Abstract
We proposed to develop a polycation lipid nanocarrier (PLN) with higher transfection efficiency than our previously described polycation nanostrucutred lipid nanocarrier (PNLC). PLN was composed of triolein, cetylated low-molecular-weight polyethylenimine, and dioleoyl phosphatidylethanolamine. The physicochemical properties of PLN and the PLN/DNA complexes (PDC) were characterized. The in vitro transfection was performed in human lung adenocarcinoma (SPC-A1) cells, and the intracellular mechanism was investigated as well. The measurements indicated that PLN and PDC are homogenous nanometer-sized particles with a positive charge. The transfection efficiency of PDC significantly increased with the content of triolein and was higher than that of PNLC and commercial Lipofectamine™ 2000. In particular, the transfection of PLN in the presence of 10% serum was more effective than that in its absence. With the help of specific inhibitors of chlorpromazine and filipin, the clathrin-dependent endocytosis pathway was determined to be the main contributor to the successful transfection mediated by PLN in SPC-A1 cells. The captured images verified that the fluorescent PDC was localized in the lysosomes and nuclei after endocytosis. Thus, PLN represents a novel efficient nonviral gene delivery vector.
Introduction
Efficient and safe gene transfer to mammalian cells remains the most challenging aspect of successful gene therapy.Citation1 Many gene delivery systems including viral and nonviral vectors have been developed for effective gene transfection.Citation2–Citation4 Viral vectors show dramatically high transfection efficiency and are commonly used as delivery modalities for in vivo gene delivery. However, their applications are limited due to their poor safety profiles for in vivo studies.Citation2,Citation5 Nonviral vectors are associated with fewer safety concerns and less immunogenicity.Citation6,Citation7 Moreover, they are easier to produce in clinically relevant quantities and have fewer restrictions on their capacity to carry DNA. In the past few years, the development of novel efficient nonviral vectors has attracted increasing attention, although their efficiency is not quite satisfactory.Citation4,Citation7
Cationic liposomes and polyethylenimine (PEI) are the most extensively investigated nonviral vectors.Citation8–Citation11 Cationic liposomes are composed of cationic lipids and dioleoyl phosphatidylethanolamine (DOPE). DOPE is essential in cationic liposomes because it can form the inverted hexagonal (HII) phase at low pH and destabilize the endosomal membrane, thereby enhancing the transfection efficiency.Citation12,Citation13 On the other hand, PEI is one of the most effective gene delivery polymers with efficient gene transfer activity.Citation10,Citation11 Uptake of the PEI/DNA complexes into mammalian cells is via the endosomal pathway, and they are released into the cytoplasma from endosomes due to the proton sponge effect.Citation14,Citation15 LPDII vectors and polycation liposomes have been developed by combining the advantages of liposomes and polycation,Citation16–Citation18 which provides an effective strategy for the development of a novel nonviral gene delivery system.
Triolein is a hydrophobic molecule that shows little interaction with water and perturbs phospholipids planar bilayers. Measured effects of triolein on the structure and properties of phosphatidylethanolamine membrane have verified that triolein could enhance the formation of the HII phase, which may be beneficial to destabilizing the endosomal membrane and enhancing gene transfection.Citation19 Artificial lipoprotein has been developed with triolein and palmitoyl poly-L-lysine for gene delivery, and the transfection efficiency was comparable with that of commercial Lipofectamine™.Citation20,Citation21 In our laboratory we have developed a polycation nanostructured lipid nanocarrier (PNLC) composed of cetylated PEI, triolein, and egg yolk phosphatidylcholine, and the transfection efficiency was increased with the content of triolein, which implied the potential effect of triolein on gene delivery.Citation22 However, the facilitating effect of triolein on the formation of the HII phase in phosphatidylethanolamine membrane has not been utilized in a nonviral gene delivery system.
In the present study, polycation lipid nanocarrier (PLN) was developed with DOPE, cetylated PEI, and triolein for efficient gene delivery. We hypothesized that the incorporation of triolein into PLN could favor the formation of the HII phase, thereby enhancing the gene transfection efficiency. The physicochemical properties of PLN and the PLN/DNA complexes (PDC) were characterized, and the transfection efficiency was evaluated in comparison with the commercial Lipofectamine™ 2000. Moreover, the intracellular mechanism of PDC and the cytotoxicity were evaluated in human lung adenocarcinoma (SPC-A1) cells to verify the effectiveness of PLN in efficient gene delivery.
Materials and methods
Materials
Triolein was purchased from Sinopharm Chemical Reagent Co Ltd (Shanghai, China), branched low-molecular PEI (1200 Da, Sigma-Aldrich, St Louis, MO) was lyophilized before use, DOPE was provided by Lipoid, Lipofectamine™ 2000 and RPMI1640 were purchased from Invitrogen (Carlsbad, CA), and fetal bovine serum (FBS) was supplied by Hyclone Co Ltd (Beijing, China). MTT (3-[4,5-dimethylthiazolyl-2]-2,5-diphenyl tetrazolium bromide) and Hoechst 33258 were supplied from Sigma- Aldrich (Shanghai, China). Ethanol and other chemicals were analytical grade and used without further purification. Plasmid pEGFP-N2 encoding the enhanced green fluorescent protein (GFP) and plasmid pGL3-luc encoding the luciferase were used as reporter genes to access transfection efficiency. The plasmids were amplified using Escherichia coli DH5α and purified using the Endo Free Kit to remove the bacterial endotoxins.
Cell lines
SPC-A1 were purchased from the Shanghai Cell Institute, Chinese Academy of Sciences. The cells were maintained at 37°C with 5% CO2 and fully humidified conditions. SPC-A1 cells were cultured with RPMI1640 medium, supplemented with 10% FBS (Hyclone), 100 unit/mL penicillin, and 100 μg/mL streptomycin (Gibco).
Preparation and characterization of PLN
The cetylated PEI 1200 (c-PEI) was synthesized as previously described.Citation18 The synthesized c-PEI contained, on average, 28 ethylene units and six cetyl groups. PLN was prepared by an emulsification solvent evaporation method. In brief, c-PEI, triolein, and DOPE (0.1:0.8:1, molar ratio) were dissolved in dichloromethane and then dropped into three volumes of double-distilled water under agitation. The mixture was emulsified by sonication with probe at 200 w for 5 minutes (JYD 650, China), and the organic solvent was evaporated under reduced pressure (Heidolph Laborata 4000, Germany) at 37°C until the solution was clear to yield PLN.
The size distribution and zeta potential values of PLN were measured by dynamic light scattering (DLS) using a NICOMP™ ZLS 380 instrument (Particle Sizing System). PLN was diluted with three volumes of double-distilled water and measured at room temperature. The scattering angle was 90°, and the results were displayed in a Gaussian model. Analysis was performed with the accompanied zpw 388 software (Particle Sizing System).
The surface morphology of PLN was characterized by atomic force microscopy (AFM) (SPM-9500, Shimazu, Japan) in air at room temperature. Briefly, 3 μL of diluted PLN was pipetted on to the freshly cleaved mica, dried over-night at room temperature, and then imaged under AFM. The AFM measurements were performed using tapping mode probes with constant amplitude. The images were recorded at the resonance frequency of the cantilever with a scan rate of 1 Hz and a resolution of 256 samples per line.
Preparation and characterization of PLN/DNA complex
The PDC were prepared as previously described.Citation11,Citation18 Briefly, 1 μg of plasmid DNA was diluted in sterile distilled water, and PLN was mixed with the DNA solution at different N/P ratios (ratios of nitrogen atoms on PEI to phosphates on plasmid DNA). The mixture was incubated for 20 minutes at room temperature prior to further experiments. The formation of PDC at different N/P ratios was examined using agarose gel analysis. Samples equivalent to 250 ng plasmid DNA were loaded into each well of the agarose gel (0.8% in Tris- Acetate-EDTA buffer). Electrophoresis was conducted for 50 minutes at 100 volts at room temperature, and the mobility of PDC was visualized under an EC3 imaging system (UVP). The morphologic examination of PDC (N/P = 10) (1 μg/mL) was performed by AFM with tapping mode as described previously. In addition, the size distribution and zeta potential values of PDC (1 μg/mL) in double-distilled water were measured by the NICOMP™ ZLS 380 instrument as well.
Cell transfection
SPC-A1 cells were seeded into 24-well plates at 1 × 105 cells/well in 0.5 mL of culture medium 24 hours prior to the transfection. The culture medium was replaced with 1 mL FBS free RPMI 1640 medium before the transfection experiments. PDC equivalent to 1 μg of plasmid DNA at various N/P ratios were added to each well and incubated for 4 hours at 37°C and 5% CO2. Then, the incubation medium was removed and 1 mL of fresh RPMI 1640 with 10% FBS was added to each well and incubated for a further 48 hours. Meanwhile, transfection with Lipofectamine™ 2000/DNA complexes (LDC) was performed according to the manufacturer’s protocol as positive control. The terminal concentration of plasmid DNA in each well was about 1 μg/mL. In addition, to evaluate the effect of serum on the transfection, PDC (N/P = 10) was incubated with cells for 4 hours in RPMI 1640 culture media with 10% FBS. Afterwards, the procedure was performed as described previously. All transfection experiments were performed in triplicate.
Evaluation of transfection efficiency
The GFP expression in SPC-A1 cells was directly observed under fluorescence microscope (Olympus IX71, Japan). Quantitative assay of expressed GFP in SPC-A1 cells was determined as previously described.Citation18 Cells were washed twice with PBS in the culture plate, solubilized with 1% Triton X-100 (Sigma) for 30 minutes at room temperature, and then centrifuged at 3000 × g for 10 minutes. The fluorescence intensity in the supernatant was measured on a fluorescence spectrometer (LS55, Perkin Elmer). The excitation wavelength was 493 nm, and the emission one was 510 nm. The lysate of SPC-A1 cells without any treatment was used as a negative control.
Moreover, the transfection of plasmid pEGFP-N2 mediated by PDC was analyzed by flow cytometry.Citation23 After 48 hours’ incubation, cells were washed with cold PBS and harvested by the addition of trypsin-EDTA solution. Then, 1 mL of PBS was added and cells were centrifuged at 1000 × g for 5 minutes. The cell pellets were washed three times with PBS and fixed in 2% para-formaldehyde solution for 30 minutes at 4°C. The fixed cells were washed twice with PBS and resuspended in 0.1% para-formaldehyde for subsequent analysis. The GFP expressed cells were measured using a FACScan flow cytometer (BD Biosciences) and analyzed with CellQuest software. A total of 10,000 cells were collected for each sample, and determination of GFP positive events was performed by a standard gating technique.
In addition, transfection of plasmid pGL3-luc in SPC-A1 cells by PDC was also preceded as described previously. After an additional 48 hours’ incubation, SPC-A1 cells were lysated with 1% Triton X-100 at 4°C for 30 minutes, and the cell lysates were centrifuged at 3000 × g for 10 minutes. The expressed luciferase activity in the supernatant was measured by the bioluminescence assay kit according to the protocol (Biotherma). Where indicated, luciferase activity was normalized for protein content using the BCA protein assay (Pierce). The relative transfection efficiency was expressed as the ratio between the expressed luciferase activity in SPC-A1 cells mediated by PDC and that transfected by LDC in the absence of serum.
Intracellular transfer mechanism
To investigate the endocytosis pathway of PDC, the transfection of PDC in SPC-A1 cells was performed in the presence of specific inhibitors.Citation24,Citation25 Chlorpromazine could disturb the intracellular clathrin processing and was used to inhibit the clathrin-dependent endocytosis. On the other hand, filipin III could complex with membrane cholesterol and interferes with the cholesterol-sensitive process, thereby blocking the caveolae-mediated endocytosis.Citation26,Citation27 SPC-A1 cells were preincubated with 10 μg/mL chlorpromazine or 1 μg/mL filipin III for 1 hour in serum free RPMI 1640 media. PLN was mixed with plasmid pGL3-luc for 20 minutes at room temperature to prepared PDC (N/P ratio = 10). Then, PDC was added to each well and incubated for another 2 hours at 37°C. Afterwards, the incubation media were removed and cells were cultured with FBS-supplemented medium without any inhibitors. After further incubation for 48 hours, cells were lysated and the luciferase activity in the supernatant was determined as described previously. All transfection experiments were performed in triplicate. The expressed luciferase activity in SPC-A1 cells without the treatment of any inhibitor was set as 100%.
Laser confocal scanning microscopy
To visualize the localization of PDC in SPC-A1 cells, plasmid DNA was fluorescently labeled with Label IT® TM-Rhodamine Labeling Kit (Mirus) according to the manufacturer’s protocol, and the fluorescently labeled PDC were prepared as described previously. Approximately 5 × 105 cells were seeded into a 35 mm glass-bottom dish (MatTek) and cultured overnight prior to the experiments. Cells were incubated in FBS containing RPMI1640 media without phenol red (Gibco). Before transfection experiments, the medium was replaced with FBS free culture media without phenol red. The fluorescently labeled PDC was added and incubated at 37°C for 2 hours. Immediately before imaging, the lysosomes were labeled with LysoTracker™ Green DND-26 (Molecular Probes), and the nuclei were stained with Hoechst 33258. Cells were washed twice with RPMI1640 media without phenol red prior to observation under laser confocal scanning microscopy (Leica Sp-5, Germany).Citation28
Cell cytotoxicity assay
The cytotoxicity of PDC in SPC-A1 cells was ascertained by MTT colorimetric assay.Citation22,Citation29 Cells were seeded in a 96-well cell culture plate at an initial density of 1 × 104 cells/well and incubated for 24 hours prior to the test. PDC at various N/P ratios was added to each well at 100 ng/well of plasmid DNA in 200 μL of serum free growth media, and then the process proceeded as described previously. Meanwhile, the cytotoxicity of different PEI (1200Da)/DNA complexes was determined as positive control. After 24 hours, cells were performed for MTT assay. The absorbance at 570 nm of each well was measured by a microplate reader (BioTek ELx800). The viability of SPC-A1 cells without any treatment was set as 100%, and the cytotoxicity of PDC was calculated thereof.
Results
Preparation and characterization of PLN
PLN was constructed with cetylated low-molecular-weight PEI (1200 Da) (c-PEI), triolein, and DOPE, which could be composed of lipid core and hydrophilic surface at the outer shell (). The hydrophobic groups of amphiphilic c-PEI, DOPE, and lipophilic Triolein could form the lipid core due to the hydrophobic interaction, whereas the hydrophilic groups of c-PEI and DOPE could surround at the outer surface. The lipophilic cetyl groups of c-PEI could facilitate its incorporation into the lipid nanocarrier and locate the cationic hydrophilic PEI on the surface. The mean diameter of PLN was 136 ± 44.8 nm with a narrow polydispersivity of 0.108 (). Meanwhile, the AFM measurements revealed that PLN was quasispherical nanometric particles (), and the particle size was in accordance with that measured by DLS. In addition, the zeta potential of PLN was 34.32 ± 2.13 mv, which could facilitate the compaction of PLN with anionic plasmids DNA for subsequent gene transfection. The cationic potential of PLN implicated that the hydrophilic cationic PEI groups of c-PEI could locate at the surface of the lipid nanocarrier.
Figure 1 Characteristics of polycation lipid nanocarrier (PLN) and PLN/DNA complexes (PDC). (A) Scheme of PLN composed of cetylated polyethylenimine (PEI) 1200 (c-PEI), dioleoyl phosphatidylethanolamine (DOPE), and triolein; (B) size distribution of PLN; (C) atomic force microscopy image of PLN, scale bar = 1.0 μm; (D) size distribution of PDC (N/P ratio = 10); (E) atomic force microscopy image of the PDC (N/P ratio = 10), scale bar = 1.0 μm; (F) agarose gel electrophoresis of the complexes of PLN with plasmid DNA stained with ethidium bromide.
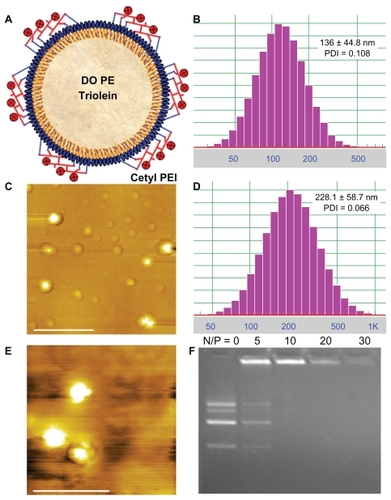
After PLN was mixed with plasmid DNA to form the PDC, the mean diameter of PDC (N/P ratio = 10) was 228.1 ± 58.7 nm with a narrow polydispersivity of 0.066 (), and the zeta potential value was 11.92 ± 1.12 mv. In addition, no aggregation or precipitation was observed in the measurements. The AFM measurements confirmed that PDC (N/P ratio = 10) had nanometer-sized particles (), and no free PLN or plasmid DNA was observed. The particle size of PDC measured by AFM was accorded with that determined by DLS. Considering the narrow size distribution and positive potential value of PDC, the plasmid DNA could be condensed at the surface of PLN. The nanometric size and positive surface charge of PDC could be beneficial to the cellular uptake and gene transfection. Moreover, the formation of PDC at various N/P ratios was analyzed by gel retardation assay (). The electrophoresis images revealed that the bands of plasmids DNA disappeared in PDC when the N/P ratio was more than 10, which indicated that the anionic plasmid DNA molecules could be condensed by PLN.
Transfection efficiency of PDC
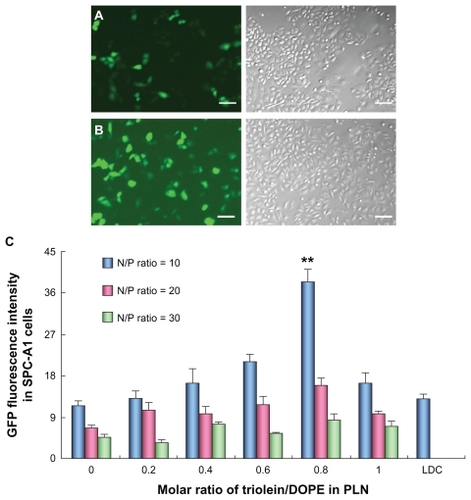
The GFP expression in SPC-A1 cells after transfection with the PDC was observed under fluorescence microscopy. The experimental results indicated that GFP was expressed 24 hours after transfection with the highest expression from the second to the fourth day. Many cells were fluorescence positive after treated with the LDC or PDC (). The fluorescence intensity expressed GFP in SPC-A1 cells was quantified by fluorescence spectrum (). The GFP fluorescence intensity in SPC-A1 cells transfected by PDC at an N/P ratio of 5 was much lower than that at an N/P ratio of 10 (data not shown), which could result from the incomplete condensation of plasmid DNA at an N/P ratio of 5. Moreover, the transfection efficiency of PDC was highest at an N/P ratio of 10 and significantly decreased with the increase in ratio. The reason may lie in the appropriate protection of plasmid DNA by PDC against degradation, unpackaging of the complexes, and its cytoplasmic transfer character. When no triolein was introduced to the formulation, the GFP fluorescence intensity of PDC (N/P = 10) was comparable with that of commercial LDC. However, after the incorporation of triolein into the PLN formulation, the GFP fluorescence intensity was increased with the content of triolein in PLN and reached the highest when the molar ratio of triolein/DOPE was 0.8, which was two-fold higher than that of LDC (P < 0.01). However, the transfection efficiency was decreased unexpectedly when the molar ratio of triolein/DOPE was over 0.8, which could be correlated with the effect of triolein on DOPE and the physicochemical properties of PLN.
Figure 2 Transfection of pEGFP-N2 in human lung adenocarcinoma (SPC-A1) cells mediated with Lipofectamine™ 2000/DNA complexes (LDC) and polycation lipid nanocarrier/DNA complexes (PDC) (N/P = 10). (A) Images of SPC-A1 cells transfected by LDC, scale bar = 100 μm; (B) images of SPC-A1 cells mediated by PDC (N/P = 10), scale bar = 100 μm; (C) fluorescence intensity of expressed green fluorescent protein in SPC-A1 cells mediated by different PDCs with various N/P ratios (**P < 0.01) (n = 3).
Furthermore, the transfection efficiency of PDC in SPC-A1 cells was quantified by monitoring GFP positive cell counts compared with those of LDC. The flow cytometry measurements indicated that GFP positive cells transfected by PDC reached about 40%, which was significantly higher than that achieved by LDC (P < 0.05) (). In the presence of 10% serum, the transfection efficiency of PDC in SPC-A1 cells was 199% of that in its absence (P < 0.05), whereas that of LDC was reduced by about 70% compared with that in the absence of serum (P < 0.05). Likewise, the gene expression intensity was quantified by measuring the activity of expressed luciferase in SPC-A1 cells. It could be noticed that the gene expression intensity mediated by PDC was 3.8-fold higher than that of LDC () (P < 0.01). The expressed luciferase activity in SPC-A1 cells transfected by PDC in the presence of 10% serum was 222% of that in its absence (P < 0.05).
Figure 3 Transfection efficiency of polycation lipid nanocarrier/DNA complexes (PDC) (N/P = 10) in human lung adenocarcinoma (SPC-A1) cells compared with those of Lipofectamine™ 2000/DNA complexes (LDC). (A) Transfection efficiency of plasmid pEGFP-N2 determined by flow cytometer; (B) relative luciferase activity in SPC-A1 cells treated with PDC in comparison with that of LDC; (C) the cell viability of PDCs in SPC-A1 cells compared with that of polyethylenimine (PEI)/DNA complexes at various N/P ratios (*P < 0.05, **P < 0.01) (n = 3).
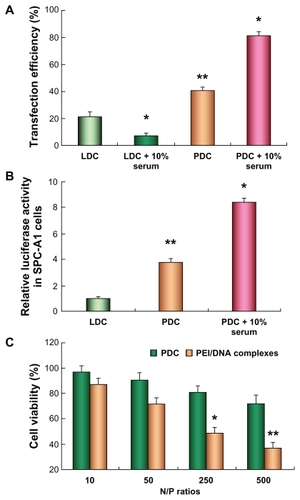
To evaluate the potential cytotoxicity of PDC in SPC-A1 cells, the cell viability was tested by MTT colorimetric assay after cells were treated with PDC at various N/P ratios.Citation23 Cells incubated with pure culture media were considered controls. The cell viability of PDC was over 70% when the N/P ratio was less than 250, and was significantly higher than that of PEI/DNA complexes at various N/P ratios, which indicated that PDC exhibited minimal cytotoxicity in SPC-A1 cells.
Intracellular transfer mechanism in SPC-A1 cells
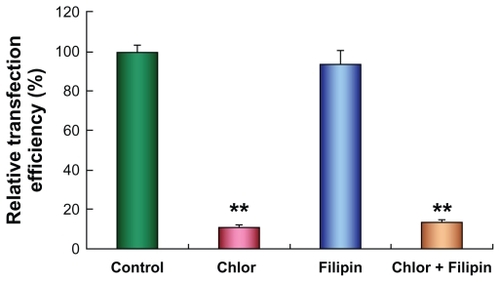
The internalization pathway of PDC could affect their intracellular processing and subsequent gene expression as well.Citation24,Citation25 To determine the internalization mode of PDC in SPC-A1 cells, transfection of plasmid pGL3-luc was performed in the presence of specific inhibitors of chlorpromazine (Chlor) and filipin III. Because the specificity and toxicity of the endocytic inhibitors varied with the cell lines, the viability of SPC-A1 cells treated with the endocytic inhibitors was over 80%, and their specificity in SPC-A1 cells was also confirmed in our preliminary experiments. Interestingly, the transfection efficiency of PDC evidently decreased by about 90% in the presence of chlorpromazine () (P < 0.01) but was not significantly reduced after the treatment of filipin III () (P > 0.05). Moreover, treatment with both the inhibitors led to 86% reduction of the transfection efficiency in comparison with that in their absence () (P < 0.01). Now it appears that PDC was mainly endocytosed via the clathrin-mediated pathway in SPC-A1 cells.
Figure 4 Transfection efficiency of polycation lipid nanocarrier/DNA complexes (PDC) in human lung adenocarcinoma (SPC-A1) cells in the presence of chlorpromazine (10 μg/mL) (**P < 0.01) or filipin III (1 μg/mL) (P > 0.05) at 48 hours after transfection. Data were corrected by protein content, and luciferase activity of cells without inhibitor treatment was set as 100% (n = 3).
Internalization of PDC in SPC-A1 cells
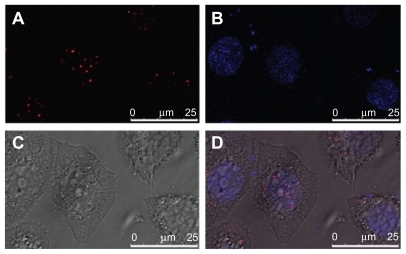
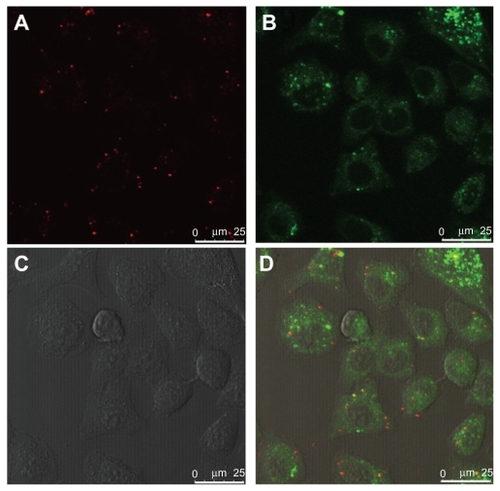
The localization of internalized PDC in SPC-A1 cells was investigated to clarify its intracellular mechanism. Plasmid DNA was labeled with red TM-Rhodamine, and the fluorescent PDC (N/P = 10) was prepared as described for direction observation under laser scanning confocal microscopy (LCSM). As a control, the late endosomes/lysosomes were stained with LysoTracker Green DND-26 (green), and the nuclei were counterstained with Hoechst 33258 (blue). Because the red fluorescence of TM-Rhodamine labeled PDC () could be easily distinguished from the green fluorescence of LyosTracker Green DND-26 (), colocalization of PDCs with the late endosomes/lysosomes would yield yellow spots (combination of red and green). Two hours after incubation, yellow spots were obviously visible in the cytoplasma (), which confirmed the colocalization of PDCs with late endosomes/lysosomes. However, no fluorescently labeled PDC was observed in the nuclei within 2 hours. The spots with different colors may exhibit the different distribution of PDC in SPC-A1 cells. Furthermore, after 6 hours’ incubation, the LCSM images revealed that the TM-Rhodamine labeled PDC was obviously located in the nucleus ().
Figure 5 Colocalization of polycation lipid nanocarrier/DNA complexes (PDC) (N/P = 10) with late endosomes/lysosomes in human lung adenocarcinoma (SPC-A1) cells under laser scanning confocal microscopy at 2 hours after transfection. (A) TM-Rhodamine labeled plasmid DNA (red); (B) LysoTracker Green DND26 labeled late endosomes/lysosomes (green); (C) phase-contrast image of S PC-A1 cells; (D) merged image, scale bar = 25 μm.
Figure 6 Colocalization of polycation lipid nanocarrier/DNA complexes (PDC) (N/P = 10) with nucleus in human lung adenocarcinoma (SPC-A1) cells under laser scanning confocal microscopy at 6 hours after transfection. (A) TM-Rhodamine labeled plasmid DNA (red); (B) Hoechst 33258 labeled nuclei (blue); (C) phase-contrast image of SPC-A1 cells; (D) merged image, scale bar = 25 μm.

Discussion
Colloidal particle systems have many advantages as potential candidates for efficient nonviral gene delivery.Citation4,Citation8,Citation30,Citation31 The nano-based nonviral vectors, including artificial lipoprotein, polycation liposome, and LPDII vectors, could offer an effective method to integrate the advantages of lipids and cationic polymers. Recently, we have developed nanostructured lipid carriers modified with c-PEI for gene delivery, and the transfection efficiency was comparable with LDC.Citation22 DOPE, an essential component of cationic liposomes, could form the HII phase and destabilize the endosomal membrane, thereby enhancing the transfection efficiency.Citation12,Citation13 In particular, triolein could facilitate the formation of the HII phase in the phosphatidylethanolamine membrane,Citation19 which could avail the destabilization of the endosomal membrane and improvement of the gene delivery activity. On the other hand, PEI could deliver DNA to the nucleus after escaping from the endosomal pathway due to the proton sponge effect.Citation4,Citation14,Citation15 Alkylation or carboxyalkylation could be effective methods to modify PEI for higher transfection efficiency and lower cytotoxicity.Citation4,Citation14,Citation32,Citation33 In the present study, PLN was developed by the combination of the advantages of cetylated PEI, triolein, and DOPE, which could integrate the effect of the HII phase and the proton sponge effect to destabilize the endosomal membrane for effective gene delivery.
From the transfection results of plasmid pEGFP-N2 and pGL3-luc in SPC-A1 cells, we learned that the transfection efficiency of PLN was significantly improved after the incorporation of triolein in the formulation. When no triolein was introduced in the formulation, the nanocarrier was similar to the previously reported polycation liposomes, and the transfection efficiency was only about 30% of that of PLN (0.1:0.8:1, molar ratio). Interestingly, the transfection efficiency of PLN was significantly increased with the addition of triolein in PLN, which obviously verified the effectiveness of triolein in enhancing the gene transfection efficiency of PLN. Triolein could perturb the structure of phospholipids planar bilayers and facilitate the formation of the HII phase,Citation19 which could destabilize the endosomal membrane and enhance the gene transfection efficiency. Moreover, the gene delivery activity of PLN was more effective than our previously described PNLC composed of c-PEI, triolein, and egg yolk phosphatidylcholine,Citation22 which identified the essentiality of DOPE in PLN. Additionally, the transfection efficiency of PLN was much higher than that of commercial Lipofectamine™ 2000, consisting of cationic lipid and DOPE, which certified the outstanding effect of cetylated PEI and triolein in PLN-mediated gene delivery. Taken together, this evidence indicated that PLN could combine the advantages of cetylated PEI, triolein, and DOPE for effective gene delivery.
Surprisingly, the transfection efficiency of PLN was obviously augmented in the presence of 10% serum, but that of the commercial Lipofectamine™ 2000 was sharply decreased, which indicated the PLN’s potential for in vivo gene delivery. Previous reports suggested that dodecylation and hexadecylation of PEI (2000 Da) could enhance the gene transfection in the presence of serum by five- to six-fold compared with the unmodified PEI (25,000 Da).Citation15 Moreover, the resistance against serum has been observed in the transfection of previously described PNLC with cetylated PEI (1200 Da) and polycation liposomes containing cetylated PEI (600 Da or 1800 Da).Citation17,Citation18,Citation22 The enhanced transfection of PLN in the presence of serum could result from c-PEI involvement in the PLN formulation.
To clarify internalization pathways of PDC in efficient gene delivery, we performed reporter gene transfection in SPC-A1 cells in the presence of specific inhibitors.Citation26,Citation27 Chlorpromazine was commonly used to inhibit clathrin-dependent endocytosis, and filipin III could specifically block caveolae-mediated uptake. The experimental results indicated that the transfection efficiency of PDC was remarkably reduced in SPC-A1 cells treated with chlorpromazine but not significantly decreased in the presence of filipin III. Thereby, it could be deduced that PDC could be predominantly internalized through a clathrin-dependent endocytosis pathway in SPC-A1 cells for efficient gene transfection. The LCSM images indicated that PDC was colocalized with the late endosome/lysosomes after their endocytosis in SPC-A1 cells. These results suggested that PDC could be endocyted through the clathrin-dependent pathway, which was different from the reported PEI polyplexes. Citation26 It has been reported that only caveolae/lipid-raft-mediated uptake contributes to efficient transfection of PEI polyplexes.Citation26 However, PLN was a hybrid nanocarrier system composed of cetylated PEI, triolein, and DOPE, rather than simple PEI. Moreover, Gersdorf et alCitation27 have demonstrated that the internalization route resulting in successful gene expression depended on both cell lines and PEI polyplex type. The cell line could be another substantial reason for the different internalization pathway of PDC in SPC-A1 cells. In our laboratory we have proven that the internalization pathway of PDC in SPC-A1 cells was different from that in Chinese hamster ovary cells (data not shown). Additionally, the LCSM images revealed that PLN could facilitate the gene entry into the nucleus to promote the transfection efficiency, which could contribute to the effect of cetylated PEI as per previously reported results.Citation34
The cytotoxicity of nonviral vectors should also be considered for gene delivery. The toxicity of PEI was correlated with its molecular weight and cationic charge density. Various modifications have been performed to minimize the cytotoxicity of PEI, eg, alkylation, carboxyalkylation, or PEGylation.Citation10,Citation11,Citation15,Citation32,Citation33 In the present formulation, the cetylation of low-molecular PEI (1200 Da) could significantly reduce the cytotoxicity; moreover, the involved DOPE and triolein were biocompatible components, which could result in the low toxicity of PLN. The measured results have indicated that the cytotoxicity of PLN was much lower than the unmodified PEI (1200 Da), which was in agreement with that of previously described dodecylation of PEI (2000 Da) or polycation lipsosomes.Citation14,Citation15,Citation17,Citation18
Conclusion
In summary, PLN was developed by combining the advantages of cetylated PEI, triolein, and DOPE for effective gene delivery. PLN and PDC were homogenous nanometric particles with a positive surface charge. The transfection efficiency of PLN in SPC-A1 cells was significantly improved after the incorporation of triolein and much higher than the commercial levels of Lipofectamine™ 2000. With the help of specific inhibitors and images of LCSM, PDC was determined to be internalized into SPC-A1 cells by the clathrin-mediated pathway and then transferred to the nucleus for successful transfection. Thus, the triolein-based PLN demonstrated a novel effective gene delivery system.
Acknowledgments
The work was sponsored by National Natural Science Foundation of China (30901862, 30901866, 30873177), Shanghai Rising-Star Program (10QA1400800, 11QA1407900), and the Scientific Research Foundation for the Returned Overseas Chinese Scholar.
Disclosure
No conflict of interest was reported by the authors of this paper.
References
- JacksonDAJuranekSLippsHJDesigning nonviral vectors for efficient gene transfer and long term gene expressionMol Ther20061461362616784894
- GiaccaMVirus-mediated gene transfer to induce therapeutic angiogenesis: where do we standInt J Nanomed20072527540
- ParkTGJeongJHKimSWCurrent status of polymeric gene delivery systemsAdv Drug Deliver Rev200658467486
- PackDWHoffmanASPunSStaytonPSDesign and development of polymers for gene deliveryNat Rev Drug Discov2005458159316052241
- LehrmanSVirus treatment questioned after gene therapy deathNature199940151751810524611
- GaoYLiuXLLiXRResearch progress on siRNA delivery with nonviral carriersInt J Nanomed2011610171025
- PoutonCWSeymourLWKey issues in non-viral gene deliveryAdv Drug Deliver Rev200146187203
- PathakAVyasSPGuptaKCNano-vectors for efficient liver specific gene transferInt J Nanomed200833149
- GeusensBLambertJDe SmedtSCUltradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytesJ Control Release200913321422018973779
- DehshahriAOskueeRKShierWTGene transfer efficiency of high primary amine content, hydrophobic, alky-oligoamine derivatives of polyethylenimineBiomaterials2009304187419419464054
- ChenXAZhangLJHeZJPlasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into rat mesenchymal stem cellsInt J Nanomed20116843853
- RädlerJOKoltoverISaldittTSafinyaCRStructure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimesScience19972758108149012343
- KoltoverISaldittTSafinyaCRAn inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and deliveryScience199828178819651248
- ThomasMKlibanovAMEnhancing polyethylenimine’s delivery of plasmid DNA into mammalian cellsProc Nat Acad Sci U S A2002991464014645
- GodbeyWTWuKKMikosAGTracking the intracellular path of polyethylenimine/DNA complexes for gene deliveryProc Nat Acad Sci U S A19999651775181
- SunXProvodaCLeeKDEnhanced in vivo gene expression mediated by listeriolysin O incorporated anionic LPDII: its utility in cytotoxic T lymphocyte-inducing DNA vaccineJ Control Release201014821922520620181
- OkuNYamazakiYMatsuuraMA novel non-viral gene transfer system, polycation liposomesAdv Drug Delver Rev200152209218
- YamazakiYNagoMMatsuuraMPolycation liposomes, a novel nonviral gene transfer system, constructed from cetylated polyethylenimineGene Ther200071148115510918482
- PradesJFunariSSEscribáPVBarcelóFEffects of unsaturated fatty acids and triacylglycerols on phosphatidylethanolamine membrane structureJ lipid Res2003441720172712810821
- AlanaziFFuZFLuDREffective transfection of rabies DNA vaccine in cell culture using an artificial lipoprotein carrier systemPharm Res20042167568215139525
- PanGShawerSOieSLuDRIn vitro gene transfection in human glioma cells using a novel and less cytotoxic artificial lipoprotein delivery systemPharm Res20032073874412751628
- ZhangZShaXShenAPolycation nanostructured lipid carrier, a novel nonviral vector constructed with triolein for efficient gene deliveryBiochem Biophys Res Commun200837047848218395002
- GaoYZhangZChenLSynthesis of 6-N,N,N-trimethyltriazole chitosan via “Click Chemistry” and evaluation for gene deliveryBiomacromolecules2009102175218219722556
- ZielloJEHuangYJovinISCellular endocytosis and gene deliveryMol Med20101622222920454523
- KuoWTHuangHYHuangYYIntracellular trafficking, metabolism and toxicity of current gene carriersCurr Drug Metab20091088589420214583
- RejmanJBragonziAConeseMRole of clathrin and caveolae mediated endocytosis in gene transfer mediated by lipo-and polyplexesMol Ther20051246847415963763
- GersdorfKVSandersNNVandenbroucheRThe internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex typeMol Ther20061474575316979385
- SukJSSuhJLaiSKHanesJQuantifying the intracellular transport of viral and nonviral gene vectors in primary neuronsExp Biol Med2007232461469
- LiangBHeMLXiaoZPSynthesis and characterization of folate-PEG-grafted-hyperbranched-PEI for tumor-targeted gene deliveryBiochem Biophys Res Commun200836787488018201560
- LiuYWangTHeFAn efficient calcium phosphate nanoparticle-based nonviral vector for gene deliveryInt J Nanomed20116721727
- TabattKKneuerCSametiMTransfection with different colloidal systems: comparison of solid lipid nanoparticles and liposomesJ Control Release20049732133215196759
- OskueeRKDehshahriAShierWTRamezaniMAlkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicityJ Gene Med20091192193219634133
- OskueeRKPhilippADehshahriAThe impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA deliveryJ Gene Med20101272973820683834
- SugiyamaMMatsuuraMTakeuchiYPossible mechanism of polycation liposome (PCL)-mediated gene transferBiochim Biophys Acta20041660243014757217