?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The aim of this study was to develop new systems for transdermal delivery of paeonol, in particular microemulsion gel and cubic gel formulations.
Methods
Various microemulsion vehicles were prepared using isopropyl myristate as an oil phase, polyoxyethylated castor oil (Cremophor® EL) as a surfactant, and polyethylene glycol 400 as a cosurfactant. In the optimum microemulsion gel formulation, carbomer 940 was selected as the gel matrix, and consisted of 1% paeonol, 4% isopropyl myristate, 28% Cremophor EL/polyethylene glycol 400 (1:1), and 67% water. The cubic gel was prepared containing 3% paeonol, 30% water, and 67% glyceryl monooleate.
Results
A skin permeability test using excised rat skins indicated that both the cubic gel and microemulsion gel formulations had higher permeability than did the paeonol solution. An in vivo pharmacokinetic study done in rats showed that the relative bioavailability of the cubic gel and microemulsion gel was enhanced by about 1.51-fold and 1.28-fold, respectively, compared with orally administered paeonol suspension.
Conclusion
Both the cubic gel and microemulsion gel formulations are promising delivery systems to enhance the skin permeability of paeonol, in particular the cubic gel.
Introduction
Paeonol, the active constituent of Cortex moutan, is widely used in traditional Chinese medicine as a pain reliever, anticoagulant, antibiotic, and antiatherosclerotic agent. In recent years, many studies have shown that paeonol has a number of therapeutic effects, including antianalgesic, antihypertensive, anticoagulant, antibacterial, anti-inflammatory, immunoregulatory, antiatherosclerotic, and central nervous system inhibitory activities.Citation1–Citation3
A microemulsion is a nanosized formulation in which active drug is dispersed in a mixture of water, oil, and surfactant, frequently in combination with a cosurfactant, and is an optically clear, stable, isotropic liquid, containing particles with diameters of 100 nm and less.Citation4,Citation5 Incorporation of a gel matrix into an o/w microemulsion provides the opportunity to obtain properties comparable with those attainable using hydrogels. Formulations of microemulsion gel were first reported in 1986,Citation6,Citation7 and since then their structure and physicochemical properties have been described.Citation8–Citation10 Microemulsion gels can increase the permeability of some drugs.
A cubic gel is formed spontaneously when amphiphilic lipids are placed in an aqueous environment. A cubic gel consists of a curved bicontinuous lipid bilayer and has a thermodynamically stable structure. Glyceryl monooleate/water systems are an example. They can incorporate small-molecule drugs and large proteins,Citation11,Citation12 and have the ability to enhance permeability.Citation13
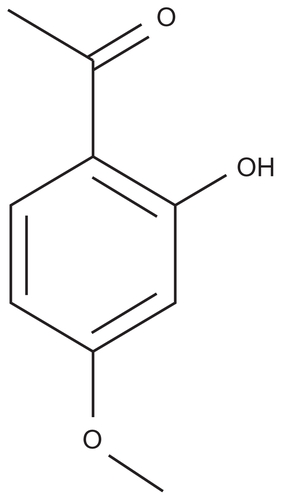
Currently commercialized preparations of paeonol include a cyclodextrin injection, oral solution, and ointment.Citation14 Although paeonol preparations of gel and emulsion are being studied, combinations of paeonol with cubic gel and microemulsion gel have been less widely reported. Paeonol is a small phenolic compound showing white crystalline needles. It has the characteristics of a low melting point, volatility, and poor water solubility. The molecular weight of paeonol is 166.18, and its chemical structure is 2-hydroxy-4-methoxy acetophenone (). Paeonol is absorbed rapidly after oral administration, and its poor oral bioavailability is suggested to be due to rapid and complete first-pass metabolism of the drug.Citation15 Paeonol is more suitable for transdermal delivery. In order to enhance the permeability and stability of paeonol, the microemulsion gel and cubic gel formulations were selected for investigation as drug delivery systems. The purpose of this work was to prepare a microemulsion gel and cubic gel for transdermal delivery of paeonol, to study the skin permeability of paeonol in microemulsion gel and in cubic gel, and evaluate whether the microemulsion gel and cubic gel formulations could improve the bioavailability of paeonol compared with oral administration.
Materials and methods
Materials
Paeonol (99% purity) was obtained from Nanjing Qingze Pharmaceutical Technology Development Co Ltd (Nanjing, China). Glyceryl monooleate (>99%) was supplied by Danisco (Copenhagen, Denmark). Carbomer 940 was purchased from Shanghai Xietai Chem Co Ltd (Shanghai, China). Transcutol® P and Labrafac® were kindly donated by Gattefosse (St Priest, France). Cremophor® EL (Crel) and Cremophor® RH 40 (CRH40) were donated by BASF (Ludwigshafen, Germany). Isopropyl myristate, oleic acid, ethyl oleate, propylene glycol, castor oil, ethanol, p-octy polyethylene glycol phenylether, polyethylene glycol 400, and Tween80 were purchased from Sinopharm Chemical Reagent Corporation (Shanghai, China). Soybean oil was obtained from Tieling North Asia Medicinal Oil Co Ltd (Tieling, China).
Pseudoternary diagrams
To determine the composition of microemulsions, pseudoternary phase diagrams were constructed by titrating a series of oil, surfactant, and cosurfactant with water mixtures at ambient temperature (25°C). Surfactants and cosurfactants were blended at different mass ratios (1: 2, 1:1, 2:1, 4:1), then each surfactant-cosurfactant mixture was mixed with oil, and the ratio of oil to the surfactant and cosurfactant mixture was varied at 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9 (w/w). Water was added drop by drop to the mixture under vigorous stirring. During titration, the isotropic, liquid crystalline, and coarse emulsion phases were observed with turbidity-to- transparency or transparency-to-turbidity transitions occurring. Clear and transparent formulations were indicative of a stable microemulsion, and their compositions were recorded. The physical states were marked on a pseudoternary phase diagram representing the surfactant and cosurfactant mixture, with the other two axes representing oil and water.
Preparation of microemulsions
Based on the phase diagram, different formulae were selected from the microemulsion region (). Exactly 1% w/w of paeonol was dissolved in the oil, surfactant, and cosurfactant mixtures, and water was added drop by drop under magnetic stirring at room temperature. The control solution was prepared by dissolving paeonol in phosphate-buffered saline at pH 7.4 to achieve a concentration 400 μg/mL and then stirring the solution at 1000 rpm.
Table 1 Compositions of various topical formulations for paeonol delivery
Microemulsion droplet size and morphology analysis
Microemulsion particle size was determined using photo-correlation spectroscopy (Malvern, Worcestershire, UK) at 25°C. Droplet morphology was observed using transmission electron microscopy (TEM) (JEM-2010; JEOL, Tokyo, Japan). One drop of each diluted sample was deposited on a film-coated 200-mesh copper specimen grid and allowed to stand for 10 minutes, after which any excess fluid was removed with filter paper. The grid was then stained with one drop of 3% phosphotungstic acid and dried for 5 minutes before examination with an electron microscope.
Preparation of microemulsion gel
Carbomer 940 0.5%–2.0% (w/w) swelled after addition of water, and pH was adjusted using triethanolamine 0.5%–2.0% (w/w). The oily solution was prepared by mixing Crel, polyethylene glycol 400, isopropyl myristate, and paeonol, and was then swelled with carbomer 940 and vigorously mixed until a homogeneous dispersion was obtained.
Preparation of cubic gel
Paeonol 3% w/w was mixed with melted glyceryl monooleate in glass vials at 42°C until completely dissolved or dispersed. Distilled water preheated to 42°C was incorporated gradually into the mixture at 30% w/w. Samples were kept at room temperature in the dark for 3 days.
In vitro permeability studies
The abdominal skins of male Wistar rats weighing 220 ± 20 g were used for the permeability experiments, which were carried out in accordance with the guidelines of the animal ethics committee at Shanghai Jiao Tong University. The abdominal skin was carefully removed leaving the fat tissue behind. The skin was examined under a magnifying lens for damage or disease, and any skin with a disrupted barrier was excluded. Freshly prepared abdominal skin was used in the diffusion experiments. Vertical Franz-type diffusion cells (Shanghai Institute of Organic Chemistry) with a diffusional surface area of 0.754 cm2 and a 5 mL cell volume were used to study the permeability of the microemulsion gel and cubic gel formulations. The skin was placed between the donor and receptor compartments of the cells, with the stratum corneum side facing the donor compartment. The receiver compartment was filled with 5 mL of phosphate-buffered saline at pH 7.4, with temperature maintained at 32°C ± 0.5°C using a thermostatic water bath (Variomag, Munich, Germany) and stirring at 600 rpm throughout the experiment. Microemulsion 1 mL and gel 1 g samples containing paeonol were placed into each donor compartment, and 0.2 mL of the receptor solution was withdrawn at predetermined time points (hours 1, 2, 3, 4, 5, 6, 7, and 8), then replaced with an equivalent volume of phosphate-buffered saline to maintained a constant volume. The samples collected were filtered through a 0.45 μm membrane and assayed by high-pressure liquid chromatography. The cumulative amount of paeonol that permeated through the rat skins was calculated using the equation:
where Q was the accumulated amount at a given time t, Cn was the drug concentration of the n sample at the sampling time, ∑Cp was the sum of the drug concentration of all the n–1 samples measured, and Cnc was the transdermal concentration after correction. The cumulative amount (Q) of paeonol permeated was plotted as a function of time (t) for each formulation. The skin permeation rate at steady-state (Jss, μg/cm2/hour) was calculated from the slope of the linear portion of the plots of Q versus time. The permeability coefficient was calculated by dividing Jss by the initial concentration of drug in the donor cell (C0):Citation16
The penetration-enhancing effect of paeonol was calculated in terms of the enhancement ratio, and was calculated using the following equation:Citation17
where Kp is the respective formulation and K0 represents the control solution.
In vivo permeability studies
Male Wistar rats weighing 200–230 g were obtained from the Animal Center of Shanghai Jiao Tong University, and fasted overnight with free access to water before drug administration. Hairs at the abdominal site were shaved off and the skin area washed with distilled water the day before the experiment. Cubic gel and microemulsion gel (30 mg/kg) were applied on the skin surface (7.9 cm2), which was then bound with gauze. Blood samples (0.25 mL) were collected at hours 0, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, and 12.0 after transdermal administration. For the oral pharmacokinetic studies, paeonol was suspended in 0.5% sodium carboxymethyl cellulose and administered (30 mg/kg) as the control. Blood samples were collected from the jugular vein at 0, 0.08, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, and 12.0 hours after dosing.
All collected samples were immediately centrifuged at 4000 rpm for 10 minutes. Plasma 0.1 mL was added to cinnamic acid 10 μL and vortex-mixed with acetonitrile 2 mL for 1 minute and centrifuged at 10,000 rpm for 10 minutes. The supernatant was transferred to a clean test tube and evaporated to dryness at 25°C under a stream of nitrogen. The residue was dissolved in methanol 1 mL, and 20 μL of the sample solution was injected into the high-pressure liquid chromatography system.
Analysis of paeonol
Paeonol was analyzed using a reversed phase high-pressure liquid chromatography system. The chromatographic system consisted of a Waters 717 Plus autosampler (Waters, Milford, MA) and a Waters 2487 dual λ absorbance detector equipped with a prepacked Hypersil ODS column (150 mm × 4.6 mm, 5 μm, Dalian Elite Analytical Instruments Co Ltd, Dalian, China). The mobile phase for analysis of paeonol consisted of methanol, water, and acetic acid (53:47:0.01, v/v). The eluent was pumped at a flow rate of 1.0 mL/min. The detector wave-length was set at 274 nm. The injection volume was 20 μL.
Statistical analysis
All skin permeability experiments were repeated three times, and the results expressed as means ± standard deviation. Statistical significance was assessed using the Student’s t-test for multiple comparison. A P value < 0.05 was considered to indicate statistical significance.
Results and discussion
Pseudoternary diagrams
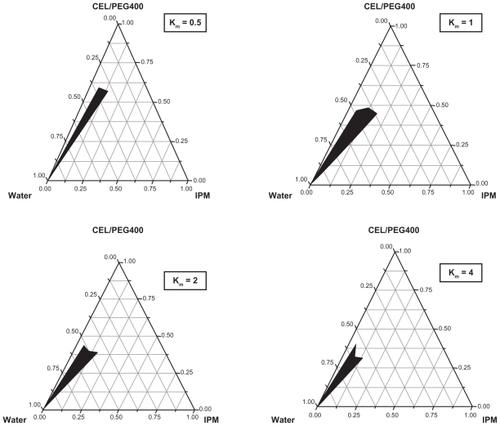
Pseudoternary phase diagrams of the system containing isopropyl myristate, Crel, polyethylene glycol 400, and water are shown in . The black areas indicate the clear o/w microemulsion region. The microemulsion domain was determined by visual inspection for clarity and fluidity as well as through a cross polarizer for the absence of a liquid crystalline phase. The rest of the region in the phase diagram represents turbid and conventional emulsions based on visual observation. No liquid crystalline structure was observed using the cross polarizer. Phase studies were used to estimate the effect of surfactant/cosurfactant (S/Cos) in stable o/w microemulsion regions. As shown in , the monophasic zone of o/w microemulsion reaches a maximum at a S/Cos of 1.
Figure 2 Pseudoternary phase diagrams of microemulsion composed of isopropyl myristate, surfactant (Crel), cosurfactant (PEG400) and water.
Note: Black area represents o/w microemulsion region; Km = S/Cos.
Abbreviations: CEL, Cremophor EL®, polyoxyethylated castor oil; Crel, Cremophor EL; IPM, isopropyl myristate; o/w, oil in water; PEG 400, polyethylene glycol.
Characteristics and physicochemical properties of the microemulsions
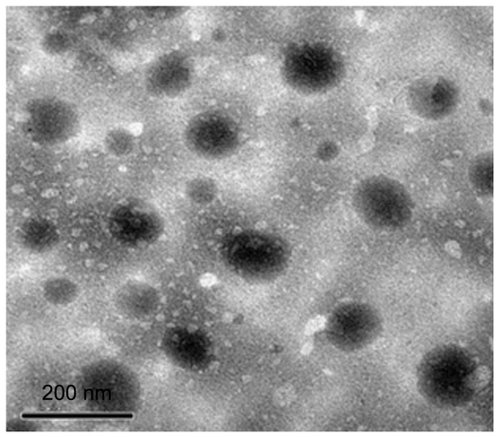
The particle size of the microemulsion was one of the most important properties. All the microemulsions (A–F) had small average droplet diameters of 27–60 nm. As a result, the droplet size of formulation A was 39.80 ± 4.6 nm. The morphology of microemulsion formulation A was characterized using transmission electron microscopy (), and showed a spherical shape and uniform droplet size. Samples were diluted with distilled water before testing to avoid multiscattering phenomena. After that, the droplet size of the diluted microemulsion was not significantly changed. The mean particle size remained consistent for 3 months at room temperature.
Preparation of gels
In this work, carbomer 940 was added to vehicle A to prepare the microemulsion gel. We found that carbomer 940 could increase the viscosity of the microemulsion, maintain the microemulsion structure, and provide a good matrix for the microemulsion gel.
Microemulsion gel containing 0.5% carbomer 940 had relatively high fluidity. The viscosity of the microemulsion gel increased with increasing concentrations of carbomer 940. However, 2% carbomer 940 resulted in excessive microemulsion gel viscosity. Microemulsion gel containing 1% carbomer 940 has suitable viscosity for topical application.Citation18 As far as we know, glyceryl monooleate, monoolein, phosphatidylethanolamine, phospholipids, and PEGylated phospholipidsCitation19–Citation22 are used to form the cubic phases. In this study, glyceryl monooleate was selected to prepare the cubic gel. After 3 days of equilibration, the cubic gel developed into glass-clear, nonbirefingent, and stiff cubic phase forms, and had greater transparency than the microemulsion gel. Both microemulsion gel with 1% carbomer 940 and the cubic gel were stable at 30°C. No changes in phase separation or paeonol degradation were observed over 3 months. The centrifuge test showed that all the gels still maintained good physical stability.
In vitro permeability studies
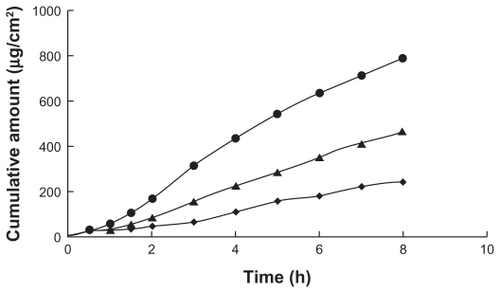
In vitro skin studies were performed to compare drug permeability from six different microemulsion formulations, and the percutaneous parameters, including permeability coefficient and enhancement ratio, were calculated following zero order release kinetics. A steady increase in the amount of permeated drug in the receptor compartment with time was observed. The permeation coefficient ranged from 1.68 ± 0.31 to 6.53 ± 1.08 cm/hour × 10−3, and the highest value was for vehicle A and the lowest was for vehicle C, so vehicle A was selected to prepare the microemulsion gel.
As shown in , the permeability coefficient of the microemulsion gel was 5.88 ± 0.28 cm/hour × 10−3, which was less than that for vehicle A. Vehicle A and the microemulsion gel produced a significantly higher permeability coefficient, ie, a 2.13-fold and 1.92-fold increase, respectively, compared with the control solution. Peltola et al reported that carbomer 940 might influence the permeability of estradiol. The permeability of paeonol decreased with the addition of carbomer 940 to the microemulsion, possibly because of increased viscosity and the changing structure of the vehicle.Citation23,Citation24 In this work, the addition of 1% carbomer 940 had the same influence on the permeability of paeonol.
Table 2 Permeability coefficient and enhancement ratio of paeonol through excised rat skin from different vehicles
shows the permeability of paeonol through rat skin. The cumulative amount of paeonol in the cubic gel was significantly higher (P < 0.05) than that of paeonol in the microemulsion gel and control solution. With a permeability coefficient of 8.34 ± 0.49 cm/hour × 10−3, cubic gel was a better carrier for transdermal delivery of paeonol than the microemulsion gel and control solution. There may be two factors accounting for this. Firstly, the content of paeonol in the cubic gel was higher than in the microemulsion gel, which means that the cubic gel had better drug solubility, which could contribute to improved absorption of the drug.Citation25,Citation26 Secondly, the structure of the cubic gel might be better than that of the microemulsion gel for drug delivery. The structure of the cubic phase is unique and consists of a curved bicontinuous lipid bilayer, which resembles the structure of lipid membranes found in nature,Citation27,Citation28 so might result in good adhesiveness to the skin and good delivery of the drug.
In vivo permeability studies
The mean plasma concentration versus time profiles for paeonol after oral and transdermal administration are shown in , and the mean pharmacokinetic parameters calculated by DAS2.1.1 are given in . These show that paeonol is rapidly absorbed, with a maximum concentration of 1.64 ± 0.14 μg/mL at 0.25 ± 0.06 hours after a single oral dose. The time taken to reach maximum concentration of paeonol in the cubic gel and microemulsion gel was 3 hours and 2 hours, respectively, after transdermal administration. This result is similar to the phenomenon reported by Sun et al, who detected the maximum concentration at 5 minutes after an oral dose.Citation29 This was due to dermal accumulation of paeonol because of the barrier properties of skin. An increase in the area under the concentration-time curve (AUC) for paeonol was observed when the cubic gel and microemulsion gel were studied. The relative bioavailability of paeonol in cubic gel and microemulsion gel was 1.51 and 1.28 times higher, respectively, than after oral administration of the drug. Mean residence times following a transdermal dose of cubic gel and microemulsion gel were 5.63 ± 1.32 hours and 5.62 ± 1.11 hours, respectively, which were higher than that for paeonol suspension.
Table 3 Pharmacokinetic parameters obtained after oral and transdermal administration of paeonol in rats
Figure 5 Plasma concentration of paeonol following oral and transdermal administration to rats.
Notes: Means ± standard deviation; n = 6; -▴-, oral administration; -●-, microemulsion gel transdermal administration; -■-, cubic gel transdermal administration.
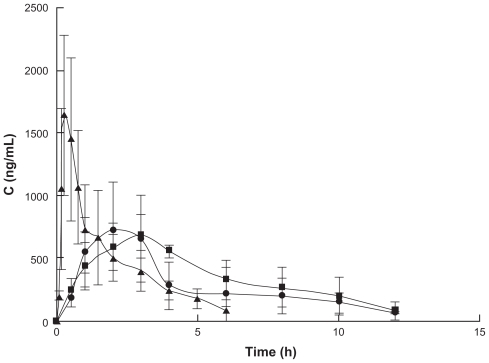
Compared with oral administration, transdermal administration had a longer time to reach maximum plasma concentration, a lower maximum plasma concentration, higher AUC, and higher mean residence time, because of avoidance of the substantial amount of hepatic first-pass metabolism associated with oral administration.
The mechanism for the enhanced transdermal penetration of microemulsion gel and cubic gel for paeonol might be attributable to several factors. Firstly, the high concentration (1%) of paeonol in the microemulsions resulted in high concentration gradients, which might be the main mechanism by which paeonol in a microemulsion gel penetrates the skin.Citation26
Microemulsions could act as drug reservoirs whereby drug is released from an inner phase to an outer phase and then into the skin.Citation24 Secondly, due to the small droplet size, droplets settling in close contact with the skin might penetrate the skin surface.Citation24
In the case of cubic phase gel, glyceryl monooleate could intercalate with the extracellular lipid matrix of the skin, and disrupt its well organized compact structure.Citation30 Some studies have reported that enhanced transdermal delivery of drugs may depend on a disordered state, or depend on a different order of lipids, given that intercellular lipids can rearrange into a cubic phase under specific circumstances.Citation31
In both the in vitro permeability and in vivo bioavailability experiments, a correlation was obtained between the outcomes of the in vitro permeability model and the in vivo bioavailability data. The in vitro permeability experiments indicate that the cubic gel formulation yielded the highest permeability coefficient of the three tested formulations, and this was also the case in the in vivo assessment model, with the AUC of the cubic gel formulation being higher than that of the other two formulations.
Conclusion
In this study, novel microemulsion gel and cubic gel formulations for transdermal delivery of paeonol were developed. Our results indicate that both the cubic gel and microemulsion gel formulations had a higher permeability coefficient than the control group. An in vivo pharmacokinetic study in rats showed that the relative bioavailability of the cubic gel and the microemulsion gel was enhanced by about 1.51-fold and 1.28-fold, respectively, compared with oral administration of paeonol suspension. These data collectively support the hypothesis that cubic gel and microemulsion gel formulations are promising delivery systems to enhance the skin permeability of paeonol, especially the cubic gel.
Acknowledgments
The work was supported by the Shanghai Municipal Committee of Science and Technology (grant No. 08DZ1971304), the National Basic Research Program of China (No. 2007CB936004), and the National Pharmaceutics Technology Platforms for Innovative Drugs (No. 2009ZX09310-007).
Disclosure
The authors report no conflicts of interest in this work.
References
- RileyCMRenTCSimple method for the determination of paeonol in human and rabbit plasma by high-performance liquid chromatography using solid-phase extraction and ultraviolet detectionJ Chromatogr19894894324372753967
- ChouTCAnti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesiaBr J Pharmacol20031391146115212871833
- KimSHKimSAParkMKKimSHParkYDNaHJPaeonol inhibits anaphylactic reaction by regulating histamine and TNF-alphaInt Immunopharmacol2004427928714996419
- El MaghrabyGMTransdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactantsInt J Pharm200835528529218243604
- KawakamiKYoshikawaTHayashiTNishiharaYMasudaKMicroemulsion formulation for enhanced absorption of poorly soluble drugs: II. In vivo studyJ Control Release200281758211992680
- HaeringGLuisiPLHydrocarbon gels from water-in-oil microemulsionsJ Phys Chem19869058925895
- QuelletCEickeHFMutual gelation of gelatin and water-in oil microemulsionsChimia (Aarau)198640233238 German
- AtkinsonPJRobinsonBHHoweAMHeenanRKStructure and stability of microemulsion-based organo-gelsJ Chem Soc Faraday Trans19918733893397
- ReesGDNascimentoMGJentaTRRobinsonBHReverse enzyme synthesis in microemulsion-based organo-gelsBiochim Biophys Acta199110734935011707672
- JentaTRBattsGReesGDRobinsonBHBiocatalysis using gelatin microemulsion-based organogels containing immobilized Chromobacterium viscosum lipaseBiotechnol Bioeng19975312113118633956
- NorlingTLandingPLarssonKKrogNNissenSSFormulation of a drug delivery system based on a mixture of monoglycerides and triglycerides for use in the treatment of periodontal diseaseJ Clin Periodontol1992196876921447387
- GeraghtyPBAttwoodDColletJHDandikerYThe in vitro release of some antimuscarinic drugs from monoolein/water lyotropic liquid crystalline gelsPharm Res199613126512718865324
- HanIHChoiSUNamDYIdentification and assessment of permeability enhancing vehicles for transdermal delivery of glucosamine hydrochlorideArch Pharm Res20103329329920195831
- XiJLiuSLiuCThe study of paeonol formulation and clinical applicationLishizhen Medicine and Materia Medica Research20051666
- WuXChenHChenXHuZDetermination of paeonol in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies following oral administration of Moutan cortex decoctionBiomed Chromatogr20031750450814648606
- ModamioPLastraCFMarinoELTransdermal absorption of celiprolol and bisoprolol in human skin in vitroInt J Pharm1998173141148
- BabarASolankiUDCutieAJPiroxicam release from dermatological bases: In vitro studies using cellulose membrane and hairless mouse skinDrug Dev Ind Pharm199016523540
- LapasinRGrassiMCoceaniNEffects of polymer addition on the rheology of o/w microemulsionsRheol Acta200140185192
- LeslieSBPuvvadaSRatnaBRRudolphASEncapsulation of hemoglobin in a bicontinuous cubic phase lipidBiochim Biophys Acta199612852462548972709
- TenchovBRappoltMKoynovaRRappGNew phases induced by sucrose in saturated phosphatidylethanolamines: an expanded lamellar gel phase and a cubic phaseBiochim Biophys Acta199612851091228948481
- ErikssonPOLindblomGLipid and water diffusion in bicontinuous cubic phases measured by NMRBiophys J1993641291368431537
- KoynovaRTenchovBRappGLow amounts of PEG-lipid induce cubic phase in phosphatidylethanolamine dispersionsBiochim Biophys Acta199713261671709218547
- TrottaMInfluence of phase transformation on indomethacin release from microemulsionsJ Control Release19996039940510425344
- PeltolaSSaarinen-SavolainenPKiesvaaraJSuhonenTMMicroemulsions for topical delivery of estradiolInt J Pharm20032549910712623186
- ParkESChangSYHahnMChiSCEnhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofenInt J Pharm200020910911911084251
- ChenHBChangXLDuDRXuHBYangXLMicroemulsion-based hydrogel formulation of ibuprofen for topical deliveryInt J Pharm2006315525816600540
- LarssonKFontellKKrogNStructural relationships between lamellar, cubic and hexagonal phases in monoglyceride-water systems. Possibility of cubic structures in biological systemsChem Phys Lipids198027321328
- LarssonKCubic lipid-water phases: Structures and biomembrane aspectsJ Phys Chem19899373017314
- SunYCSunGPShenYXChenLMQuJWeiWStudy the pharmacokinetics of paeonol in mice in vivoChin Hosp Pharm J200626543545
- KwonTKKimJCIn vitro skin permeation of monoolein nanoparticles containing hydroxypropyl beta-cyclodextrin/minoxidil complexInt J Pharm201039226827320362653
- FoldvariMBadeaIWettigSTopical delivery of interferon alpha by biphasic vesicles: Evidence for a novel nanopathway across the stratum corneumMol Pharm2010775176220349952