?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to formulate and evaluate nano lipid vesicles of methotrexate (MTX) for its anti-rheumatoid activity.
Methods
In this study the principle of both active as well as passive targeting using MTX-loaded stealth liposomes as per the magic gun approach was followed. Stealth liposomes of MTX were prepared by thin-film hydration method using a PEGylated phospholipid-like DSPE-MPEG 2000. Similarly, conventional liposomes were prepared using phospholipids like DPPC and DSPC. Conventional liposomes were coated with a hydrophilic biocompatible polymer like chitosan. They were investigated for their physical properties and in vitro release profile. Further, in vivo screening of the formulations for their anti-rheumatoid efficacy was carried out in rats. Rheumatoid arthritis was induced in male Wistar-Lewis rats using complete Freund’s adjuvant (1 mg/mL Mycobacterium tuberculosis, heat killed in mineral oil).
Results
It was found that chitosan coating of the conventional liposomes increased the physical stability of the liposomal suspension as well as its entrapment efficiency. The size of the unsonicated lipid vesicles was found to be in the range of 8–10 μm, and the sonicated lipid vesicles in the range of 210–260 nm, with good polydispersity index. Further, chitosan-coated conventional liposomes and the PEGylated liposomes released the drug for a prolonged period of time, compared to the uncoated conventional liposomes. It was found that there was a significant reduction in edema volume in the rat group administered with the test stealth liposomal formulations and chitosan-coated conventional liposomes (PEGylated and chitosan-coated conventional) compared to that of the control and standard (administered with free MTX) group of rats. PEGylated liposomes showed almost equal efficacy as that of the chitosan-coated conventional liposomes.
Conclusion
Lipid nano vesicles of MTX can be administered by intravenous route, whereby the drug selectively reaches the target site with reduced toxicity to other organs.
Introduction
Liposomes are lipid vesicles which are under extensive investigation as drug carriers for improving the delivery of therapeutic agents. They are composed of relatively biocompatible and biodegradable materials, and consist of an aqueous volume entrapped by one or more bilayers of natural and/or synthetic lipids. Liposomes were discovered in the early 1960s by Bangham and collaegues (CitationBangham et al, 1965) and subsequently became the most extensively explored drug delivery system.Citation1
The availability of liposomes in the market (Doxil® [doxorubicin, Sequus Pharmaceuticals USA]; DaunoXome® [daunorubicin, Sequus Pharmaceuticals USA], ableet, amphotech [amphotericin B, Sequus Pharmaceuticals USA], and am-BiSome® [amphotericin B, NeXstar Pharmaceuticals], etc) makes it obvious that liposome technologies can mature into highly sophisticated pharmaceutical products. There was a continuous increase in sales of these products in 1999. Total sales of over one-quarter of a billion dollars makes the development of other liposome-based pharmaceutical products attractive.Citation2 They are microscopic aggregates of highly ordered lipid molecules which are normally dispersed in a hydrophilic solvent, typically water.Citation3 Lipid vesicles are micro-particulate or colloidal carriers, usually 0.05–5.0 μm in diameter which form spontaneously when certain lipids are hydrated in aqueous media.Citation4 Drugs with widely varying lipophilicities can be encapsulated in lipid vesicles, either in the phospholipid bilayer, in the entrapped aqueous volume, or at the bilayer interface. Drugs used in the treatment of diseases like cancer and rheumatoid arthritis usually have a narrow therapeutic index (TI) and can be highly toxic to normal tissues. The toxicity of these drugs may be minimized by decreasing delivery to critical normal organs. It has been shown that even a small reduction in distribution of the drug to critical organs by encapsulation in lipid vesicles can significantly reduce the drug toxicity. Lipid vesicles are taken up poorly by tissues such as heart, kidney, and GI tract, which are major sites for toxic side effects of a variety of antineoplastic drugs. Thus lipid vesicle formulations may improve the TI by altering the bio-distribution of drugs away from drug sensitive normal tissues.Citation5 Methotrexate (MTX) is the most commonly used DMARD (disease modifying anti-rheumatic drug) for the treatment of rheumatoid arthritis. MTX has gained popularity among doctors as a second-line drug because of its effectiveness. MTX as an antimetabolite-folate antagonist is also used in the treatment of metastatic breast cancer. The drug side-effect profile includes mouth sores, stomach upset, and low white blood counts. MTX can cause severe toxicity of the liver, kidneys, and bone marrow, which require regular monitoring with blood tests. It can cause headache and drowsiness, itching, skin rash, dizziness, and hair loss. A dry, non-productive cough can be a result of rare lung toxicity, which often results in cessation of therapy. To decrease the systemic side-effects and optimize the local anti-inflammatory effect, intra-articular free MTX has been administered, but the efficacy was low due to rapid clearance of the drug from the joint cavity.Citation6 This can be overcome by encapsulating MTX in lipid vesicles with the aim of increasing the retention time in the joint and with the added advantage of targeting the damaged synovial membrane in the joints. PEGylated lipid vesicles of MTX can be prepared which are “stealth” lipid vesicles that evade detection and destruction by phagocytes by virtue of their cloaks of hydrated PEG (polyethylene glycol) molecules. The other main advantage of PEGylated lipid vesicles lies in the possibility of active-targeted delivery of drugs to the tissues or organs that need them most. Not only does this maximize delivery efficiency for the agent in question, but it also minimizes the chances of toxicity to other organs. The objective of the present study is to formulate and optimize the stable conventional and PEGylated lipid vesicles of MTX and in vivo screening of the formulations in rats for its anti-rheumatoid efficacy.
Materials and methods
Materials
MTX was gifted by Khandelwal Laboratories Pvt Ltd, Mumbai, India. DSPE-MPEG 2000 (1,2-Distearoyl-phosphatidyleth-anolamine-methyl-polyethyleneglycol conjugate-2000) was a gift from Lipoid GmBH, Ludwigshafen, Germany. DSPC (1,2-Distearoyl-sn-glycero-3-phosphocholine) and DPPC (1,2-Dipalmitoyl-sn-glycero-3-phosphocholine) were also gifted as samples from Genzyme Pharmaceuticals, Liestal, Switzerland. Cholesterol was obtained from HiMedia Laboratories Pvt Ltd, Mumbai, India. All other chemicals used were of analytical grade.
Preparation of lipid nano vesicles
Lipid nano vesicles were prepared by the passive-loading technique thin-film hydration method as per the method described by Bangham et al in 1965.Citation4 The molar ratios of lipids (phospholipids-MPEG-DSPE/DSPC/DPPC and cholesterol) were accurately weighed and dissolved in a minimal quantity (about 2 mL) of a mixture of chloroform:methanol (2:1) in a 250 mL round bottom flask with a ground glass neck to obtain a clear solution (typically lipid solutions were prepared at 10–20 mg lipid/mL of the organic solvent). The round bottom flask was rotated at 60 rpm while immersed in a water bath with a thermostat set at a temperature above the phase transition temperature (Tm) of the phospholipids to obtain a thin dry lipid film. Hydration of the dry lipid film was accomplished by adding the MTX in PBS pH 7.4 buffer at a concentration of 1.5 mg/mL and the temperature of the hydrating medium was maintained above the gel-liquid crystal transition temperature (Tm) of the phospholipid with the highest Tm, before adding to the dry lipid. Once a stable multi lamellar vesicles (MLV) suspension was produced, it was then subjected to ultra-probe sonication by transferring the suspension into a glass vial. Sonication was done in two cycles – first the liposomal suspension was sonicated at 80% amplitude with a pulse of 0.5 cycles per second for a period of 3 minutes, followed by 3 minutes rest (excess heat may be generated during probe sonication, which may damage the lipids). After 3 minutes, the second cycle was processed for 3 minutes at 80% amplitude with 0.5-second pulse for another 3 minutes. After sonication, the heterogeneous liposomal suspension of SUVs (small, unilamellar vesicles) was converted to a homogenous suspension of SUVs by passing it through a 0.22 μm syringe filter, which further improves the polydispersibility index (PDI) and also achieved the sterilization of the liposomal suspension which can be administered intravenously (IV). Different molar ratios of lipids were used to formulate the lipid vesicles (DSPC/DPCC:cholesterol, 10:1, 10:2 for conventional lipid vesicles and for stealth lipid vesicles DSPC/DPCC:MPEG-DSPE:cholesterol, 10:0.1:2; 10:0.2:2; 10:0.4:2; 10:0.5:2, etc) and drug-to-lipid ratio were tested (D/L; 0.2, 0.25, 0.3, 0.35, 0.4, 0.5, etc).
Coating of the lipid vesicles containing the drug with a cationic hydrophilic polymer chitosan
Coating of MLVs was done by mixing an aliquot of the liposomal suspension with the chitosan solution in 0.5% v/v of glacial acetic acid.Citation7 Chitosan solution (containing 0.1% w/v, 0.2% w/v, 0.4% w/v, and 0.6% w/v) was added drop-wise into the respective lipid vesicle suspensions under controlled stirring (50 rpm) using a magnetic stirrer at room temperature. After coating the lipid vesicles, the samples were incubated at 10°C in the refrigerator for 1 hour in a 50 mL beaker. The concentration of chitosan solution was optimized to 0.1% w/v. This was sonicated at 80% amplitude, with 0.5-second pulses for 3 minutes with a rest period of 3 minutes, followed by sonication for a further 3 minutes.
The optimized ratios of DSPC:cholesterol (F3) or DPCC:cholesterol (F4) are 10:2, respectively for both conventional lipid vesicles.
In vitro characterization of the lipid vesicles
Differential scanning calorimetry (DSC)
DSC of the phospholipid samples (solid mixture) was performed in order to determine the exact transition temperature (Tm [°C]) of the mixtures of phospholipids: (1) mixture of DSPC with MPEG 2000-DSPE, (2) mixture of DPPC with MPEG 2000-DSPE.
Optical photomicroscopy
The MLV suspension (100 μL) was placed on a clean glass slide; a cover slip was placed on it, taking care to prevent air bubble formation. This was focused under 45× magnification using a MOTIC digital photographic microscope to view the MLVs. The size (in μm) of the MLVs was also measured using the microscopic scale ().
Average particle size and size distribution
Average particle size (in nanometers) and size distribution (as the PDI) of the liposomal suspension (SUVs) was measured using a Malvern nano zetasizer instrument (Zetasizer 3000 HAS; Malvern Instruments Ltd, Worcestershire, UK).
Zeta potential
Zeta potential measurement of the chitosan-coated liposomal formulation (SUVs) was done by using a Malvern nano zetasizer instrument (Zetasizer 3000 HAS; Malvern Instruments Ltd, Worcestershire, UK).
Entrapment efficiency (EE)
Entrapment efficiency of MTX in the lipid vesicles was determined as follows: After sonication, 1 mL of the liposomal suspension (SUVs) was taken in a 1 mL micro centrifuge tube, centrifuged at 80,000 rpm for 1 hour at 4°C in a cold centrifuge to get a white pellet of SUVs which settled at the bottom of the centrifuge tube. The supernatant was separated as it contained unentrapped MTX which is highly soluble in PBS pH 7.4 buffer, using a micropipette. To the remaining pellet in the centrifuge tube, 500 μL of 0.1 N NaOH (as MTX is highly soluble in 0.1 N NaOH) was added and vortexed thoroughly for 3 minutes. After vortexing, a white suspension was obtained and 1 mL of this suspension was transferred to a test tube using a micropipette. To this 5 mL, methanol was added which resulted in a clear solution; this was further vortexed for 2 minutes (to ensure that the lipid vesicles are lysed completely to release the drug).Citation8 This solution (1 mL) was further diluted with methanol and the absorbance was determined using a UV-VIS spectrophotometer (UV-1700 Pharmaspec; Shimadzu Corporation, Kyoto, Japan) at λmax of 303 nm.
The entrapment efficiency (EE) was calculated using the following formula:
In vitro release studies
The liposomal suspension (1 mL) of SUVs was placed on one side of the Sigma dialysis membrane (MWCO – 20 kDa) in a vertical Franz diffusion cell. The other side of the membrane was in contact with the dissolution medium. The entire dissolution assembly was placed on a magnetic stirrer at 37°C. The dissolution medium was 50 mL of PBS pH 7.4 buffer. Aliquots (5 mL) of dissolution medium was withdrawn at different time intervals: 5, 10, 15, 30, 45, and 60 minutes, and 2, 4, 8, 12, and 24 hours. Whenever a sample was withdrawn, an equal volume of fresh dissolution medium was added to the beaker to maintain a constant volume. Drug concentrations in the dissolution medium were determined using UV spectrophotometry, at λmax of 303 nm. All the experiments were carried out in triplicate.
Stability studies
For stability testing, the sonicated liposomal suspension of SUVs was stored away from light in sealed 2 mL micro centrifuge eppendroff tubes in the refrigerator (4°C–8°C) for 3 months.
Sampling was done by withdrawing 100 μL of the supernatant using a micropipette at days 2, 4, 10, 20, 40, 45, 60, 80, and 90. Suitable dilutions were made with PBS pH 7.4 buffer. Whenever a sample was withdrawn, UV absorbance was determined at λmax of 303 nm and drug content was estimated.
In vivo studies
Anti-rheumatoid efficacy studies
Male Wistar-Lewis rats (5–6 weeks old weighing 200 ± 10 g) were used. Animal groups were selected with the same initial body weight. Animals were kept under controlled environmental conditions (22°C ± 0.5°C with relative humidity 40%–60%), alternate light–dark cycles, food and water. The animals were allowed to acclimate for 1 week before the experiment. They were housed in cages in which the floor was covered with sawdust to minimize the possibility of painful contact with a hard surface. Adjuvant arthritis was induced as per the method described by Pearson and Wood, by injecting 0.6 mL (1 mg/mL) of complete Freund’s adjuvant (CFA, heat killed and dried, Mycobacterium tuberculosis in 0.85 mL mineral oil, 0.15 mL mannide mono-oleate) to the subplantar region of the left hind paw.
The parameter of interest of adjuvant-induced arthritis is the swelling of the right paw, which is typically established in 19 days after induction. Rats were divided into five groups containing six animals in each group: normal control (neither induction of arthritis nor treatment with MTX), CFA-control (induction of arthritis with CFA), CFA+ standard, CFA+ test 1, and CFA+ test 2. CFA-control group received only 0.6 mL of complete Freund’s adjuvant. The CFA+ test 1 group of rats received CFA to the left hind paw + PEGylated MTX liposomal formulation (DSPC: MPEG-DSPE: CH-10:0.2:2 at a dose of 0.13 mg/kg MTX iv), the CFA+ test 2 group of rats received chitosan-coated conventional lipid vesicles (DSPC: CH-10:2 at a dose of 0.13 mg/kg MTX iv), and the standard group received MTX solution (0.13 mL of MTX injection [15 mg/mL] equivalent to 0.13 mg MTX IV) as a single IV injection through the tail vein on day 0. The onset day of arthritis was determined as the day on which right hind paw swelling or its redness was detectable. For determining the arthritic reaction, a marking was made in the tebio-tarsal joint of the right and left hind paws and the paw volume (in mL) of each paw was determined on day 0, 5, 10, 21, 26, and 29 after induction of adjuvant arthritis, using a water displacement digital plethysmometer. The severity of the induced adjuvant disease was determined by measurement of the non-injected right paw (secondary lesion) with a plethysmometer and by measuring the body weight every 3 days after arthritis induction.Citation9,Citation10
where ‘VT’ is edema volume of treated animal and ‘VC’ edema volume of control animal.
Arthritis assessments
The rats were assessed daily for signs of arthritis between days 7 and 25, after the administration of CFA. During the treatment with MTX formulations, paw volume was measured every second day with a plethysmometer. Blood was collected from the animals by retro-orbital puncture for estimation of biochemical parameters. Serum aspartate transaminase (AST) as SGOT levels, alanine transaminase (ALT) as SGPT levels, alkaline phosphatase (ALP), and total proteins (TP) were assessed. This study was performed not only to assess the extent of recovery of animals from the effects of CFA treatment, but also to study the level of side effects of MTX, as reported earlier in the introduction. The animals were sacrificed by cervical dislocation for the determination of organ weight.Citation11
Statistical analysis
The data were presented as mean ± SEM one-way analysis of variance (ANOVA) followed by post Dunnet multiple comparison tests to compare the efficacy of the formulations using GraphPad Prism software (v 4.03.354; GraphPad Software, Inc, San Diego, CA).
Results and discussion
Preparation of lipid vesicles
Lipid vesicles were prepared by the thin-film hydration method as per the method described by Bangham et al, 1965. Drug to lipid ratio was optimized as 0.2. ie, D/L = 0.2, means 1/L = 0.2 or 1/0.2 = L, hence L = 5, ie, drug:lipid ratio is 1:5. The hydration volume was optimized to 6 mL, based on the amount of phospholipid taken. The hydration medium contained 9 mg of MTX dissolved in 6 mL PBS 7.4. The optimized ratios of stealth lipid vesicles are, F1;DSPC:MPEG-DSPE:CH-10: 0.2:2 and F2;DPPC:MPEG-DSPE: CH-10: 0.2:2.
Differential scanning calorimetry (DSC) analysis
The phase transition temperature (Tm) of the mixture of DSPC with MPEG-2000 DSPE was found to be 76.28°C, whereas Tm for the mixture of DPPC with MPEG-2000 DSPE was found to be 69.28°C ().
Particle size analysis
Particle size analysis of the sonicated lipid vesicles – SUVs was determined using a Malvern zetasizer instrument. It was found that the average particle size of stealth lipid vesicles (F1 and F2) was found to be 210 nm, whereas the average particle size of conventional chitosan-coated lipid vesicles (F3-coated and F4-coated) was found to be 253 nm. The size range for unsonicated lipid vesicles was found to be around 8–10 μL (). The PDI remained in the range of 0.2–0.3, indicating good dispersion of uniformly sized lipid vesicles.
Zeta potential analysis
The zeta potential of the coated formulations was compared with that of the uncoated formulation; it was found that the zeta potential of the chitosan-coated formulation – F3 was positive (+39.5 mv), compared to the zeta potential of the uncoated formulation – F3 which was found to be negative (−2.78 mv). This finding indicated the successful coating of the formulation with chitosan.
Entrapment efficiency
Entrapment efficiency of the lipid vesicles was determined after sonication. Entrapment efficiency of the SUVs was determined by lysing the SUVs in methanol. Entrapment of the drug in the uncoated lipid vesicles was found to be in the range 23%–31%, whereas for coated lipid vesicles it was in the range 43%–53%. The increase in size of the lipid vesicles also increased the entrapment efficiency. It was found that the chitosan-coated conventional lipid vesicles showed significantly higher entrapment efficiency compared to that of the PEGylated lipid vesicles and the uncoated conventional lipid vesicles. This indicated that chitosan coating of the conventional lipid vesicles increases entrapment efficiency. More recently, the most accepted theory with respect to lipid vesicles–chitosan interaction was that chitosan covers the surface of the liposomal formulation.Citation12,Citation13 In this study, chitosan coating of the conventional lipid vesicles produced cationic lipid vesicles (CHITOSOMES). Chitosan has strong affinity for the phospholipid of the vesicle bilayer and covers the surface of the lipid vesicles by forming an ion complex with phosphatidyl choline in the liposomal formulation. MTX, being negatively charged, does not compete with positively charged chitosan to interact with the phospholipid of the bilayer. Hence, there is a possibility of interaction between the drug and chitosan as well as chitosan and phospholipids, and thus, there was increased retention of the drug, thereby increasing entrapment efficiency.
In vitro release studies
The in vitro release profile of the drug is given in . The initial burst release was observed for all formulations, owing to their surface hydrophilicity. PEGylated (F1 and F2) and chitosan-coated formulations (F3-coated and F4-coated) showed a significantly higher burst release compared to the other formulations. However, PEGylated formulations showed a higher burst release compared to that of the chitosan-coated formulations. This burst release was observed due to the presence of the drug on the surface in the adsorbed form. All the PEGylated formulations (F1 and F2) and the chitosan-coated formulations (F3 and F4) released the drug for 24 hours. PEGylated formulations (F1 and F2) also released the drug for a period of 24 hours. The conventional formulations F3 and F4 released the drug for a period of 12 hours. Formulation F3 released 88.41% of the drug in 12 hours, whereas formulation F4 released 90.45% of the drug in 12 hours. Formulation F3-coated (conventional-coated) released 70.63% of the drug in 24 hours, whereas formulation F4-coated (conventional-coated) released 75.67% of the drug in 24 hours. These results indicate that the release of MTX follows a slow release profile, which suggests that it takes time for MTX to be released once encapsulated in the lipid vesicles because the lipid bilayers are stabilized by cholesterol. Thus, a depot effect could be achieved using lipid vesicles, especially in the coated liposomal formulations.
Anti-rheumatoid and anti-arthritic efficacy
Adjuvant-induced arthritis in rats is a well-established experimental model that has features similar to human rheumatoid arthritis. In addition, it is a good chronic inflammatory model for the development of potential analgesic and/or anti-inflammatory drugs useful for arthritis treatment. Adjuvant arthritis is characterized by chronic proliferative and inflammatory reactions in synovial membranes, producing pain, disability, and eventually destruction of joints. Although the etiology of this disease is unknown, it is thought that autoimmune processes are involved. Experimental evidence suggests that an autoimmune process involving T lymphocytes is responsible for the generation of adjuvant arthritis.Citation14,Citation15
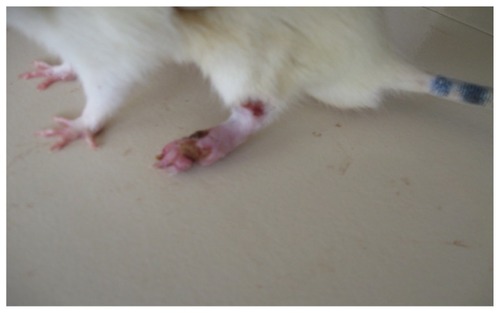
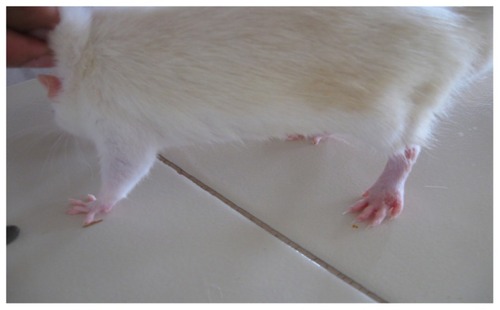
In the present study, the challenge with CFA resulted in mono-articular arthritis, which is evident from the significantly increased paw volume in arthritis control, whereas the reduction of paw volume with the test-1 and test-2 formulations after 21 days indicated the effectiveness of the anti-inflammatory activity of the formulations. The arthritic lesions, ie, swelling of the right hind paw and lesions on the forepaw, appeared from day 19 onwards in CFA-control rats and the standard group of rats, whereas the lesions were observed from day 21, after arthritis induction in the test group (both CFA+ test 1 and CFA+ test 2) of rats treated with the lipid vesicle formulations (–).
Figure 6 CFA+ test 2 group rat showing only moderate inflammation of the left hind paw on day 26.
Abbreviation: CFA, complete Freund’s adjuvant.
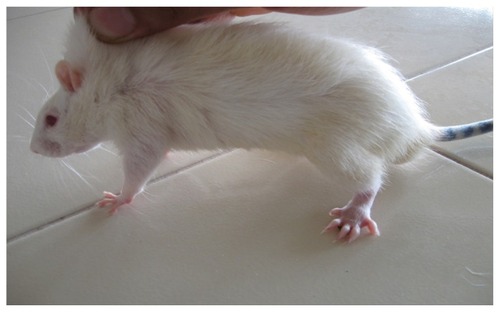
Figure 7 CFA+ test 1 group rat showing complete recovery of the paws with completely suppressed secondary lesion and minimal paw inflammation on day 26.
Abbreviation: CFA, complete Freund’s adjuvant.
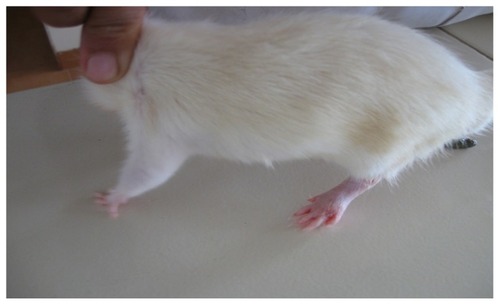
The following observations were made during the study (–): in the CFA-control group of rats, the total edema volume of four paws gradually increased as the days passed. Volume displaced reached a peak on day 21, followed by a gradual decrease for CFA-treated groups CFA+ test 1, CFA+ test 2, and the standard group of rats. Rats treated with CFA+ test 1 formulation showed a significantly lower peak value on day 21 compared with that of the CFA+ test 2, and the standard groups, whereas the rat group treated with the CFA+ test 2 formulation showed a peak lower than the standard. The CFA+ test 1 group of rats showed a highly significant (P < 0.01) percentage decrease in edema volume on day 26 and day 29 compared to the CFA-control group of rats. The CFA+ test 2 group of rats showed a significant (P < 0.05) percentage decrease in edema volume on days 26 and 29, compared to the CFA-control group of rats. Formulation CFA+ test 1 (PEGylated lipid vesicles) showed the highest anti-rheumatoid efficacy, followed by CFA+ test 2 (chitosan-coated liposomal formulation), and the standard-free MTX (the effectiveness of polymer-coated lipid vesicles is not as high as PEG-derivative-containing lipid vesicles in terms of retention in the bloodstream after iv injection).Citation13 However, formulation CFA+ test 2 showed an anti-rheumatoid efficacy better than that of the standard. Assessment of the serum levels of AST, ALT, and ALP provides an excellent and simple tool to measure the anti-arthritic activity of the drug. The activities of aminotransferases and ALP increased significantly in arthritic rats, since these are good indices of liver and kidney impairment, which is also considered a feature of adjuvant arthritis. Serum AST and ALT has been reported to play a vital role in the formation of biologically active chemical mediators such as bradykinins in inflammatory process. Increased levels of serum ALP in adjuvant-induced arthritic rats can be due to increases in the liver and bone fractions or due to the increased levels of both isoenzymes. This in turn indicates localized bone erosion and peri-articular osteopenia, as the enzyme is released into the circulation during the course of bone formation and resorption.Citation16 It was reported that adjuvant administration in rats immunologically alters the hepatic biochemistry. Most of the literature has suggested an increase in the baseline serum ALP level. It has also reported a decrease in serum total protein in rheumatoid arthritis.Citation16
Table 1 Volume of water displaced in the plethysmograph
Table 2 Edema volume of different groups of animals on various days
Table 3 Percentage decrease in edema volume in various animal groups
In the present study, the challenge with CFA significantly (P < 0.001) elevated the serum AST and ALT estimated as serum SGOT and SGPT level and decreased the total protein level (), whereas the effect on ALP was insignificant (P < 0.001) in arthritis control. It was suggested that the development of inflammation in CFA-induced arthritis may be related to the biochemical changes in hepatic enzymes. The serum SGOT and SGPT levels (P < 0.01 level significance compared to CFA control) in animal groups treated with MTX lipid vesicle formulations (CFA+ test 1 and CFA+ test 2), indicated the effectiveness of the lipid vesicles in inflammatory conditions. However, the level of SGOT and SGPT in animal groups treated with standard formulations of MTX remained above the normal level (upper limit is 60 units/L). This may be due to the fact that MTX itself may cause damage to the liver as reported earlier. Though the extent of inflammation is reduced with the standard formulation of MTX, the effect of MTX on liver was reflected in the level of serum SGOT and SGPT.
Table 4 Effect of MTX formulations on serum enzyme level and total protein level of CFA-induced arthritic animals
The effect of MTX formulations on spleen and kidney was further confirmed by changes in their weight. The challenge with CFA significantly increased the weight of kidney, spleen, and liver organs in arthritis control. The MTX standard formulation significantly (P < 0.05) increased the liver weight. However, the effect on kidney weight and spleen weight was insignificant (P < 0.05; ). The effect of MTX in the form of lipid vesicles on organ weight, especially the weight of the liver indicated the safety margin of the formulations (). The spleen is an important lymphoid organ involved in immune responses against all types of antigens that appear in the circulation and it provides a readily available source of cells known to be involved in adjuvant arthritis. Increased cellularity in the spleen of adjuvant injected rats engendered interest as to the potential for concomitant classical antibody formation where the increased antibody titer in arthritic animals further supports the hyper immune status by humoral immunity. Further, the increase in spleen weight in the adjuvant- induced arthritic rats has been reported to be associated with splenomegaly, generalized lymphadenopathy, and altered hepatic function.Citation17 Injection of CFA significantly increased spleen weight in arthritis control and showed no effect on spleen weight with MTX formulations (). Thus, these findings suggested that the inhibition of lymphocytes and decreased immunological response may be responsible for the anti-arthritic potential of MTX formulations.
Table 5 Effect of MTX formulations on organ weight of CFA-induced arthritic animals
Hence in treating rheumatoid arthritis, instead of using intra-articular (can lead to joint inflammation with pain) or oral delivery of MTX, (where MTX gets uniformly distributed to all the tissues in the body leading to unwanted adverse effects), alternative delivery of MTX using stealth lipid vesicles can be administered by IV route, whereby the drug selectively reaches the target site with reduced toxicity to other organs and may offer reduced doses, decreasing dosing frequency, thereby improving patient compliance.
Stability study
As the liposomes are thermodynamically unstable systems, they tend to fuse and grow into bigger vesicles, resulting in breakage of the liposomes on storage, which poses a problem of drug leakage from the vesicles. Unsaturated phospholipids undergo oxidation easily. Hence, in the present work, only saturated phospholipids like DPPC, DSPC, and MPEG-DSPE were used to formulate the liposomes, to avoid oxidation. Therefore, no antioxidants such as α-tocopherol were used. Since the saturated phospholipids have a high Tm they exhibited good physical stability. The physical stability of sonicated liposomes on storage was studied by monitoring the amount of leaked drug from liposomes into the supernatant and by the size of the liposomes. The presence of divalent metal ions such as calcium and magnesium in the aqueous buffer causes the aggregation of liposomes, particularly those with negative charge. Therefore, aqueous buffer used for the preparation of liposomes was made using double-distilled water.
The stability data of liposomes at 4°C–8°C is given in . Stealth liposomes (F1 and F2) were found to be more stable than the conventional liposomes (F3 and F4) and showed much less drug leakage compared to the conventional liposomes. However, chitosan-coated liposomes (F3-coated and F4-coated) were highly stable compared to the stealth and conventional liposomes. Chitosan-coated liposomes also showed significantly less drug leakage when compared to the stealth and conventional liposomes. Chitosan-coated liposomes showed the best physical stability because of the steric repulsion created by the positive surface charge on the liposomes leading to steric stabilization of the colloidal suspension.
Table 6 Stability data of various liposomal formulations
Conclusion
MTX-loaded nano lipid vesicles were prepared for the treatment of rheumatoid arthritis and compared with the MTX standard (solution form). The nano lipid vesicles enhanced the targeting of MTX in vivo, and effectively promoted the retention of MTX in the inflamed tissue. It can be concluded that in treating rheumatoid arthritis, lipid nano vesicles of MTX can be more effective than intra-articular or oral delivery of MTX, which can lead to joint inflammation with pain and in the case of oral delivery (where MTX gets uniformly distributed to all the tissues in the body), can lead to unwanted adverse effects. Delivery of MTX using stealth lipid vesicles can be administered by iv route, whereby the drug selectively reaches the target site with reduced toxicity to other organs.
Disclosure
The authors declare no conflicts of interest in relation to this paper.
References
- SawarbrikJBoylanJCLipid vesicles as pharmaceutical dosage formsSwarbrickJBoylanJCEncyclopedia of Pharmaceutical TechnologyNew YorkMarcel Dekker Inc1994131
- BarenholzYLipid vesicles application: problems and prospectsCurr Opin Colloid Interface Sci200166677
- KirbyCJGregoriadisGLipid vesiclesCollinsGEncyclopedia of Pharmaceutical Controlled Drug DeliveryNew YorkJohn Wiley and Sons Inc1999461489
- BanghamADStandishMMWatkinsJCDiffusion of univalent ions across the lamellae of swollen phospholipidsJ Mol Biol1965132382525859039
- SharmaASharmaUSReview Lipid vesicles in drug delivery: progress and limitationsInt J Pharm1997154123140
- Methotrexate Available from: http://www.drugs.com/methotrexate.htmlAccessed November 16, 2011
- Gonzalez-RodriguezMLBarrosLBPalmaJGonzalez-RodriguezPLRabascoAMApplication of statistical experimental design to study the formulation variables influencing the coating process of lidocaine lipid vesiclesInt J Pharm20073371–233634517317050
- GulatiMGroverMSinghMSinghSStudy of azathioprine encapsulation into lipid vesiclesJ Microencapsul19981544854949651870
- ZhangRXFanAYZhouANExtract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in ratsJ Ethnopharmacol2009121336637119100323
- VogelGHAdjuvant arthritis in ratsGerhard VogelHDrug Discovery and EvaluationPharmacological Assays2nd edNew YorkSpringer-Verlag Berlin Heidelberg2002802803
- OtariKVSheteRVUpasaniCDAdakVSBagadeMYHarpalaniANEvaluation of anti-inflammatory and anti-arthritic activities of ethanolic extract of Vernonia anthelmintica seedsJ Cell Tissue Res201010222692280
- GuoJPingQJiangGHuangLTongYChitosan-coated liposomes: characterization and interaction with leuprolideInt J Pharm2003260216717312842337
- TakeuchiHMatsuiYYamamotoHKawashimaYMucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to ratsJ Control Release2003862–323524212526820
- HoloshitzJNaparstekYBen-NunACohenIRLines of T lymphocytes induce or vaccinate against autoimmune arthritisScience1983219458056586336851
- FrancischiJNYokoroCMPooleSTafuriWLCunhaFQTeixeiraMMAnti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritisEur J Pharmacol20003992–324324910884526
- CellaJHWatsonJManual of Laboratory TestsVirener Kumar Arya, India1991145147
- IsmailMFEL-MaraghySASadikNAStudy of the immunomodulatory and anti-inflammatory effects of evening primrose oil in adjuvant arthritisAfrican J Biochem Res2008237480