?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to fabricate camptothecin/iron(III) oxide (CPT/Fe2O3)-loaded poly(D,L-lactide-co-glycolide) (PLGA) composite mats to modulate the CPT release and to improve the structural integrity and antitumor activity of the released drug. The CPT/Fe2O3-loaded PLGA ultrafine fibers were prepared for the first time by electrospinning a composite solution of CPT/Fe2O3 and neat PLGA (4 weight percent). The physicochemical characterization of the electrospun composite mat was carried out by scanning electron microscopy, energy dispersive X-ray spectroscopy, electron probe microanalysis, thermogravimetry, transmission electron microscopy, ultraviolet-visible spectroscopy, and X-ray diffraction pattern. The medicated composite fibers were evaluated for their cytotoxicity on C2C12 cells using Cell Counting Kit-8 assay (Sigma-Aldrich Corporation, St Louis, MO). The in vitro studies indicated a slow and prolonged release over a period of 96 hours with mild initial burst. Scanning electron microscopy, thermogravimetry, and X-ray diffraction studies confirmed the interaction of CPT/Fe2O3 with the PLGA matrix and showed that the crystallinity of CPT decreased after loading. Incorporation of CPT in the polymer media affected both the morphology and the size of the CPT/Fe2O3-loaded PLGA composite fibers. Electron probe microanalysis and energy dispersive X-ray spectroscopy results confirmed well-oriented composite ultrafine fibers with good incorporation of CPT/Fe2O3. The cytotoxicity results illustrate that the pristine PLGA did not exhibit noteworthy cytotoxicity; conversely, the CPT/Fe2O3 composite fibers inhibited C2C12 cells significantly. Thus, the current work demonstrates that the CPT/Fe2O3-loaded PLGA composite fibers represent a promising chemotherapeutic system for enhancing anticancer drug efficacy and selectively targeting cancer cells in order to treat diverse cancers.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Cancer is a major growing public problem and is the second main cause of deaths after cardiovascular diseases. It is a disease characterized by unregulated cell proliferation. Cancer can develop in almost any organ or tissue of the body, however, the efficient cure of cancer is still a challenge in disease treatments. Camptothecin (CPT) is a potent cytotoxic agent and shows anticancer activity for solid malignancies. CPT and its analogs show anticancer activity against colon, breast, liver, lung, prostate, and pancreatic cancer cells. It inhibits DNA topoisomerase I.Citation1,Citation2 Two CPT analogs, Hycamtin® and Camptosar®, have received Food and Drug Administration approval for the treatment of ovarian and lung cancers and for colorectal cancer, respectively.Citation3,Citation4 CPT was first isolated by Wall et alCitation5 from the wood Camptotheca acuminate Decaisne (Nyssaceae), a plant native to mainland China. After its successful isolation and characterization from the oriental tree C. acuminata, it aroused tremendous interest due to its splendid antitumor activity. Despite its high activity, it has limited therapeutic use. This is due to its poor aqueous solubility, serious side effects, and opening of the lactone ring at physiological pH to yield the carboxylate form which is inactive.Citation6,Citation7 CPT is commonly given as a sodium salt of the carboxylate form in the clinic to overcome the poor solubility of the lactone form; this requires higher dosing which may lead to additional toxic reactions. To overcome the solubility and stability problems, a series of CPT analogs have been synthesized and a few have entered the clinic.Citation8 Simultaneously, considerable interest has been devoted towards the design of new drug delivery systems with the aim to specifically target the drug to a tumor site, such that the drug is released at a controlled rate and at the desired time.
Nanotechnology offers promising applications in cancer treatments due to the unique properties of nanostructures. Drug-coated polymer nanoparticles can efficiently increase the intracellular accumulation of anticancer drugs.Citation9 Although diverse delivery systems have been developed for the insoluble lactone form of CPT and its derivatives,Citation10,Citation11 the possibility of iron(III) oxide (Fe2O3) loaded with CPT embedded in poly(D,L-lactide-co-glycolide) (PLGA) ultrafine fibers (CPT/Fe2O3-PLGA) for controllable release has hitherto not been investigated. The purpose of the current study was to encapsulate CPT in a novel carrier leading to low and controllable release to maintain the structural integrity and antitumor activity of drug as long as possible. PLGA was selected as it is a Food and Drug Administration-approved biodegradable and biocompatible copolymer. PLGA, with different glycolic acid to lactic acid ratios, produces fibers with suitable mechanical properties and a wide range of diameters and degradation rates.Citation12 The electrospinning process provides marvelous opportunities for fabricating fibers with a diameter from nanometers to a few micrometers.Citation13–Citation15 In addition to the widespread applications in tissue engineering, electrospun nanofibers can also be used as a drug delivery system. Drug delivery with polymer nanofibers is based on the principle that the dissolution rate of the drug increases with increased surface area of both the drug and the corresponding carrier. Besides their large surface area to volume ratio, polymer nanofibers also have other additional advantages. For example, unlike common encapsulation involving some complicated preparation process, therapeutic compounds can be easily incorporated into the carrier polymers using simple electrospinning. These electrospun nanofibers may be beneficial as cancer treatments through passive tumor targeting due to the enhanced permeability and retention effect.Citation16,Citation17 Furthermore, for biomedical applications the use of particles that present superparamagnetic behavior at room temperature is preferred. The magnetic nanoparticles of iron oxides, ie, magnetite and maghemite, represent the suitable candidates for preparation of magnetic nanocomposites owing to their unique applications such as vehicles for drug delivery,Citation18 nontoxicity, biocompatibility, biodegradability properties, and low price. To use these magnetic nanoparticles in biomedical applications, they often have to be modified with biocompatible compounds.Citation19 Scientists have accomplished this by either coating the magnetic nanoparticles with a layer of biodegradable polymers or evenly distributing a polymer matrix throughout the nanoparticles.Citation20 Magnetic drug targeting has been used to improve localized drug delivery and also enhance drug-therapeutic efficiency in various tumors.Citation21
Considering the unique properties of Fe2O3 nanoparticles and PLGA nanofibers, the current study attempted to synthesize CPT/Fe2O3-PLGA composite ultrafine fibers via simple and cost-effective electrospinning technique. By blending a drug into a polymeric carrier matrix, it is dispersed, meaning that the crystal lattice energy has already been overcome. In addition, the polymer carrier can stabilize the formed amorphous drug dispersion through specific molecular interactions and the tendency for the drug to recrystallize is made kinetically unfavorable for a long enough period to make the material pharmaceutically useful.Citation22 Thus, the combination of the chemotherapeutic agent (CPT) with composite solution (Fe2O3 magnetic nanoparticles and PLGA) is an attractive strategy to overcome the limitations of conventional cancer treatment. Moreover, the present project demonstrates the possibility of a designed composite matrix for enhanced adsorption of an anticancer drug in target cancer cells. To the best of the authors’ knowledge, an efficient delivery system that uses a novel composite of PLGA ultrafine fibers and Fe2O3 magnetic nanoparticles was developed here for the first time in order to realize the efficient accumulation of the anticancer drug CPT in target cancer cells. The resulting medicated nanofibers were characterized with regard to morphology, drug release behavior, and cytotoxicity on mouse myoblast C2C12 cells.
Material and methods
Materials
Iron(III) nitrate nonahydrate (Fe(NO3)3 · 9H2O, >98.5%) and ammonia solution (28%–30%) were purchased from Samchun Chemical Co, Ltd (Seoul, Korea). Cetyltrimethylammonium bromide (98%) was purchased from Sigma-Aldrich Corporation (St Louis, MO). PLGA (82:18 to 88:12 molar ratio; L-lactide: glycolide; inherent viscosity 2.5–3.5 dL/g) was obtained from Boehringer Ingelheim Pharma GmbH (Ingelheim, Germany). Dichloromethane and N,N-dimethylformamide (analytical grade; Showa Chemicals, Tokyo, Japan) were used as solvents without further purification. CPT was purchased from Sigma-Aldrich (95%). All other chemicals and solvents were of analytical grade and purchased from Sigma-Aldrich unless otherwise indicated.
Preparation of magnetic Fe2O3 nanoparticles
Fe2O3 nanoparticles were prepared by the hydrothermal method as described elsewhereCitation17 with suitable modifications. In a typical procedure, 5.0 g iron(III) nitrate nonahydrate was dissolved in 100 mL distilled deionized water. Then, 0.5 g cetyltrimethylammonium bromide was added to the aqueous iron nitrate solution and mixed under vigorous magnetic stirring for 30 minutes at room temperature. The solution was maintained at pH ~7 by adding ammonia. The suspension (100 mL) was subsequently transferred into a Teflon-lined stainless steel autoclave (R-201 Series, Reaction Engineering, Inc., South Korea) (200 mL capacity), sealed, and maintained at 150°C for 24 hours. The reaction mixture was cooled to room temperature and the precipitate was washed with distilled water. The precipitate was then separated by filtration and dried in an oven at 80°C overnight. Finally, the as-synthesized material was calcined at 600°C for 5 hours.
Preparation of pristine and CPT/Fe2O3- PLGA composite ultrafine fiber mats
The synthesis of CPT/Fe2O3-PLGA composite ultrafine fibers was carried out by using as-synthesized Fe2O3 nanoparticles and analytical grade extra pure (95%) CPT. Typically, PLGA (4 weight percent) was dissolved in dichloromethane: N,N-dimethylformamide (80:20 volume/volume) under magnetic stirring at room temperature overnight. A predetermined amount of CPT (~5 mg) and synthesized Fe2O3 (5% weight/ weight based on the polymer) were added into the PLGA solution. The composite solution was stirred for ~2 hours at room temperature for proper mixing. The blend solution of the polymer, Fe2O3 and CPT was transferred to a 10 mL syringe. A copper pin connected to a high voltage generator was inserted into the solution as a positive terminal whereas a ground iron drum covered by polyethylene sheet served as counter electrode. The solution was kept in the capillary by adjusting the inclination angle. A voltage of 15 kV was applied to this solution. The distance between the syringe needle tip and collector was fixed at 10 cm. The as-spun (pristine and composite) mats were initially dried at room temperature and thereafter maintained in a vacuum for 24 hours to remove residual solvents and finally stored at 4°C away from light for further analysis.
Characterization
X-ray diffraction (XRD) analysis
The XRD patterns of synthesized Fe2O3 nanoparticles and pristine, medicated CPT/Fe2O3-PLGA composite ultrafine fibers were recorded on a X-ray diffractometer (D/MAX 2500, Rigaku Corporation, Tokyo, Japan) with copper Kα radiation (λ = 1.540 Å) over Bragg angles ranging from 10–80 degrees. The operating voltage and current was maintained at 30 kV and 40 mA, respectively.
Morphology of Fe2O3 nanoparticles and pristine CPT/Fe2O3-PLGA composite fibers
The surface morphology of Fe2O3 nanoparticles, pristine PLGA ultrafine fibers, and the medicated composite was studied by using scanning electron microscopy (SEM) (S-7400; Hitachi High Technologies, Tokyo, Japan). The samples were uniformly sprayed on carbon tape, platinum coating was applied for 10 seconds onto the synthesized nanofibers, and the images were acquired at various magnifications. The fiber diameter was measured directly from SEM images. The microscopic features of the composite fibers were examined under transmission electron microscopy (TEM) (H-7650; Hitachi). The samples for analysis were prepared by placing copper grids near to the syringe microtip opening for a few seconds to collect the fibers.
Drug loading content and encapsulation efficiency
The CPT content and encapsulation efficiency in the CPT/ Fe2O3-PLGA composite mat was determined by following a previously established methodCitation23 with suitable modifications. Briefly, an exactly weighed amount of composite fibers was dissolved in dichloromethane: N,N-dimethylformamide (4:1 volume/volume; 2 mL) solvent mixture and gently stirred for ~10 minutes at room temperature. The resulting suspension was properly diluted with the aforementioned solvent mixture (10 mL) and vortexed for ~3 minutes. From this solution, 1 mL was transferred to a test tube using a micropipette. To this 1 mL, 5 mL dichloromethane solvent was added, which resulted in a clear solution. The CPT concentration in the suspension was determined by ultraviolet (UV) absorption at the wavelength of 295 nm with reference to a calibration curve on a UV-visible (UV-Vis) spectrophotometer (HP 8453; Agilent Technologies, Santa Clara, CA) equipped with a computer. The encapsulation efficiency (%) was estimated as being the percentage of CPT incorporated into the composite fibers in relation to the amount of drug initially added to the composite solution for fiber preparation. The drug loading efficiency and entrapment efficiency was calculated using the following equations:
Electron probe microanalysis (EPMA) and energy dispersive X-ray analysis
The chemical composition of the CPT/Fe2O3-PLGA composite fibers was analyzed by energy dispersive X-ray spectrometer (S-7400, Hitachi High technologies, Tokyo, Japan) equipped with SEM apparatus, whereas the distribution of elements was measured using EPMA (S-7400, Hitachi High technologies, Tokyo, Japan).
Thermogravimetric analysis (TGA)
The thermal stability of the samples was characterized by TGA (Pyris 1 TGA, PerkinElmer, Waltham, MA) under nitrogen with a flow rate of 20 mL/minute. The samples (~2–5 mg) were heated in a platinum pan from 25°C–450°C at a scanning rate of 10 °C/minute.
Fourier transform infrared (FTIR)
The composition of samples was characterized by FTIR (Spectrum RX1, PerkinElmer) spectroscopy in the wave number range of 500–4000 cm−1 at a resolution of 4 cm−1. The dried samples were ground into powder by a fiber microtome and then blended with potassium bromide before pressing the mixture into ultrathin pellets. The change in chemical structure of electrospun mats was investigated by FTIR spectroscopy.
In vitro drug release, matrix degradation, and stability of encapsulated CPT
The CPT/Fe2O3-PLGA composite mat was exactly weighed (0.1 g) and immersed in 40 mL 0.1 M Gibco® phosphate buffered saline (PBS: pH 7.4; Invitrogen Life Technologies, Carlsbad, CA) in order to obtain sink conditions. The suspension was kept in a thermostated shaker (SI-300R; Jeio Tech Co, Ltd, Seoul, Korea) that was maintained at 37°C and 100 rpm. At predetermined time intervals, 2 mL of the released solution was removed for analysis, and an equal amount of fresh buffer solution was added back in. The CPT concentration in the release media was detected by UV-Vis spectrophotometer and the spectra obtained were analyzed by spectrophotometer software (Probe 2.21, UV-Vis ChemStation; Agilent Technologies). The analysis was carried out in duplicate and the CPT release (%) versus time (hours) profiles were then plotted. To evaluate the change in morphology of the CPT/Fe2O3-PLGA composite mat after drug release in PBS, a fraction of composite mat was taken out at a predetermined time interval (after 30 days), rinsed with pure water to remove residual buffer salts, and dried in a vacuum desiccator (JEIOTECH Co. Ltd, South Korea). The morphology was observed by SEM as described above. To determine the stability of encapsulated CPT in the release medium, the composite mat was incubated for 3 weeks in PBS at 37°C with constant stirring. Aliquots (1 mL) were taken from the medium for scanning with UV-Vis spectrophotometry according to a previously described method.Citation24 The ratios (A355/A368) were calculated from the intensity of the characteristic UV absorption of CPT lactone form (λmax 355 nm) and carboxylate form (λmax 368 nm), and used to evaluate the stability of encapsulated CPT.
In vitro cytotoxicity assay
The CPT/Fe2O3-PLGA composite fibers and pristine PLGA fibers were sterilized under UV for 10 minutes. Mouse myoblast C2C12 (CRL 1772™; American Type Culture Collection, Manassas, VA) cells were used as model cell lines and the cell viability was evaluated by Cell Counting Kit-8 (Sigma-Aldrich) reagent. Briefly, cells were cultured in Dulbecco’s modified Eagle medium (pH 7.4, with 10% fetal bovine serum and 1% penicillin–streptomycin) in a humidified incubator (RCO3000T-5-VBC, Kendro Laboratory Products, Asheville-NC, USA) at 37°C with 5.0% carbon dioxide and 95% air environment. The cell density of 1 × 104 cells/well was seeded in a 96-well tissue culture plate (Becton Dickson and Company, Franklin Lakes, NJ) and allowed to attach and grow in wells overnight before CPT/Fe2O3-PLGA composite treatment. When C2C12 reached ~40% confluence, cells were treated with different concentrations (5 μg and 10 μg) of encapsulated CPT in composite matrix for a specific time (24, 48, and 72 hours) duration. Cell proliferation without drug treatment was set as the control. Cell viability was evaluated using a Cell Counting Kit-8 assay, in which 10 μL of water-soluble tetrazolium-8 (Cell Counting Kit-8) solution in each well (100 μL medium) was added and incubated for 4 hours at 37°C according to the manufacturer’s instructions. At the end of the experiment, absorbance was measured at 450 nm for each well by a microplate spectrophotometer (model 680; Bio-Rad Laboratories, Hercules, CA). Morphological alterations and cell damage were qualitatively investigated using a light phase contrast microscope (CX41; Olympus Corporation, Tokyo, Japan) at magnification 40×, and the photos were taken at different time intervals by computerized color FOculus® IEEE 1394 digital camera (NET New Electronic Technology GmbH, Finning, Germany) using the DIXI image solution software (v1.0; DIXI Optics, Daejeon, Korea).
Results and discussion
Characterization
represents the preparation of the CPT/Fe2O3-PLGA composite fibers. show the SEM micrographs of the synthesized Fe2O3 nanoparticles, plain PLGA, and the CPT/Fe2O3-PLGA system at different magnifications. Electrospinning of PLGA solutions containing 4 weight percent of the polymer afforded well-defined ultrafine fibers with an average diameter of about 750 ± 30 nm (). The pristine PLGA electrospun fibers had a smooth surface and appeared well defined without any interconnection (point bonding) between the fibers. The CPT/Fe2O3-PLGA system appeared smooth with Fe2O3 nanoparticles, but no drug crystals, on the polymer surface (). This suggested that the drug was dispersed homogeneously in the electrospun fibers. The drug-loaded fibers showed increased diameter size as compared to drug-free fibers. Furthermore, the incorporation of the drug produced ultrafine fibers with cross linking between the fibers. The fiber diameter was in the range of 1 μm (± 30 nm) on incorporation of the drug, and the formation of point-bonded structures was observed throughout the mats. Enlargement of the fibers obtained from the CPT/Fe2O3-PLGA colloid is preliminary evidence for CPT incorporation inside the polymeric ultrafine fibers. The drug loading and encapsulation efficacy of the composite mat was found to be ~3.8% and 75%, respectively. Earlier workers have reported that the affinity of loaded drugs to the polymeric matrix significantly affects drug loading capacity.Citation25,Citation26 In view of this, the promising values of encapsulation efficiency obtained for CPT in the current study could be related to the higher binding affinity of the Fe2O3-embedded PLGA matrix to CPT.
Figure 1 Illustration for the preparation of camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite fibers by electrospinning process.
Abbreviations: CPT, camptothecin; Cu, copper; Fe2O3, iron(III) oxide; PLGA, poly(D,L-lactide-co-glycolide).
Figure 2 Scanning electron microscopic images of (A and B) pristine poly(D,L-lactide-co-glycolide), (C and D) camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite ultrafine fibers, and (E) iron(III) oxide nanoparticles at different magnifications (yellow circles represent point bonding). (F) Energy dispersive X-ray spectrum of camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite. The inset photographic images represent the (i) pristine and (ii) composite nano-fibrous mats under ultraviolet fluorescence (λmax 256 nm).
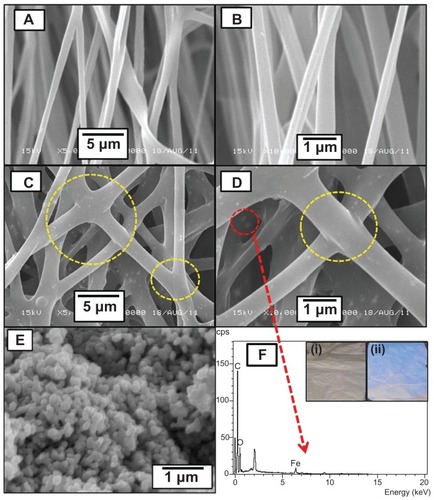
The morphology of the Fe2O3 nanoparticles examined by SEM was spherical () and the average nanoparticle size was found to be around 125 ± 15 nm. shows the energy dispersive X-ray spectrum of the CPT/ Fe2O3-PLGA composite fibers which contained carbon, oxygen, and iron; no other elemental impurity was detected, indicating that the final product was free of impurity and composed of CPT and Fe2O3. The inoculation of CPT into electrospun fibers was analyzed by UV fluorescence at wavelength 256 nm. As shown in the inset of , the electrospun composite mat emitted fluorescent light, suggesting the presence of CPT in the CPT/Fe2O3-PLGA composite mat. Noteworthy is that CPT nanoparticles could not be observed on the fiber surface, which indicates full encapsulation of the drug nanoparticles. The shift from the nonbonded to the point-bonded fiber morphology of the mat significantly affects the mechanical strength and stability of the mat, which is desirable for various applications.
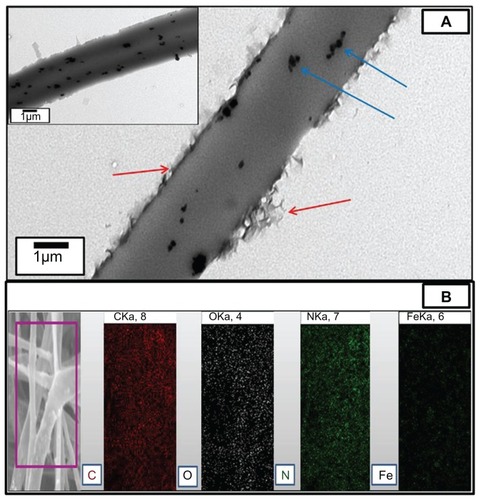
In order to get indepth insight into the structural features of electrospun composite fibers, TEM analysis was performed. shows the TEM image of the composite mat. The TEM image clearly demonstrates that the composite fibers contained Fe2O3 nanoparticles on the surface and exhibited a distinct uniform stratum due to the presence of CPT on or near the surface of the composite fibers. CPT is basically hydrophobic in nature and the CPT/ polymer solution would carry excess charges and move to the outer region of the electrospinning jet due to the charge repulsion. As a result, an outer layer was formed in the CPT/ Fe2O3 composite fibers but no such layer was observed in pristine PLGA fibers. The successful blending of CPT and Fe2O3 with PLGA ultrafine fibers was further confirmed by EPMA (). The EPMA image clearly shows that carbon, oxygen, and nitrogen are the main components and Fe2O3 is also uniformly dispersed on the surface of the CPT/ Fe2O3-PLGA composite fibers.
Figure 3 (A) Representative transmission electron microscopic image of the camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite. The inset image shows the uniform distribution of iron(III) oxide nanoparticles. Red and blue arrows demonstrate the distinct camptothecin layer and iron(III) oxide nanoparticles, respectively. (B) Electron probe microanalysis mapping result of the camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite. The purple square represents the selected area.
Abbreviations: C, carbon; Fe, iron; N, nitrogen; O, oxygen.
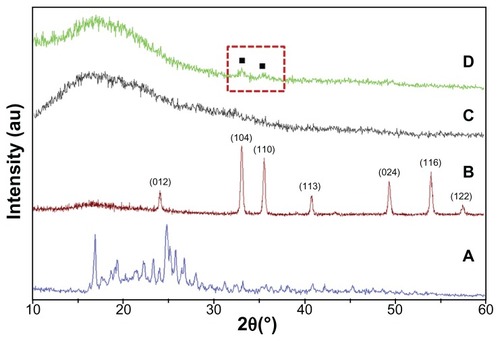
The crystalline state of the incorporated drug in the electrospun composite fibers was examined by XRD. summarizes the results. Fe2O3 has rhombohedral structure (Joint Committee on Point Diffraction Standards number 892810) and pure CPT was crystalline with major peaks at 16.97 and 24.8 with many other minor peaks, whereas the PLGA polymer had no prominent peaks. The CPT/Fe2O3-PLGA also showed the same spectrum as that of plain PLGA except for two small peaks at 34.0 and 36.0, which confirms the presence of Fe2O3 nanoparticles. However, no distinct peak was observed for CPT. During the electrospinning process, the large surface area that is associated with nanofibers allowed fast and efficient solvent evaporation, which gave the incorporated drug limited time to recrystallize and favored the formation of an amorphous state.
Figure 4 X-ray diffraction patterns of (A) free camptothecin, (B) iron(III) oxide nanoparticles, (C) electrospun pristine poly(D,L-lactide-co-glycolide), and (D) camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite fibers.
Note: The red dashed square represents the iron(III) oxide nanoparticle signals.
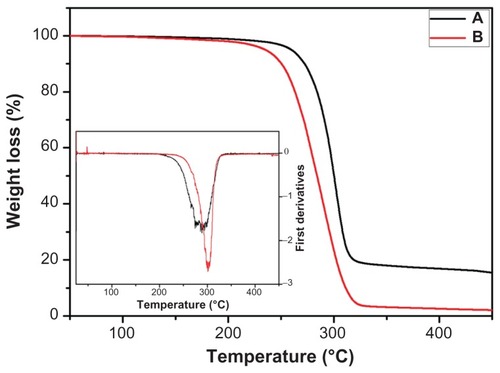
The TGA thermograms of the pristine and CPT/Fe2O3-PLGA ultrafine fibers are shown in . As pointed out by the TGA, pure PLGA fibers decomposed up to 94.1% while CPT/Fe2O3-PLGA fibers showed 81.2% degradation in the temperature range of 200°C–350°C. This result shows that CPT/Fe2O3-PLGA fibers have relatively higher thermal stability than pristine PLGA, which may be due to the higher decomposition temperature of CPT and Fe2O3 nanoparticles. This shift towards a higher temperature due to the addition of CPT/Fe2O3 also supports that there is point-bonded structure in the composite mat due to the homogenous mixing of CPT with PLGA, as shown in the SEM images (). To properly investigate the thermal properties, the first derivative plots of the TGA curves were established. A major peak was observed in both the pristine and CPT/Fe2O3-PLGA composite fibers ( inset), which is due to the decomposition of the polymer.
Figure 5 Thermogravimetric analysis graphs of (A) camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite and (B) pristine poly(D,L-lactide- co-glycolide) ultrafine fibers.
Note: The inset graph represents the corresponding first derivatives in nitrogen atmosphere.
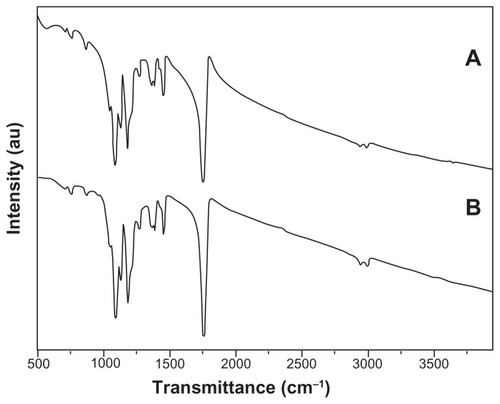
FTIR spectroscopy was used to further characterize the composite fibrous mats. It is clear from the current results that the FTIR spectra () of pristine and composite electrospun mats were very similar. The peaks around 2995 cm−1 and 2948 cm−1 were attributed to aliphatic carbon– hydrogen stretching vibrations. Meanwhile, the strong peak at 1755 cm−1 was related to the absorption by an ester carbonyl (carbon = oxygen) stretch from PLGA. The copolymer PLGA also had characteristic peaks at 1455 cm−1 (methyl group carbon–hydrogen stretching), two peaks at 1422 cm−1 and 1383 cm−1 (wagging vibrations from saturated carbon– hydrogen bonds), and carbon–oxygen peaks at 1265, 1180, 1090, and 1048 cm−1.Citation27,Citation28 A broad peak at ~571 cm−1 shows the presence of an iron–oxygen bond in the composite fibers (), which further confirms the incorporation of Fe2O3 in the composite mat.
In vitro drug release and degradation of CPT/Fe2O3-PLGA composite mat
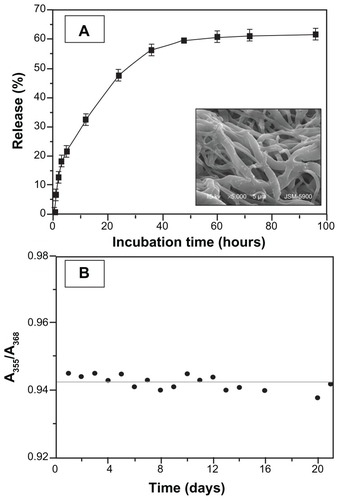
In order to investigate the drug release characteristics from the CPT/Fe2O3-PLGA electrospun mats, experiments were carried out using PBS buffer as the release medium at a controlled temperature and rpm as described previously. Since CPT is a hydrophobic substance with scarce aqueous solubility, the release investigation was carried out under sink conditions, keeping the drug concentration below its solubility limit; duplicate samples were incubated in PBS. At intervals, samples (2 mL) were removed and the medium replenished to maintain the sink conditions. The amount of drug in the samples was measured by UV-vis spectra with a range of 200–500 nm. The release of CPT from the composite fibers versus time of incubation is shown in . The release kinetics can be illustrated in two stages: an initial burst release followed by a constant slow release. CPT released from the composite fibers showed a burst release during the first 5 hours, releasing a considerable amount of drug. Afterwards, a gradual increase in the cumulative release followed over the next few hours, reaching a plateau at ~60 hours. This burst release could be attributed to the presence of CPT on or near the surface of the composite fibers (). This portion of CPT could be easily diffused into the release medium and lead to burst effect. As depicted in , a ~19% initial burst release from the CPT/Fe2O3-PLGA composite followed by sustained release was observed during the 4-day incubation.
Figure 7 In vitro release profile of (A) camptothecin from electrospun camptothecin/ iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite fibers and (B) A355/A368 ratios of camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co- glycolide) composite mat against incubation time in phosphate buffered saline.
Note: The inset scanning electron microscopic image shows the camptothecin/ iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite after incubation in phosphate buffered saline at 37°C for 30 days.
Different researchers have developed a number of delivery systems such as microparticles, nanoparticles, liposomes, micelles, and miniemulsions to overcome the solubility and stability problems of CPT, besides chemical modifications.Citation29–Citation33 The need of a system to further improve the therapeutic efficacy of the drug and to reduce its toxic effects is continuously attracting research attention. Different types of solid lipid nanoparticles have been developed for CPT deliveryCitation34 showing a higher cytotoxicity on cells than the free control. A novel biodegradable and biocompatible CPT/polymer implant based on chitosan for sustained intratumoral release of the drug has been described.Citation35 An approach to overcome the shortcomings of CPT and its analogs, especially their high systemic toxicity, is to load it into a delivery system such as CPT/Fe2O3-PLGA electrospun composite fibers which will protect the drug from hydrolysis and control its release over a prolonged period. Since the active drug is dispersed and not solubilized, there is no possibility of chemical reaction between the active drug and the PLGA matrix.
shows the CPT release profiles from the synthesized blend system. A significant amount released from the CPT-loaded electrospun nanofibers; however, after an initial burst release, a sustained release was observed. CPT is highly hydrophobic and the CPT/polymer solution would carry excess charges and move to the outer region of the electrospinning jet due to the charge repulsion. The initial burst release is due to the drug molecules enriched close to the fiber surface during the electrospinning process or loosely associated with the fiber matrix. For the delivery of antineoplastic drugs, a certain amount of initial burst is actually required to achieve enough of the initial dosage. Of course, for the cancer cells that survive the initial stage, sustained drug release is necessary. The current investigation indicated the advantages of electrospun fibrous mats in enhancing the constant release of hydrophobic drugs due to the significantly higher surface area for the drug dissolution and carrier erosion. Additionally, the electrospun mats can be cut to almost any size and fabricated into other shapes using different target geometries for clinical applications.Citation36
The degradation behavior of the CPT/Fe2O3-PLGA blend system was determined in buffer solution with respect to the morphological changes of the fibrous mats. Prominent changes (swollen and diffused fibers) were observed in the morphology after incubation for 30 days in PBS buffer ( inset). Since carboxylate conversion limits the bioavailability and efficacy of CPT, maintenance of the lactone structure during preparation, storage, and release is a prerequisite for improved therapy. To know the stability of CPT in the composite matrix, the characteristic UV absorption of encapsulated CPT was monitored in PBS (pH 7.4). Native CPT was used as a control. The characteristic UV absorption (λmax) of CPT lactone and carboxylate form in CPT/Fe2O3-PLGA was observed at 355 nm and 368 nm, respectively, even after 3 weeks of incubation with PBS (), which suggests the stability of CPT in CPT/Fe2O3-PLGA composite. To further check the stability of CPT in the composite matrix, the A355/A368 ratios were calculated from the intensity of the maximum UV absorption of CPT lactone and carboxylate form. The results showed that the A355/A368 ratios of encapsulated CPT remained the same with increased incubation time. Moreover, the surface of the composite matrix was hydrophobic and CPT encapsulated in the ultrafine fibers was prevented from being exposed to the liquid medium. It is suggested that electrospun fibers could effectively release the lactone form of CPT which is beneficial for the enhancement of anticancer activity.
In vitro cytotoxicity assay
The cytotoxicity of the CPT/Fe2O3-PLGA composite mat was tested on C2C12 cell lines; free CPT and the pristine PLGA composite mat were kept as controls. summarizes the cell viability after 24, 48, and 72 hours of incubation. As depicted in , ~40% and 56% inhibition was observed after 24 hours of incubation in free CPT and CPT/Fe2O3-PLGA samples (10 μg/well), respectively. No significant cytotoxicity of the unloaded PLGA mat was observed, indicating that the toxicity towards the cells was a consequence of the CPT molecule. However, a slight decline was observed at 72 hours with pristine PLGA. Moreover, it was also observed that the toxicity increased with an increase in concentration. Approximately 58% and 62%, 87% and 97% inhibition was observed at 5 μg/well and 10 μg/well with free CPT and CPT/Fe2O3-PLGA blend system, respectively, after 72 hours of incubation time. The data lead to the conclusion that the growth inhibition is concentration and time dependent. In particular, the CPT/Fe2O3-PLGA blend system showed a higher inhibition effect with respect to free CPT. The increased activity observed for the medicated composite fibers compared to that of free CPT could be explained by the increased stability of CPT caused by the interaction with Fe2O3/PLGA matrix. The complexed CPT is less prone to hydrolysis and the lactone ring is protected, which is essential for passive diffusion of the drug into cancer cells.
Figure 8 In vitro cytotoxicity of (A) free camptothecin and camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite. Untreated C2C12 cells and cells treated with pristine poly(D,L-lactide-co-glycolide) were used as a control. Representative phase contrast images of C2C12 cell lines (B) unexposed to, (C) exposed to 5 μg, and (D) exposed to 10 μg camptothecin/iron(III) oxide-embedded poly(D,L-lactide-co-glycolide) composite.
Note: Magnification 40×.
Abbreviations: CPT, camptothecin; PLGA, poly(D,L-lactide-co-glycolide).
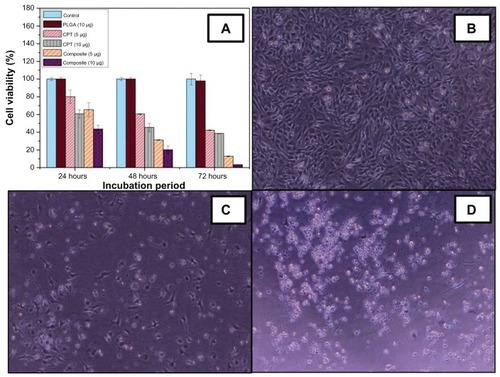
As is already known, the targeted delivery of antitumor agents adsorbed on the surface of magnetic nanoparticles is a promising alternative to conventional chemotherapy. The particles loaded with the drug are concentrated at the target site with the aid of an external magnet. The drugs are then released on the desired area.Citation37 Therefore, the incredible enhancement of cell inhibition rates by the CPT/Fe2O3- PLGA composite is due to the synergistic effect of novel Fe2O3/PLGA. The results from the current study indicate that the composite (CPT/Fe2O3-PLGA) system facilitated the accumulation and cellular uptake of CPT. Morphological alterations induced by CPT/Fe2O3-PLGA was also tested in C2C12 cells. Untreated cells were thin and elongated with two tapering ends (). The incubation with CPT/ Fe2O3-PLGA composite fibers showed that C2C12 cells lost their common elongated shape and appeared in a form of numerous roughly rounded cells of variable size (). Areas devoid of cells were also recorded (). After incubation with CPT/Fe2O3-PLGA (10 μg/well), the cells fragmented to minute vesicles. Few rounded cells and some areas devoid of cells were also noticed in the same culture medium. The treatment led to the aggregation of dense irregular cellular debris. No intact cells were recognized in this medium, which indicates the occurrence of widespread cell death ().
Conclusion
To sum up, pristine PLGA and CPT/Fe2O3-PLGA composite mats were successfully prepared by a simple, cost-effective electrospinning technique. For the current investigation, CPT and C2C12 cells were chosen as the model drug and cell lines, respectively. The release profile of CPT from the Fe2O3-loaded PLGA composite fibrous mat and the matrix degradation behavior were also studied. The characterization of the synthesized CPT/Fe2O3-PLGA composite mat was carried out by SEM, energy dispersive X-ray spectrometry, EPMA, TEM, TGA, and XRD analysis. Moreover the medicated electrospun fibers were evaluated for their cytotoxicity against C2C12 cells. The cytotoxicity assay indicated that the as-spun composite fibers had superior in vitro inhibition than the pristine CPT. The most probable reason for the enhanced activity is the synergistic effect of Fe2O3 and PLGA. The combination of magnetic and anticancer features exhibited by the CPT/Fe2O3-PLGA composite can be exploited in medicine, where they can be used for targeted transport of the anticancer agent (CPT) and its subsequent removal by an external magnetic field. Physicochemical characterization results confirm that the loaded drug retained its biological functionality even after it had been subjected to a high electrical voltage, indicating that the composite medicated fibers developed in the current study have great potential in drug delivery and is a promising material for cancer chemotherapy. However, the in vivo behavior of the drug release from the electrospun composite fibers needs to be confirmed in future studies.
Acknowledgments
This work is supported by the grant from the Korean Ministry of Education, Science, and Technology (The Regional Core Research Program/Center for Healthcare Technology and Development, Chonbuk National University, Jeonju, Korea).
Disclosure
The authors report no conflicts of interest in this work.
References
- RedinboMRStewartLKuhnPChampouxJJHolWGCrystal structures of human topoisomerase I in covalent and noncovalent complexes with DNAScience19982795356150415139488644
- StakerBLHjerrildKFeeseMDBehnkeCABurginABJrStewartLThe mechanism of topoisomerase I poisoning by a camptothecin analogueProc Natl Acad Sci U S A20029924153871539212426403
- LiSYAdairKTCamptotheca acuminata Decaisne (Xi Shu, Chinese Happy Tree): A Promising Antitumor and Antiviral Tree for the 21st CenturyNacogdoches, TXStephen F Austin State University1994
- OberliesNHKrollDJCamptothecin and taxol: historic achievements in natural products researchJ Nat Prod200467212913514987046
- WallMEWaniMCCookCEPalmerKHMcPhailATSimGAPlant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminataJ Am Chem Soc1966881638883890
- FassbergJStellaVJA kinetic and mechanistic study of the hydrolysis of camptothecin and some analoguesJ Pharm Sci19928176766841403703
- ChourpaIMillotJMSockalingumGDRiouJFManfaitMKinetics of lactone hydrolysis in antitumor drugs of camptothecin series as studied by fluorescence spectroscopyBiochim Biophys Acta1998137933533669545598
- VendittoVJSimanekEECancer therapies utilizing the camptothecin: a review of in vivo literatureMol Pharm20107230734920108971
- LvGHeFWangXNovel nanocomposite of nano Fe3O4 and polylactide nanofibers for application in drug uptake and induction of cell death of leukemia cancer cellsLangmuir20082452151215618193905
- LallooAChaoPHuPSteinSSinkoPJPharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecinsJ Control Release2006112333334216650910
- ZhangLYangMWangQ10-Hydroxycamptothecin loaded nanoparticles: preparation and antitumor activity in miceJ Control Release2007119215316217400320
- ZahediPRezaeianIRanaei-SiadatSOJafariSHSupapholPAA review on wound dressings with an emphasis on electrospun nano-fibrous polymeric bandagesPolym Adv Technol20102127795
- RenekerDHChunINanometer diameter fibers of polymer produced by electrospinningNanotechnology19967216223
- HuangZMZhangYZKotakiMRamakrishnaSA review on polymer nanofibers by electrospinning and their applications in nanocompositesCompos Sci Technol2003631522232253
- GreinerAWendorffJHElectrospinning: a fascinating method for the preparation of ultrathin fibersAngew Chem Int Ed Engl200746305670570317585397
- MatsumuraYMaedaHA new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancsCancer Res19864612 Pt 1638763922946403
- DuncanRThe dawning era of polymer therapeuticsNat Rev Drug Discov20032534736012750738
- JainTKMoralesMASahooSKLeslie-PeleckyDLLabhasetwarVIron oxide nanoparticles for sustained delivery of anticancer agentsMol Pharm20052319420515934780
- LubbeASAlexiouCBergemannCClinical applications of magnetic drug targetingJ Surg Res20089520020611162046
- MartinaMSWilhemCLesieurSThe effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cellsBiomaterials200829304137414518667235
- ScarberryKEDickersonEBMcDonaldJFZhangZJMagnetic nanoparticle- peptide conjugates for in vitro and in vivo targeting and extraction of cancer cellsJ Am Chem Soc200813031102581026218611005
- LeunerCDressmanJImproving drug solubility for oral delivery using solid dispersionEur J Pharm Biopharm2000501476010840192
- ZhuAYuanLJinWPolysaccharide surface modified Fe3O4 nanoparticles for camptothecin loading and releaseActa Biomater2009551489149819286431
- ZhaoHLeeCSaiP20-O-acylcamptothecin derivatives: evidence for lactone stabilizationJ Org Chem200065154601460610959865
- LeeJChoECChoKIncorporation and release behavior of hydrophobic drug in functionalized poly(D,L-lactide)-block-poly(ethylene oxide) micellesJ Control Release2004942–332333514744484
- AllenCMaysingerDEisenbergANano-engineering block copolymer aggregates for drug deliveryColloids Surf B Biointerfaces1999161–4327
- LiuFGuoRShenMWangSShiXEffect of processing variables on the morphology of electrospun poly(lactic acid)-co-(glycolic acid) nanofibersMacromol Mater Eng200929410666672
- ArmentanoIDottoriMPugliaDKennyJMEffects of carbon nanotubes (CNTs) on the processing and in-vitro degradation of poly(DL-lactide-co-glycolide)/CNT filmsJ Mater Sci Mater Med20081962377238718158616
- HatefiAAmsdenBCamptothecin delivery methodsPharm Res200219101389139912425455
- ShenderovaABurkeTGSchwendemanSPStabilization of 10-hydroxycamptothecin in poly(lactide-co-glycolide) microsphere delivery vehiclesPharm Res19971410140614149358554
- CortesiREspositoEMaiettiAMenegattiENastruzziCFormulation study for the antitumor drug camptothecin: liposomes, micellar solutions and a microemulsionInt J Pharm1997159195103
- KuniiROnishiHMachidaYPreparation and antitumor characteristics of PLA/(PEG-PPG-PEG) nanoparticles loaded with camptothecinEur J Pharm Biopharm200767191717337172
- DavisMEDesign and development of IT-101, a cyclodextrin containing polymer conjugate of camptothecinAdv Drug Deliv Rev200961131189119219682514
- HuangZRHuaSCYangYLFangJYDevelopment and evaluation of lipid nanoparticles for camptothecin delivery: a comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsionActa Pharmacol Sin20082991094110218718178
- BerradaMSerreqiADabbarhFOwusuAGuptaALehnertSA novel non-toxic camptothecin formulation for cancer chemotherapyBiomaterials200526142115212015576186
- VerreckGChunIRosenblattJIncorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymerJ Control Release200392334936014568415
- Fernandez-PachecoRMarquinaCValdiviaJGMagnetic nanoparticles for local drug delivery using magnetic implantsJ Magn Magn Mater20073111318322