Abstract
Background
Saquinavir mesylate (SQR) tablets are widely used against human immunodeficiency virus. SQR has bioavailability issues owing to its poor aqueous solubility, extensive first-pass metabolism, and even low gastrointestinal tract permeability and absorption.
Objective
An in-depth optimization process was carried out using factorial design to improve the permeation parameters and thereby the bioavailability of SQR by formulating self-nanoemulsifying drug delivery system (SNEDDS)-loaded polymeric transdermal films.
Methods
The solubility of SQR in different nanoemulsion components was examined. Various combinations of selected components were prepared in an extreme vertices mixture design to identify the useful nanoemulsion zone and to develop SNEDDS with minimum globule size. The optimized SQR-SNEDDS was loaded in polyvinyl alcohol (PVA)-based transdermal films. The Box-Behnken design was used to optimize and evaluate SQR permeability. The prepared films were characterized for thickness, tensile strength, elongation, folding endurance, and accelerated stability studies. The optimized film was examined for ex vivo skin permeation and in vivo pharmacokinetic parameters.
Results
The optimized SQR-SNEDDS was prepared in proportions of 0.1, 0.55, and 0.35 of clove oil, labrasol, and Transcutol, respectively. The implemented Box-Behnken design indicated the optimized film consisted of 1.0% PVA, 0.25% propylene glycol, and clove oil as the oil phase. The tensile strength, thickness, percent elongation, and folding endurance of the optimized SQR-SNEDDS film were 0.93 ± 0.013 kg/cm2, 0.22 ± 0.006 mm, 43.1 ± 0.022%, and >200 times, respectively. A higher Cmax and double the AUC were observed for SQR-SNEDDS–loaded film in comparison to pure SQR-loaded films.
Conclusion
Implementation of a two-step design to optimize and control experimental factors in the preparation of SQR-SNEDDS and its loading onto PVA-based transdermal films was achieved. The films indicated improved ex vivo skin permeation, enhanced bioavailability, and overcame the limitations of the oral dosage form.
Introduction
HIV is a chronic infection that attacks and destroys the CD4 cells of the immune system. The loss of CD4 cells makes it hard for the body to fight infections and certain cancers.Citation1 Currently, there is no recognized cure for HIV, and those who have contracted the virus must receive medical treatment for the rest of their life. Without treatment, HIV can progressively destroy the immune system and advance to AIDS.Citation2,Citation3 A momentous advancement in understanding HIV replication and its pathogenesis has helped in the identification of diverse pharmacological targets and a large number of antiretroviral (ARV) drugs.Citation4 Antiretroviral therapy (ART) has developed from monotherapy with azidothymidine (AZT) to a combination of 2 ARVs from nucleoside reverse transcriptase inhibitors to highly active antiretroviral therapy. Novel drugs with a unique mechanism of action, such as maturation inhibitors, CD4 receptor attachment inhibitors, capsid assembly inhibitors, pharmacokinetic enhancers, and lens epithelium growth factor inhibitors are still under development.Citation5,Citation6
Oral bioavailability of medication depends predominantly on aqueous solubility, but dissolution rate, first-pass metabolism, drug permeability, and susceptibility to efflux mechanisms are also significant parameters.Citation7–Citation9 Therefore, enhancing the dissolution rate and bioavailability of poorly soluble drugs is an imperative challenge to pharmaceutical scientists.Citation10–Citation12 The bioavailability of BCS class II drugs is likely to be limited by dissolution rate, but owing to their high permeability, these drugs have been in the spotlight for solubility enhancement.Citation13–Citation15
The discovery of HIV protease inhibitors has introduced new and efficient first-line therapies for HIV/AIDS, but many of these drugs still suffer from reduced bioavailability owing to low aqueous solubility and/or membrane permeability.Citation16,Citation17 Such drugs are structurally related “peptidomimetics” compounds, with high molecular weights in contrast to conventional drugs; comparatively high lipophilic character, as demonstrated by their octanol-water partition coefficients (ie, LogP); and characteristic pH-dependent solubility.Citation17 Saquinavir mesylate (SQR), an archetypal protease inhibitor, has LogP (4.1), molecular weight (767.0 g/mol), and permeability coefficient values similar to those of other drugs in its class. Its aqueous solubility has been reported (73 mg/L at a pH of 6.5; 36 mg/L at a pH of 7.4), along with at least two ionization constants (pKa 7.0 and 5.5), but conflicting reports can still be found in the literature.Citation18–Citation21 As a result, the oral bioavailability of SQR is reported to be very low (0.7–4.0%), depending on the dosage form considered. In addition to its poor solubility, SQR is metabolized by both human hepatic and small intestinal microsomes;Citation22–Citation31 therefore, pre-systemic elimination also plays a critical role in its low bioavailability.
Much effort has been made to increase the drug’s solubility and dissolution properties, including the use of water-soluble carriers, surfactants, solid dispersions, and polymeric conjugates. In recent years, the most prominent methodology is the incorporation of the active, poorly water-soluble component into lipid vehicles such as microemulsions, surfactant dispersions, mucoadhesive, nanoparticles, nanoemulsion, self-emulsifying formulations, self-microemulsifying formulations, nanoscale solid lipid particles, liposomes, solid lipid nanoparticles, and self-nanoemulsifying formulations.Citation32
Self-nanoemulsifying drug delivery systems (SNEDDS) may offer an improvement in dissolution rates and extent of absorption, resulting in more reproducible blood–time profiles due to the nanometer-sized droplets present. SNEDDS can minimize the effect of pH variability and improve release performance.Citation33 SNEDDS disperse in aqueous media as a fine emulsion with globules in the nanosize range; the drug thus remains in solution, consequently overcoming one of the main barriers in drug absorption, the dissolution step.Citation34 In addition, the emulsification process of SNEDDS in the aqueous gastrointestinal tract (GIT) medium improved the permeability of the drug across the GIT membrane, which improves its bioavailability.Citation35 Pre-dissolving the drugs in a blend of lipidic and emulsifying excipients omits the disintegration/dissolution steps, which are potential rate-limiting factors for oral absorption of poorly water-soluble drugs.Citation36 Solid SNEDDS is considered to be preferred over the liquid form, plausibly due to better portability, improved stability, higher drug loading, and above all higher patient compliance.Citation37
Systematic optimization of isotropic systems using the design of experiments (DoE) has become routine practice in both industrial and academic milieus.Citation38 The recent systematic approach of “formulation by design” (FbD), based on the salient principles of DoE and quality by design, provides rational understanding of the plausible interaction(s) among the variables. This approach helps in selecting the best formulation with less expenditure of time and effort and lower developmental costs compared with the traditional one factor at a time approach. The FbD methodology involves defining the quality target product profile and identifying critical material attributes, critical quality attributes, and critical process parameters using risk assessment and screening. Optimization data analysis featuring DoE, modelization, and optimum search through response surface methodology was used to postulate and design a control strategy for continuous improvement.Citation39
One approach to avoid the first-pass effect is the formulation of transdermal drug delivery systems. The transdermal mode offers several distinct advantages over the oral route, including avoidance of hepatic first-pass metabolism and variability in gastrointestinal absorption, controlled release, and reduction of the typical dosing schedule, hence improving patient compliance.Citation40,Citation41 The non-invasiveness of this route and the ability to quickly discontinue treatment by removing the system have attracted research on innovative technologies to deliver drugs.Citation42,Citation43 This work aimed to implement a two-step optimization to understand the experimental parameters that affect the preparation of SQR-loaded SNEDDS incorporated into transdermal films and to investigate both ex vivo and in vivo SQR enhancement. Physical evaluation of transdermal films also is performed.
Materials And Methods
SQR was kindly supplied by SAJA Pharmaceutical Company (Jeddah, Saudi Arabia). Clove oil, Labrasol, and Transcutol were kind gifts from Nikko Chemicals Co., Ltd. (Tokyo, Japan). Coated liners and backing membranes were generous gifts from 3M (St Paul, MN). Polyvinyl alcohol (PVA), propylene glycol, 2-pyrrolidone, DMSO, and oleic acid were obtained from TEDIA company, Inc. (Fairfield, OH). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Determination Of SQR Solubility
SQR’s solubility in various oils, surfactants, and co-surfactants was tested to determine the ideal components for the preparation of SQR-SNEDDS. Briefly, an excess amount of SQR was added to 3 mL of respective components in sealed vials and left under shaking in a water bath at 25±0.5°C for 72 hrs. After attainment of equilibrium, the individual samples were centrifuged at 3500 rpm for 16 min. The obtained supernatant was filtered (0.45 µm) and diluted with mobile phase and quantified at 238 nm using high-performance liquid chromatography (HPLC), as previously described.Citation44
Preparation Of SQR-Loaded SNEDDS
Based on the solubility of SQR in various components, clove oil, Labrasol, and Transcutol were selected as oil, surfactant, and co-surfactant components, respectively, for the mixture optimization studies to prepare the SNEDDS (data not shown). The amount of SQR was kept constant at about 10 mg per gram of SNEDDS to prevent precipitation from the mixture.Citation19
Optimization Of SQR-Loaded SNEDDS
As per the data obtained from the SNEDDS formulations, the various compositions of mixture components indicating a clear, stable system were placed in an Extreme Vertices mixture experimental design.Citation32 The design was framed to elucidate the three-component effects in 14 runs overall () to establish a SQR-SNEDDS composition with minimum globule size and maximum stability. Clove oil (X1), Labrasol (X2), and Transcutol (X3) were used in various proportions in a randomized order; a range from 0.1 to 0.2 was used for clove oil, 0.4 to 0.7 for Labrasol, and 0.2 to 0.5 for Transcutol. The mean SQR-SNEDDS globule size in nanometers was chosen as the response variable (Y1) and stability index (Y2). The stability index was calculated using the below equation:
Table 1 Formulation Runs As Per The Extreme Vertices Design, Along With Observed Globule Size And Stability Index
*Change in size in the equation is the difference between the size before and after the stability test (consisting of three freeze-thaw cycles, each cycle being freezing at −20°C for 12 hrs followed by thawing at +25°C for 12 h).Citation46
Both dependent and independent variables were related using a regression equation in Statgraphics plus, version 4 (Manugistic Inc., Rockville, MD). The final optimized formulation was used in the preparation of SQR transdermal films.
Preparation Of SQR-SNEDDS Transdermal Films Using An Experimental Design
Transdermal films were prepared using the optimized SQR-SNEDDS. To determine the effect of independent variables on the amount of SQR permeation (Y) from the formulated films, a Box-Behnken design was used in 15 runs total. The variables evaluated in this work were percentage of film-forming polymer PVA (X1) (1–3%) and skin permeation enhancer (X2) at three levels of concentration (0.25, 0.625, and 1.0), along with clove oil and oleic acid as oils (X3) (0.0, 0.5, and 1.0, where 0 is only clove oil, 0.5 is a 1:1 mixture of clove oil and oleic acid, and 1 is only oleic acid). The levels of the factors selected for the experimental design were established based on the preliminary studies carried out earlier. The polynomial equation and overall optimization were executed using JMP software (version 11.0.0, SAS Institute Inc., Cary, NC). The complete observed response along with the composition of the 15 formulations is depicted in .
Table 2 SQR-SNEDDS–Loaded Transdermal Films As Per The Box-Behnken Design
Preparation Of SQR-SNEDDS And SQR-SNEDDS–Loaded Transdermal Films
A calculated amount of optimized SQR-SNEDDS, equivalent to 0.6 mg of drug per square centimeter of dish area, was carefully dispersed in 50 mL of distilled water. Film-forming polymer PVA (1–3% w/v), plasticizer-like propylene glycol (1% w/v), and the respective level of permeation enhancer as per the design were added to the dispersion medium. The final casting solution was gently stirred and placed at 7°C for 24 hrs. The gelling medium then was transferred to silicon-coated Petri dishes (9 cm diameter). To completely evaporate any water, the dishes were placed in a hot air oven at 40°C. The backing membrane was used to cover the films and cut into specific dimensions of 202 ± 24 mm with uniform thickness and stored in a desiccator.Citation45 The same amount of SQR was used to prepare pure SQR-loaded transdermal films using the same protocol described earlier.
Evaluation Parameters
Accelerated Physical Stability Studies Of SQR-SNEDDS
The inherent stability of the prepared SQR-SNEDDS was determined using a combination of heating-cooling cycles, centrifugation, and freeze-thaw cycle stress tests. Namely, the samples were maintained between 45°C and room temperature (25 ± 2°C) for 24 hrs with six cycles at each temperature. The process was followed by centrifugation at 6000 rpm for 25 min and then by six cycles of freezing at –20°C and thawing at room temperature (25 ± 2°C) for 24 hrs.Citation46 The SQR-SNEDDS formulation that was stable in these processing conditions was used for further studies.
Evaluation Parameters Of SQR-SNEDDS–Loaded Transdermal Film
Determination Of Folding Endurance Of The Prepared Film
A specific area (2 × 2 cm) of the strip was cut evenly and subjected to repeated folding at the same site until it broke. The average number of times the film was folded at same site without any breakage was used to determine the folding endurance of the film.Citation47
Determination Of Tensile Strength Of The Prepared Film
A tensiometer (Erection and instrumentation, India) was used to evaluate the tensile strength of the prepared film. The instrument has two movable load cell grips, lower fixed and upper. A 2 × 2 cm strip of the prepared film was fixed between the grips, and force was gradually applied until film breakage appeared. The total tensile strength (in kg) was depicted on the dial of the instrument.Citation47
Determination Of Percentage Elongation Break Test
The percentage stretching or elongation break was elucidated by reading the length just before the breakpoint. The following formula was used to determine the percentage elongationCitation47
where L1 and L2 are the final length and initial length of each strip, respectively.
Determination Of Thickness
The prepared SQR-SNEDDS film thickness was determined using a digital micrometer screw gauge (Mitutoyo 293-821-30, India) at three different places. The mean value was calculated and noted.Citation47
Ex Vivo Skin Permeation Study Of SMV From Transdermal Films
An automated Franz diffusion cell apparatus (Hanson Research, MicroettePlus, Chatsworth, CA) was used to determine the diffusion of SQR from the SNEDDS-based films. The cells had a diffusion area of 1.76 cm2 with a 7 mL receptor chamber. A 2 × 2 cm area of full skin thickness was isolated from Wistar rats (234 g) to be used as a membrane. The dermal side of the skin was placed in direct contact with the receptor medium. The prepared film was placed between the donor and receptor chamber. The diffusion medium used was phosphate-buffered saline (pH 7.4) maintained at 32±0.5°C under constant stirring (400 rpm). At regular time intervals, aliquots were withdrawn by auto sampler and parallel analyzed using the HPLC method.Citation44
Pharmacokinetic Study Of The Optimized SQR-SNEDDS Transdermal Film
The study was performed using male Wistar rats (200–230 g), given a standard diet and maintained at ambient temperature (24±2°C) and humidity conditions (30–70%). The experimental study was approved by IRBPCR (Institutional Review Board for Preclinical & Clinical Research) and ensured that the care and use of animals conformed to the guidelines in the Declaration of Helsinki, the Guiding Principle in Care and Use of Animals (DHEW publication NIH 80–23), and the Principles of Laboratory Animal Care (NIH publication #85-23, revised in 1985).
The study animals were divided into three groups and had the film applied to a 3.0 cm2 area. The first group (negative control) received pure PVA films, the second group (positive control) received pure SQR PVA film, and the third group received the optimized SQR-SNEDDS–loaded PVA film containing a 2 mg/kg dose. Plain adhesive patches were used to cover the applied films. Blood collection was performed as per the protocol, and serum SQR concentrations were used to calculate the pharmacokinetic parameters. An earlier reported HPLC method was used to determine the plasma SQR concentrations.Citation48 The mobile phase in the system used was methanol/0.05M potassium dihydrogen orthophosphate buffer (pH 5.0; 85/15 v/v). Pharmacokinetic parameters such as area time point of maximum plasma concentration under the plasma concentration curve (AUC), maximum plasma concentration (Cmax), and elimination rate constant (ke) were calculated using Kinetica (version 4, Thermo Electron Corporation, Waltham, MA)
Statistical Analysis
Unpaired Student’s t-test was used to assess the significance of the difference between pharmacokinetic parameters (AUC, Cmax, tmax, and ke). Two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test was used to assess the significance of the difference between quantitative variables. A P value <0.05 was considered to be statistically significant. Statistical analysis for the in vivo data, expressed as mean±SD, was conducted using GraphPad Prism 6 (GraphPad Software, San Diego, CA).
Results And Discussion
The aim of the present study was to develop an SQR-SNEDDS formulation loaded onto a polymeric PVA-based transdermal film using a two-step optimization to control the process variables. One of the steps was to rationalize, optimize, and select SQR-SNEDDS components using the extreme vertices mixture design. The second step, optimization, was performed using a Box-Behnken design to select the components of the transdermal film.
SQR Solubility Studies
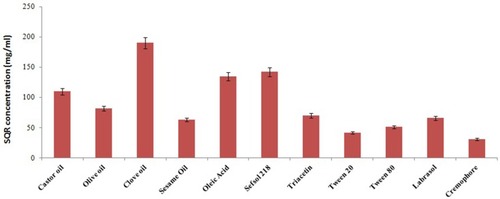
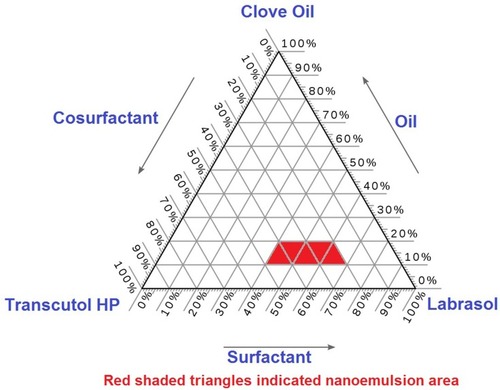
SQR’s solubility in various oils, surfactants, and cosurfactants is depicted in . Because of their effect in enhancing the solubility of SQR, clove oil, Labrasol, and Transcutol were selected as oil, surfactant, and cosurfactant for the preparation of the SQR-SNEDDS formulation; this was done using a pseudo-ternary phase diagram (). Alcohols such as ethanol yielded good solubility of SQR (126 mg/mL) (data not shown) but were not chosen for the study because of instability during the formation of the nanoemulsion. Transcutol has better stability and less volatility in comparison to the other co-solvents used in the emulsion system. The HLB value of Transcutol more than 4 and is decorated with both diethylene glycol (hydrophilic) and ethyl ether (lipophilic) structures that aids in formation of larger and stable emulsion than other co-solvents and greatly contributed to the solubilization of SQR. Having a longer alcohol molecule, Transcutol can increase the rate of transport from the dermis layer in comparison to alcohol. These attribute to select Transcutol for the preparation of SQR-loaded SNEDDs.Citation48
Clove oil is one of the rare biocompatible oil used in the drug solubilization and especially in preparation or emulsion system. The droplet size of clove oil formed with water or other medium is in range of 10–20 nm in comparison to other oils. Even studies have indicated good permeation enhancement of drug when it is used in topical preparations in comparison to other organic or vegetable oils.Citation49
Clove oil, Labrasol, and Transcutol P were selected because SQR had the highest solubility due to the presence of eugenol in clove oil (). These components were optimized using the mixture design to attain optimum SQR loading and minimize the precipitation of SQR upon dilution.Citation50 A mathematical relationship was devised per the extreme vertices design to establish the proper factor levels for optimal globule size and good stability index. The ratio of SNEDDS ingredients needed to obtain minimal globule size was predicted from the obtained regression equation, and the optimized SQR-SNEDDS composition was loaded onto the polymeric transdermal films for topical delivery.
Optimization Of SQR-Loaded SNEDDS
As per the mixture experimental design, different mean responses have been obtained as a result of different factor combinations. The levels of each factor, their combinations, and experimental runs, along with the mean globule size and stability index, are depicted in . A linear model was used to conduct the regression analysis of the obtained data. The P-value was <0.01 for globule size by ANOVA, indicating a statistically consequential relationship between components and response at the 99% confidence level. The model as fitted explains 99.784% of the variability in globule size as per the R-squared statistics (99.64% per the adjusted R-squared statistics). The regression equation was developed using equation 1; 0.1, 0.55, and 0.35 were the calculated proportions for the clove oil, Labrasol, and Transcutol, respectively. The optimized formulations using these proportions were prepared for further characterization, such as globule size.
To rationalize and study the maximum SQR permeation from the transdermal films, a factorial design was implemented. Factors with P < 0.05 had a significant effect on the amount of SQR permeation. Synergistic and antagonistic effects of factors on SQR permeation (Y) are indicated by positive and negative signs, respectively. Coefficient (X1-X3) values and their effect were related to the amount of SQR permeation. Interaction terms were identified by coefficients having more than one factor. Polymer percentage (X1), type of oil (X3), and the interaction between type of oil (X2, X3) and enhancer percentage had a notable effect (P < 0.05) on the model’s performance in predicting the amount of SQR permeation after 12 hrs. By substituting the X1–X3 values in Equation (4), the theoretical values of SQR permeation after 12 hrs were obtained.
Optimization Of SQR-SNEDDS–Loaded Transdermal Film
The optimized SQR-SNEDDS was selected and incorporated into the transdermal film formulation. Fifteen formulations were proposed by the Box-Behnken design. The observed response (i.e., amount of drug permeation after 12 h) for the different factor combinations generated by the experimental design is shown in . The cumulative amount of SQR permeation ranged from 58.3±9.2 (run 5) to 198.3±1.7 mg/cm2 (run 8). Evidence of a regression effect is deemed significant at P values of ≤0.05 as per the ANOVA. Independent factors were determined to have a significant effect on response (Y) at a P value of 0.001, as indicated in .
Table 3 Analysis Of Variance For The Amount Of SQR Permeation After 12 hrs
The factor estimates for the amount of drug permeation after 12 hrs along with mathematical relationships are listed in .
Table 4 Factor Estimates For The Amount Of Drug Permeation After 12 hrs
The relationship between the amount of SQR permeation (Y) and the quantitative effect of factors is given by Equation 5.
The relationship between the independent and dependent variables was revealed by the contour and response surface plots shown in . explains the interaction of the effect of percentage polymer (X1), enhancer (X2), and type of oil (X3) on Y at the middle level. Y decreases from 173.6 mg/cm2 (run 14) to 78.3 mg/cm2 (run 9) at low levels of percentage enhancer, especially at 0.25. Y gradually decreases from 198.3 mg/cm2 (run 8) to 58.3 mg/cm2 (run 5) when the level of X3 increases from 0.0 to 1. Conversely, when X1 increases from 1% to 3%, Y decreases from 159.3 mg/cm2 (run 4) to 118.6 mg/cm2 (run 7) at a low level of X2 (0.25).
Figure 3 (A) Pareto chart showing amount of SQR permeation after 12 hrs. (B) Estimate response surface plot showing the effect of the studied variables on the amount of SQR permeation after 12 hrs. (C) Contour plot showing the effect of different variables on the amount of SQR permeation after 12 hrs.
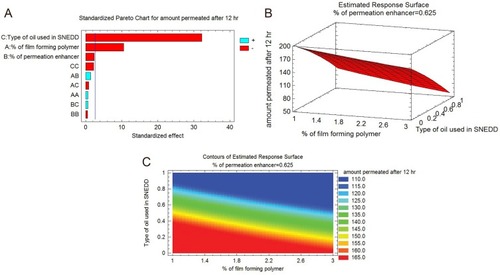
To investigate the main effect of factors on the response, a Pareto chart was used (). In the interaction plots, the percentage of enhancer used had little effect. The amount of SQR permeation after 12 hrs decreased when 3% (high level) of polymer was used. There was no change in SQR permeation after 12 hrs when changing the type of enhancer in the films, especially at the low level. SQR permeation gradually increased as the percentage enhancer was increased to the maximum level (3). It is clear from the interaction plots that use of oleic acid at the 0.5 and 1.0 level decreased the SQR permeation after 12 hrs even at high levels of enhancer and polymer.
Ex Vivo Skin Permeation Study Of The Optimized SQR-SNEDDS–Loaded Transdermal Film
The cumulative amount of SQR permeation through the excised skin from the optimized SQR-SNEDDS–loaded transdermal film in comparison to the pure SQR-loaded film is depicted in . The results in indicate enhancement of the permeation attributes of SQR with the optimized SQR-SNEDDS–loaded film. In comparison to the pure SQR-loaded transdermal film, the optimized SQR-SNEDDS–loaded film exhibited a 2-fold increase in SQR skin flux, mostly because of the enhanced solubility and permeation across skin due to the presence of eugenol in clove oil.Citation50
Figure 4 In vitro skin permeation studies from pure SQR-loaded and SQR-SNEDDS–loaded transdermal film.
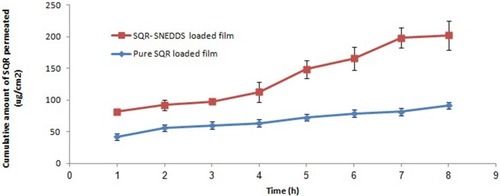
Table 5 Optimized SQR-SNEDDS–Loaded And Pure SQR-Loaded Ex Vivo Skin Permeation Parameters
With an increase in the polymer concentration (X1), the amount of SQR diffused was reduced at a low level of percent enhancer (X2); this was evidenced by the obtained response and contour plots (), and is mostly caused by increased polymer concentrations obstructing SQR from diffusing out of the film.Citation51 Conversely, increasing the percentage of Labrasol (X2) while maintaining the same level of polymer increased SQR diffusion (Y), indicating Labrasol’s potential to enhance permeation by reversibly decreasing the skin barrier’s resistance, thereby easing SQR diffusion.
The Pareto chart () illustrates the factors and their main effects on SQR permeation after 12 hrs. A significant effect is indicated by the bar length exceeding the vertical line (P < 0.05). The significant effects of factors such as type of oil (X3), interaction effect of percentage enhancer (X2 and X3), and percentage polymer (X1) are indicated in . The Box-Behnken design, desirability functions, and computer optimization were used to determine the levels of the independent variables and their effect on SQR permeation. The predicted response value was 133.96 mg/cm2 at X1 (1.0% PVA), X2 (0.625% enhancer), and X3 (clove oil as oil phase for SNEDDS) levels, respectively. The same proportions used in run 8, indicated in , were repeated to prepare a fresh formulation and confirm the optimization.
Approximately 198.3 mg/cm2 of SQR permeated from the formulation prepared using optimized levels of individual factors. The predicted and observed values were in close agreement (P < 0.05). Overall findings imply the importance of factorial design and its use in predicting SQR permeation from transdermal film. In comparison to the SQR-SNEDDS–loaded film, the pure SQR-loaded transdermal film had low SQR permeation with a plateau after 1 hr. This phenomenon is mainly due to the SQR release from the superficial layer of film, followed by retarded diffusion from the inner layers ().Citation48,Citation52
Pharmacokinetic Study Of Optimized SQR-SNEDDS–Loaded Transdermal Film
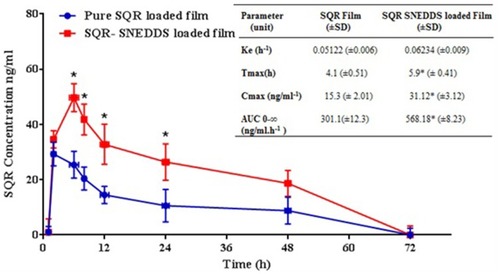
There was a significant (P < 0.05) increase in time vs plasma concentration from SQR-SNEDDS in comparison to the pure SQR-loaded film. A significantly (p < 0.05) higher Cmax and relative AUC were observed from PVA-optimized SQR films in comparison to the pure SQR-loaded film (). No significant difference in Ke was observed for either transdermal film. From the ex vivo and in vivo pharmacokinetic data, PVA-optimized films loaded with SQR-SNEDDS had enhanced SQR absorption in comparison to pure SQR-loaded films, mostly because nanostructured SQR with clove oil containing eugenol as an anti-oxidant exhibits improved solubility and bioavailability. The type of oil (i.e., clove oil) used alters the polar pathway of SQR and lipid, leading to better absorption and deepening penetration of SQR into the skin and directly into the bloodstream. This composition of oil phase and surfactant is the reason for enhanced permeation of SQR from SNEDDS in comparison to pure SQR-loaded films.Citation53 As an additive effect, the small globule size of SNEDDS acts as an efficient carrier for enhanced percutaneous penetration of SQR, with more nano-sized vesicles that can interact on a fixed area of stratum corneum.Citation54
Figure 5 Means of plasma concentration-time profiles from pure SQR- and SQR-SNEDDS–loaded film groups. (Inset) In vivo pharmacokinetic parameters of SQR and optimized SQR-SNEDDS–loaded film. *P < 0.05.
Physical Characterizations Of Film (Folding Endurance, Percent Elongation, Tensile Strength, And Thickness)
The results of all four parameters in the factorial design formulations are listed in . Satisfactory values for folding endurance were obtained for all films prepared using PVA at a concentration of 1% w/v, which yielded optimum flexibility without brittleness. The tensile strength of the films formulated using PVA was found to be between 0.46±0.011 and 0.93±0.013 kg/cm2 for the SQR-SNEDDS and pure SQR-loaded films, respectively. Increases in PVA concentration resulted in gradual enhancement of the tensile strength of the films.Citation55 The percent elongation was in the range of 27.68 ± 0.018% to 43.1 ± 0.022%. Minimum percent elongation was observed in pure SQR-loaded films. The results established an inverse relationship between the percent elongation and the tensile strength of the film. Both films ranged between 0.18 ± 0.003 and 0.22 ± 0.006 mm in thickness. All of the prepared films were uniform, and all the batches had an SD of <0.01.
Table 6 Evaluation Parameters For SQR-SNEDDS–Loaded And Pure SQR-Loaded Transdermal Film. Values Expressed As Mean ± SD, N = 3
Accelerated Stability Studies
Among the formulation runs subjected to accelerated stability studies, the formulation with a minimum percentage of surfactant and cosurfactant had better stability. provides the composition of these formulations that passed the stability studies.Citation56 It is almost impossible to eliminate the meta-stable state in an emulsion system due to time constraints imposing a physical limit on the length of time that can help equilibrate the system.
Slight turbidity was observed when the SQR-SNEDDS was stored at 21°C. Low temperature aids in coagulation of the internal phase, due to which instability was observed; however, the SNEDDS showed recovery at ambient temperature. A few reports have shown that SNEDDS should be stored at least 15°C above room temperature.Citation57 Formulations that did not pass the accelerated physical stability tests were discarded. The presence of eugenol as an important constituent in clove oil greatly contributed to the physical stability of the nanoemulsion at elevated temperatures, as reported earlier.Citation58 The process of emulsification is non-spontaneous in the case of macroemulsion; in comparison, nanoemulsion features low interfacial tension and less interfacial energy, making the free energy zero or negative. This phenomenon explains the stability of a nanoemulsion very well. The four formulations (runs 4, 6, 8, and 14) in having a 0 level of X3, or only clove oil, were more stable than the others.
Conclusion
Two-step optimization to develop SQR-SNEDDS and design SNEDDS-loaded polymeric PVA transdermal films was successfully performed. The optimized SQR-SNEDDS–loaded film had minimal globule size and a good stability index. The folding endurance and tensile strength of SNEDDS-loaded film were good in comparison to pure SQR-loaded film. Improved ex vivo skin permeation was observed in clove oil-based SNEDDS because eugenol, an alcoholic constituent, aided in dual action by providing maximum SQR solubility, acting as a penetration enhancer for high skin flux, and even improving physical stability. Higher AUC and Cmax with better stability were observed for SQR-SNEDDS–loaded film in comparison to pure SQR-loaded film. The results obtained form a strong rationale for further preclinical studies and indicate the potential of SNEDDS-loaded transdermal film as an alternative to oral delivery of SQR with enhanced bioavailability and patient compliance and minimal side effects.
Disclosure
The author reports no conflicts of interest in this work.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah (under grant No. DF-774-166-1441). The author, therefore, gratefully acknowledges DSR technical and financial support.
References
- Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi:10.1056/NEJM1998032633813019516219
- Sterne JAC, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–384. doi:10.1016/S0140-6736(05)67022-516054937
- Lewden C. Responders to antiretroviral treatment over 500 CD4/mm3 reach same mortality rates as general population: APRICO and Aquitaine Cohorts. 10th European Aids Conference; 11 17–20; 2005; Dublin.
- Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510. doi:10.1016/S0140-6736(06)69158-716890837
- Hull MW, Montaner J. Antiretroviral therapy: a key component of a comprehensive HIV prevention strategy. Curr HIV/AIDS Rep. 2011;8(2):85–93. doi:10.1007/s11904-011-0076-621445551
- Desai M, Iyer G, Dikshit RK. Antiretroviral drugs: critical issues and recent advances. Indian J Pharmacol. 2012;44(3):288. doi:10.4103/0253-7613.9629622701234
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi:10.1023/A:10162128042887617530
- Williams HD, Trevaskis NL, Charman SA, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.23383426
- Krishnaiah YSR. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J Bioequiv Availab. 2010;2(2):28–36.
- Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10. doi:10.1016/j.ijpharm.2011.08.03221884771
- Hu J, Johnston KP, Williams III RO. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm. 2004;30(3):233–245. doi:10.1081/DDC-12003042215109023
- Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133. doi:10.1016/S0928-0987(01)00095-111297896
- Kumar S, Bhargava D, Thakkar A, Arora S. Drug carrier systems for solubility enhancement of BCS class II drugs: a critical review. Crit Rev Ther Drug Carr Syst. 2013;30(3). doi:10.1615/CritRevTherDrugCarrierSyst.2013005964
- Onoue S, Kojo Y, Aoki Y, Kawabata Y, Yamauchi Y, Yamada S. Physicochemical and pharmacokinetic characterization of amorphous solid dispersion of tranilast with enhanced solubility in gastric fluid and improved oral bioavailability. Drug Metab Pharmacokinet. 2012;1201120342.
- Urbanetz NA. Stabilization of solid dispersions of nimodipine and polyethylene glycol 2000. Eur J Pharm Sci. 2006;28(1–2):67–76. doi:10.1016/j.ejps.2005.12.00916472995
- Sharma P, Garg S. Pure drug and polymer-based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62(4–5):491–502. doi:10.1016/j.addr.2009.11.01919931328
- Williams GC, Sinko PJ. Oral absorption of the HIV protease inhibitors: a current update. Adv Drug Deliv Rev. 1999;39(1–3):211–238. doi:10.1016/S0169-409X(99)00027-710837775
- Branham ML, Moyo T, Govender T. Preparation and solid-state characterization of ball milled saquinavir mesylate for solubility enhancement. Eur J Pharm Biopharm. 2012;80(1):194–202. doi:10.1016/j.ejpb.2011.08.00521906676
- Hosny KM, Hassan AH. Intranasal in situ gel loaded with saquinavir mesylate nanosized microemulsion: preparation, characterization, and in vivo evaluation. Int J Pharm. 2014;475(1–2):191–197. doi:10.1016/j.ijpharm.2014.08.06425178831
- Al-Subaie MM, Hosny KM, El-Say KM, Ahmed TA, Aljaeid BM. Utilization of nanotechnology to enhance percutaneous absorption of acyclovir in the treatment of herpes simplex viral infections. Int J Nanomedicine. 2015;10:3973.26109856
- Pathak SM, Musmade P, Dengle S, Karthik A, Bhat K, Udupa N. Enhanced oral absorption of saquinavir with methyl-beta-cyclodextrin—preparation and in vitro and in vivo evaluation. Eur J Pharm Sci. 2010;41(3–4):440–451. doi:10.1016/j.ejps.2010.07.01320656025
- Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25(2):256–266.9029057
- Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44(2):190–194. doi:10.1046/j.1365-2125.1997.00644.x9278209
- Chaumeil JC. Micronization: a method of improving the bioavailability of poorly soluble drugs. Methods Find Exp Clin Pharmacol. 1998;20(3):211–216.9646283
- Agharkar S, Lindenbaum S, Higuchi T. Enhancement of solubility of drug salts by hydrophilic counterions: properties of organic salts of an antimalarial drug. J Pharm Sci. 1976;65(5):747–749. doi:10.1002/jps.2600650533932950
- Amin K, Dannenfelser R, Zielinski J, Wang B. Lyophilization of polyethylene glycol mixtures. J Pharm Sci. 2004;93(9):2244–2249. doi:10.1002/jps.2013515295785
- Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1. doi:10.1007/s11095-006-9132-017109211
- Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11. doi:10.1016/j.ijpharm.2006.10.04417137734
- Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25(1):103–128. doi:10.1016/S0169-409X(96)00494-2
- Vella S, Floridia M. Saquinavir. Clin Pharmacokinet. 1998;34(3):189–201. doi:10.2165/00003088-199834030-000029533981
- Nolan D. Metabolic complications associated with HIV protease inhibitor therapy. Drugs. 2003;63(23):2555–2574. doi:10.2165/00003495-200363230-0000114636077
- Ahmed TA, El-Say KM, Hosny KM, Aljaeid BM. Development of optimized self-nanoemulsifying lyophilized tablets (SNELTs) to improve finasteride clinical pharmacokinetic behaviour. Drug Dev Ind Pharm. 2018;44(4):652–661. doi:10.1080/03639045.2017.140597729139305
- Singh B, Bandopadhyay S, Kapil R, Singh R, Katare OP. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carr Syst. 2009;26(5). doi:10.1615/CritRevTherDrugCarrierSyst.v26.i5.10
- Khalid M, Ahmed TA, Ahmed OAA, Hosny KM, Abd-Allah FI. Self-nanoemulsifying lyophilized tablets for flash oral transmucosal delivery of vitamin K: development and clinical evaluation. J Pharm Sci. 2017;106(9):2447–2456. doi:10.1016/j.xphs.2017.01.00128087316
- El Maghraby GM. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: effect of phase transition. Colloids Surf B Biointerfaces. 2010;75(2):595–600. doi:10.1016/j.colsurfb.2009.10.00319892531
- Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63(3):288–294. doi:10.1016/j.ejpb.2005.12.00516527467
- Tan A, Rao S, Prestidge CA. Transforming lipid-based oral drug delivery systems into solid dosage forms: an overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm Res. 2013;30(12):2993–3017. doi:10.1007/s11095-013-1107-323775443
- Singh B, Singh S. A comprehensive computer program for the study of drug release kinetics from compressed matrices. Indian J Pharm Sci. 1998;60(6):358.
- Lionberger RA, Lee SL, Lee L, Raw A, Lawrence XY. Quality by design: concepts for ANDAs. Aaps J. 2008;10(2):268–276. doi:10.1208/s12248-008-9026-718465252
- Cho C-W, Choi J-S, Shin S-C. Enhanced transdermal controlled delivery of glimepiride from the ethylene-vinyl acetate matrix. Drug Deliv. 2009;16(6):320–330. doi:10.1080/1071754090303108419606946
- Ah Y-C, Choi J-K, Choi Y-K, Ki H-M, Bae J-H. A novel transdermal patch incorporating meloxicam: in vitro and in vivo characterization. Int J Pharm. 2010;385(1–2):12–19. doi:10.1016/j.ijpharm.2009.10.01319833177
- Alexander A, Dwivedi S, Giri TK, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40. doi:10.1016/j.jconrel.2012.09.01723064010
- Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29. doi:10.1111/j.2042-7158.2011.01369.x22150668
- Caon T, Kratz JM, Kuminek G, et al. Pharmacokinetics of saquinavir mesylate from oral self-emulsifying lipid-based delivery systems. Eur J Drug Metab Pharmacokinet. 2017;42(1):135–141. doi:10.1007/s13318-016-0321-x26846485
- Li L, Fang L, Xu X, Liu Y, Sun Y, He Z. Formulation and biopharmaceutical evaluation of a transdermal patch containing letrozole. Biopharm Drug Dispos. 2010;31(2–3):138–149. doi:10.1002/bdd.69820140970
- Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015;22(4):455–466. doi:10.3109/10717544.2013.86699224329559
- Prajapati ST, Patel CG, Patel CN. Formulation and evaluation of transdermal patch of repaglinide. ISRN Pharm. 2011;2011.
- Guo RX, Fu XX, Chen JX, Zhou LX, Chen GX. Preparation and characterization of microemulsions of myricetin for improving its antiproliferative and antioxidative activities and oral bioavailability. J Agric Food Chem. 2016;64(32):6286–6294. doi:10.1021/acs.jafc.6b0218427455843
- Gupta S, Moulik S. Biocompatible microemulsions and their prospective uses in drug delivery. J Pharm Sci. 2008;97(1):22–45. doi:10.1002/jps.2117717887122
- Eggadi V, Ponna SK, Kankanala SR, Sheshagiri SBB, Gaddam SR. Determination of simvastatin and diltiazem in rat plasma by HPLC and pharmacokinetic studies. Int J Pharm Sci Res. 2013;21:53–56.
- Aqil M, Kamran M, Ahad A, Imam SS. Development of clove oil based nanoemulsion of olmesartan for transdermal delivery: box–behnken design optimization and pharmacokinetic evaluation. J Mol Liq. 2016;214:238–248. doi:10.1016/j.molliq.2015.12.077
- Fu XC, Wang GP, Liang WQ, Chow MSS. Prediction of drug release from HPMC matrices: effect of physicochemical properties of drug and polymer concentration. J Control Release. 2004;95(2):209–216. doi:10.1016/j.jconrel.2003.11.00714980769
- Wahab AFA, Hussein AK, Khaled KA, Ahmed OAA. Meloxicam depot parenteral bio-degradable microspheres: preparation, characterization and in-vivo evaluation. Int J Pharm Sci Rev Res. 2013;21(2):38–45.
- Pathan IB, Setty CM. Enhancement of transdermal delivery of tamoxifen citrate using nanoemulsion vehicle. Int J Pharm Tech Res. 2011;3(1):287–297.
- Thacharodi D, Rao KP. Transdermal absorption of nifedipine from microemulsions of lipophilic skin penetration enhancers. Int J Pharm. 1994;111(3):235–240. doi:10.1016/0378-5173(94)90346-8
- Patel RP, Patel G, Patel H, Baria A. Formulation and evaluation of transdermal patch of aceclofenac. Res J Pharm Dos Forms Technol. 2009;1(2):108–115.
- Chen H, Chang X, Weng T, et al. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release. 2004;98(3):427–436. doi:10.1016/j.jconrel.2004.06.00115312998
- Chen H, Jin X, Li Y, Tian J. Investigation into the physical stability of a eugenol nanoemulsion in the presence of a high content of triglyceride. RSC Adv. 2016;6(93):91060–91067. doi:10.1039/C6RA16270C