?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The objective of this study was to develop pegylated poly lactide-co-glycolide acid (PLGA) immunonanocarriers for targeting delivery of docetaxel to human breast cancer cells.
Methods
The polyethylene glycol (PEG) groups on the surface of the PLGA nanoparticles were functionalized using maleimide groups. Trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2) antigens of cancer cells, used as the targeting moiety, was attached to the maleimide groups on the surface of pegylated PLGA nanoparticles. Nanoparticles prepared by a nanoprecipitation method were characterized for their size, size distribution, surface charge, surface morphology, drug-loading, and in vitro drug release profile.
Results
The average size of the trastuzumab-decorated nanoparticles was 254 ± 16.4 nm and their zeta potential was −11.5 ± 1.4 mV. The average size of the nontargeted PLGA nanoparticles was 183 ± 22 nm and their zeta potential was −2.6 ± 0.34 mV. The cellular uptake of nanoparticles was studied using both HER2-positive (SKBR3 and BT-474) and HER2-negative (Calu-6) cell lines.
Conclusion
The cytotoxicity of the immunonanocarriers against HER2-positive cell lines was significantly higher than that of nontargeted PLGA nanoparticles and free docetaxel.
Introduction
An important goal of chemotherapy is minimizing dose-dependent side effects because of nonspecific biodistribution of chemotherapeutic agents. Many nanoparticulate drug delivery systems have been designed to deliver chemotherapy agents to the site of interest, minimize side effects, and improve the efficacy of anticancer agents, and to enhance biocompatibility, serum stability, and the in vivo drug delivery profile.Citation1 Improved drug selectivity, capability to carry a high concentration of drug to target sites, protection of drugs from enzymatic attack, and the possibility to use a similar carrier to encapsulate various drugs with no covalent conjugation to therapeutic agents, are some of the advantages of nanoparticles. Citation2 The surface modification of nanoparticles with peptides, nucleic acids, antibodies, aptamers, or small molecules that bind to antigens on the surface of cells or tissues may be considered for targeted delivery of drugs to tumor cells or malignant tissue.Citation3
Among the targeting moieties, monoclonal antibodies have been those used most often for the targeting of nanoparticles to tumor sites. Similar to metastatic tumors, primary tumors usually overexpress antigens on their surfaces (such as epidermal growth factor receptor in lymphoma cancer and human epidermal growth factor receptor 2 (HER2) in breast cancer). Therefore, monoclonal antibodies specific to certain antigens on the surface of cancer cells may be considered as targeting moieties against tumors.Citation4 The HER2 receptor, over-expressed in about 20%–25% of breast cancers, is one of the major targets for the design of anticancer drugs. Trastuzumab (Herceptin®), a monoclonal antibody against HER2-positive cells, is licensed by the US Food and Drug Administration.Citation5 HER2 is a transmembrane receptor that is readily accessible to antibody-based therapy. These receptors are internalized by receptor-mediated endocytosis. This is beneficial when the purpose is active targeting. Certainly, this characteristic promotes intracellular accumulation of anti-HER2-covered immunonanocarriers, including anticancer drugs.Citation2
Poly lactide-co-glycolide (PLGA), from the polyester family of biodegradable polymers used frequently as controlled drug delivery systems, has been used by many researchers for passive and active targeting of anticancer agents.Citation6 This polymer has been widely used in biomedical manufacturing because of its biodegradability and biocompatibility.Citation7 Due to the hydrophobic nature of PLGA molecules, hydrophobic drugs, including most anticancer agents, can be easily loaded into PLGA nanoparticles.Citation8 Surface treatments of PLGA nanoparticles are carried out using different functional groups for various purposes, such as targeted drug delivery and a long blood circulation time. Most often, carboxylic acid or amine groups are attached to the nanoparticle surfaces and used for further conjugation purposes. For this reason, the technique usually employed to conjugate trastuzumab to nanoparticles is amine coupling involving carboxyl activation via the highly water-soluble sulfo-N-hydroxysuccinimide (NHS) ester. Eghtedari et al have reported a novel technique to functionalize gold nanorods, enabling in vivo targeting of breast cancer tumors by attachment of trastuzumab and polyethylene glycol (PEG) on the surfaces of nanoparticles.Citation9
In another study, PLGA immunonanoparticles conjugated with monoclonal antibodies were prepared by a double emulsion and adsorption technique using 1-ethyl-3-(3- dimethylaminopropyl)-carbodiimide (EDC) as a reversible crosslinker. The immunonanoparticles were internalized directly into an MCF-10A neoT cell line, indicating that the system can be used to build up targeted and stable carrier systems.Citation10
The length of the crosslinker is very important, because the accessibility of the ligand is directly related to the length of the crosslinker, and the use of an extended spacer arm can greatly reduce steric hindrance. In many cases, short distances between the targeting moieties and the surface of the nanoparticles lead to poor receptor binding affinities and thus poor biological effects.Citation11 To resolve this problem, we used PEG-maleimide (PEG-MAL) as a linker between PLGA and the antibody. PEG-MAL is more reactive with thiol groups. Alternatively, trastuzumab could be thiolated with suitable agents, such as 2-iminothiolane, which will conjugate to the free amine groups of the antibody.Citation11,Citation12
In our previous studies, we investigated different approaches for the delivery of docetaxel, a leading chemotherapeutic drug for breast carcinoma, ovarian cancer, head and neck, and lung cancer, using nanoparticulate systems.Citation13–Citation17 In this study, we report on the preparation and characterization of trastuzumab-decorated pegylated PGLA nanoparticles as a targeted delivery system for docetaxel. The quantity of monoclonal antibodies attached to the surface of the nanoparticles was characterized. Their ability to target and enter antigen-rich BT-474 cells was studied and compared with Calu-6 cells as a negative control.
Materials and methods
Materials
Docetaxel was obtained from Cipla (Mumbai, India). PLGA (lactide:glycolide, 50:50; Resomer RG 504H, molecular weight 48,000) was purchased from Boeringer Ingelheim (Ingelheim, Germany). Polyvinyl alcohol (molecular weight 22,000) and bifunctional NH2-PEG-OH (average molecular weight 5000) were purchased from Sigma-Aldrich (St Louis, MO). EDC, NHS, 2-iminothiolane (Traut’s reagent), 5,5′-dithio-bis(2-nitro-benzoic acid (Ellman’s reagent), hydroxylamine hydrochloride, and cysteine hydrochloride were obtained from Fluka (Buchs, Switzerland). 4-Aminobenzoic acid p-aminobenzoic acid and 3-(4,5- dimethylthiazol-2-yl)-(2,5-diphenyl tetrazolium bromide) (MTT) was purchased from Sigma-Aldrich.
Cell culture
The breast cancer cell lines, BT-474 and SKBR3, and a Calu-6 lung cell line (American Type Culture Collection) were obtained from the Pasteur Institute (Tehran, Iran). The cell lines were cultivated in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a humidified incubator with 5% CO2. The cells were maintained in an exponential growth phase by periodic subcultivation.
Synthesis of PLGA-PEG
Hydrixylate-functionalized copolymer PLGA-PEG was synthesized by conjugation of OH-PEG-NH2 to PLGA-COOH. NHS (135 mg, 1.1 mmol) in the presence of EDC (230 mg, 1.2 mmol) was added to a stirred solution of PLGA-COOH (5 g, 0.28 mmol) in methylene chloride (10 mL). The mixture was stirred for 3 hours at 25°C. PLGA-NHS was precipitated with ethyl ether (5 mL) and repeatedly washed in an ice-cold mixture of ethyl ether and methanol to remove the residual NHS. After drying under vacuum, PLGA-NHS (1 g, 0.059 mmol) was dissolved in chloroform (4 mL) followed by addition of NH2-PEG-OH (250 mg, 0.074 mmol) and N,N-diisopropylethylamine (28 mg, 0.22 mmol). The copolymer was precipitated with cold methanol after 12 hours and washed with the same solvent (35 mL) to remove unreacted PEG. The resulting PLGA-PEG block copolymer was dried under vacuum and used for nanoparticle preparation without further treatment. The following are the main nuclear magnetic resonance (NMR) peaks of the sample:
1H-NMR (CDCl3 at 300 Hz) d 5.2 (m, (OCH(CH3) C(O)OCH2C(O)n-(CH2CH2O)m), 4.8 (m, (OCH(CH3)C(O) OCH2C(O)n-(CH2CH2O)m), 3.7 (s, (OCH(CH3)C(O)OCH2C(O) n-(CH2CH2O)m), 1.6 (d, (OCH(CH3)C(O)OCH2C(O)) n-(CH2CH2O)m.
Synthesis of p-maleimidobenzoic acid
The benzoic acid derivative was synthesized according to the procedure described by Yushika et al. 4-Aminobenzoic acid (4.26 g, 31 mmol) was suspended in 30 mL of acetone by addition of 5 mL of methanol. A solution of maleic anhydride (3.66 g, 37 mmol) in 10 mL of acetone was added dropwise and the resulting precipitate was stirred for 20 minutes. The material was suction-filtered, washed with acetone, and vacuum-dried to yield a yellow powder (6.36 g). The residue was dissolved in acetic anhydride (13 mL), treated with sodium acetate (1.08 g), and refluxed at 50°C for 2 hours. The volatiles were then removed under reduced pressure, and the residue was taken up in 150 mL of water and heated at 70°C for 2.5 hours. The resulting white precipitate was suction-filtered, washed with water, and vacuum-dried overnight to yield P-maleimidobenzoic acid (4.7 g, 70%). The following are the NMR main peaks of the sample:
1H-NMR (CDCl3 at 300 Hz): d 10.0–8.5 (br s, 1 H, CO2H), 7.95 (d, J = 9 Hz, 2 H, Ar-H ortho to maleimide), 7.32 (d, J = 9 Hz, 2 H, Ar-H ortho to carboxylic acid), 6.8 (s, 2 H, maleimide vinyl).
Synthesis of p-maleimidobenzoy1 azide
P-maleimidobenzoic acid (215 mg, 1 mmol), sodium azide (651 mg, 1 mmol), and excess N-hydroxysuccinimide (123 mg, 1 mmol) were refluxed in 50 mL acetonitrile for 2 hours in the presence of EDC (230 mg, 1.2 mmol). The solvent was removed under reduced pressure. The following are the NMR main peaks of the sample:
lH-NMR (CDCl3 at 300 Hz): 6 8.0 (d, J = 8 Hz, 2 H, Ar-H ortho to maleimide), 7.45 (d, J = 8 Hz, 2 H, Ar-H ortho to acyl azide), 6.75 (8, 2 H, maleimide vinyl).
Synthesis of PEG-PLGA with maleimide functional group
Dry toluene 150 mL was added to the residue and refluxed under nitrogen for 1 hour and 20 minutes. Toluene was decanted, and the copolymer of PLGA-PEG was added to it and stirred at room temperature overnight. The yielded polymer was filtered, washed with water, and dried. The following are the NMR main peaks of the sample:
1H-NMR (CDCl3 at 300 Hz) d 5.2 (m, ((OCH(CH3) C(O)OCH2C(O))n-(CH2CH2O)m), 4.8 (m, ((OCH(CH3) C(O)OCH2C(O))n-(CH2CH2O)m), 3.7 (s, ((OCH(CH3) C(O)OCH2C(O))n-(CH2CH2O)m), 1.6(d, ((OCH(CH3)C(O) OCH2C(O))n-(CH2CH2O)m), 6 8.0 (d, J = 8 Hz, 2 H, Ar-H ortho to maleimide), 7.45 (d, J = 8 Hz, 2 H, Ar-H ortho to PLGA-PEG), 6.75 (8, 2 H, maleimide vinyl).
Preparation of docetaxel-loaded pegylated PLGA nanoparticles
PLGA-PEG copolymer nanoparticles loaded with docetaxel were prepared using a nanoprecipitation method. Briefly, copolymer and the drug were dissolved in acetone. This organic phase was emulsified with an aqueous solution of polyvinyl alcohol (0.5% w/v) by sonication using a probe sonicator (Cole Parmer) at amplitude 5 for 60 seconds. Nanoparticles were formed immediately, and gently stirred at room temperature for 5 hours to evaporate the organic solvent. The resulting nanoparticle suspension was then ultracentrifuged at 15,000 rpm for 20 minutes. After centrifugation, the nanoparticle precipitate was washed using the same volume of distilled water as the supernatant, and again centrifuged at 15,000 rpm for 15 minutes. The washing process was repeated three times in order to remove the adsorbed drugs. The washed nanoparticles were then freeze- dried using the FreeZone system (Labconco, FreeZone Plus 6) for 48 hours.
Thiolation of trastuzumab: quantification of thiol groups
Trastuzumab was thiolated with a 50-fold molar excess of 2-iminothiolane for 2 hours and purified as described earlier, ie, an aliquot (1.0 mL) of trastuzumab solution at a concentration of 1 mg/mL in phosphate buffer pH 8.0 was thiolated with 40.2 mL of 2-iminothiolane solution for 2 hours. The antibody was purified by Microcons 100,000 microconcentrators (Amicon, Beverly, MA).
The concentration of antibody solution obtained from the purification step was about 1.1 mg/mL. Aliquots (250.0 mL) of concentrated trastuzumab solution were incubated with 6.25 mL of Ellman’s reagent (8.0 mg in 2.0 mL phosphate buffer, pH 8.0) for 15 minutes at 25°C. Afterwards, the samples were measured photometrically in UVette (Eppendorf AG, Hamburg, Germany) at 412 nm. The number of thiol groups introduced was calculated relative to L-cysteine standard solutions that were treated in the same manner. The concentration of antibody calculated by a spectrophotometric method (λ = 280 nm, Hewlett Packard, Model 8453, Germany) was used, assuming an extinction coefficient of 1.4 M−1 cm−1.
Immunoreactivity of thiolated trastuzumab
To examine the binding affinity and specificity of thiolated trastuzumab, 1 × 104 HER2-overexpressing breast cancer cells (BT-474) and negative cells (Calu-6) were incubated with 1 μL of 2 mg/mL trastuzumab as a control and thiolated trastuzumab in 0.5 mL phosphate-buffered saline for 1 hour at 4°C. The cells were then pelleted by centrifugation, suspended in 0.5 mL phosphate-buffered saline, and incubated with fluorescein isothiocyanate (FITC)-labeled sheep antihuman immunoglobulin as a secondary antibody for 1 hour. Thereafter, the cells were washed and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Covalent coupling of thiolated trastuzumab to PLGA nanoparticles
For the coupling reaction, an aliquot (500.0 μL) of sulfhydryl- reactive nanoparticle suspension (about 10 mg of nanoparticles) was incubated with 500.0 μL of thiolated trastuzumab (about 250 μg) for at least 12 hours at 20°C under constant shaking at 600 rpm. The samples were purified from the unreacted antibody by three cycles of centrifugation (18,000 × rpm, 15 minutes, 4°C). The amount of trastuzumab conjugated to a nanoparticle was determined indirectly by measuring uncoupled monoclonal antibodies in the supernatant after the centrifugation step. A spectrophotometric method (λ = 280 nm, Hewlett Packard, Model 8453, Germany) was used, assuming an extinction coefficient of 1.4 M−1 cm−1. The total amount of trastuzumab bound to the nanoparticle surface was calculated as the difference between the total amount of antibody obtained after thiolation and purification and the amount of antibody determined in the supernatant obtained after the conjugation step. The following equation was used to calculate the number of monoclonal antibodies molecules conjugated on the surface of a single nanoparticle:
where n is the number of trastuzumab molecules per nanoparticle, a is the mol of monoclonal antibodies per g PLGA, d is the density of nanoparticles (estimated to be 1.5 g/cm3 based on the polymer density), r is the mean radius of nanoparticles, and N is Avogadro’s number.Citation18
Determination of monoclonal antibodies on the surface of nanoparticles
A secondary antibody, FITC-labeled sheep antihuman immunoglobulin, was used to identify the presence of monoclonal antibodies on the surface of the nanoparticles. Monoclonal antibody-modified nanoparticles were treated with 0.5 μL of labeled sheep antihuman immunoglobulin at room temperature for 2 hours. The volume ratio between the solution of secondary monoclonal antibodies and dispersion of immunonanocarriers was 1:1000. Samples were centrifuged at 13,200 rpm for 15 minutes and washed twice with 1 mL of phosphate-buffered saline to eliminate the excess secondary monoclonal antibody. After a final washing, the sediment was redispersed in phosphate-buffered saline at pH 7.4, and the fluorescence intensity of fluorescent dye (fluorescein, λ ex 494 nm and λ em 525 nm) was measured using a microplate reader (Safire2™, Tecan, Switzerland). The fluorescence intensity was compared with that of the noncoated nanoparticles, FITC-labeled sheep antihuman immunoglobulin, and phosphate-buffered saline at pH 7.4 as the median.
Size and zeta potential measurements
The mean size of the PLGA nanoparticles and targeted nanoparticles was determined by laser light scattering (Malvern Zetasizer ZS, Worcestershire, UK). Each freezedried sample (100 μg) was diluted with water (10 mL), filtered to avoid multiscattering phenomena, and placed in a quartz cuvette. Size analysis of each sample consisted of 13 measurements, and the result was expressed as mean size ± standard deviation. Zeta potential measurements were performed on the same samples prepared for size analysis. Zeta limits ranged from −120 to 120 mV.
Surface morphology
Scanning electron microscopy (SEM, Philips XL 30, Philips, The Netherlands) was used to determine the shape and surface morphology of the nanoparticles produced. Particles were coated with gold under vacuum before scanning electron microscopy.
Drug loading efficiency
Lyophilized nanoparticles (2.5 mg) were dissolved in 1 mL of acetonitrile and shaken lightly followed by sonication for 6 minutes. Thereafter, 2 mL of methanol was added to precipitate the polymer. The sample was filtered and the drug quantity in the filtrant was determined by high-pressure liquid chromatography. Drug loading was determined as the relative amount of drug content of nanoparticles to the whole weight of the nanoparticles.
In vitro docetaxel release
The docetaxel release profile at pH 7.4 in phosphate-buffered saline and at pH 5.5 in acetate buffer was studied. 5 milligrams of freeze-dried docetaxel-loaded nanoparticles was poured into screw-capped tubes and suspended in 10 mL of phosphate-buffered saline pH 7.4 or acetate buffer pH 5.5. The tubes were placed in a water bath maintained at 37°C ± 0.5°C and shaken at 90 cycles/minute. At fixed time intervals, the tubes were taken from the water bath and centrifuged at 21,000 g for 15 minutes. The nanoparticles were resuspended in 10 mL of fresh buffer and placed back into the water bath to continue the release measurements. An aliquot of 9 mL was taken from the supernatant. A volume of 1 mL of methanol was added to precipitate any remaining PLGA, centrifuged for 15 minutes at 21,000 g, and analyzed by high-pressure liquid chromatography. The experiments were carried out in triplicate.
High-pressure liquid chromatography
High-pressure liquid chromatography was performed at room temperature using a Knauer apparatus model K-1001, WellChrom (Berlin, Germany) equipped with a reversed- phase C18 column (25 cm × 0.46 cm internal diameter, pore size 5 μm; Teknokroma, Barcelona, Spain) and eluted isocratically with acetonitrile/water (65/35 v/v). The flow rate was fixed at 1 mL/min, with ultraviolet detection at 230 nm. The linear regression coefficient determined in the range 0.05–10 μg/mL was 0.9994 (n = 6). The method sensitivity was 0.05 μg/mL (signal to noise ratio, 3:1).
Cell targeting and internalization of immunonanoparticles
Cellular targeting and uptake of trastuzumab and immunonanoparticles were investigated using monocultured SKBR3 and BT-474 as HER2-overexpressing cells and negative Calu-6 cells. The cells were seeded on chambered eight-well coverslips (Nunc, Naperville, IL) at a density of 2 × 104 cells per well. The next day, the spent growth medium was removed. The cells were incubated with 500 μL of 0.5 mg/mL FITC-labeled trastuzumab-modified nanoparticles in serum-free medium, and 500 μL of 5 μg/mL trastuzumab in serum-free medium at 37°C for 1 hour. The cells were washed three times with cold phosphate-buffered saline to remove any nanoparticles not internalized by the cells, and were fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 minutes. The cells were then washed again three times with cold phosphate-buffered saline. Thereafter, 4,6-diamidino-2-phenylindole (DAPI, Invitrogen) was used to counterstain the nuclei of the cells. The cells were then washed with phosphate-buffered saline. Finally, the cells were imaged using an Olympus IX/81 inverted fluorescence microscope (ultraviolet visible) and the images were analyzed using CellR Imaging software. Each measurement was performed in triplicate.
Additionally, internalization of FITC-loaded noncoated and immunonanoparticles into SKBR3 and Calu-6 cells was evaluated using flow cytometry. Cells were placed into six well plates and left to attach; 200 μL of fluorescein-loaded immunonanoparticles or noncoated nanoparticles (0.5 mg/mL) were then added to each well and incubated for 15, 30, 45, and 60 minutes. As a control, cells were grown separately in the absence of nanoparticles. Flow cytometry was performed on a FACSCalibur (Becton Dickinson Inc, Franklin Lakes, NJ).
In vitro cell viability
The in vitro cytotoxicity of the following docetaxel formulations was tested on BT-474 cells using the MTT test: NP-DTX-HER, NP-DTX, and free docetaxel. Unloaded nanoparticles and IgG isotype monoclonal antibodies were conjugated to the surface of the nanoparticles to act as a control for the corresponding anti-HER2 conjugated nanoparticles, and tested to determine the effects of the polymer on cell viability.
BT-474 cells were seeded in 96-well plates (Costar, Chicago, IL) at the density of 1 × 104 viable cells/well and incubated for 24 hours to allow cell attachment. The medium was replaced by 100 μL of the formulation at concentrations of 1–200 nM for 48 hours. For free docetaxel, a stock solution was prepared in dimethyl sulfoxide (1 mg/mL docetaxel). The dimethyl sulfoxide concentration in the medium was lower than 0.5%, at which level it has no effect on cell proliferation. The diluent for preparing the working solution for free docetaxel drug and nanoparticles was RPMI-1640 culture medium. At designated time intervals, 20 μL MTT (5 mg/mL in phosphate-buffered saline) was added to each well, and the culture medium containing MTT solution was removed after 3–4 hours. The formazan crystals were dissolved in 100 μL dimethyl sulfoxide and read at 570 nm by a microplate reader. Cell viability was calculated using the following equation:
where Ints is the colorimetric intensity of cells incubated with the samples, and Intcontrol is the colorimetric intensity of cells incubated with the phosphate-buffered saline only (positive control).
Statistical analysis
One-way analyses of variance were performed for comparison of the results. P values of 0.05 were considered to be statistically significant.
Results and discussion
Cancer is among the top causes of morbidity and mortality in humans worldwide. Development of new drugs and chemotherapy agents has opened up new horizons for the treatment of tumors. Optimum concentration of drugs at a tumor site is presently only possible at the cost of severe side effects. Nanocarriers with tumor-targeting moiety attachments, such as epidermal growth factor,Citation19 RGD peptide, folate,Citation20 transferrin,Citation21 or antibodies and antibody fragments, can maximize tumortargeted delivery and limit drug side effects. In this study docetaxel-loaded nanoparticles conjugated with the anti- HER2 monoclonal antibody, trastuzumab, were prepared.
Synthesis of PLGA-PEG-MAL
PEG-PLGA with the functional group maleimide was synthesized and characterized. The basic chemical structure of PLGA-PEG copolymer was confirmed by 1H-NMR. One of the prominent features is a peak at 3.7 ppm, matching the methylene groups of PEG. Overlapping doublets at 1.6 ppm are attributed to the methyl groups of the D- and L-lactic acid repeat units. The multiples at 5.2 ppm and 4.8 ppm correspond to the lactic acid —CH and the glycolic acid —CH, respectively, with the high complexity of the peaks resulting from different D-lactic, L-lactic glycolic acid sequences in the polymer backbone. Proton signals from phenyl and maleimide groups can be also detected. The maleimide group located at the end terminal of the hydrophilic PEG block is available for surface chemistry on the nanoparticle surface.
Preparation and characterization of docetaxel-loaded nanoparticles
At first, docetaxel was encapsulated in the pegylated PLGA nanoparticles with maleimide end groups by the nanoprecipitation method. The physicochemical characteristics of the nanoparticles are summarized in . The NPs-DTX-HER nanoparticles were prepared by covalent coupling of monoclonal antibodies to the NP-DTX using a two-step reaction. Covalent reactions are a useful method for attaching the ligands irreversibly to nanocarriers, because the connection formed is very stable and reproducible. The covalent binding between nanoparticles and the ligand must not affect the biological activity of the ligand. The amount of monoclonal antibody conjugated is approximately 195 anti-HER2 per nanoparticle.
Table 1 Physicochemical characteristics of poly lactic-co-glycolic acid nanoparticles and targeted nanoparticles (n = 3)
Immunoreactivity of thiolated trastuzumab
Thiolation of the antibody is an essential step for preparation of immunonanoparticles. Sulfhydryl groups were introduced into the monoclonal antibody molecule to provide a reactive site for the conjugation with maleimide groups onto the nanoparticle surface. Sulfhydryl groups were attached to trastuzumab molecules by a ring opening reaction of the primary amino groups of trastuzumab using 2-iminothiolane. These groups are at risk of oxidative disulfide bridge formation, leading to dimers or even higher oligomers of trastuzumab. These byproducts could affect biological activity so are undesirable. Steinhauser et al evaluated different thiolation conditions and determined the degree of antibody dimerization. They analyzed the thiolated antibody by size exclusion chromatography and identified the best thiolation procedure for the preparation of trastuzumab-conjugated nanoparticles.Citation22 We used their optimized conditions to produce thiolated trastuzumab and studied the immunoreactivity of thiolated anti-HER2 antibodies on BT-474 cells using FITC-labeled monoclonal antibodies. There was no significant difference in activity between native and thiolated anti-HER2 monoclonal antibodies.
Determination of monoclonal antibodies on the surface of nanoparticles
In order to verify the covalent linkage of the antibody to nanoparticles, an FITC-labeled sheep antihuman antibody coupling to anti-HER2 monoclonal antibody experiment were performed using increasing fluorescent intensity of the antibody-modified nanoparticles compared with unmodified nanoparticles (). The increase in fluorescent intensity of the antibody-modified nanoparticles indicated that the monoclonal antibody was covalently attached to the surface of the nanoparticles. Therefore, the concentration of FITC-labeled sheep antihuman antibody on the nanoparticle surface was increased.
Table 2 Fluorescent intensity of nanoparticles, targeted nanoparticles, phosphate-buffered saline as solution, and sheep antihuman antibody conjugated with fluorescein-5-isothiocyanate
Characterization of immunonanoparticles
The most important characteristic of nanoparticle systems is their size and size distribution. The biodistribution, toxicity, and targeting ability of these systems is determined by their size. Pore sizes in tumor microvasculature vary between 100 nm and 780 nm.Citation22 Many studies have recognized the suitable size of these carriers for extravasation and accumulation in solid tumors below 400 nm.Citation23 As shown in , the nontargeted nanoparticles had a size of 181 ± 3.5 nm, whereas the monoclonal antibody-targeted nanoparticles were larger at 254 ± 16.4 nm. Conjugation may be a reason for the larger size of the targeted nanoparticles. Several steps of the centrifugation and freeze-drying process may be another reason for the larger size of the targeted nanoparticles. Citation24 Scanning electron microscopy showed that both the nanoparticles and targeted nanoparticles were spherical and rather homogeneous in size ().
Figure 1 Scanning electron microscopic image of (A) poly lactic-co-glycolic acid nanoparticles containing docetaxel and (B) targeted nanoparticles.

For nanoparticles and monoclonal antibody-targeted nanoparticles, the zeta potential was −2.63 ± 0.34 mV and −11.5 ± 1.4 mV, respectively. This result shows that the mean zeta potential of the nanoparticles is lower than that previously reported for empty PLGA nanoparticles (−25 mV), indicating that the maleimide end groups of the polymer are located on the surface of the nanoparticle. After coupling of the anti-HER2 monoclonal antibodies to the nanoparticles, containing several ion groups, the negative value of the zeta potential is increased, suggesting that conjugation of monoclonal antibodies to the nanoparticles leads to an increase in the negative surface charge of the targeted nanoparticles.
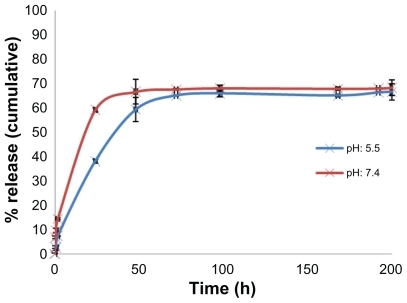
In vitro docetaxel release
The profile in vitro release of docetaxel from nanoparticles in the acetate buffer (pH 5.5) and phosphate-buffered saline (pH 7.4) within 200 hours is depicted in . During the first day of release, an early burst release greater than 60% was detected. A slow and steady release of docetaxel was observed after the burst release. The burst release may be due to the release of docetaxel loaded on the surface or just beneath the surface of the nanoparticles. The sustained docetaxel release following the initial burst release may be the result of diffusion of docetaxel through the polymeric matrix.Citation25 The main objective of using nanocarriers as drug delivery systems against cancer cells is targeted delivery rather than sustained delivery. Therefore, the fast drug release from these immunonanocarriers is not a negative feature.
Cell targeting and internalization of immunonanoparticles
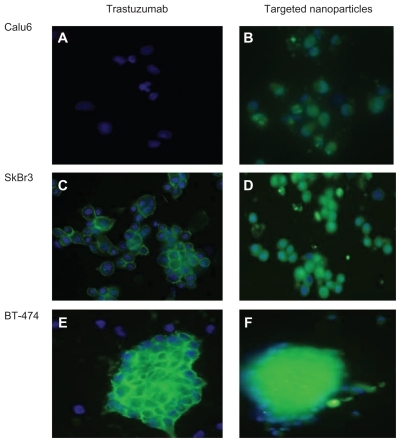
Cellular uptake of immunonanocarriers was studied in three different cell lines via confocal microscopy, and compared with pure trastuzumab. As shown in , SKBR3 and BT-474 cell lines with overexpression of HER2 antigen showed higher uptake of the immunonanocarriers (, green regions in the cytosol and blue regions in the nucleus). These results are in line with other studies demonstrating specific binding and internalization of anti-HER2 nanoparticulate drug delivery systems, such as immunoliposomes or immunonanoparticles. Citation18 However, fewer nanoparticles were internalized by HER2-negative Calu-6 cells ().
Figure 3 (A) Fluorescence microscope image of HER 2-overexpressing SKBR3 and BT-474 cells and (B) HER 2-negative Calu-6 cells, incubated with FITC-conjugated trastuzumab and docetaxel-PLGA nanoparticles coated with FITC-conjugated monoclonal antibody after 1 hour of incubation.
Abbreviations: HER2, human epidermal growth factor receptor 2; FITC, fluorescein isothiocyanate; PLGA, poly lactic-co-glycolic acid.
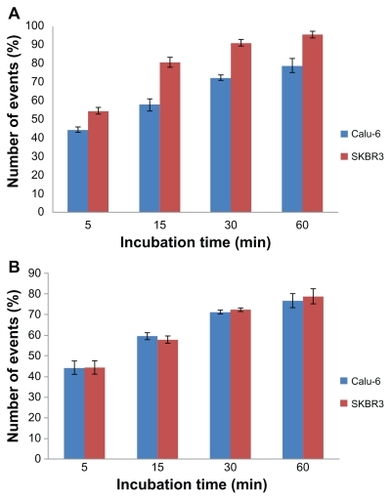
The results of flow cytometry also showed that uptake of the FITC-loaded immunonanoparticles by SKBR3 was higher than that by Calu-6 cells. Higher uptake of targeted nanoparticles by SKBR3 cells was in agreement with a previous study.Citation26 These results also suggest that immunonanoparticles may be taken up more rapidly than nontargeted nanoparticles.
Percentage cell gating of SKBR3 was significantly higher than for Calu-6 for targeted nanoparticles () confirming that the immunonanoparticles may attach to and be internalized by those cells with high expression of antigens (SKBR3 cells) compared with cells without expression of antigens (Calu-6). As shown in , for nontargeted nanoparticles cellular uptake by both cell lines is the same.
In vitro cellular cytotoxicity assay
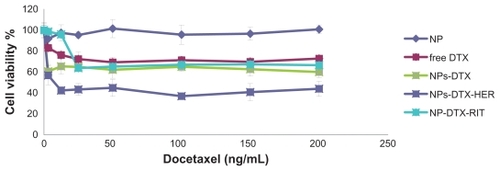
A series of in vitro cytotoxicity assays was performed to evaluate the anticancer potential of docetaxel-encapsulated targeted nanoparticles using BT-474 (HER2-positive) cells in comparison with that of free docetaxel. Nontargeted and trastuzumab-conjugated nanoparticles with no drug loading were also tested to determine the effects of polymerization and conjugation on cell viability. Statistical analysis showed that the unloaded nanoparticles did not influence cell viability. As shown in , docetaxel-loaded nanoparticles were highly cytotoxic to BT-474 cells which express the HER2 antigen on their surface. They were also more toxic than free docetaxel in the BT-474 cells, indicating the potential of these nanoparticles to treat human breast cancer. The greater efficiency of the immunonanoparticles can be explained by their specific interaction with HER2 antigens on the surface of BT-474 cells.Citation18
Figure 5 Viability of BT-474 cells overexpressing HER2, with docetaxel formulations after 48 hours. Different concentrations of docetaxel ranging from 1 to 200 ng/mL either as solution (free docetaxel) or encapsulated in nanoparticles (NP-DTX) or targeted (NP-DTX-HER or NP-DTX-RIT) were tested. Unloaded nanoparticles was used as controls.
Abbreviations: DTX, docetaxel; HER, herceptin; RIT, irrelevant mAb; HER2, human epidermal growth factor receptor 2; NP, nanoparticle.
Conclusion
In this study, trastuzumab-decorated pegylated PLGA nanoparticles with suitable characterization were successfully prepared for targeted delivery of docetaxel. Their size, size distribution, surface charge, surface morphology, drug loading, and in vitro drug release were characterized. In vitro cytotoxicity studies of docetaxel-loaded immunonanocarriers showed greater cytotoxicity effects against BT-474 HER2- positive breast cancer cells. These results suggest that monoclonal antibody-targeted nanoparticles are potentially useful as targeting delivery systems for chemotherapeutic agents.
Disclosure
The authors report no conflicts of interest in this work.
References
- YokoyamaMDrug targeting with nano-size carrier systemsJ Artif Organs20058778416094510
- Cirstoiu-HapcaABucheggerFLangeNBenefit of anti- HER2- coated paclitaxel-loaded immuno-nanoparticles in the treatment of disseminated ovarian cancer: Therapeutic efficacy and biodistribution in miceJ Control Release201014432433120219607
- ZhangLRadovic-MorenoAFAlexisFCo-delivery of hydrophobic and hydrophilic drugs from nanoparticle–aptamer bioconjugatesChemMedChem200721268127117600796
- MishraBPatelBBTiwariSColloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug deliveryNanomedicine2010692419447208
- HarbeckNPegramMDRüschoffJMöbusVTargeted therapy in metastatic breast cancer: The HER2/neu oncogeneBreast Care (Basel)201053720847829
- DinarvandRSepehriNManoochehriSRouhaniHAtyabiFPolylactide- co-glycolide nanoparticles for controlled delivery of anticancer agentsInt J Nanomedicine2011687789521720501
- AcharyaSSahooSKPLGA nanoparticles containing various anticancer agents and tumor delivery by EPR effectAdv Drug Deliv Rev20116317018320965219
- ManchandaRFernandez-FernandezANagesettiAMcGoronAJPreparation and characterization of a polymeric (PLGA) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agentsColloids Surf B Biointerfaces20107526026719775872
- EghtedariMLiopoAVCoplandJAOraevskyAAMotamediMMEngineering of hetero-functional gold nanorods for the vivo molecular targeting of breast cancerNano Lett2009928729119072129
- KocbekPObermajerNCegnarMKosJKristlJTargeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibodyJ Control Release2007120182617509712
- NobsLBucheggerFGurnyRAllémannECurrent methods for attaching targeting ligands to liposomes and nanoparticlesJ Pharm Sci2004931980199215236448
- AnhornMGWagnerSKreuterJLangerKvon BriesenHSpecific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticlesBioconjug Chem2008192321233118937508
- EsmaeiliFGhahremaniMHOstadSNFolate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugateJ Drug Target20081641542318569286
- EsmaeiliFDinarvandRGhahremaniMHDocetaxel-albumin conjugates: Preparation, in vitro evaluation and biodistribution studiesJ Pharm Sci2009982718273018972321
- YousefiAEsmaeiliFRahimianSAtyabiFDinarvandFPreparation and in vitro evaluation of a pegylated nano-liposomal formulation containing docetaxelSci Pharm200977453464
- EsmaeiliFDinarvandRGhahremaniMHOstadSNEsmailyHAtyabiFCellular cytotoxicity and in-vivo biodistribution of docetaxel poly (lactide-co-glycolide) nanoparticlesAnticancer Drugs201021435219809300
- SaremiSHAtyabiFAkhlaghiSPOstadSNDinarvandRThiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluationInt J Nanomedicine2011611912821289989
- Cirstoiu-HapcaABossy-NobsLBucheggerFGurnyRDelieFDifferential tumor cell targeting of anti-HER2 (Herceptin) and anti- CD20 (Mabthera) coupled nanoparticlesInt J Pharm200733119019617196347
- MilaneLDuanZFAmijiMPharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancerNanomedicine182011 [Epub ahead of print.]
- EbrahimnejadPDinarvandRNomaniARAziziEPreparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell linesNanomedicine2010647848519836467
- GanCWFengSSTransferrin-conjugated nanoparticles of poly(lactide)-D-alpha-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrierBiomaterials2010317748775720673685
- SteinhauserISpaBStrebhardtKLangerKTrastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cellsAnalysis20062749754983
- HobbsSKMonskyWLYuanFRegulation of transport pathways in tumor vessels: Role of tumor type and microenvironmentProc Natl Acad Sci U S A199895460746129539785
- IshidaOMaruyamaKSasakiKIwatsuruMSize-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing miceInt J Pharm1999190495610528096
- EsmaeiliFAtyabiFDinarvandRPreparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion methodDaru200816196202
- ChittasuphoCXieS-XBaoumAICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cellsEur J Pharm Sci20093714115019429421