Abstract
Single-walled carbon nanotubes (SWNTs) have been identified as an efficient drug carrier. Here a controlled drug-delivery system based on SWNTs coated with doxorubicin (DOX) through hydrazone bonds was developed, because the hydrazone bond is more sensitive to tumor microenvironments than other covalent linkers. The SWNTs were firstly stabilized with polyethylene glycol (H2N-PEG-NH2). Hydrazinobenzoic acid (HBA) was then covalently attached on SWNTs via carbodiimide-activated coupling reaction to form hydrazine-modified SWNTs. The anticancer drug DOX was conjugated to the HBA segments of SWNT using hydrazine as the linker. The resulting hydrazone bonds formed between the DOX molecules and the HBA segments of SWNTs are acid cleavable, thereby providing a strong pH-responsive drug release, which may facilitate effective DOX release near the acidic tumor microenvironment and thus reduce its overall systemic toxicity. The DOX-loaded SWNTs were efficiently taken up by HepG2 tumor cells, and DOX was released intracellularly, as revealed by MTT assay and confocal microscope observations. Compared with SWNT-DOX conjugate formed by supramolecular interaction, the SWNT-HBA-DOX featured high weight loading and prolonged release of DOX, and thus improved its cytotoxicity against cancer cells. This study suggests that while SWNTs have great potential as a drug carrier, the efficient formulation strategy requires further study.
Introduction
Although most of the existing anticancer drugs are very potent small molecules, their efficacy is constrained not only by their side effects but also as a result of drug resistance and limited cellular entry. Thus it is important to develop efficient delivery systems to enhance cellular uptake of existing drugs. Carbon nanotubes (CNTs) have received considerable attention in biomedical applications due to their unique optical, electronic, chemical, and physiological properties.Citation1,Citation2 They have been shown to deliver various biomolecules including protein,Citation3 DNA, and RNACitation4–Citation6 into cells by endocytosis.Citation7 Functionalized water-soluble CNTs (f-CNTs) were suggested to be compatible and nontoxic at the cellular level.Citation8,Citation9 Furthermore, the one-dimensional structure of CNTs may have advantages over existing delivery vectors, including an enhanced capacity to penetrate cellular membranes and high surface area to provide sites to carry multiple moieties at high density.Citation10–Citation12 These properties made f-CNTs a promising candidate for drug delivery system.
There have been a number of promising reports in recent years on the conjugation of f-CNTs with drug molecules for delivery applications. Doxorubicin (DOX) has been noncovalently bound to CNTs by means of π–π stacking interactions, hydrophobic interactions,Citation13–Citation15 and electrostatic interaction.Citation16 Since the noncovalent interaction is not as robust as covalent linkage, the early release of the drug before reaching the pharmacologic tissues should be inevitable. Small drug molecules have been covalently conjugated to f-CNTs through amide bonds,Citation12 disulfide bonds,Citation17 ester bonds,Citation18,Citation19 and carbamate bondsCitation20 for in-vitro and in-vivo delivery. To improve the cellular uptake of tumor cells, drug molecules were linked to the same nanotubes together with tumor-targeting molecules that could specifically recognize cancer-specific receptors on the cell surface and induce receptor-mediated endocytosis. Compared with the tumor-associated receptors, tumor microenvironment indicators including acidosisCitation21 and hypoxiaCitation22 are universal phenomena of solid tumors, regardless of the tumor types or its developmental stages. The acidic extracellular environment is perhaps the most pervasive of tumor microenvironments. Therefore, a drugdelivery system that is responsive in the physiologically acidic pH range (4.5–6.9) is promising for the drug release inside tumor cells.Citation23
Despite excellent progress in using single-walled carbon nanotubes (SWNTs) as drug-delivery vehicles, more research is needed to further optimize their application to selective accumulation in diseased tissues and to release their toxic payload in a controlled manner. Hydrazones, formed by condensation of the C-13 ketone of DOX with a hydrazine, have been used for attaching drugs to polymersCitation24–Citation26 and nanoparticles.Citation27,Citation28 However, there is no report on the covalent immobilization of anticancer drug onto the surfaces of functionalized SWNTs through hydrazone linkage. Presented herein is the preparation of the SWNT-hydrazone-DOX delivery system, in which the antitumor agent DOX was covalently linked to SWNTs through biocleavable hydrazone linkage. The strategies presented in this paper may lead to improved tumor-targeting efficacy via the enhanced permeability and retention effect and pH-controlled DOX release, thereby improving the efficacy of cancer therapy. This study provides the fundamental characteristics of a pH-responsive SWNT-based system for improved intracellular drug delivery.
Materials and methods
Chemicals and materials
SWNTs were purchased from Nanostructured and Amorphous Materials Inc (Houston, TX). DOX hydrochloride (DOX·HCl), N-hydroxy succinimide (NHS), (N-(3- dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT), and polyoxyethylene bis(amine) (NH2-PEG-NH 2) (with a molecular weight [MW] of 3350) were purchased from Sigma-Aldrich (St Louis, MO) at reagent grade and used without further purification. 4-Hydrazinobenzoic acid (HBA) was purchased from International Laboratory Ltd (San Bruno, CA). Microcon centrifugal filter device (YM-100, regenerated cellulose 10,000 MW cutoff [MWCO]) was purchased from Millipore (Bellerica, MA). Spectra/Por® dialysis membrane (MW 12,000–14,000) was purchased from Spectralabs (Rancho Dominguez, CA).
Characterization
The morphologies and structures of SWNT, SWNT-PEG-NH 2, and SWNT-HBA-DOX conjugates were characterized by a transmission electron microscopy (Philips Tecnai 20, FEI Co, Eindhoven, The Netherlands) with an accelerating voltage of 200 kV. Samples were prepared by drying droplets of a sample in ethanol dispersed onto carbon-coated copper grids. Ultraviolet-visible (UV-Vis) spectra were recorded using a UV-Vis-near-infrared spectrophotometer (UV-3150; Shimadzu Co, Kyoto, Japan). Fluorescence spectra were measured on a fluorescence spectrometer (F-7000; Hitachi, Tokyo, Japan).
Preparation of DOX-conjugated SWNTs through hydrazone linkage
The PEGylated SWNTs (SWNT-PEG-NH2) were synthesized according to a previous report.Citation29 The DOX attached to SWNTs through hydrazone bond was then prepared according to the following steps. The SWNT-PEG-NH2 (5 mL) was suspended with HBA (30 mg) in a pH 7.4 phosphate buffered saline (PBS) solution (5 mL), EDC · HCl (48 mg), and NHS (20 mg) was then added. The mixture was allowed to react at room temperature for 24 hours, after which the conjugate was dialyzed against H2O to remove unreacted reagent to obtain SWNT-HBA complex. Finally, the solution was freeze-dried to obtain the hydrazine modified SWNTs as a fine powder. DOX can be bound to SWNTs by the reaction of SWNT-HBA with DOX · HCl in dimethyl sulfoxide (DMSO) in the dark according to the method previously described in the literature. Citation30 Five milligrams of freeze-dried hydrazine-modified SWNTs was dissolved in 5 mL anhydrous DMSO, and excess amount of DOX · HCl (10 mg) was added. The mixture solution was stirred at room temperature in the dark for 24 hours. After the reaction, the solution was transferred into a dialysis tubing (MWCO of 12,000 Da) and dialyzed against Milli-Q® (Millipore) water for 3 days to remove the excess amount of unbounded DOX molecules and DMSO. The amount of DOX loaded onto SWNTs was measured by the absorbance peak at 490 nm (characteristic of DOX, after subtracting the absorbance of SWNTs at that wavelength) based on a standard curve of DOX. The DOX weight loading was defined as the weight ratio of the attached DOX to the SWNTs.
Evaluation of pH sensitivity
The rate of DOX released from SWNTs was measured as a function of time during incubation in 0.1 M PBS at pH 7.4 and pH 5.5. Triplicate samples of 0.4 mL of SWNT-HBA-DOX and SWNT-DOX (the detail of preparation of SWNT-DOX is described in ) were suspended in 10 mL PBS in a microcentrifuge tube. The samples were then incubated at room temperature. The nanotubes were centrifuged and 4 mL of supernatant was periodically removed and the same volume of fresh PBS solution was added afterward. The amount of released DOX was analyzed by UV-vis spectrophotometry at 490 nm.
Cell culture and intracellular DOX trafficking
Human hepatocellular carcinoma cells (HepG2, American Type Culture Collection [ATCC] No: HB8065) and human cervical adenocarcinoma cells (HeLa, ATCC No: CCL2) were purchased from ATCC (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Cat # 12800-017; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Cat # 10082-147; Invitrogen) and 100 U/mL penicillin and 100 μg/mL streptomycin (Cat # 15070-0; Invitrogen). The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and regularly passaged by trypsinization with 0.05% trypsin in PBS (pH 7.4).
The cellular uptake and distribution of DOX from the SWNT-HBA-DOX conjugates were examined under a Leica TCS SPE confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany). Both bright view differential interference contrast images and confocal images were obtained with a 40× (oil immersion, numerical aperture 1.25) objective. For confocal microscope observation, HepG2 cells were grown on coverslips in a 6-well plate 1 day before the experiment. Cells were treated with SWNT-DOX and SWNT-HBA-DOX (DOX concentration 15 μg/mL) for 2 hours. The cells were then thoroughly washed with PBS and incubated in fresh culture medium. The intracellular distribution of DOX was then examined under the confocal microscope. The fluorescence intensity was measured and analyzed with MetaMorph (Universal Imaging, Downingtown, PA).
Cell viability
The cytotoxicity of SWNT-DOX and SWNT-HBA-DOX against HepG2 cells was assessed using the MTT assay. HepG2 cells were seeded into a 96-well microtiter plate at a density of 1 × 104 cells/well in 150 μL DMEM medium 24 hours before treatment. The cells were then exposed to a series of concentrations of SWNTs, SWNT-DOX, and SWNT-HBA-DOX conjugates for 24 hours. The dose of SWNT-DOX and SWNT-HBA-DOX was set according to the contained DOX dose in the conjugates within a range of 0.25–10.0 μg/mL (0.43–17.2 μM) of DOX. After incubation for 24 hours, the cells were washed three times with warm PBS and incubated for another 4 hours containing 0.5 mg/mL of MTT solution. After discarding the culture medium, 100 μL of 50% DMSO: 50% ethanol was added to dissolve the formazan crystals, and the resulting solution was measured for absorbance at 570 nm using a microplate reader. Cells incubated with blank cell culture medium were used as reference for 100% viability. The mean percentage of cell survival relative to that of untreated cells was estimated from data from five replicates. The results were expressed as viability (%) relative to a control without any treatment.
To further evaluate the drug release efficiency from SWNTs, HepG2 cells were incubated with a specific concentration (DOX concentration: 17.2 μM) of SWNT-HBA-DOX and SWNT-DOX in culture medium at 37°C for 2 hours. The medium was then replaced with fresh medium and incubated again for 24, 48, and 72 hours. The cells were washed three times with warm PBS before further analysis by MTT.
Statistical analysis
All data are presented as mean ± standard deviation from 3–6 experiments. Significant differences between mean values were determined using two-way analysis of variance (ANOVA) and Tukey’s post-hoc test. The level of statistical significance in all cases was P < 0.05. Percentage data was log transformed before analysis. ANOVA was performed using SPSS (v 19.0; IBM SPSS, Chicago, IL) software.
Results and discussion
Synthesis and characterization of SWNT-HBA-DOX nanoconjugates
DOX is one of the widely used chemotherapeutic agents in the treatment of cancers. The exact mechanism of its antitumor activity still remains unclear. It is known, however, that DOX intercalates into DNA, which results in the blocking of topoisomerase II activity, preventing DNA replication and cell division.Citation31–Citation33 For DOX-loaded nanoconjugates, the efficacy depends not only on cellular uptake but also on their release efficiency inside the cancer cells. The controlled release is mainly achieved through cleavable linkers. Hence, discovery of a reliable method to load and release DOX from SWNTs is attractive.
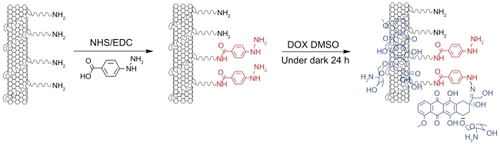
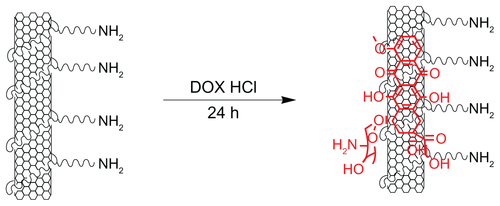
Hydrazone bond was employed in this work to connect DOX, as it is more sensitive to tumor microenvironments than other covalent linkers. The reaction scheme for the synthesis of SWNT-HBA-DOX conjugate is shown in . Functionalization of SWNTs with H2N-PEG-NH2 (PEG) is based on the authors’ previously described method.Citation29 To conjugate DOX onto SWNT-PEG-NH2 through hydrazone bond, the amine groups on SWNT-PEG-NH2 were firstly coupled with the carboxylic acid terminal groups of HBA to form hydrazine-modified SWNTs using EDC and NHS as the catalysts. Thereafter, DOX was conjugated to the SWNTs by forming acid-sensitive hydrazone bonds (SWNT-HBA-DOX) between the hydrazine moiety attached to SWNTs and ketonic groups of DOX. For comparison, DOX was also attached to SWNTs through noncovalent interaction (SWNT-DOX) by simply mixing the SWNT-PEG-NH2 with DOX in aqueous solution (). Note that free DOX in the SWNT solution was removed thoroughly by repeated filtrations with a 10 kD molecular cutoff to obtain SWNT-HBA-DOX and SWNT-DOX complexes.
Figure 1 Schematic illustration of the SWNT-HBA-DOX. The DOX (blue color) was attached to SWNT through hydrazone linkage (red color) and supramolecular interaction, respectively.
Abbreviations: DMSO, dimethyl sulfoxide; DOX, doxorubicin; EDC, (N-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; HBA, hydrazinobenzoic acid; NHS, N-hydroxy succinimide; SWNT, single-walled carbon nanotube.
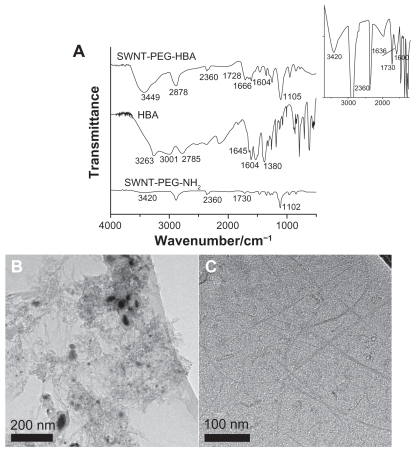
To investigate the immobilization of HBA on SWNTs, the infrared (IR) spectroscopy was applied for the characterization of SWNT-PEG-HBA, HBA, and SWNT-PEG-NH2. The measurement results of the prepared samples are presented in , with the IR region from 4000 to 500 cm−1. The Fourier transform IR (FTIR) spectrum for HBA displays bands at 1645 and 3263 cm−1, which are attributed to the C=O and NH2 groups, and various bands between 3000 and 2700 cm−1, which represent the intermolecular hydrogen bond between the carbonyl and the amino groups.Citation34 The SWNT-PEG-NH2 sample shows peaks at 3420, 1730, and 1102 cm−1. The bands at 1730 cm−1 are attributed to the C=O bonds from carboxylic acid groups, and the bands at 3420 and 1102 cm−1 represent the stretching frequency of -O-H groups and C-O stretching frequencies. It also shows an unobvious shoulder at 1636 cm−1 (inset in ) which is attributed to the carbonyl group. The weak signal is due to the low amount of carbonyl group on SWNT-PEG-NH2 and the detection limit of the FTIR instrument. According to one report,Citation35 the N-H peaks should appear at ~3409 cm−1. However, in this present case, it was difficult to discern the -O-H (3420 cm−1) and N-H (3409 cm−1) peaks for SWNT-PEG-NH2 sample because of the overlay of these two peaks and the detection limit of the instrument. After the reaction of HBA with SWNT-PEG-NH2, the peaks at 1666 cm−1 in spectrum of SWNT-HBA can be assigned to amide bond, suggesting chemical complexation of carboxylic acid groups on HBA with amine groups on SWNTs. Additionally, the band at 2360 cm−1 is the typical IR bands of CO2 likely adsorbed on SWNTs. The morphology of SWNTs was also investigated by transmission electron microscopy (TEM). and C show the TEM images of raw SWNTs and SWNT-PEG-NH2. The SWNT-PEG-NH2 appeared to be rough and without impurity compared with raw SWNTs, which indicate that metal particles and amorphous carbon have been removed. The morphologies of SWNT-HBA- DOX and SWNT-DOX were also analyzed by TEM. No detectable change was found in comparison with that of SWNT-PEG-NH2.
Figure 2 (A) Infrared spectra of SWNT-PEG-HBA, HBA, and SWNT-PEG-NH2 composites. Inset: enlargement of of infrared spectrum of SWNT-PEG-NH2. TEM images of (B) raw SWNT and (C) SWNT-PEG-NH2.
Abbreviations: HBA, hydrazinobenzoic acid; PEG, polyethylene glycol; SWNT, single-walled carbon nanotube; TEM, transmission electron microscopy.
DOX loading on the modified SWNTs
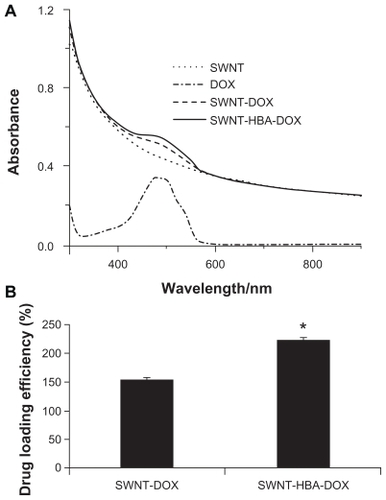
All the modified SWNTs were quite soluble in aqueous medium, which attribute to the high density of the hydrophilic glycol chains on the surface of SWNTs. The SWNT-HBA-DOX and SWNT-DOX dispersed in H2O both displayed a UV-Vis absorption peak at 490 nm, characteristic of DOX (). Consequently, the amount of DOX loaded onto SWNTs was measured by the absorbance peak at 490 nm based on a standard curve of DOX in SWNTs solution. The DOX weight loading on SWNT-HBA was obtained to be 220%, which was higher than that on SWNTs of 160% (). Previously, it has been shown that DOX can be adsorbed onto the sidewalls of SWNTs via π–π stacking interactions,Citation11 by simply mixing the SWNTs with DOX. In contrast, DOX was conjugated on HBA-coated SWNTs via not only hydrazone bond but also via π–π stacking interactions, thereby allowing the higher loading of DOX on SWNT-HBA-DOX than SWNT-DOX conjugate. The result indicated that hydrazone bond as well as π–π stacking interactions played an important role with respect to DOX loading capacity on SWNTs.
Figure 3 (A) Ultraviolet-visible absorbance spectra of PEGylated SWNTs (dotted line), free DOX (dash-dotted line), SWNT-DOX (dash line), and SWNT-HBA-DOX (solid line) in H2O. (B) Drug weight loading of the DOX-conjugated SWNTs.
Notes: All values are mean ± standard deviation; *denotes P < 0.05.
Abbreviations: DOX, doxorubicin; HBA, hydrazinobenzoic acid; PEG, polyethylene glycol; SWNT, single-walled carbon nanotube.
DOX release from modified SWNTs
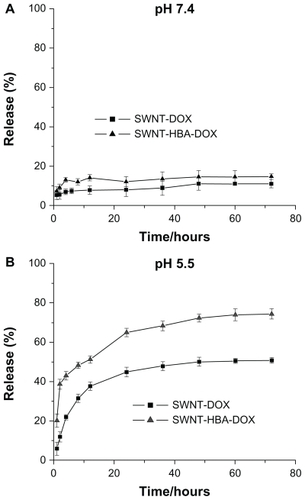
The drug-release profiles of SWNT-HBA-DOX and SWNT-DOX were investigated under simulated physiological conditions (PBS, pH 7.4) and in an acidic environment (PBS, pH 5.5) at room temperature, which assess the release efficacy of using SWNTs as the drug carrier. As shown in , the rate and amount of DOX were dependent on the pH of the medium. It showed that the DOX on modified SWNTs supports was stable in PBS buffer at pH 7.4. In slightly acidic conditions, such as pH 5.5, corresponding to lysosomal pH, an appreciable release DOX from the materials was observed over 72-hour periods. The release profile of SWNT-HBA-DOX and SWNT-DOX was similar except to the rate and efficiency. SWNT-HBA-DOX and SWNT-DOX conjugates released out of the conjugated DOX about 51% and 37% after 12 hours, respectively. However, the released portion of SWNT-DOX did not exceed 50% of the total amount of loaded drug during the experimental period, indicating relatively high stability of the π–π stacking interaction in the buffer solution. SWNT-HBA- DOX liberated 73%, which was higher than that of SWNT-DOX of 50% after 60 hours incubation at the same conditions. This result showed that the DOX release efficiency from the SWNT-HBA-DOX conjugates was higher than that from SWNT-DOX conjugate. There is a strong possibility that hydrazone bond may be more sensitive than π–π stacking interaction to the lysosome or endosome membrane at pH 4.0–5.0.
Intracellular DOX from SWNT-DOX and SWNT-HBA-DOX conjugates
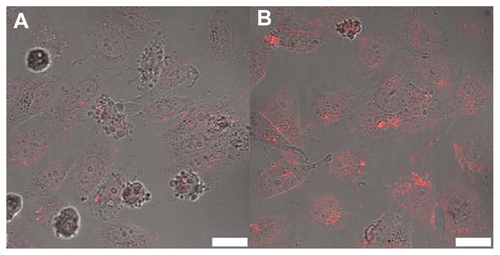
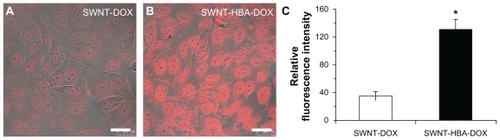
The intracellular trafficking of DOX was studied in HepG2 cells using confocal microscopy. shows the intracellular DOX profile with its intrinsic fluorescence after incubating HepG2 cells with SWNT-HBA-DOX and SWNT-DOX for 2 hours. The red fluorescence was observed in all treated cells, indicating fast cellular internalization of DOX. Moreover, when incubating HepG2 cells with equivalent concentration of DOX from both formulations and for the same period of time, stronger DOX fluorescence intensity was detected in both cytoplasm and nuclei from SWNT-HBA-DOX-treated cells than that of SWNT-DOX conjugate. The fluorescent intensity of DOX at each group of treated cells was further quantitatively analyzed. The average intracellular fluorescent intensity from SWNT-HBA-DOX- treated cells was significantly greater than that of SWNT-DOX conjugate (). It is difficult to discern the difference in cellular uptake of SWNT-HBA-DOX and SWNT-DOX nanoconjugates. However, DOX released from SWNT-HBA-DOX was significantly improved because the intracellular DOX was stronger than that from SWNT-DOX conjugate. It was indicated that more DOX was uptaken by cells with the help of SWNTs modified with hydrazone bond. This result implied that a delivery system comprising hydrazone linkage between DOX molecules and SWNTs results in more efficient DOX release from lysosomesCitation28,Citation36 which then moves to the nucleus.
Figure 5 Confocal microscope images of HepG2 cells after incubation with (A) SWNT-DOX and (B) SWNT-HBA-DOX (DOX concentration: 15 μg/mL) at 37°C for 2 hours. (C) Average intracellular DOX fluorescence intensity (20 cells) in SWNT-DOX- and SWNT-HBA-DOX-treated HepG2 cells.
Notes: Scale bar: 25 μm. All values are mean ± standard deviation; *denotes P < 0.05, analysis of variance.
Abbreviations: DOX, doxorubicin; HBA, hydrazinobenzoic acid; SWNT, single-walled carbon nanotube.
Cytotoxicity of DOX-loaded SWNT conjugates
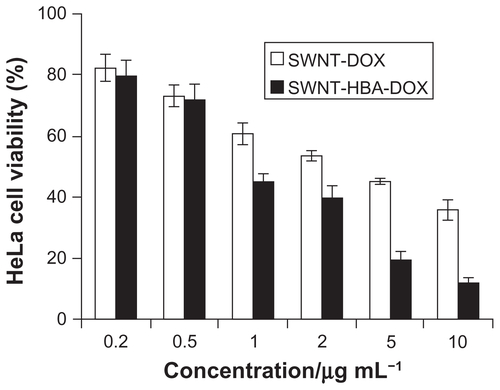
The cytotoxicities of SWNT-HBA-DOX and SWNT-DOX with different DOX concentrations against HepG2 after 24 hours were evaluated using the MTT assay. As shown in , SWNT-HBA-DOX exhibited stronger cytotoxicity than that of SWNT-DOX conjugate at all tested concentrations to HepG2 cells. Furthermore, when DOX concentration was higher than 5 μg/mL, SWNT-HBA-DOX induced significantly enhanced cytotoxicity to HepG2 cells than that of SWNT-DOX. The half-maximal inhibitory concentration values for SWNT-HBA-DOX and SWNT-DOX in HepG2 cells were about 2.8 μg/mL (4.8 μM) and 4.3 μg/mL (7.4 μM), respectively. This finding is consistent with the confocal observation that greater intracellular DOX was observed in SWNT-HBA-DOX-treated cells than those treated with SWNT-DOX conjugate. Of note, SWNTs themselves at the concentrations used for SWNT-HBA-DOX and SWNT-DOX formulations did not affect the survival of HepG2 cells after exposure to PEGylated SWNTs for up to 72 hours (data not shown), which is similar to previous reports.Citation9,Citation29 The cytotoxicity of two-drug formulations against HeLa cells was also evaluated using MTT assay (). The SWNT-HBA-DOX also showed higher cytotoxicity to HeLa cells than that of SWNT-DOX conjugate under the same experimental conditions.
Figure 6 Cytotoxicity of the DOX-conjugated SWNTs. (A) Cytotoxicity of SWNT-DOX and SWNT-HBA-DOX against HepG2 cells. (B) Viability of HepG2 cells treated with SWNT-DOX and SWNT-HBA-DOX (DOX concentration: 10 μg/mL) for 2 hours, washed with phosphate buffered saline, then continued incubating in fresh culture media for another 24, 48, and 72 hours. (C) Average intracellular DOX fluorescence intensity (20 cells) after release for 24 hours in fresh medium in SWNT-DOX- and SWNT-HBA-DOX-treated HepG2 cells.
Notes: All values are mean ± standard deviation; *denotes P < 0.05, analysis of variance.
Abbreviations: DOX, doxorubicin; HBA, hydrazinobenzoic acid; SWNT, singlewalled carbon nanotube.
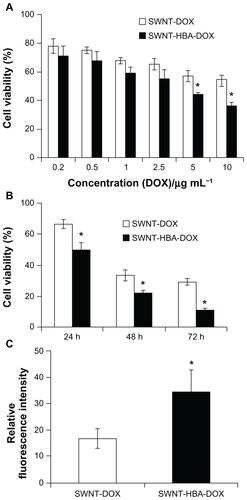
To further evaluate the drug-release efficiency from the SWNTs, HepG2 cells were firstly incubated with the SWNT-HBA-DOX and SWNT-DOX complexes (DOX concentration: 10 μg/mL, 17.2 μM) for 2 hours, as hyperthermic chemotherapy is often delivered for 1–2 hours. After the treatment, the treated cell samples were further incubated for 24, 48, and 72 hours with fresh culture medium, respectively. The cytotoxicity profiles for all these agents are illustrated in . After incubation with fresh medium without drug for 24 hours, the cell viability of HepG2 cells exposed to SWNT-HBA-DOX for 2 hours was significantly lower than that of SWNT-DOX conjugate (DOX concentration: 10 μg/mL, 17.2 μM). The cell viability decreased with the increase of the incubation time (24–72 hours) for both conjugates. The cell viability of HepG2 cells exposed to SWNT-HBA-DOX was always lower than that of SWNT-DOX conjugate within all tested time ranges, which may imply improved drug release by SWNT-HBA-DOX conjugate (DOX concentration: 10 μg/mL, 17.2 μM). Confocal microscope images of the treated HepG2 cells () also showed that the DOX was still retained inside the cells after release for 24 hours in fresh medium. The DOX fluorescence intensity, as shown in , decreased after release for both SWNT-HBA-DOX and SWNT-DOX conjugates. However, stronger DOX fluorescence was still observed in HepG2 cells treated with SWNT-HBA-DOX than that of SWNT-DOX conjugate, suggesting the prolonged release of DOX from SWNT-HBA-DOX conjugate. The enhanced cytotoxicity of SWNT-HBA-DOX is probably due to the enhanced cellular uptake of DOX as well as the persistent intracellular releases of DOX from SWNT-HBA-DOX conjugate.
Conclusion
A highly effective drug-delivery system based on SWNTs coated with DOX through hydrazone bond was developed, which improved the loading and release efficiency of DOX. The prepared SWNT-HBA-DOX conjugate exhibited a dramatic pH responsive drug-release behavior and was stable at physiological conditions. The cellular uptake of DOX can be enhanced by employing a hydrazone linkage between DOX molecules and SWNTs. Consequently, with enhanced intracellular accumulation of the drugs, SWNT-HBA-DOX conjugate demonstrated significantly improved cytotoxicity as compared with SWNT-DOX formed through supramolecular interaction. These results suggest that the hydrazone bond appears to be beneficial in developing more efficient drug release from SWNT.
Acknowledgments
This study was supported by a grant from the Research Grants Council of the Hong Kong SAR, China to SHC and WTW (Project # CityU 160108) and the Research Scholarship to YJ Gu from the CityU Research Scholarship Enhancement Scheme.
Supplementary material
Preparation of SWNT-DOX
In brief, DOX was attached onto SWNT-PEG-NH2 by simply mixing 3.6 mg DOX with the PEGylated SWNTs at a nanotube concentration of 0.05 mg/mL at pH 7.4 PBS buffered solution for 24 hours at room temperature. Unbound DOX was removed by dialysis against H2O in a 12–14 K MWCO membrane for 3 days. The formed complex (denoted SWNTDOX) was stored at 4°C.
Cytotoxicity of HeLa cells by MTT methods
HeLa cells were also tested with SWNT-HBA-DOX and SWNT-DOX complex. A similar result was observed as for HepG2 cells. The cell viability of the HeLa cells after incubating in the medium containing SWNT-HBA-DOX (2–10 μg/mL) was in the range of 10%–40%, which was lower than that of the HeLa cells after incubation with 10 μg/mL SWNT-DOX (~40%).
Figure S1 Schematic illustration of the SWNT-DOX. The DOX can be attached to SWNT through supramolecular bond.
Abbreviations: SWNT, single-walled carbon nanotube; DOX, doxorubicin.
Disclosure
The authors report no conflicts of interest in this work.
References
- FoldvariMBagonluriMCarbon nanotubes as functional excipients for nanomedicines. II Drug delivery and biocompatibility issuesNanomedicine20084317318218550451
- LiuZTabakmanSWelsherKDaiHCarbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug deliveryNano Res2009228512020174481
- KamNWDaiHCarbon nanotubes as intracellular protein transporters: generality and biological functionalityJ Am Chem Soc2005127166021602615839702
- PantarottoDSinghRMcCarthyDFunctionalized carbon nanotubes for plasmid DNA gene deliveryAngew Chem Int Ed2004433952425246
- LiuYWuDCZhangWDPolyethylenimine-grafted multiwalled carbon nanotbue for secure noncovalent immobilization and efficient delivery of DNAAngew Chem Int Ed2005443047824785
- KamNWLiuZDaiHFunctionalization of carbon nanotbues via cleavable disulfide bonds for efficient intracelluar delivery of siRNA and potent gene silencingJ Am Chem Soc200512736124921249316144388
- KostarelosKLacerdaLPastorinGCellular uptake of functionalized carbon nanotubes in independent of functional groups and cell typeNat Nanotechnol2007210811318654229
- LiuZDavisCCaiWHeLCheXDaiHCirculation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopyProc Natl Acad Sci200810551410141518230737
- ChengJShiralFernandokMonicaKAVecaLReversible accumulation of PEGylated single-walled carbon nanotubes in the mammalian nucleus ACSNano200821020852094
- DharSLiuZThomaleJDaiHLippardSJTargeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing deviceJ Am Chem Soc200813034114671147618661990
- KostarelosKThe long and short of carbon nanotubes toxicityNat Biotech2008267774776
- PastorinGWuWWieckwskiSDouble functionalisation of carbon nanotubes for multimodal drug deliveryChem Comm2006111182118416518484
- LiuZSunXNakayama-RatchfordNDaiHSupramolecular chemistry on water-soluble carbon nanotubes for drug loading and deliveryACS Nano200711505619203129
- Ali-BoucettaHAl-JamalKTMcCarthyDPratoMBiancoAKostarelosKMultiwalled carbon nanotube-doxorubicin supramolecular complexes for cancer therapeuticsChem Comm2008445946118188467
- LiuZFanACRakhraKSupermolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapyAngew Chem Int Ed2009484176687672
- ZhangXMengLLuQFeiZDysonPJTargeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubesBiomaterials200930306041604719643474
- ChenJChenSZhaoXKuznetsovaLVWongSSOjimaIFunctionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug deliveryJ Am Chem Soc200813049167781678519554734
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res200868166652666018701489
- WuWLiRBianXCovalently combining carbon nanotubes with anticancer agent: preparation and antitumor activityACS Nano2009392740275019702292
- ChaudhuriPSoniSSenguptaSSingle-walled carbon nanotube-conjugated chemotherapy exhibits increased therapeutic index in melanomaNanotechnology20102102510219955607
- GatenbyRAGilliesRJWhy do cancers have high aerobic glycolysis?Nat Rev Cancer200441189189915516961
- DenkoNCHypoxia, HIF 1 and glucose metabolism in the solid tumorNat Rev Cancer20088970571319143055
- LiCXiaJWeiXYanHSiZJuSpH-activated near-infrared fluorescence nanoprobe imaging tumors by sensing the acidic microenvironmentAdv Funct Mater2010201422222230
- EtrychTJelínkováMŘíhováBUlbrichKNew HPMA copolymers containing doxorubicin bound via pH sensitive linkage: synthesis and preliminary in vitro and in vivo biological propertiesJ Controlled Release200173189102
- LeeCCCramerATSzokaFCJrFréchetJMJTumor-targeting, pHresponsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapyBioconjugate Chem2006173496504
- YangXGrailerJJPillaSSteeberDGongSTumor-targeting, pH-responsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapyBioconjugate Chem2010213496504
- AryalSGrailerJJPillaSSteeberDAGongSDoxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriersJ Mater Chem20091978797884
- PrabaharanMGrailerJJPillaSSteeberDAGongSGold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug deliveryBiomaterials200930306065607519674777
- ChengJGuYJWangYChengSHWongWTNanotherapeutics in angiogenesis: synthesis and in vivo assessment of drug efficacy and biocompatibility in zebrafish embryosInt J Nanomedicine201162007202121976976
- ŘíhováBEtrychTŠírováMSynergistic action of doxorubicin bound to the polymeric carrier based on N-(2-hydroxypropyl) methacrylamide copolymers through an amide or hydrazone bondMol Pharmacol20107410271040
- BodleyALiuLFIsraelMSeshadriRKosekiYGiulianiFCDNA topoisomerase II-mediated interaction of doxorubicin and daunorubicin conjugates with DNACancer Res19894921596959782551497
- CummingsJSmythJFDNA topoisomerase I and II as targets for rational design of new anticancer drugsAnn Oncol1993475335438395870
- FornariFARandolphJKYalowichJCRitkeMKGewirtzDAInterference by doxorubicin with DNA unwinding in MCF-7 breast tumor cellsMol Pharmacol19944546496568183243
- AbdElWahedMGAbdElWaneesSGamel ElMAbdElHaleemSPhysical studies of some hydrazinobenzoic acid complexesJ Mater Sci Technol2005211140144
- JainAKDubeyVMehraNKCarbohydrate-conjugated multiwalled carbon nanotubes: development and characterizationNanomedicine2009543244219341818
- ŘíhováBEtrychTPecharMDoxorubicin bound to a HPMA copolymer carrier through hydrazone bond is also effective in a cancer cell line with a limited content of lysosomeJ Controlled Release2001741–3225232