Abstract
Background
To develop a lipid-stabilized contrast formulation containing gadobenate dimeglumine for clear visualization of the mucosal surfaces of the larynx and trachea for early diagnosis of disease by magnetic resonance imaging.
Methods
The contrast formulation was prepared by loading gadobenate dimeglumine into egg phosphotidylcholine, cholesterol, and sterylamine nanoliposomes using the dehydration-rehydration method. The liposomal contrast formulation was ultrasonically nebulized, and the deposition and coating pattern on explanted pig laryngeal and tracheal segments was examined by inductively coupled plasma atomic emission spectroscopy. The sizes of the nebulized droplets were characterized by photon correlation spectroscopy. The contrast-enhanced mucosal surface images of the larynx and trachea were obtained in a 3.0T magnetic resonance scanner using a T1-weighted spectral presaturation inversion recovery sequence.
Results
Various cationic liposome formulations were compared for their stabilization effects on the droplets containing gadobenate dimeglumine. The liposomes composed of egg phosphotidylcholine, cholesterol, and sterylamine in a molar ratio of 1:1:1 were found to enable the most efficient nebulization and the resulting droplet sizes were narrowly distributed. They also resulted in the most even coating on the laryngeal and tracheal lumen surfaces and produced significant contrast enhancement along the mucosal surface. Such contrast enhancement could help clearer visualization of several disease states, such as intraluminal protrusions, submucosal nodules, and craters.
Conclusion
This lipid-stabilized magnetic resonance imaging contrast formulation may be useful for improving mucosal surface visualization and early diagnosis of disease originating in the mucosal surfaces of the larynx and trachea.
Introduction
There are many diseases that involve abnormalities occurring on the mucosal surfaces of the larynx and trachea.Citation1 Among them, squamous cancer of the larynx is the most commonly occurring head and neck cancer.Citation2 In addition, neoplasms involving the mucosal surfaces of the trachea are also abundant, but easily misdiagnosed as bronchitis or asthma because of a lack of specific symptoms.Citation3 Endoscopic examination has been widely used to detect these mucosal abnormalities. However, disease-related submucosal spread and infiltration into the surrounding tissues are not visible at endoscopy.Citation1 Some bulky tumors are also reported to hinder distal endoscopic visualization, particularly in the subglottic region.Citation4 As a complementary tool, cross-sectional imaging methods, such as magnetic resonance imaging (MRI) may be used to detect abnormal soft tissue thickening and cartilage invasion or fat infiltration, and thus help in accurate depiction of the disease state.Citation2,Citation4,Citation5
Although MRI has excellent soft tissue contrast, the imaging qualities of the lung and airway have always been poor. The low proton density of the airway and pulmonary parenchyma results in low signal-to-noise ratios.Citation6 In addition, complex air-soft tissue interfaces produce an inhomogeneous magnetic field for imaging, and artifacts are common. Citation6 In order to improve the quality of MRI in the airway, researchers had used hyperpolarized gas, such as 3HeCitation7 or 129Xe,Citation8 or nebulized gadolinium solutions.Citation9 However, these agents were designed mainly to evaluate lung ventilation and do not deposit on the lumen surfaces. Therefore, it has been difficult to visualize the mucosal surfaces using MRI.Citation2 To address this problem, we developed a contrast formulation that can coat the mucosal surfaces of the larynx and trachea evenly, thus helping visualization and detection of small foci in early stages of disease development.
Mucoadhesive systems have been widely used for intranasal,Citation10 buccal,Citation11 peroral,Citation12,Citation13 and airwayCitation14,Citation15 drug delivery. Longer retention of drugs adhering to mucosal surfaces has been shown to improve in situ or systemic therapeutic effects significantly. The most commonly used mucoadhesive excipients are hydrophilic macromolecules, such as carbomers,Citation16 sodium alginate,Citation11 chitosan,Citation15 and cellulose derivatives.Citation17 However, these can be highly viscous and unsuitable for airway administration. In some recent research, cationic lipids were also shown to promote adhesion through interactions between cationic vehicles and the negatively charged mucosal surface.Citation18 Lipids are also useful for modulating the aerodynamic diameters of nebulized droplets for optimal distribution in the respiratory tract and deposition on the laryngeal and tracheal surfaces.Citation19 In this study, in order to achieve even coating of the mucosal surfaces, we included egg phosphotidylcholine and cholesterol in a formulation for stabilizing gadobenate dimeglumine droplets, and added stearylamine to improve mucoadhesiveness. The formulations were optimized to provide significant and even imaging contrast enhancement of the mucosal surfaces.
Materials and methods
Materials
Egg phosphatidylcholine of 99% purity was purchased from Lipoid GmbH (Ludwigshafen, Germany), and cholesterol and stearylamine of 97% and 99% purity, respectively, were purchased from Sigma-Aldrich Co Ltd (St Louis, MO). Gadobenate dimeglumine injection (MultiHance®) was obtained from Shanghai Bracco Sine Pharmaceutical Corporation Ltd (Shanghai, China). The other reagents used in this study were of analytical grade and purchased from Shanghai Chemical Reagent Co Ltd (Shanghai, China).
Preparation of lipid-stabilized contrast formulation
The lipid-stabilized contrast agent was prepared by lyophilization-rehydration. Egg phosphatidylcholine, sterylamine, and cholesterol in different ratios were dissolved in tert-butanol. MultiHance injection was slowly added to the lipid solution and mixed well. The resulting transparent solutions were dispersed in vials and lyophilized (0.100 mbar, 36–48 hours). The lipid-stabilized contrast formulations were obtained by rehydration of the lyophilized powder with water for injection in 50°C to a concentration of 12 mg Gd/mL, and homogenized in an ultrasonic water bath.
Measurement of particle size and zeta potential
The particle size and zeta potential of the gadobenate dimeglumine encapsulated/complexed liposomes were measured by photon correlation spectroscopy (Zetasizer 3000HSa, Malvern, Worcestershire, UK) at 25°C. The samples were diluted with deionized water. The measurements were carried out three times and the results were averaged.
Nebulization of lipid-stabilized contrast formulations
The lipid-stabilized contrast formulations in 30 mL volume were put into an ultrasonic nebulizer (YUYUE402, Shanghai Medical Instrument Company, Shanghai, China) and nebulized using the following parameters: frequency 1.5 mHz, water bath temperature 50°C, nebulization volume 4 mL/min, and medium airstream flow.
Measurement of droplet size of nebulized contrast formulations
The droplet sizes of the nebulized lipid-stabilized contrast formulations were measured using a Spraytec RTSizer (Malvern) at room temperature in auto mode. The measurement parameters were: path length 12.00 mm, scattering rings 3–31, size bins 0–59, and mesh factor 1, with multiple scattering enabled. Diluted MultiHance injection in the same concentration (12 mg Gd/mL) was used as the control.
Preparation of explanted pig larynx and trachea segments
Freshly explanted pig tracheas with an intact larynx were ordered from a slaughter house. The blood on the segments was carefully cleaned with cotton balls and the tracheas were cut to be around 30 cm in length.
Three diseased tissue models with representative mucosal abnormalities were prepared. The intraluminal protrusion model was prepared by implanting three lean pork balls with diameters of 5 mm, 10 mm, and 15 mm inside the tracheal lumen. The submucosal nodule model was prepared by injection of 1% methyl cellulose under the tracheal mucosal surface to form “lumps” of about 3 mm, 5 mm, and 8 mm in diameter, respectively. For preparation of the submucosal crater lesion model, a small area of tracheal tissue was resected to about 1–2 mm in depth, and then the tracheal tissue was sewn up.
Characterization of contrast agent deposition on luminal surface
The lipid-stabilized contrast formulations were nebulized and delivered to the horizontally placed ex vivo pig tracheal segments from the direction of the larynx. The tracheas were stretched softly during delivery. For each trachea, nebulization delivery lasted for two minutes. Diluted MultiHance injection in the same concentration (12 mg Gd/mL) was nebulized for the comparison.
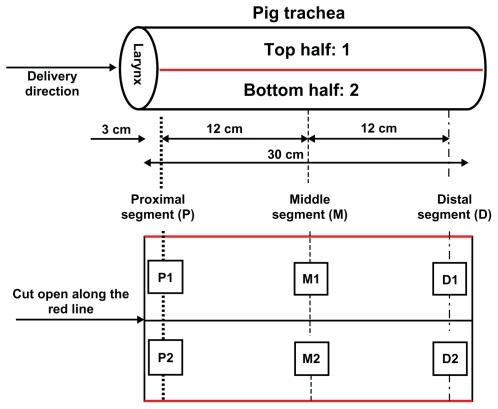
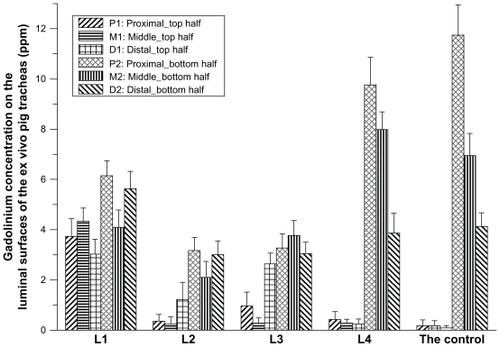
After nebulization delivery, the pig tracheal segments were cut open horizontally and divided into top half and bottom half segments (). Each half segment was further divided into three areas, based on their distances to the larynx, ie, the proximal area (3 cm to the larynx), the middle area (15 cm to the larynx), and the distal area (27 cm to the larynx). For each area, a 1 cm2 trachea sample in a square shape was collected. The samples were homogenized and treated with nitric acid. Gadolinium concentrations in each tracheal sample were measured using inductively coupled plasma atomic emission spectroscopy (IRIS/AP, Thermo Fisher Scientific, Waltham, MA).
Magnetic resonance imaging of larynx and trachea
The nebulized lipid-stabilized contrast formulation was delivered to the ex vivo pig tracheal segments using the same method as described earlier. The tracheal samples were numbered and enwrapped inside fresh lean meat to eliminate the interference of air on imaging. MRI examination of the tracheas before and after contrast agent delivery was performed on a Philips Intera Achieva 3.0 Tesla MR scanner (Philips, The Netherlands) with a SENSE head loop using a T1-weighted spectral presaturation inversion recovery sequence.
Statistical analysis
The data were presented as the mean ± standard deviation. Statistical analysis was performed using the Student’s t-test, and differences were judged to be significant at P < 0.05.
Results
Preparation and characterization of lipid-stabilized contrast formulation
We determined in preliminary studies that gadolinium concentrations in the range of 6–12 mg/mL were optimal for contrast enhancement on mucosal surfaces. Based on this concentration, four different lipid formulations were designed () and prepared by the lyophilization-rehydration method. After rehydration, while some of the gadobenate dimeglumine molecules could be encapsulated, some could be simply adsorbed onto the liposome surfaces due to the excessive amount of negative ions of gadobenic acid. The resulting liposomes had mean particle sizes of around 200 nm. The zeta potentials ranged from −39 to −64 mV.
Table 1 Particle sizes and zeta potentials of the lipid-stabilized contrast agents
Nebulization of lipid-stabilized contrast formulation
The liposomal contrast solution was nebulized by ultrasonication. The sizes of the resulting droplets were measured using a Spraytec RTSizer based on the laser diffraction method. As shown in , for all the five groups, droplets with a volume mean diameter D (4,3) of around 5–7 μm suitable for upper airway deposition were generated via ultrasonic nebulization. However, the size distribution was affected considerably by the lipid components. The lipid-stabilized droplets had much smaller Dv10 and Dv90 diameters than that of the control (), and concentrated in only one peak in the cumulative volume percent curve except for L4 (). The results suggest that the lipid components were helpful for generation and stabilization of small and uniform droplets during nebulization. In addition, increasing the ratio of sterylamine in the lipid formulation seemed to have a further stabilization effect for the smaller droplets (). More droplets smaller than 2.15 μm were observed for formulation L1 (33% sterylamine) compared with formulation L2 (20% sterylamine). Formulation L3 with 50% sterylamine also produced more smaller droplets after nebulization considering that the total lipids (25 mg/mL) were less in the formulation. In comparison, the L4 formulation free of sterylamine produced larger droplets after ultrasonic nebulization and two different size peaks were detected. For the control, the droplets were generally bigger, without the stabilization effects of the lipids.
Figure 2 Average diameter (A), diameter distribution (B), and percentage of small droplets (C) of lipid-stabilized droplets and the control after ultrasonic nebulization. In B, the blue columns represent the volume frequency percentage and the red curve represents the cumulative volume percentage; in the cumulative volume overlay graph, pink indicates L1, black indicates L2, green indicates L3, blue indicates L4 and red indicates the control.
Deposition of nebulized contrast formulations on lumen surface of pig trachea
Deposition of the nebulized contrast formulations on the mucosal surface of the larynx and trachea was studied by measuring gadolinium concentrations at different sampling sites using inductively coupled plasma atomic emission spectroscopy. As shown in , the addition of sterylamine to the formulation greatly improved the deposition of gadolinium at the top luminal surfaces. Gadolinium concentrations from L1, L2, and L3 reached on the top half of the trachea lumen were significantly higher than that from L4 and the control. At the same time, more lipid-stabilized droplets successfully distributed to the distal segments. As a comparison, the droplets of the control and L4 formulations accumulated mainly in the bottom parts of the tracheas in the proximal segment just adjacent to the larynx, and gadolinium concentrations decreased significantly with distance. Of the four lipid-stabilized contrast agents, the L1 formulation showed the most even deposition on the tracheal mucosal surface. It was therefore used in the MRI study.
MRI study of larynx and tracheal lumen surface
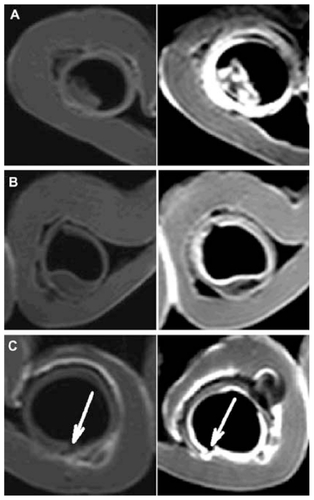
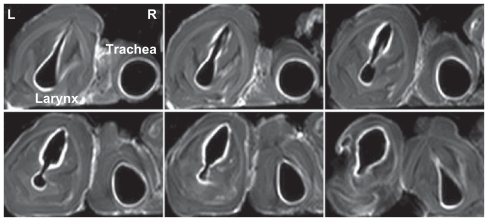
The lipid-stabilized gadobenate dimeglumine formulation, L1, was nebulized and delivered to the freshly explanted pig trachea segments. The mucosal surface visualization effect was evaluated. In imaging sequence screening, the T1 weighted-spectral presaturation inversion recovery sequence showed the best visualization effect and was used in the MRI studies. As shown in , even contrast enhancement on the laryngeal and tracheal mucosal surface was achieved with the lipid-stabilized contrast formulation. The mucosal surfaces were effectively “lightened up” with an even coating of gadolinium and were very clearly visualized. The bright signals along the lumen surface in both the larynx and trachea was generally uniform, as shown in the transverse images of different layers in successive scanning (). In comparison, only parts of the larynx and tracheas (especially the areas in the bottom half of the lumens) were effectively contrasted with the control ().
Figure 4 Transverse magnetic resonance images of ex vivo pig laryngeal (A1 and B1) and tracheal (A2 and B2) segments before and after nebulization delivery of lipid-stabilized contrast agent and the control. Left images, before nebulization; right images, after nebulization.
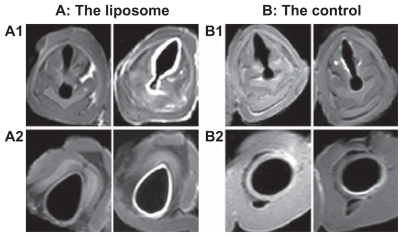
Figure 5 Transverse magnetic resonance images of ex vivo pig laryngeal and tracheal lumen in successive scanning layers after nebulization delivery of the lipid-stabilized contrast agent. In each image, the left is the larynx, and the right is the tracheal lumen.
The mucosal surface visualization effect of the lipid-stabilized contrast formulation was further evaluated in the three diseased tissue models (). With the nebulized lipid-stabilized contrast formulation, the small intraluminal protrusion and the submucosal nodule were clearly depicted with bright signals. In , one small crater (as pointed out by the arrow) on the luminal surface was also clearly visualized.
Discussion
MRI has been proven to be very useful in the diagnosis of laryngeal and tracheal tumors because of excellent soft tissue contrast and high sensitivity to cartilaginous invasion. However, due to the very low proton density and large air-soft tissue interfaces in the airway, visualization of the mucosal surfaces by MRI is not satisfactory. Small foci or alternations on the mucosal surface are reported to be hard to detect or invisible.Citation2 Because most malignant laryngeal and tracheal tumors occur in the epithelium with alterations on the mucosal surface, we tried to improve the sensitivity of MRI for early detection of mucosal lesions by direct clear visualization of the mucosal surfaces. In order to enhance the signals on the mucosal surface, we designed a lipid-stabilized formulation for a gadolinium contrast agent. The nebulized contrast formulation could coat evenly on the mucosal surface, and light up the surface for MRI. Some earlier studies have used aerosolized gadolinium solutions to evaluate pulmonary ventilation,Citation9,Citation20–Citation22 but there has been no report on mucosal coating contrast agents specifically designed to visualize the laryngeal and tracheal surfaces for MRI.
In order to achieve an even coating effect on the laryngeal and tracheal mucosal surfaces for imaging, we selected lipids as the stabilization and mucoadhesion agent. Lipids and liposomes have been widely used for pulmonary delivery of therapeutic proteins or chemical compounds.Citation23–Citation25 In recent years, cationic liposome systems were developed for improved gene delivery to airway mucosal surfaces.Citation18,Citation26,Citation27 Compared with other materials, such as polymers, lipids have good safety and biocompatibility. Therefore, we used egg phosphatidylcholine as the main lipid component for formation of fluid phase membrane vesicles, and cholesterol in high molar ratios ranging from 25% to 50% was included.
We included sterylamine in the formulations to improve the mucoadhesive effect of the droplets. The mucosal surface is covered by a thin layer of mucus, with major components of mucin glycoproteins, lipids, inorganic salts, and water.Citation28 Because most mucin glycoproteins have a strongly negatively charged surface due to a high sialic acid and sulfate content,Citation29 various positively charged materials could be used to enhance the electric interactions with the mucosal surfaceCitation28 and help the vehicles to adhere to the mucosal surface effectively. In our study, sterylamine was positively charged at neutral pH, and its single fatty acid chain could easily insert into the phospholipid bilayers.
The gadolinium agent was loaded into the lipid vehicles using the lyophilization-rehydration method, and particles with a mean diameter of about 200 nm were generated. Interestingly, the particles were negatively charged, with zeta potentials ranging from −39 mV to −64 mV. We believe this to be because of the excessive negative charges in the gadobenate dimeglumine molecules adsorbed on the vehicle surfaces. As a rather toxic ion, gadolinium in free form (Gd3+) must be chelated to form a stable complex for in vivo imaging applications. For gadobenate dimeglumine, the gadolinium ion is chelated with 4-carboxy-5,8,11-tris(carboxymethyl)- 1-phenyl-2-oxa-5,8,11-triazatridecan-13-oic acid to form gadobenic acid [Gd(bopta)(H2O)]2−.Citation30 The two extra negative charges on each gadobenic acid molecule may interact with the positively charged amine of sterylamine and create a negatively charged surface. Therefore, the particles had negative zeta potentials.
In addition to mucoadhesive properties, the droplets should also be of a suitable size range for effective deposition on the laryngeal and tracheal mucosal surface. Deposition of nebulized droplets in the respiratory tract was significantly affected by their sizes. Droplets larger than 5 μm would impact on the larynx and pharynx after inhalation. Droplets with diameters of 0.5–5 μm would sediment in the airway, and droplets smaller than 0.5 μm would be in Brownian motion and settle very slowly in the airway lumen.Citation19 In our study, we characterized the sizes of nebulized droplets stabilized with various lipid formulations using the laser diffraction method. This method has been used in aerosol formulation studies and proven to be a very fast and highly reliable technique. Citation31 Compared with the more commonly used cascade impactor analysis, the size distributions measured by the laser diffraction method are independent of the flow rate and could be automatically recorded as a function of inhalation time.Citation31 As shown in , the mean volume diameter of the droplets was around 4–6 μm. As expected, the amphiphilic lipid components effectively helped to generate droplets in a narrow size distribution.
Deposition of the nebulized droplets on the mucosal surface of the trachea was studied by measuring gadolinium concentrations at different sites in the lumen. In order to evaluate the mucoadhesive effect of the droplets, we positioned the pig tracheal segments horizontally during nebulization delivery, then cut them open vertically to compare deposits on the top and bottom segments. The delivery time was set to be two minutes to ensure effective coating without droplet coagulation or flowing on the surface. As shown in , in all the groups, gadolinium concentrations in the bottom half of the mucosal surface of the trachea were always higher than those at the top half, in part due to the gravity effect. However, the three lipid formulations containing sterylamine (L1, L2, and L3) effectively improved adsorption of droplets to the top half of the mucosal surface. According to Smart,Citation28 the mucoadhesion process would include two steps, ie, contact and consolidation. The droplets or particles would firstly come into intimate contact with the mucosal surface, and then a strengthened adhesive joint would form through various physicochemical interactions, leading to a prolonged adhesion effect. On this basis, we believe that the improved deposition profiles of the three lipid-stabilized droplets may be attributable to the amphiphilic properties of the lipids and the positive surface charges on the cationic lipid, sterylamine. The presence of amphiphilic lipids would improve contact of the droplets with the mucosal surface by decreasing the surface energy of the droplets. The adhesive process was further consolidated by the charge interactions between the positively charged sterylamine and negatively charged mucosal surface.
We also believe that the smaller droplets in the three formulations helped their mucoadhesion. As shown in , the addition of sterylamine resulted in smaller droplets with a diameter of less than 2.15 μm after nebulization. The smaller droplets with large surface area to volume ratios and higher attractive forcesCitation28 would move in the lumen with the direction of the airstream and adhere to the mucosal surface whenever the interactions were strong enough. We noticed that in the proximal parts of the lumen where the gas flow changed dramatically due to the cross-sectional diameter moving from the larynx to the trachea,Citation32 the smaller particles seemed to have more chance to interact with the mucosal surface, and better deposition was observed for L2 and L3. For the same reason, in the distal part at the end of the tracheal segments, better deposition by L2 and L3 was also achieved. Therefore, we think the improved deposition effect of L1 was not merely due to the presence of sterylamine in the formulation. The lipid ratios and total amounts of lipid also played an important role in the generation, stabilization, and mucoadhesion of the droplets via nebulization.
For the formulations free of sterylamine, the droplets mainly deposited in the bottom half of the mucosal surfaces of the trachea, and gadolinium concentrations were much higher on the proximal surface. This is probably because of the larger droplets in these two groups. The medium-sized droplets were more likely to deposit onto the middle and distal areas along the tracheal lumen. It should be noticed that the amount of gadolinium delivered with the L4 formulation and the control was much higher than that with the other three lipid groups. This could be explained by the higher nebulization efficiency of L4 and the control due to their relatively low viscosities in the five groups. There were more droplets obtained for the group with low viscosity (the droplet volume concentrations for L1, L4, and the control were 7.4, 25.3, and 77.0 ppm, respectively).
In the MRI study, the mucosal surface of freshly explanted pig laryngeal and tracheal segments was very clearly visualized using the lipid-stabilized contrast agent. The mucosal surface was outlined smoothly and evenly with bright signals. Considering the complex anatomic structures in the larynx and the complicated gas flows in the upper airway, the even coating and contrast enhancement with the nebulized agent was a significant improvement. We further evaluated the visualization effects for three common airway lesions. The shapes and locations of all the representative tissue abnormalities were successfully depicted. These preliminary results suggest that a lipid-stabilized contrast agent would be beneficial for improvement of MRI in early diagnosis of diseases of the larynx and trachea.
Conclusion
In this study, a liposomal gadobenate dimeglumine contrast formulation was successfully developed for clear MRI visualization of the mucosal surface on the larynx and trachea via nebulization. Various cationic liposome formulations were compared for their stabilization effects on gadobenate dimeglumine-containing droplets. Liposomes composed of egg phosphotidylcholine, cholesterol, and stearylamine in a molar ratio of 1:1:1 were found to have the most efficient nebulization effect, and had the most narrowly distributed droplet sizes. The nebulized droplets were of mean diameter 4–6 μm after ultrasonic nebulization. They evenly coated on the laryngeal and tracheal lumen surfaces and produced significant contrast enhancement along the mucosal surface. Such contrast enhancement enabled clearer visualization of several disease states, such as intraluminal protrusions, submucosal nodules, and craters in ex vivo pig laryngeal and tracheal segments. The liposomal MRI contrast formulation may be useful for improving visualization of the mucosal surface and early diagnosis of diseases originating from the mucosal surfaces of the larynx and trachea.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors are grateful to Shi Xu from the Analytical Center of Shanghai Jiao Tong University for performing the inductively coupled plasma atomic emission spectroscopy measurements, and to Tom Qin of Malvern, China, for his assistance with measurement of nebulized droplet sizes.
References
- BeckerMBurkhardtKDulguerovPAllalAImaging of the larynx and hypopharynxEur J Radiol200866346047918495402
- HermansRStaging of laryngeal and hypopharyngeal cancer: value of imaging studiesEur Radiol200616112386240016733680
- MathisenDPrimary tracheal tumor managementSurg Oncol Clin N Am19998230710339648
- ConnorSLaryngeal cancer: how does the radiologist help?Cancer Imaging2007719317535777
- DeliyskiDDHillmanREState of the art laryngeal imaging: research and clinical implicationsCurr Opin Otolaryngol Head Neck Surg201018314715220463479
- KauczorHUKreitnerKFMRI of the pulmonary parenchymaEur Radiol1999991755176410602947
- GastKKEberleBSchmiedeskampJKauczorHUMagnetic resonance imaging using hyperpolarized He-3-gasAcad Radiol200310101119113114587630
- PatzSHersmanFWMuradianIHyperpolarized Xe-129 MRI: a viable functional lung imaging modality?Eur J Radiol200764333534417890035
- HaagePKaraagacSSpuntrupETruongHTSchmidtTGuntherRWFeasibility of pulmonary ventilation visualization with aerosolized magnetic resonance contrast mediaInvest Radiol2005402858815654252
- VyasSPPawarDGoyalAKEvaluation of mucoadhesive PLGA microparticles for nasal immunizationAAPS J201012213013720077052
- PongjanyakulTSuksriHAlginate-magnesium aluminum silicate films for buccal delivery of nicotineColloids Surf B Biointerfaces200974110311319643587
- ErjavecVPavlicaZSentjurcMPetelinMIn vivo study of liposomes as drug carriers to oral mucosa using EPR oximetryInt J Pharm200630711816257157
- MajumdarDKThakralNKRayARBar-ShalomDErikssonAHThe quest for targeted delivery in colon cancer: mucoadhesive valdecoxib microspheresInt J Nanomed2011610571068
- AlonsoMJOyarzun-AmpueroFABreaJLozaMITorresDChitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthmaInt J Pharm2009381212212919467809
- AntimisiarisSGZaruMMancaMLFaddaAMChitosan-coated liposomes for delivery to lungs by nebulisationColloids Surf B Biointerfaces2009711889519201583
- MortazaviSAMehravaranNMoghimiHThe influence of various mucoadhesive polymers on in vitro performance of the resulting artificial saliva pump spray formulationsIran J Pharm Res200981313
- GattaniSGSavaliyaPJBelgamwarVSFloating-mucoadhesive beads of clarithromycin for the treatment of Helicobacter pylori infectionChem Pharm Bull (Tokyo)201058678278720522987
- PillaiRPetrakKBlezingerPUltrasonic nebulization of cationic lipid-based gene delivery systems for airway administrationPharm Res19981511174317479833997
- AmighiKPilcerGFormulation strategy and use of excipients in pulmonary drug deliveryInt J Pharm20103921–211920223286
- MisselwitzBMuhlerAHeinzelmannIBockJCWeinmannHJMagnetic resonance imaging of pulmonary ventilation – initial experiences with a gadolinium-DTPA-based aerosolInvest Radiol199732127978019406020
- HaagePAdamGKaraagacSMechanical delivery of aerosolized gadolinium-DTPA for pulmonary ventilation assessment in MR imagingInvest Radiol200136424024311283422
- HaagePKaraagacSSpuntrupEAdamGGuntherRWMR imaging of lung ventilation with aerosolized gadolinium-chelatesRofo20031752187193 German12584617
- AntimisiarisSGZaruMMourtasSKlepetsanisPFaddaAMLiposomes for drug delivery to the lungs by nebulizationEur J Pharm Biopharm200767365566617540552
- HuangYYWangCHPulmonary delivery of insulin by liposomal carriersJ Control Release2006113191416730838
- LuDMHickeyAJLiposomal dry powders as aerosols for pulmonary delivery of proteinsAAPS Pharm Sci Tech200564E641648
- KedarEEven-OrOJosephAA new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticityVaccine201129132474248621251901
- LiuDZTsengLPChiouCJEvaluation of encapsulated newcastle disease virus liposomes using various phospholipids administered to improve chicken humoral immunityJ Biomed Mater Res B200991B2621625
- SmartJDThe basics and underlying mechanisms of mucoadhesionAdv Drug Deliv Rev200557111556156816198441
- HanesJLaiSKWangYYWirtzDMicro- and macrorheology of mucusAdv Drug Deliv Rev20096128610019166889
- WernerEJDattaAJocherCJRaymondKNHigh relaxivity MRI contrast agents: where coordination chemistry meets medical imagingAngew Chem Int Ed2008474585688580
- de BoerAHGjaltemaDHagedoornPFrijlinkHWCharacterization of inhalation aerosols: a critical evaluation of cascade impactor analysis and laser diffraction techniqueInt J Pharm20022491–221923112433450
- EhtezaziTSouthernKWAllansonDJenkinsonIO’CallaghanCSuitability of the upper airway models obtained from MRI studies in simulating drug lung deposition from inhalersPharm Res200522116617015771244