, cREDV-ALG stimulated new vessel formation and increased blood vessel density in animal models.Citation156,Citation157 Altogether, findings from these studies demonstrated the potential use of AuNPs as a promising modality for targeted drug and gene delivery, both systemically and topically.
AuNPs for medical imaging
The application of AuNPs in radiology is aimed at overcoming some of the limitations of traditional imaging techniques, such as shallow penetration along with reduced sensitivity and specificity.Citation158 Tumor tissues have shown higher retention of AuNPs compared to traditional contrast agents thus improving tumor detection as the clearance of AuNPs from the blood is slower compared to iodine agents, allowing for longer imaging times.Citation139 Clark et al reported on the potential use of AuNPs as contrast agents for computed tomography (CT) using a primary mouse model of soft-tissue sarcoma.Citation159 The protocol identified changes and improved limits of detectability for tumor vasculature in vivo.Citation159 Furthermore, AuNPs were applied as signal amplifiers in multispectral optoacoustic tomography (MSOT) to visualize gastrointestinal cancer in an animal model.Citation160 Results showed that PEGylated AuNPs have the capacity to penetrate tumors and provide high-resolution signal amplification for optoacoustic imaging.Citation160
AuNPs have been modified with radioactive material for in vivo imaging. Radioactive iodine-labeled, cyclic RGD-PEGylated AuNP probes were designed to test for selective targeting on tumors in experimental models.Citation161 Intravenously injected probes revealed rapid and effective accumulation of the probes within 10 min after injection, which was increased with time. Analysis of tumor tissue revealed uptake of 125I-cRP-AuNPs via integrin αvβ3-receptor specific endocytosis.Citation161 Similarly, Morales-Avila et al showed effective accumulation of technetium-99m-labeled AuNPs conjugated to RGD [99mTc-AuNP-RGD] into tumor tissue after both intravenous and intraperitoneal injections.Citation162 Intravenous administration of 99mTc-AuNP-RGD however resulted in a faster tumor uptake when compared to the intraperitoneal mode of administration.Citation162 Pan et al reported that photoacoustic tomography combined with αvβ3-gold nanobeacons demonstrated high resolution of in vivo angiogenesis imaging.Citation163 Using a mouse Matrigel-plug model of angiogenesis, αvβ3-gold nanobeacons enhanced contrast and detection of both existing and sprouting neovessels. Interestingly, the nanoparticles showed specific homing towards immature neovessels but not mature ones.Citation163 AuNPs have been also examined for magnetic resonance imaging (MRI) indications. Zhang et al investigated thiol-PEG-carboxyl-stabilized Fe2O3/AuNPs targeted to CD105 for the detection of tumor angiogenesis in breast cancer xenografts. Tumor angiogenesis was detected using MRI at different time points after intravenous administration of nanoparticles to tumor-bearing mice.Citation164
AuNPs for photothermal therapy
AuNPs-mediated photothermal therapy has been thoroughly examined for cancer therapy in animal models and in clinical studies.Citation165–Citation167 Laser irradiation of AuNPs that were accumulated in tumor tissues resulted in the destruction of the tumor tissue while avoiding damage to healthy tissues as a result of selective light absorption and hyperthermia that was generated at the tumor site.Citation165,Citation167,Citation168
Gum Arabic-AuNPs associated with laser exposure produced apoptotic, anti-inflammatory, lipid peroxidation, and anti-neovascular effects in an animal model of lung cancer.Citation169 The apoptotic effects were mediated by elevations of cytochrome-c, death receptor 5, and caspase-3, while the anti-angiogenic activity was associated with a reduced VEGF-A expression in tumor tissue.Citation169 An αvβ3 integrin-targeted multi-modal nanoprobe, dendrimer-RGD (Den-RGD), was designed and intravenously injected into tumor-bearing mice xenografts at 24 h after photothermal therapy.Citation170 Authors showed that AuNPs modified with carboxylated bovine serum albumin had both anti-tumor and anti-angiogenesis effects under near-infrared laser irradiation in human tumor-bearing mice xenografts, suggesting the efficacy of AuNPs for photothermal therapy in vivo.Citation170 Interestingly, Pedrosa et al demonstrated the possibility of producing local anti-angiogenic effects through specific laser irradiation of target blood vessels that were treated with AuNPs conjugated to anti-angiogenic peptides in vivo. In this study, a green laser coupled to AuNPs generated high localized temperatures that were sufficient to precisely cauterize blood vessels to be applied in cancer therapy.Citation171 Photoacoustic imaging conducted by irradiation with RGD peptides conjugated plasmonic gold nanostars with a near-infrared pulse laser demonstrated deep imaging and homogenous resolution for early diagnosis of tumor angiogenesis.Citation172 Tumor vessel specific ultrafine AuNPs, which were anchored with anti-RhoJ antibody and conjugated to iodine (AuNP-I), were applied as targeted radiosensitizers.Citation173 Patient-derived xenograft tumor model was constructed through orthotopic transplantation of human breast tumor tissue into mice. AuNPs-I showed selective binding to tumor vessels, rather than normal vessel. Targeted photothermal treatment remarkably reduced the number of tumor blood vessels when compared to animal groups that were treated with radiation alone or with the angiogenesis inhibitor bevacizumab. The anti-angiogenic effects of targeted therapy were sustained for 30 days.Citation173 PEGylated gold nano-semicubes (PEG-GNSCs) also produced anti-angiogenic effects in an animal model of skin cancer.Citation174 Photothermal treatment with laser-stimulated PEG-GNSCs significantly reduced VEGF-A concentrations in tumor-bearing animals through down-regulation of circulating VEGF-A and reduced expression of VEGFR2, PDGFR, and HIF-1.Citation174
Toxicity of AuNPs
While a considerable number of experimental work supported the non-toxicity of AuNPs, other research raised concerns about the toxic effects of these particles.Citation175,Citation176 Toxicity of AuNPs has been shown to depend on the physicochemical properties of the particles, such as their size, charge, and surface-chemistry.Citation177,Citation178
The majority of toxicity studies revealed that AuNPs with a particle size greater than 4–5 nm in diameter are mostly non-toxic after acute exposures.Citation177 Nanoparticles smaller than 4 nm in size however become catalytically active and could be cytotoxic.Citation177 In addition, AuNPs toxicity has been shown to be related to their cellular internalization pathways. Sabella et al showed that AuNPs with stripe-like ligands were taken up by non-endocytic pathways and mainly distributed in the cytosol as opposed to unstructured AuNPs that entered the cells via endocytic pathway and co-localized within lysosomes. This was associated by a greater toxicity of the unstructured AuNPs when compared to the striped ones.Citation179
Falagan-Lotsch et al evaluated the long-term effects of AuNPs with different shapes and surface coatings under chronic and non-chronic exposure conditions.Citation177 Viability of the human dermal fibroblasts (HDFs) was not changed between AuNP-treated and non-treated cells in both exposure conditions suggesting AuNPs were generally not cytotoxic to human fibroblasts. However, long-term exposure to AuNPs induced changes in HDFs homeostasis through altered gene expression for genes regulating cell cycle and oxidative stress.Citation177 Other investigations showed that AuNPs were not cytotoxic to fetal HDFs but affected cell morphology at high concentrations.Citation180 AuNPs showed low cytotoxicity to hepatic HepG2 cells, though they can disrupt adhesion of the HepG2 cells.Citation181 Bahamonde et al assessed the toxicity of a single intravenous dose of AuNPs in rodents.Citation182 Mice exposed to the particles developed granulomas in the liver and increased serum levels of the inflammatory cytokine interleukin-18. Such effects were not observed in rats exposed to AuNPs.Citation182 In terms of AuNP biodistribution and excretion, rats showed greater particle accumulation in spleen and higher fecal excretion compared to mice.Citation182 Hepatotoxic potential of AuNPs was further investigated using rodents with healthy and stressed liver.Citation183 AuNPs were toxic to liver tissues in animals with stressed liver environment but not in animals with healthy liver function. Hepatotoxicity was mediated through increased production of reactive oxygen species, acute inflammation, and increased apoptosis in animals with stressed liver function after exposure to AuNPs.Citation183 Toxicity of AuNPs has been assessed using other in vivo models as well. Exposure to AuNPs in zebrafish model provided data about their embryonic toxicity.Citation184 Embryonic toxicity was found to be dependent on AuNPs surface function and charge and ranged from lethal effects to sublethal malformations.Citation184 Genotoxicity of AuNPs was evaluated using Drosophila melanogaster as an in vivo model.Citation185 Findings revealed that AuNPs were able to induce genetic mutations, which were further transmitted to Drosophila fly progeny.Citation185 Furthermore, genomic toxicity of AuNPs was evaluated in Caenorhabditis elegans. Whole-genome microarray identified significant differential expression of 797 genes of the nematode after exposure to AuNPs.Citation186 Collectively, toxicity of AuNPs needs further investigation to determine the safety of these formulations for human use. Clearly, the toxic effects are determined by the physicochemical properties of the particles, their biodistribution and cellular uptake, and the cell line or animal model being examined.Citation175,Citation187
Conclusions and future directions
Dysregulation of angiogenesis is the hallmark of multiple pathologic conditions. The search for new drug entities which can modify angiogenic process to treat human disease is highly warranted. Despite the availability of anti-angiogenic drugs in the market, the utilization of these drugs is limited by the lack of therapeutic benefits in specific medical conditions, adverse effect profile, and the potential for the development of drug resistance. AuNPs represent an appealing alternative as therapeutic agents to modify the angiogenic process. In this review, the majority of biomedical applications demonstrated that AuNPs are effective angiogenic inhibitors and could be further modified to facilitate targeted delivery and loading with other drug entities. Most evidence for the effectiveness of AuNPs was demonstrated by pre-clinical studies using cell culture and animal models. It is of paramount importance to test AuNPs in clinical studies to further assess their applicability and to improve and develop these formulations for specific medical applications, which may provide a new approach to overcome the limitations of the currently available angiogenic inhibitors, especially in cancer management.
Despite the potential therapeutic applications of AuNPs, several limitations for their utilization exist. Clearly, the uptake and internalization pathways for AuNPs are highly diverse and are dependent on multiple particle properties and the target tissue. Better understanding of cellular uptake mechanisms and intracellular trafficking would provide great insights into the fate of AuNPs and further their potential therapeutic benefits. In addition, clinical applications for AuNPs require careful evaluation of their toxicity. Although many studies have demonstrated that AuNPs are biocompatible and non-cytotoxic, there is an increasing demand to examine their toxicity. Therefore, it is critical to develop and/or identify appropriate in vitro and in vivo models to assess the toxicity of these particles.
Abbreviations
AGR2, anterior gradient 2; ALG, alginate; Ang-1 or-2, angiopoietin-1 or −2; AuNPs, gold nanoparticles; CAM, chick chorioallantoic membrane model; CRC, colorectal cancer; CT, computed tomography; CTAB, cetyltrimethylammonium bromide; Den-RGD, dendrimer-arginine-glycine-aspartic acid; EAC, ehrlich ascites carcinoma; ECs, endothelial cells; EGF, epidermal growth factor; EMT, epithelial-to-mesenchymal transition; EPR, enhanced permeability and retention effect; FGF, fibroblast growth factor; HCM, hydrocolloid membrane; HDFs, human dermal fibroblasts; HP, Hamelia patens; HRMECs, human retinal microvascular endothelial cells; HUVECs, human umbilical vein endothelial cells; IF-γ, interferon-γ; IL, interleukin; mCRC, metastatic colorectal cancer; MMPs, matrix metalloproteinases; MPC, monolayer protected cluster; MSOT, multispectral optoacoustic tomography; mTOR, mammalian target of rapamycin; OSCC, oral squamous cell carcinoma; pAuNPs, phytochemically stabilized AuNPs; PBMT, photo-bio-modulation therapy; PDGF, platelet-derived growth factor; PEG, polyethylene glycol, PEG-GNSCs, PEGylated gold nano-semicubes; PlGF, placental growth factor; PVA, polyvinyl alcohol; QAuNP, hybrid quinacrine-gold nanoparticle; RGD, arginine-glycine-aspartic acid peptide; rhES, recombinant human endostatin; siRNA, small interfering RNA; STB, sunitinib malate; TGF-β, transforming growth factor-beta; TIMPs, tissue inhibitors of matrix metalloproteinases; TKIs, tyrosine kinase inhibitors; TNF-α, tumor necrosis factor-α; TOAB, tetraoctylammonium bromide; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Disclosure
Figures of this review were created with the aid of Servier medical art, BioRender, and ChemDraw software. The authors report no conflicts of interest, financial or otherwise, in this work.
References
- Arboleda-Velasquez JF, D’Amore PA. Chapter 10 - Vasculogenesis and Angiogenesis In: Willis MS, Homeister JW, Stone JR, editors. Cellular and Molecular Pathobiology of Cardiovascular Disease. San Diego: Academic Press; 2014:181–196.
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389. doi:10.1038/7465110742145
- Ribatti D. The discovery of tumor angiogenesis factors: a historical overview In: Ribatti. D, editor. Tumor Angiogenesis Assays: Methods and Protocols. New York: Springer New York; 2016:1–12.
- Adair TH, Montani JP. Integrated Systems Physiology: from Molecule to Function to Disease. In: Angiogenesis. San Rafael (CA); 2010.
- Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22(7):251–256.9255066
- Mousa SA, Arias HR, Davis PJ. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis modulation In: Mousa SA, Davis PJ, editors. Angiogenesis Modulations in Health and Disease: Practical Applications of Pro- and Anti-angiogenesis Targets. Dordrecht: Springer Netherlands; 2013:55–75.
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi:10.1038/nm0603-65312778163
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi:10.1038/nature1014421593862
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi:10.1038/nature0447816355210
- Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi:10.1126/science.277.5322.559204896
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13(9):1055–1066. doi:10.1101/gad.13.9.105510323857
- Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13(11):1382–1397. doi:10.1101/gad.13.11.138210364156
- Mousa SA. Survey of Pro-angiogenesis Strategies In: Mousa SA, Davis PJ, editors. Angiogenesis Modulations in Health and Disease: Practical Applications of Pro- and Anti-angiogenesis Targets. Dordrecht: Springer Netherlands; 2013:15–18.
- Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta Rev Cancer. 2014;1846(1):161–179. doi:10.1016/j.bbcan.2014.05.002
- Mousa SA. Survey of Anti-angiogenesis Strategies In: Mousa SA, Davis PJ, editors. Angiogenesis Modulations in Health and Disease: Practical Applications of Pro- and Anti-angiogenesis Targets. Dordrecht: Springer Netherlands; 2013:95–106.
- Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9(6):1144–1155. doi:10.3748/wjg.v9.i6.114412800214
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65(10):3967–3979. doi:10.1158/0008-5472.CAN-04-242715899784
- Mousa SA, Anwar LH. Anti-angiogenesis therapy as an adjunct to chemotherapy in oncology In: Mousa SA, Davis PJ, editors. Angiogenesis Modulations in Health and Disease: Practical Applications of Pro- and Anti-angiogenesis Targets. Dordrecht: Springer Netherlands; 2013:143–155.
- Dvorak HF. Angiogenesis: update 2005. J Thromb Haemost. 2005;3(8):1835–1842. doi:10.1111/j.1538-7836.2005.01361.x16102050
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi:10.1038/nrc109312778130
- Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp. 1991;22:339–347.1726933
- Nagy JA, Vasile E, Feng D, et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–237.12858545
- Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60(11):1289–1306. doi:10.1016/j.addr.2008.03.01318501989
- Brown LF, Yeo KT, Berse B, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176(5):1375–1379. doi:10.1084/jem.176.5.13751402682
- Dvorak HF. How tumors make bad blood vessels and stroma. Am J Pathol. 2003;162(6):1747–1757. doi:10.1016/s0002-9440(10)64309-x12759232
- Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–158.9893349
- Chu H, Wang Y. Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther Deliv. 2012;3(6):693–714.22838066
- Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111(12):1556–1566. doi:10.1161/01.CIR.0000159345.00591.8F15795364
- Van Hove AH, Benoit DSW. Depot-based delivery systems for pro-angiogenic peptides: a review. Front Bioeng Biotechnol. 2015;3(102). doi:10.3389/fbioe.2015.00102
- Bader RA, Putnam DA. 2013 Engineering Polymer Systems for Improved Drug Delivery. Somerset, UNITED STATES: John Wiley & Sons, Incorporated.
- Hollister C, Li VW. Using angiogenesis in chronic wound care with becaplermin and oxidized regenerated cellulose/collagen. Nurs Clin North Am. 2007;42(3):457–465, vii. doi:10.1016/j.cnur.2007.05.00217825664
- The Angiogenesis Foundation. (2018). “Therapeutic angiogenesis.” Accessed 126, 2019, Available from: https://angio.org/learn/treatments/.
- Steed DLMD. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plastic & Reconstructive Surgery Current Concepts in Wound Healing. 2006;117(7S):143S–149S. doi:10.1097/01.prs.0000222526.21512.4c
- Papanas N, Maltezos E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 2010;33(6):455–461. doi:10.2165/11534570-000000000-0000020486728
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi:10.1016/j.ccr.2009.01.02119249681
- Griffioen AW, Mans LA, de Graaf AMA, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res. 2012;18(14):3961–3971. doi:10.1158/1078-0432.CCR-12-000222573349
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788. doi:10.1038/sj.bjc.660381317519900
- Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61(2):47–56.22760023
- Banerjee D, Harfouche R, Sengupta S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc Cell. 2011;3(1):3. doi:10.1186/2045-824X-3-321349160
- Verheul HMW, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475. doi:10.1038/nrc215217522716
- Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. 2013;4(3):253–263. doi:10.3978/j.issn.2078-6891.2013.03623997938
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi:10.1038/nrc244218650835
- Huijbers EJ, van Beijnum JR, Thijssen VL, Sabrkhany S, Nowak-Sliwinska P, Griffioen AW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat. 2016;25:26–37. doi:10.1016/j.drup.2016.02.00227155374
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771. doi:10.1038/nrd261418758474
- Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv. 2010;7(6):753–763. doi:10.1517/1742524100377701020408736
- Seo D, Song H. Synthesis of gold nanoparticles in liquid phase. In Louis C, Pluchery O. Gold Nanoparticles for Physics, Chemistry and Biology. London: Imperial College Press; 2012;103–138.
- Mori T, Hegmann T. Determining the composition of gold nanoparticles: a compilation of shapes, sizes, and calculations using geometric considerations. J Nanopart Res. 2016;18(10):295. doi:10.1007/s11051-016-3587-727766020
- Zhang -S-S, Feng L, Senanayake RD, et al. Diphosphine-protected ultrasmall gold nanoclusters: opened icosahedral Au(13) and heart-shaped Au(8) clusters. Chem Sci. 2017;9(5):1251–1258. doi:10.1039/c7sc03566g29675171
- Huang R, Wen Y-H, Shao G-F, Zhu -Z-Z, Sun S-G. Single-crystalline and multiple-twinned gold nanoparticles: an atomistic perspective on structural and thermal stabilities. RSC Adv. 2014;4(15):7528–7537. doi:10.1039/c3ra46631k
- Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2003;298:2176–2179. doi:10.1126/science.1077229
- Zhang J, Langille MR, Personick ML, Zhang K, Li S, Mirkin CA. Concave cubic gold nanocrystals with high-index facets. J Am Chem Soc. 2010;132(40):14012–14014. doi:10.1021/ja106394k20853848
- Skrabalak SE, Chen J, Sun Y, et al. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 2008;41(12):1587–1595. doi:10.1021/ar800018v18570442
- Zhang Q, Zhou Y, Villarreal E, Lin Y, Zou S, Wang H. Faceted gold nanorods: nanocuboids, convex nanocuboids, and concave nanocuboids. Nano Lett. 2015;15(6):4161–4169. doi:10.1021/acs.nanolett.5b0128625927399
- Personick ML, Langille MR, Zhang J, Harris N, Schatz GC, Mirkin CA. Synthesis and isolation of {110}-faceted gold bipyramids and rhombic dodecahedra. J Am Chem Soc. 2011;133(16):6170–6173. doi:10.1021/ja201826r21452816
- Wu H-L, Chen C-H, Huang MH. Seed-mediated synthesis of branched gold nanocrystals derived from the side growth of pentagonal bipyramids and the formation of gold nanostars. Chem Mater. 2009;21(1):110–114. doi:10.1021/cm802257e
- Wu S, Yang X, Luo F, et al. Biosynthesis of flower-shaped Au nanoclusters with EGCG and their application for drug delivery. J Nanobiotechnology. 2018;16(1):90. doi:10.1186/s12951-018-0417-330424776
- Sau TK, Murphy CJ. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc. 2004;126(28):8648–8649. doi:10.1021/ja047846d15250706
- Chen S, Wang ZL, Ballato J, Foulger SH, Carroll DL. Monopod, bipod, tripod, and tetrapod gold nanocrystals. J Am Chem Soc. 2003;125(52):16186–16187. doi:10.1021/ja038927x14692749
- Shakiba A, Zenasni O, Marquez M, Randall Lee T. Advanced drug delivery via self-assembled monolayer-coated nanoparticles. AIMS Bioeng. 2017;4:275–299. doi:10.3934/bioeng.2017.2.275
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53(46):12320–12364. doi:10.1002/anie.20140303625294565
- Webb JA, Bardhan R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale. 2014;6(5):2502–2530. doi:10.1039/c3nr05112a24445488
- McIntosh CM, Esposito EA 3rd, Boal AK, Simard JM, Martin CT, Rotello VM. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J Am Chem Soc. 2001;123(31):7626–7629. doi:10.1021/ja015556g11480984
- Conde J, Ambrosone A, Sanz V, et al. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS Nano. 2012;6(9):8316–8324. doi:10.1021/nn303022322882598
- Wu Z, Liu GQ, Yang XL, Jiang JH. Electrostatic nucleic acid nanoassembly enables hybridization chain reaction in living cells for ultrasensitive mRNA imaging. J Am Chem Soc. 2015;137(21):6829–6836. doi:10.1021/jacs.5b0177825969953
- Lin J, Zhang H, Chen Z, Zheng Y. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;4(9):5421–5429. doi:10.1021/nn101079220799717
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. doi:10.1039/df9511100055
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241(105):20–22. doi:10.1038/physci241020a0
- Frens G. Particle size and sol stability in metal colloids. Kolloid-Zeitschrift und Zeitschrift für Polymere. 1972;250(7):736–741. doi:10.1007/BF01498565
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun. 1994;7:801–802. doi:10.1039/C39940000801
- Shah M, Badwaik V, Kherde Y, et al. Gold nanoparticles: various methods of synthesis and antibacterial applications. Front Biosci. 2014;19:1320. doi:10.2741/4284
- Giersig M, Mulvaney P. Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir. 1993;9(12):3408–3413. doi:10.1021/la00036a014
- Hostetler MJ, Templeton AC, Murray RW. Dynamics of place-exchange reactions on monolayer-protected gold cluster molecules. Langmuir. 1999;15(11):3782–3789. doi:10.1021/la981598f
- Murphy CJ, Jana NR. Controlling the aspect ratio of inorganic nanorods and nanowires. Adv Mater. 2002;14(1):80–82.
- Shankar SS, Bhargava S, Sastry M. Synthesis of gold nanospheres and nanotriangles by the Turkevich approach. J Nanosci Nanotechnol. 2005;5(10):1721–1727. doi:10.1166/jnn.2005.19216245535
- Bridges CR, DiCarmine PM, Fokina A, Huesmann D, Seferos DS. Synthesis of gold nanotubes with variable wall thicknesses. J Mater Chem A. 2013;1(4):1127–1133. doi:10.1039/C2TA00729K
- Shao L, Susha AS, Cheung LS, Sau TK, Rogach AL, Wang J. Plasmonic properties of single multispiked gold nanostars: correlating modeling with experiments. Langmuir. 2012;28(24):8979–8984. doi:10.1021/la204809722353020
- Jana NR, Gearheart L, Murphy CJ. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater. 2001;13(18):1389–1393. doi:10.1002/(ISSN)1521-4095
- Jana NR, Gearheart L, Murphy CJ. Seeding growth for size control of 5−40 nm diameter gold nanoparticles. Langmuir. 2001;17(22):6782–6786. doi:10.1021/la0104323
- Isaeva EI, Svistunova OB, Gorbunova VV, Boitsova TB. Photochemical synthesis of gold nanoparticles in elastomer films of poly(butyl acrylate) latex. Russ J Gen Chem. 2007;77(12):2113–2116. doi:10.1134/S1070363207120067
- Marin ML, McGilvray KL, Scaiano JC. Photochemical strategies for the synthesis of gold nanoparticles from Au(III) and Au(I) using photoinduced free radical generation. J Am Chem Soc. 2008;130(49):16572–16584. doi:10.1021/ja803490n19049456
- Niidome Y, Nishioka K, Kawasaki H, Yamada S. Rapid synthesis of gold nanorods by the combination of chemical reduction and photoirradiation processes; morphological changes depending on the growing processes. Chem Commun. 2003;18:2376–2377. doi:10.1039/B307836A
- Yusof NS, Ashokkumar M. Sonochemical synthesis of gold nanoparticles by using high intensity focused ultrasound. Chemphyschem. 2015;16(4):775–781. doi:10.1002/cphc.20140269725598360
- Gutiérrez-Wing C, Esparza R, Vargas-Hernández C, Fernández García ME, José-Yacamán M. Microwave-assisted synthesis of gold nanoparticles self-assembled into self-supported superstructures. Nanoscale. 2012;4(7):2281–2287. doi:10.1039/c2nr12053d22398420
- Chen Y-S, Hung Y-C, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4(8):858–864. doi:10.1007/s11671-009-9334-620596373
- Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12(7):2313–2333. doi:10.1007/s11051-010-9911-821170131
- Aljabali AAA, Akkam Y, Al Zoubi MS, et al. Synthesis of gold nanoparticles using leaf extract of ziziphus zizyphus and their antimicrobial activity. Nanomaterials (Basel). 2018;8(3):174. doi:10.3390/nano8030174
- Husen A. Gold nanoparticles from plant system: synthesis, characterization and their application. In: Ghorbanpour M, Manika K, Varma A, eds. Nanoscience and Plant–Soil Systems Cham: Springer International Publishing; 2017;48:455–479.
- Elia P, Zach R, Hazan S, Kolusheva S, Porat ZE, Zeiri Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine. 2014;9:4007–4021. doi:10.2147/IJN.S5734325187704
- Singh PK, Kundu S. Biosynthesis of gold nanoparticles using bacteria. Proc Natl Acad Sci India Sect B Biol Sci. 2014;84(2):331–336. doi:10.1007/s40011-013-0230-6
- Zhang X, Qu Y, Shen W, et al. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf A Physicochem Eng Asp. 2016;497:280–285. doi:10.1016/j.colsurfa.2016.02.033
- Pourali P, Badiee SH, Manafi S, Noorani T, Rezaei A, Yahyaei B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. ELECTRON J BIOTECHN. 2017;29:86–93. doi:10.1016/j.ejbt.2017.07.005
- Pei X, Qu Y, Shen W, et al. Green synthesis of gold nanoparticles using fungus Mariannaea sp. HJ and their catalysis in reduction of 4-nitrophenol. Environ Sci Pollut Res Int. 2017;24(27):21649–21659. doi:10.1007/s11356-017-9684-z28752308
- González-Ballesteros N, Prado-López S, Rodríguez-González JB, Lastra M, Rodríguez-Argüelles MC. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surf B Biointerfaces. 2017;153:190–198. doi:10.1016/j.colsurfb.2017.02.02028242372
- Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem. 2017;10:S3029–S3039. doi:10.1016/j.arabjc.2013.11.044
- Shao Y, Jin Y, Dong S. Synthesis of gold nanoplates by aspartate reduction of gold chloride. Chem Commun. 2004;9:1104–1105. doi:10.1039/b315732f
- Maruyama T, Fujimoto Y, Maekawa T. Synthesis of gold nanoparticles using various amino acids. J Colloid Interface Sci. 2015;447:254–257. doi:10.1016/j.jcis.2014.12.04625591824
- Tetgure SR, Borse AU, Sankapal BR, Garole VJ, Garole DJ. Green biochemistry approach for synthesis of silver and gold nanoparticles using Ficus racemosa latex and their pH-dependent binding study with different amino acids using UV/Vis absorption spectroscopy. Amino Acids. 2015;47(4):757–765. doi:10.1007/s00726-014-1906-925618751
- Gholami-Shabani M, Shams-Ghahfarokhi M, Gholami-Shabani Z, et al. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: a green eco-friendly approach. Process Biochem. 2015;50(7):1076–1085. doi:10.1016/j.procbio.2015.04.004
- Abraham S, Narine SS. A facile synthesis of lipid stabilized gold nanoparticles: a step towards biodegradable biosensors. J Nanosci Nanotechnol. 2011;11(8):7027–7032. doi:10.1166/jnn.2011.487822103117
- Rasch MR, Rossinyol E, Hueso JL, Goodfellow BW, Arbiol J, Korgel BA. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: membrane-loaded and janus vesicles. Nano Lett. 2010;10(9):3733–3739. doi:10.1021/nl102387n20731366
- Sharma D. A biologically friendly single step method for gold nanoparticle formation. Colloids Surf B Biointerfaces. 2011;85(2):330–337. doi:10.1016/j.colsurfb.2011.03.00521459561
- Kunoh T, Takeda M, Matsumoto S, et al. Green synthesis of gold nanoparticles coupled with nucleic acid oxidation. ACS Sustain Chem Eng. 2018;6(1):364–373. doi:10.1021/acssuschemeng.7b02610
- Anstaett P, Zheng Y, Thai T, Funston AM, Bach U, Gasser G. Synthesis of stable peptide nucleic acid-modified gold nanoparticles and their assembly onto gold surfaces. Angew Chem Int Ed Engl. 2013;52(15):4217–4220. doi:10.1002/anie.20120968423460137
- Das APDR, Nath SS, Bhattacharjee R. Preparation of linoleic acid capped gold nanoparticles and their spectra. Physica E Low Dimens Syst Nanostruct. 2010;43:224–227. doi:10.1016/j.physe.2010.07.008
- Kitaoka T, Yokota S, Opietnik M, Rosenau T. Synthesis and bio-applications of carbohydrate–gold nanoconjugates with nanoparticle and nanolayer forms. Mat Sci Eng C Mater. 2011;31(6):1221–1229. doi:10.1016/j.msec.2010.10.009
- Tagad CK, Rajdeo KS, Kulkarni A, More P, Aiyer RC, Sabharwal S. Green synthesis of polysaccharide stabilized gold nanoparticles: chemo catalytic and room temperature operable vapor sensing application. RSC Adv. 2014;4(46):24014–24019. doi:10.1039/c4ra02972k
- Pandey S, Goswami GK, Nanda KK. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: an efficient ammonia sensor. Carbohydr Polym. 2013;94(1):229–234. doi:10.1016/j.carbpol.2013.01.00923544533
- Mao Z, Zhou X, Gao C. Influence of structure and properties of colloidal biomaterials on cellular uptake and cell functions. Biomater Sci. 2013;1(9):896–911. doi:10.1039/c3bm00137g
- Panzarini E, Mariano S, Carata E, Mura F, Rossi M, Dini L. Intracellular transport of silver and gold nanoparticles and biological responses: an update. Int J Mol Sci. 2018;19(5):1305. doi:10.3390/ijms19051305
- Zhu M, Nie G, Meng H, Xia T, Nel A, Zhao Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res. 2013;46(3):622–631. doi:10.1021/ar300031y22891796
- Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi:10.1021/nl052396o16608261
- Ko WK, Heo DN, Moon HJ, et al. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J Colloid Interface Sci. 2015;438:68–76. doi:10.1016/j.jcis.2014.08.05825454427
- Fernandez TD, Pearson JR, Leal MP, et al. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials. 2015;43:1–12. doi:10.1016/j.biomaterials.2014.11.04525591956
- Le Guevel X, Perez Perrino M, Fernandez TD, et al. Multivalent glycosylation of fluorescent gold nanoclusters promotes increased human dendritic cell targeting via multiple endocytic pathways. ACS Appl Mater Interfaces. 2015;7(37):20945–20956. doi:10.1021/acsami.5b0654126329370
- Saha K, Kim ST, Yan B, et al. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small. 2013;9(2):300–305. doi:10.1002/smll.20120112922972519
- Fytianos K, Rodriguez-Lorenzo L, Clift MJ, et al. Uptake efficiency of surface modified gold nanoparticles does not correlate with functional changes and cytokine secretion in human dendritic cells in vitro. Nanomedicine. 2015;11(3):633–644. doi:10.1016/j.nano.2014.11.00425555350
- Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7(1):3827. doi:10.1038/s41598-017-04229-z28630477
- Pan F, Yang W, Li W, et al. Conjugation of gold nanoparticles and recombinant human endostatin modulates vascular normalization via interruption of anterior gradient 2-mediated angiogenesis. Tumour Biol. 2017;39(7):1010428317708547. doi:10.1177/101042831770854728714365
- Balakrishnan S, Bhat FA, Raja Singh P, et al. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016;49(6):678–697. doi:10.1111/cpr.1229627641938
- Saber MM, Bahrainian S, Dinarvand R, Atyabi F. Targeted drug delivery of Sunitinib Malate to tumor blood vessels by cRGD-chiotosan-gold nanoparticles. Int J Pharm. 2017;517(1–2):269–278. doi:10.1016/j.ijpharm.2016.12.01627956189
- Pan Y, Ding H, Qin L, Zhao X, Cai J, Du B. Gold nanoparticles induce nanostructural reorganization of VEGFR2 to repress angiogenesis. J Biomed Nanotechnol. 2013;9(10):1746–1756.24015504
- Shen N, Zhang R, Zhang HR, et al. Inhibition of retinal angiogenesis by gold nanoparticles via inducing autophagy. Int J Ophthalmol. 2018;11(8):1269–1276. doi:10.18240/ijo.2018.08.0430140628
- Jo DH, Hong JW, Kim JH, Han SW, Kim JH. Gold nanocrystals with well-defined crystallographic {111} facets suppress pathological neovascularization. J Biomed Nanotechnol. 2016;12(7):1520–1526.29337491
- Pan Y, Wu Q, Qin L, Cai J, Du B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed Res Int. 2014;(2014:418624.24987682
- Reva GV, Reva IV, Yamamoto T, et al. Reaction of dermal structures to subcutaneous injection of gold nanoparticles to CBA mice. Bull Exp Biol Med. 2014;156(4):491–494. doi:10.1007/s10517-014-2382-724771435
- Kemp MM, Kumar A, Mousa S, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20(45):455104. doi:10.1088/0957-4484/20/45/45510419822927
- Song HB, Wi JS, Jo DH, et al. Intraocular application of gold nanodisks optically tuned for optical coherence tomography: inhibitory effect on retinal neovascularization without unbearable toxicity. Nanomedicine. 2017;13(6):1901–1911. doi:10.1016/j.nano.2017.03.01628400160
- Tan G, Onur MA. Cellular localization and biological effects of 20nm-gold nanoparticles. J Biomed Mater Res A. 2018;106(6):1708–1721. doi:10.1002/jbm.a.3637329468810
- Vimalraj S, Ashokkumar T, Saravanan S. Biogenic gold nanoparticles synthesis mediated by Mangifera indica seed aqueous extracts exhibits antibacterial, anticancer and anti-angiogenic properties. Biomed Pharmacother. 2018;105:440–448. doi:10.1016/j.biopha.2018.05.15129879628
- Pan F, Li W, Yang W, et al. Anterior gradient 2 as a supervisory marker for tumor vessel normalization induced by anti-angiogenic treatment. Oncol Lett. 2018;16(3):3083–3091. doi:10.3892/ol.2018.899630127899
- Li W, Li X, Liu S, et al. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-mesenchymal transition inhibition. Int J Nanomedicine. 2017;12:3509–3520. doi:10.2147/IJN.S12880228496326
- Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11(9):3530–3534. doi:10.1158/1078-0432.CCR-04-248215867256
- Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P. Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine. 2011;7(5):580–587. doi:10.1016/j.nano.2011.01.01121333757
- Kim JH, Kim MH, Jo DH, Yu YS, Lee TG, Kim JH. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32(7):1865–1871. doi:10.1016/j.biomaterials.2010.11.03021145587
- Satapathy SR, Nayak A, Siddharth S, Das S, Nayak D, Kundu CN. Metallic gold and bioactive quinacrine hybrid nanoparticles inhibit oral cancer stem cell and angiogenesis by deregulating inflammatory cytokines in p53 dependent manner. Nanomedicine. 2018;14(3):883–896. doi:10.1016/j.nano.2018.01.00729366881
- Pivodova V, Frankova J, Galandakova A, Ulrichova J. In vitro AuNPs’ cytotoxicity and their effect on wound healing. Nanobiomedicine (Rij). 2015;2:7. doi:10.5772/6217429942372
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi:10.1002/smll.20040009317193451
- Mukherjee P, Bhattacharya R, Bone N, et al. Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): enhancing apoptosis. J Nanobiotechnology. 2007;5:4. doi:10.1186/1477-3155-5-417488514
- Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79(939):248–253. doi:10.1259/bjr/1316988216498039
- Amendola V, Pilot R, Frasconi M, Marago OM, Iati MA. Surface plasmon resonance in gold nanoparticles: a review. J Phys Condens Matter. 2017;29(20):203002. doi:10.1088/1361-648X/aa60f328426435
- Lau P, Bidin N, Islam S, et al. Influence of gold nanoparticles on wound healing treatment in rat model: photobiomodulation therapy. Lasers Surg Med. 2017;49(4):380–386. doi:10.1002/lsm.2261427859389
- Saha S, Xiong X, Chakraborty PK, et al. Gold nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano. 2016;10(12):10636–10651. doi:10.1021/acsnano.6b0223127758098
- Roma-Rodrigues C, Heuer-Jungemann A, Fernandes AR, Kanaras AG, Baptista PV. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int J Nanomedicine. 2016;11:2633–2639. doi:10.2147/IJN.S10866127354794
- Groult H, Ruiz-Cabello J, Pellico J, et al. Parallel multifunctionalization of nanoparticles: a one-step modular approach for in vivo imaging. Bioconjug Chem. 2015;26(1):153–160. doi:10.1021/bc500536y25494619
- Vilchis-Juarez A, Ferro-Flores G, Santos-Cuevas C, et al. Molecular targeting radiotherapy with cyclo-RGDFK(C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J Biomed Nanotechnol. 2014;10(3):393–404.24730235
- Li M, Li Y, Huang X, Lu X. Captopril-polyethyleneimine conjugate modified gold nanoparticles for co-delivery of drug and gene in anti-angiogenesis breast cancer therapy. J Biomater Sci Polym Ed. 2015;26(13):813–827. doi:10.1080/09205063.2015.105799126166244
- Yang Y, Zhang L, Cai J, et al. Tumor angiogenesis targeted radiosensitization therapy using gold nanoprobes guided by MRI/SPECT imaging. ACS Appl Mater Interfaces. 2016;8(3):1718–1732. doi:10.1021/acsami.5b0927426731347
- Mieszawska AJ, Kim Y, Gianella A, et al. Synthesis of polymer-lipid nanoparticles for image-guided delivery of dual modality therapy. Bioconjug Chem. 2013;24(9):1429–1434. doi:10.1021/bc400166j23957728
- Bhowmik T, Saha PP, DasGupta AK, Gomes A. Influence of gold nanoparticle tagged snake venom protein toxin NKCT1 on Ehrlich ascites carcinoma (EAC) and EAC induced solid tumor bearing male albino mice. Curr Drug Deliv. 2014;11(5):652–664.24827982
- Kim JE, Lee J, Jang M, et al. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater Sci. 2015;3(3):509–519. doi:10.1039/c4bm00390j26222294
- Chen Y, Wu Y, Gao J, et al. Transdermal vascular endothelial growth factor delivery with surface engineered gold nanoparticles. ACS Appl Mater Interfaces. 2017;9(6):5173–5180. doi:10.1021/acsami.6b1591428112909
- Chien CC, Chen HH, Lai SF, et al. Gold nanoparticles as high-resolution X-ray imaging contrast agents for the analysis of tumor-related micro-vasculature. J Nanobiotechnology. 2012;10:10. doi:10.1186/1477-3155-10-1022409971
- Son S, Kim N, You DG, et al. Antitumor therapeutic application of self-assembled RNAi-AuNP nanoconstructs: combination of VEGF-RNAi and photothermal ablation. Theranostics. 2017;7(1):9–22. doi:10.7150/thno.1604228042312
- Nethi SK, Mukherjee S, Veeriah V, Barui AK, Chatterjee S, Patra CR. Bioconjugated gold nanoparticles accelerate the growth of new blood vessels through redox signaling. Chem Commun (Camb). 2014;50(92):14367–14370. doi:10.1039/c4cc06996j25298204
- Gomez-Beloz A, Rucinski JC, Balick MJ, Tipton C. Double incision wound healing bioassay using Hamelia patens from El Salvador. J Ethnopharmacol. 2003;88(2–3):169–173.12963138
- Wang B, Wang W, Yu Y, Zhang Y, Zhang J, Yuan Z. The study of angiogenesis stimulated by multivalent peptide ligand-modified alginate. Colloids Surf B Biointerfaces. 2017;154:383–390. doi:10.1016/j.colsurfb.2017.03.04928384617
- Liu Y, Tan TTY, Yuan S, Choong C. Multifunctional P(PEGMA)–REDV conjugated titanium surfaces for improved endothelial cell selectivity and hemocompatibility. J Mater Chem B. 2013;1:(2):157–167. doi:10.1039/C2TB00014H
- Chien CC, Chen HH, Lai SF, et al. X-ray imaging of tumor growth in live mice by detecting gold-nanoparticle-loaded cells. Sci Rep. 2012;2:610. doi:10.1038/srep0038622934133
- Clark DP, Ghaghada K, Moding EJ, Kirsch DG, Badea CT. In vivo characterization of tumor vasculature using iodine and gold nanoparticles and dual energy micro-CT. Phys Med Biol. 2013;58(6):1683–1704. doi:10.1088/0031-9155/58/6/168323422321
- Bao C, Beziere N, Del Pino P, et al. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small. 2013;9(1):68–74. doi:10.1002/smll.20120177923001862
- Kim YH, Jeon J, Hong SH, et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small. 2011;7(14):2052–2060. doi:10.1002/smll.20110092721688390
- Morales-Avila E, Ferro-Flores G, Ocampo-Garcia BE, et al. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor alpha(v)beta(3) expression. Bioconjug Chem. 2011;22(5):913–922. doi:10.1021/bc100551s21513349
- Pan D, Pramanik M, Senpan A, et al. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. Faseb J. 2011;25(3):875–882. doi:10.1096/fj.10-17172821097518
- Zhang S, Gong M, Zhang D, Yang H, Gao F, Zou L. Thiol-PEG-carboxyl-stabilized Fe(2)O (3)/Au nanoparticles targeted to CD105: synthesis, characterization and application in MR imaging of tumor angiogenesis. Eur J Radiol. 2014;83(7):1190–1198. doi:10.1016/j.ejrad.2014.03.03424832501
- Kennedy LC, Bickford LR, Lewinski NA, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7(2):169–183. doi:10.1002/smll.20100013421213377
- Kharlamov AN, Feinstein JA, Cramer JA, Boothroyd JA, Shishkina EV, Shur V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017;13(4):345–363. doi:10.2217/fca-2017-000928644056
- Mesicek J, Kuca K. Summary of numerical analyses for therapeutic uses of laser-activated gold nanoparticles. Int J Hyperthermia. 2018;34(8):1255–1264. doi:10.1080/02656736.2018.144001629447018
- Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):e1449.
- Gamal-Eldeen AM, Moustafa D, El-Daly SM, et al. Gum Arabic-encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed Pharmacother. 2017;89:1045–1054. doi:10.1016/j.biopha.2017.03.00628298068
- Bai YY, Zheng S, Zhang L, et al. Non-invasively evaluating therapeutic response of nanorod-mediated photothermal therapy on tumor angiogenesis. J Biomed Nanotechnol. 2014;10(11):3351–3360.26000393
- Pedrosa P, Heuer-Jungemann A, Kanaras AG, Fernandes AR, Baptista PV. Potentiating angiogenesis arrest in vivo via laser irradiation of peptide functionalised gold nanoparticles. J Nanobiotechnology. 2017;15(1):85. doi:10.1186/s12951-017-0305-229162137
- Nie L, Wang S, Wang X, et al. In vivo volumetric photoacoustic molecular angiography and therapeutic monitoring with targeted plasmonic nanostars. Small. 2014;10(8):1585–1593, 1441. doi:10.1002/smll.20130292424150920
- Liu S, Li H, Xia L, et al. Anti-RhoJ antibody functionalized Au@I nanoparticles as CT-guided tumor vessel-targeting radiosensitizers in patient-derived tumor xenograft model. Biomaterials. 2017;141:1–12. doi:10.1016/j.biomaterials.2017.06.03628666098
- Abo-Elfadl MT, Gamal-Eldeen AM, Elshafey MM, et al. Photothermal therapeutic effect of PEGylated gold nano-semicubes in chemically-induced skin cancer in mice. J Photochem Photobiol B. 2016;164:21–29. doi:10.1016/j.jphotobiol.2016.09.01227636008
- Jia Y-P, Ma B-Y, Wei X-W, Qian Z-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin Chem Lett. 2017;28(4):691–702. doi:10.1016/j.cclet.2017.01.021
- Fratoddi I, Venditti I, Cametti C, Russo MV. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015;8(6):1771–1799. doi:10.1007/s12274-014-0697-3
- Falagan-Lotsch P, Grzincic EM, Murphy CJ. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc Natl Acad Sci U S A. 2016;113(47):13318–13323. doi:10.1073/pnas.161640011327821760
- Gerber A, Bundschuh M, Klingelhofer D, Groneberg DA. Gold nanoparticles: recent aspects for human toxicology. J Occup Med Toxicol. 2013;8:32. doi:10.1186/1745-6673-8-3224330512
- Sabella S, Carney RP, Brunetti V, et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale. 2014;6(12):7052–7061. doi:10.1039/c4nr01234h24842463
- Qu Y, Lu X. Aqueous synthesis of gold nanoparticles and their cytotoxicity in human dermal fibroblasts-fetal. Biomed Mater. 2009;4(2):025007. doi:10.1088/1748-6041/4/2/02500719258699
- Wei X-L, Mo Z-H, Li B, Wei J-M. Disruption of HepG2 cell adhesion by gold nanoparticle and Paclitaxel disclosed by in situ QCM measurement. Colloids Surf B Biointerfaces. 2007;59(1):100–104. doi:10.1016/j.colsurfb.2007.04.01617566716
- Babin C, Gagnaire P-A, Pavey SA, Bernatchez L. RAD-seq reveals patterns of additive polygenic variation caused by spatially-varying selection in the american eel (Anguilla rostrata). Genome Biol Evol. 2017;9(11):2974–2986. doi:10.1093/gbe/evx22629136139
- Hwang JH, Kim SJ, Kim Y-H, et al. Susceptibility to gold nanoparticle-induced hepatotoxicity is enhanced in a mouse model of nonalcoholic steatohepatitis. Toxicology. 2012;294(1):27–35. doi:10.1016/j.tox.2012.01.01322330258
- Kim KT, Zaikova T, Hutchison JE, Tanguay RL. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicological sciences: an official journal of the Society of Toxicology. 2013;133(2):275–288.23549158
- Vecchio G, Galeone A, Brunetti V, et al. Mutagenic effects of gold nanoparticles induce aberrant phenotypes in Drosophila melanogaster. Nanomedicine. 2012;8(1):1–7. doi:10.1016/j.nano.2011.11.00122094122
- Tsyusko OV, Unrine JM, Spurgeon D, et al. Toxicogenomic responses of the model organism Caenorhabditis elegans to gold nanoparticles. Environ Sci Technol. 2012;46(7):4115–4124. doi:10.1021/es203310822372763
- Yang Y, Qin Z, Zeng W, et al. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol Rev. 2016;6:279–289.
