 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this work was to evaluate how an aqueous micellar system containing Amphotericin B (AmB) and sodium deoxycholate (DOC) can be rebuilt after heating treatment. Also, a review of the literature on the physicochemical and biological properties of this new system was conducted. Heated (AmB-DOC-H) and unheated (AmB-DOC) micelles were then diluted at four different concentrations (50 mg · L−1, 5 mg · L−1, 0.5 mg · L−1, and 0.05 mg · L−1) to perform physicochemical studies and a pharmacotoxicity assay, in which two cell models were used for the in vitro experiments: red blood cells (RBC) from human donors and Candida parapsilosis (Cp). While potassium (K+) and hemoglobin leakage from RBC were the parameters used to evaluate acute and chronic toxicity, respectively, the efficacy of AmB-DOC and AmB-DOC-H were assessed by K+ leakage and cell survival rate from Cp. The spectral study revealed a slight change in the AmB-DOC aggregate peak from 327 nm to 323 nm, which is the peak for AmB-DOC-H. Although AmB-DOC and AmB-DOC-H exhibited different behavior for hemoglobin leakage, AmB-DOC produced higher leakage than AmB-DOC-H at high concentrations (from 5 mg · L−1). For K+ leakage, both AmB-DOC and AmB-DOC-H showed a similar profile for both cell models, RBC and Cp (P < 0.05). AmB-DOC-H and AmB-DOC also revealed a similar profile of activity against Cp with an equivalent survival rate. In short, AmB-DOC-H showed much less toxicity than AmB-DOC, but remained as active as AmB-DOC against fungal cells. The results highlight the importance of this new procedure as a simple, inexpensive, and safe way to produce a new kind of micelle system for the treatment of systemic fungal infections.
Introduction
Amphotericin B (AmB) is a water-insoluble compound widely used to effectively treat systemic fungal infections.Citation1 However, its utilization as an antifungal agent has been limited by high acute and chronic toxicity, characterized by chills, fever, nausea, vomiting, and nephrotoxicity.Citation2–Citation4
Due to the amphiphilic nature of the molecule and its poor solubility in water, AmB is able to self-associate in aqueous media and form supramolecular aggregates. However, in aqueous media, monomers and soluble and insoluble aggregates of AmB coexist, the latter being responsible for the toxic effects.Citation5–Citation8 When AmB in solution is below the minimal concentration for self-association, toxicity against fungi is still observed, while the human toxic effect decreases.Citation2,Citation3
In the current model of AmB selective toxicity, soluble monomeric AmB is able to form ion channels in ergosterol-rich fungal membranes, but a soluble self-associated oligomer damages sterol-free and cholesterol-containing membranes.Citation9,Citation10 Also, the oligomers seem to be susceptible to oxidation, which may enhance their toxicity.Citation11 Therefore, the way in which AmB is cleared from the body plays an important role in its toxicity and activity. The widely-accepted mechanism of action of AmB described above is true for micelle systems; the AmB molecules bind to a surfactant with a high degree of freedom and can easily leave the surfactant molecule and self-associate.
The micellar deoxycholate system of AmB (AmB-DOC), a well-known commercial antifungal product (Fungizon®), has been used in clinical practice for over 45 years for the treatment of several systemic fungal infections.Citation2,Citation12 Unfortunately, AmB-DOC shows a concentration-dependent profile of toxicity and its selectivity depends on its aggregation state. Clinically, nephrotoxicity is frequently observed.Citation13
In an attempt to reduce the AmB-DOC toxicity, some lipid formulations such as liposomes and other AmB lipid-associated forms were developed.Citation14–Citation18 These systems are able to immobilize the AmB monomers, preventing self-aggregation. They slowly release monomers to the media, and, therefore, are active only against fungal cells. Although these formulations exhibit a far superior therapeutic index compared to AmB-DOC, they are costly and their clinical use is inaccessible for many countries where fungal-related diseases are rampant. In fact, only AmB-DOC is widely used in some developing countries, and after the 1990s, when Fungizon® was no longer patent-protected, this product started to be manufactured for several brands as a generic product.
The literature indicates that, when subjected to controlled heat, AmB-DOC forms a new type of aggregate, which is less susceptible to oxidative degradation and less selective for the cholesterol in the mammalian cell membrane, thus making it less toxic.Citation19–Citation25 The heating of AmB-DOC solutions generates “super-aggregates,” in which the toxicity is significantly reduced while keeping their antifungal activity. However, a recent study conducted in our research group revealed that AmB micellar systems made by different companies present different profiles of activity and toxicity, probably due to the variation in the formation of the structure of the drug-surfactant micelle system as a result of changes in the manufacturing process or because of the quality of the raw materials used (our unpublished results).
The aim of this work was to evaluate the relationship between controlled heat treatment, the absorption spectra, and the pharmacotoxicity of a Brazilian brand of AmB-DOC in aqueous micellar solutions before and after heat treatment (AmB-DOC-H). In this way, a new process to rebuild a pre- formed micelle system can be achieved.
Materials and methods
Preparation of the AmB-DOC and AmB-DOC-H samples
The stock solution of AmB-DOC (Cristália, Itapira, Brazil) at 5 × 10−3 M (5,000 mg · L−1) was prepared by adding 10 mL of water for injection into the vial and submitting it to vortex shaking until complete dissolution of the lyophilized powder. Each vial of AmB-DOC contained 50 mg of AmB, approximately 41 mg of sodium deoxycholate, and phosphate buffer, pH 7.4.
Samples of AmB-DOC were prepared with different concentrations by successive dilutions 1:10 of AmB-DOC stock solution in order to obtain the concentrations of 5 × 10−5 M (50 mg · L−1), 5 × 10−6 M (5 mg · L−1), 5 × 10−7 M (0.5 mg · L−1), and 5 × 10−8 M (0.05 mg · L−1). The heated AmB (AmB-DOC-H) was prepared by treatment with controlled heat of the AmB-DOC stock solution at 70°C for 20 minutes in a thermostatic bath without stirring. The temperature and the time exposition of the bath were checked by a built-in thermometer (Incoterm, Brazil) and chronometer (Model Labor, Hanhart, Germany), respectively. Then, following the same procedure as that for AmB-DOC, dilutions were prepared to perform a similar study of concentration samples.
Spectral study
Scanning spectra of both AmB-DOC and AmB-DOC-H at the four previously mentioned concentrations were taken by using a UV-VIS Spectrophotometer (Biochrom Libra S32, Cambridge, UK). The optical path of the quartz cuvettes used was 0.1 cm for the concentration of 5 × 10−5 M (50 mg · L−1); a 1 cm path for 5 × 10−6 M (5 mg · L−1), and a 10 cm path for the concentrations of 5 × 10−7 M (0.5 mg · L−1) and 5 × 10−8 M (0.05 mg · L−1). These paths were chosen to obtain spectra with absorbance values less than 0.8. Their molar extinction coefficients (ɛ) were calculated using the Beer–Lambert equation. All spectra were recorded at 25°C ± 0.1°C with a 300–450 nm range.Citation6
Preparation of red blood cell (RBC) suspension
This study was previously approved by the Ethical Research Committee of the Federal University of Rio Grande do Norte, protocol number 002/2009. In order to minimize sources of variability, one healthy female adult donor, who gave her written informed consent before participating in the study, provided all normal human RBCs for the in vitro experiments. Five milliliters of venous blood was collected in sterile EDTA (1 mg/mL, ethylene-diamine-tetraacetate at 10% (w/v), Labtest, Lagoa Santa, Brazil) syringes and promptly centrifuged (Refrigerated centrifuge, ALC, Model PK121R, Milan, Italy) in tubes at 1,100 g for 10 minutes at 4°C. Plasma was carefully aspirated and the exposed buffy coat was removed and discarded. The RBCs were washed three times by centrifugation (1,100 g for 5 minutes at 4°C) and suspended in 5 volumes of normal saline [NaCl at 0.9% (w/v), B. Braun, São Paulo, Brazil]. They were then resuspended in 4 mL of saline, counted in a Neubauer™ chamber (Splabor, São Paulo, Brazil), and resuspended again until the desired concentration (5 × 107 cells · mL−1) was achieved. The cells were used on the day of collection.Citation17
Preparation of Candida parapsilosis suspension
A strain of Candida parapsilosis (Cp) ATCC (22019) was maintained on Sabouraud- Dextrose-Chloramphenicol agar (SDC, MicroMed, São Paulo, Brazil) at room temperature and passaged monthly. Before experiments, an inoculum from the culture was transferred to a SDC agar scope and incubated at 37°C for 16–18 hours. The fungal cells were then washed three times with normal saline, resuspended, counted in the central reticule of a Neubauer™ chamber, and resuspended again to obtain the desired cell concentration (5 × 107 cfu · mL−1).Citation17
Evaluation of the AmB-DOC and AmB-DOC-H toxicity
Because the AmB molecule binds to human cholesterol of the plasmatic membranes and also to ergosterol in fungal membranes forming hydrophilic poresCitation1, analysis of the toxicity and efficacy of the AmB-DOC and AmB-DOC-H formulations was performed using two cell models, RBCs and Cp. The potassium ions (K+) inside the cells can be released by any disturbance in the cell membrane due to the pore formation process. In RBCs the generated pore enables the output of K+, but not the release of hemoglobin, which occurs only after the total destruction of the membrane barrier. The phenomenon of K+ release from model cells has been referred to as a chronic toxicity by some authors.Citation6,Citation17 On the other hand, a strong toxicity level, which induces hemoglobin leakage, for RBCs, or cell death for Cp, has been classified as acute toxicity.Citation6,Citation17
Four milliliter samples of RBCs (5 × 107 cells · mL−1) were incubated for 1 hour at 37°C with the vehicle control or with different concentrations (50, 5, 0.5, and 0.05 mg · L−1) of both AmB-DOC and AmB-DOC-H. The RBCs were then centrifuged for 5 minutes at 1,100 g and washed three times with normal saline. The pellet of RBC was lysed by adding 4 mL of distilled water and then stirred and centrifuged (1,100 g for 10 minutes) in order to remove membranes. The K+ content of the supernatant was determined using a Flame Photometer 7000 (910M Analyser, São Paulo, Brazil) calibrated with K+ reference at 5 mEq · L−1. Hemoglobin was estimated from its absorption at 540 nm recorded on a UV-VIS Spectrophotometer (Biochrom Libra S32). The total K+ and hemoglobin content was measured for the control RBC tubes. Release was calculated as the difference between control and treated cells and expressed as a percentage of the total hemoglobin or K+ content. At least three different experiments were performed with each formulation and each experimental point was performed in triplicate.Citation26
Evaluation of the AmB-DOC and AmB-DOC-H efficacy
Two milliliter samples of fungal suspension containing 5 × 107 cfu · mL−1 were incubated for 1 hour at 37°C with both AmB-DOC and AmB-DOC-H at the concentrations of 50, 5, 0.5, and 0.05 mg · L−1. Cells were centrifuged for 10 minutes at 2,200 g and washed three times in normal saline, and 2 mL of purified water was added to the pellet of fungal cells. An aliquot of this pellet was lysed by heating for 5 minutes at 100°C and centrifuged to remove membranes, and free K+ was measured. The K+ leakage was calculated similarly to the calculation of the RBCs. For the cell viability evaluation, 100 μL aliquots of appropriate dilution of the fungal pellet were seeded, in duplicate, onto agar plates and incubated at 37°C. The number of colony-forming units (CFU) was counted at 24 and 48 hours and expressed as a percentage of those obtained from a control inoculum incubated without AmB-DOC or AmB-DOC-H. Three different experiments were performed with each formulation and each experimental point was performed in duplicate.Citation26
Statistical analyses
The statistical results were performed with ANOVA and t test to analyze the variation response in the same group and in different groups, respectively, using Prim 4 for Windows 4.02 (GraphPad Software, San Diego, CA).
Results
Aggregation state behavior of AmB-DOC and AmB-DOC-H
The AmB-DOC-H showed spectra similar to that of AmB-DOC, which were concentration-dependent ( and ). At a low concentration, such as 5.10−8 M, the AmB presented a spectrum with three well-defined absorption bands, with λmax at 363, 385, and 408 nm, and a shoulder around 347 nm, which is similar to that obtained with organic solvents such as methanol, for the monomeric AmB. However, by increasing the concentration of AmB, a new band appeared at λmax 327 nm at the expense of the bands recorded in the longer wavelengths (363, 385, and 408 nm), especially the one characteristic of the monomeric AmB species at 408 nm, which is responsible for the biological activity against fungal cells Citation10. The band at 327 nm was reported in the literature as indicative of the presence of self-associated species of AmB. This band was also sensitive to AmB concentration, being very well defined at high concentrations as in the range of 10 × 10−7 to 10−5 M.Citation10
Figure 1 Concentration-induced changes in the AmB-DOC spectra at 25°C at 5 × 10−5 M.
Abbreviation: AmB-DOC, amphotericin B with sodium deoxycholate.
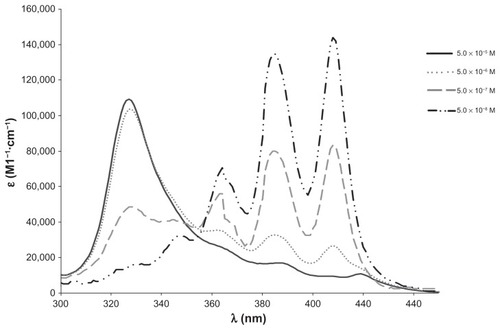
Figure 2 Concentration-induced changes in the AmB-DOC-H spectra at 25°C at 5 × 10−5 M.
Abbreviation: AmB-DOC, amphotericin B with sodium deoxycholate.
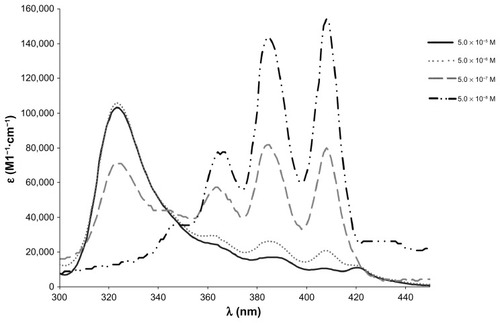
Concerning the AmB-DOC-H spectra, the band at 327 nm was slightly blue-shifted to 323 nm after heating. Several authors have stated that this band is characteristic of the formation of AmB super-aggregates.Citation20 It is important to note that the variation in the molar extinction coefficient of AmB at λmax 323 nm and the peaks at 363, 385, and 408 nm are related to variation in the equilibrium between the monomeric and aggregated species of AmB, which displays an isosbestic point at 347 nm. See Equationscheme(scheme 1) .
where the underlined letters m and sa refer to the monomeric and self-associated AmB, respectively, while K is the equilibrium constant between the AmB species.
This equilibrium shows that at low concentrations the monomeric species of AmBm prevails, but when the concentration is increased the equilibrium is shifted favoring the predominance of the self-aggregated AmBsa.
The most important difference among the AmB-DOC and AmB-DOC-H spectra occurred at the concentration of 5 × 10−7 M, in which the band at 323 nm presented a higher molar extinction coefficient (ɛ = 71,000) compared to the one assigned for AmB-DOC (ɛ = 48,666). It should be emphasized that the spectra at 5 × 10−8 M were given to illustrate the tendency of concentration dependence. In fact, they should not be considered on a quantitative basis because of the weakness of the signals at the low wavelength region (maximum absorbance at 408 nm was 0.071 for AmB-DOC).
The data for and show that the recorded spectral differences can be attributed to the different interactions of AmB with the surfactant aggregates provoked by controlled heating, and such changes remained over the whole range of concentrations. Therefore, the heated micelle process was able to produce AmB super-aggregated micelle systems that remained stable after the dilution process. Also, the physicochemical stability of such super-aggregated forms was demonstrated by the absence of changes on the spectra measured on different days over three months (results not shown).
Three decades ago, Ernst et alCitation28 showed the thermally induced increase in aggregate size of Fungizon® aqueous solution at 10−5 M. This phenomenon was more evident at 60°C and increased rapidly at higher temperatures. In an aqueous solution at 70°C, the apparent mass of the aggregates was 500-fold larger than the one at 20°C.Citation20 Therefore, the term super-aggregate used for this new species of AmB is completely justified.Citation20
In vitro toxicity assay for AmB-DOC and AmB-DOC-H
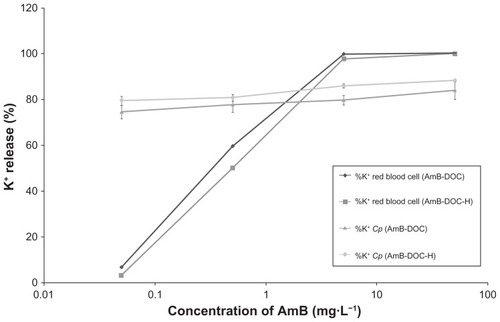
The toxicity of both AmB-DOC and AmB-DOC-H against RBCs was quite interesting. For K+ leakage, both profiles were identical (). No significant K+ leakage was observed at 0.05 mg · L−1 (5 × 10−8 M). At higher concentrations, this completely changes. At 0.5 mg · L−1 (5 × 10−7 M), both products induced strong permeability of the cell membrane as reflected by a K+ release of around 50% (59.59% ± 0.41% and 50.12% ± 0.9% for AmB-DOC and AmB-DOC-H, respectively). At the highest concentrations of 5 mg · L−1 (5 × 10−6 M) and 50 mg · L−1 (5 × 10−5 M), where the aggregate species of AmB predominates, both systems presented a total release of K+.
Figure 3 In vitro release of potassium from human RBCs and Candida parapsilosis induced by AmB-DOC and AmB-DOC-H.
Note: Each point on the figure is the mean (±SD) of three determinations.
Abbreviations: AmB-DOC, amphotericin B with sodium deoxycholate; AmB-DOC-H, amphotericin B with sodium deoxycholate, heated; RBC, red blood cells.
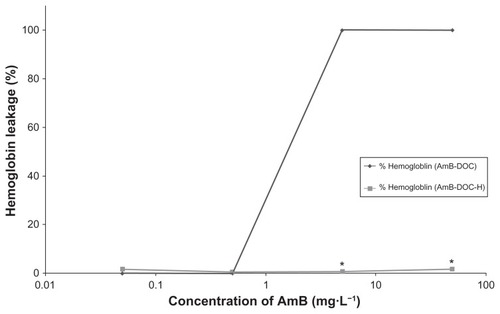
Both AmB-DOC and AmB-DOC-H produced no significant hemoglobin leakage below 0.5 mg · L−1 (). While the AmB-DOC-H showed no toxic effect on the whole range of the tested concentrations (0.05 to 50 mg · L−1, P < 0.001), the AmB-DOC revealed a sharp increase in the toxicity from 0.5 mg · L−1, reaching to fully lysed cells from 5 mg · L−1 with total hemoglobin leakage to external media.
Figure 4 In vitro release of hemoglobin from human RBCs induced by AmB-DOC and AmB-DOC-H.
Notes: Each point on the figure is the mean (±SD) of three determinations. *Significant difference between both products (P < 0.001).
Abbreviations: AmB-DOC, amphotericin B with sodium deoxycholate; AmB-DOC-H, amphotericin B with sodium deoxycholate, heated; RBC, red blood cells.
In vitro activity assay for AmB-DOC and AmB-DOC-H
The in vitro activity against Cp showed that both AmB-DOC and AmB-DOC-H were very effective () and that the profile of K+ release was quite similar (). In fact, a significant K+ leakage was found at the lowest concentration (0.05 mg · L−1), reaching around 85% at the highest concentration. In this assay AmB-DOC-H was slightly more active than AmB-DOC over the whole range of concentration. For example, at 5 mg · L−1 AmB-DOC-H presented a K+ release of 85.92% ± 0.85% while AmB-DOC showed a release of 79.75% ± 1.96%. At 50 mg · L−1 these values were 88.38 ± 0.70% and 83.98 ± 3.86%, respectively. However, such values were not statistically significant.
Figure 5 In vitro antifungal activity of AmB-DOC and AmB-DOC-H on C. parapsilosis.
Notes: Each point on the figure is the mean (±SD) of three determinations. *Significant difference between both products (P < 0.001).
Abbreviations: AmB-DOC, amphotericin B with sodium deoxycholate; AmB-DOC-H, amphotericin B with sodium deoxycholate, heated; CFU, colony-forming unit; RBC, red blood cells.
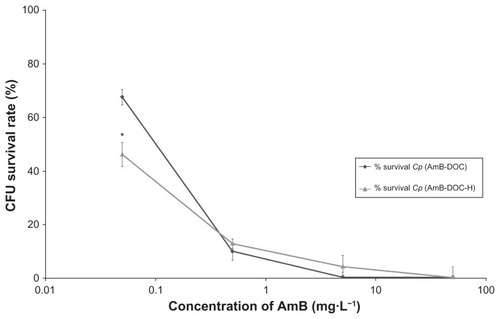
These data are in agreement with the results of the cell survival rate, which indicated for both AmB_DOC and AmB-DOC-H a significant decrease in the rate of survival cell at concentrations up to 0.5 mg · L−1. At this concentration range, the AmB-DOC-H showed higher antifungal activity than the unheated AmB. For example, at the concentration of 0.05 mg · L−1 AmB-DOC-H was able to kill 53.72% ± 5.17% whereas AmB-DOC killed only 32.31% ± 1.40%. Considering the standard deviation, in the range of concentrations from 0.5 mg · L−1 to 50 mg · L−1, the CFU survival rate profile was similar for both preparations. Finally, at 50 mg · L−1 both products were able to kill the totality of the fungal cells.
Discussion
Micelle systems have been used as nanotechnologic carriers for more than 60 years.Citation2 Particularly for AmB, this system has been used since 1957 when Bartner et al studied the incorporation of AmB molecules into a micellar sodium deoxycholate (DOC), in a molar ratio of approximately 1:2 AmB:DOC, respectively.Citation27
The idea that a micellar complex could change its physicochemical property by heating remained completely undiscovered since the first works of Ernst et al.Citation28 Almost twenty years later, Gaboriau et alCitation20 produced a major study about this phenomenon by discovering the super-aggregated form of AmB. This new AmB species not only presents an important change in the absorption and circular dichroism spectra of AmB, which is related to its physicochemical state, but also reveals a biological difference when compared to the unheated micelle.Citation21–Citation25 Therefore, it can be estimated that the industrial production of a micellar system could be strongly influenced by the manufacturing process, which can modify the surfactant-molecule binding and generate different physicochemical forms.
The results of this study show that, unlike the unheated form, the Brazilian micelle system presents a physicochemical profile quite similar to the European one when heated at 70°C for 20 minutes, generating a superaggregated spectroscopic species, which was characterized by a band at 323 nm on its spectrum. Because this band appeared at the expense of the ones at 327 and 408 nm, the presence of the superaggregated form can be suggested to be a result of the condensation of the aggregated and monomer species of the AmB. This explanation is in complete agreement with the study of Gaboriau et al using a European AmB-DOC (Fungizon®).Citation20 Unlike the AmB aggregate species, the dissociation of this super-aggregated form into monomers occurs at lower AmB concentrations.Citation29 Therefore, it can be inferred that the AmB super-aggregated species may be considered a reservoir of monomeric AmB species that releases only a limited amount of monomeric AmB in the aqueous media. As a consequence, the concentration of monomeric AmB might be below its critical aggregation concentration, which is around 10−6 M; thus, the drug can remain in its monomeric form. This form would be able to bind to the ergosterol of fungal cells, but unable to bind to the cholesterol of the RBCs.Citation10
In fact, the variation of patterns of activity against ergosterol-containing fungal cells with respect to toxicity against cholesterol-containing mammalian cells and its dependence on the AmB molecular presentation has been reported by several research groups.Citation1,Citation6,Citation26,Citation30 It is well-established that the monomeric species of AmB is less effective than the aggregated form in inducing permeability of membranes containing cholesterol, leading to potassium leakage.Citation31 The AmB aggregated form was shown to trigger permeability changes in RBC membranes and to induce cytotoxic events.Citation10 Such correlation was also maintained in several in vivo studies.Citation32–Citation34
The present study reveals that the equilibrium between the different species of AmB in aqueous media may change and, therefore, its overall activity or toxicity could be changed. The spectral evaluation of both AmB-DOC and AmB-DOC-H clearly demonstrates that the behavior of AmB molecules was modified. For example, the concentration dependence, characteristic of AmB-DOC, disappeared at the concentration of 5 × 10−7 M. In fact, at this concentration the AmB super-aggregated band, located at 323 nm, was maintained, whereas the proportion of the monomeric form remained low (). This suggests that the AmB molecules were strongly bound to the surfactant molecule and the micelle system had a greater strength than the unheated ones.Citation26 Only at the lowest concentration (5 × 10−8 M) did both products present a similar spectrum in which the monomeric band had the same shape and similar intensity, reflected by almost the same value of ɛ.
The reservoir model hypothesis suggested for the super-aggregated form of AmB was demonstrated by the in vitro studies. When the target cells were human RBCs, AmB-DOC- H was unable to induce hemoglobin leakage over the whole range of doses tested (). This demonstrated that the existing aggregated form at 5 × 10−6 M and 5 × 10−5 M was completely different from the AmB-DOC-H product. On the other hand, the activity of both products was quite similar when the cell model was a membrane containing ergosterol, as in the Candida parapisilosis.
The statistical comparison among the different concentrations of AmB for both AmB-DOC and AmB-DOC-H products by the ANOVA test was significant (P < 0.001), which confirms that the dilution process induced a decrease in the pattern of activity for all samples. Additionally, Student’s t-test showed that the toxicity assay against RBCs, represented by the release of K+ and hemoglobin, was significantly different from that for AmB-DOC-H, which was much smaller than AmB-DOC (P < 0.05).
The probable mechanism behind this pattern of activity could be the high chemical stability of the AmB super-aggregates, which are less susceptible to peroxidative process than the aggregated form and, therefore, present less affinity to membranes containing cholesterol than the unheated preparation.Citation8,Citation22 Also,Citation8,Citation12 this protection against the peroxidation process allows the AmB super-aggregated form to act as a controlled release of the monomeric AmB species, which is able to maintain its antifungal activity.Citation10 Therefore, as suggested in the literature, a complete resculpting of AmB aggregates probably occurs with the heat-inducing process, and this phenomenon induces changes in the AmB distribution and interaction with various serum fractions.Citation29
A mechanistic rationale for the similar efficacy and lower toxicity of AmB-DOC-H, compared to AmB-DOC, as previously proposed, reports that AmB-DOC can be rapidly converted from its aggregated form to a protein-bound monomer in the presence of human serum albumin, whereas AmB-DOC-H demonstrates greater stability by persisting as a stable inactive aggregate.Citation12 Moreover, the increased therapeutic index of the AmB-DOC-H may also be the result of greater phagocytosis of the super-aggregates, owing to their larger size (600 nm), which allows them to be efficiently engulfed by the macrophages and to be transferred to the site of infection in the case of internal cell infections such as leishmaniosis.Citation19 On the other hand, it was also shown that the cytotoxicity may be decreased due to a reduction of the interaction of AmB-DOC-H with the kidney cell membranes.Citation35
The benefits of the pre-heating process for AmB-DOC (Fungizon®) were also studied in persistently leukopenic mice with severe invasive candidiasis, concluding that higher dosages of AmB-DOC-H, (3.0 versus 0.8 mg · L−1 of body weight), were tolerated, compared to AmB-DOC, resulting in significantly improved therapeutic efficacy.Citation36 The cytotoxicity of AmB-DOC-H against pig kidney cells was evaluated by Bartlett et al, who discovered a decrease in the AmB renal cytotoxicity without modifying its antifungal activity.Citation25 In the same way, Bau et alCitation37 showed the benefits of the patent-free heated AmB product for use by public-health authorities or a reactive non-governmental organization for treatment of leishmaniasisCitation23 and other neglected diseases.
Other important studies have been carried out with the AmB-DOC (Fungizon®) heated form. For example, a recent work evaluated the fluorescence of AmB aggregates for the heated and unheated form. The authors concluded that not only the monomer and dimer AmB states but also the aggregate and super-aggregate forms present different spectra.Citation38 Therefore, the fluorescence technique could also be used to characterize the different AmB state forms. Rogers et al studied the cytokine and chemokine response elicited by AmB-DOC-H in comparison to AmB-DOC in the human monocyte cell line THP-1.Citation24 They concluded that AmB-DOC produced dose-dependent increases in interleukin (IL)-1β, IL-1α, tumor necrosis factor-α, macrophage inflammatory protein (MIP)-1α, and MIP-1β and that AmB-DOC-H induced cytokine and chemokine production at a lower level than those observed with the corresponding concentrations of AmB-DOC.Citation24
Conclusion
Heat treatment of Fungizon® was shown to modify the aggregation state of the AmB desoxicholate micellar system, generating an AmB super-aggregate species whose appearance is concomitant with the disappearance of both normal aggregates and monomeric AmB species.
All the results together suggest that the AmB-DOC-H, from a Brazilian industrial company, was less toxic than the unheated form (AmB-DOC) probably due to changes in the AmB aggregation state. It is possible to rebuild micelle systems and generate new entities at a nanoscale level by simple heating. Therefore, we can speculate that this strategy opens the way to creating nanocarriers by changing the manufacturing parameters and process of micelle production.
In short, heat treatment of Fungizon® solutions has been shown to be a simple and inexpensive alternative for treating patients with systemic fungal infections. AmB-DOC-H has been demonstrated to be less toxic to mammalian cells while keeping its activity against fungal cells and protozoan microorganisms. This hypothesis is supported not only by the results presented here, but also by the results found in the literature, which were extensively discussed in this paper.
Acknowledgments
This work was financially supported by the grant numbers 301979/04-9, 473882/04-3, and 47836/01-7-NV from CNPq and also by UFRN-PPG-PIBIC-CNPq-PPGFar. The authors were grateful to Glenn Hawes, from the University of Georgia, American Language Program, for editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BolardJHow do the polyene macrolide antibiotics affect the cellular membrane-propertiesBiochim Biophys Acta19868643–42573043539192
- HartselSBolardJAmphotericin B: new life for an old drugTrends Pharmacol Sci199617124454499014498
- BrajtburgJBolardJCarrier effects on biological activity of amphotericin BClinical Microbiology Reviews1996945125318894350
- HiemenzJWWalshTJLipid formulations of amphotericin B: Recent progress and future directionsClin Infec Dis199622S1331448722841
- AdamsMLKwonGSRelative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block-poly (N-hexyl-L-aspartamide)-acyl conjugate micelles: effects of acyl chain lengthJ Control Release2003871–3233212618020
- EgitoESTAraujoIBDamascenoBPriceJCAmphotericin B/emulsion admixture interactions: an approach concerning the reduction of amphotericin B toxicityJ Pharm Sci200291112354236612379920
- KleinbergMWhat is the current and future status of conventional amphotericin B?Int J Antimicrob Agents200627Suppl 1S1216
- SelvamSMishraAKDisaggregation of amphotericin B by sodium deoxycholate micellar aggregatesJ Photochem Photobiol B2008932667018725181
- BolardJLegrandPHeitzFCybulskaBOne-sided action of amphotericin-B on cholesterol-containing membranes is determined by its self-association in the mediumBiochemistry19913023570757152043613
- LegrandPRomeroEACohenBEBolardJEffects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytesAntimicrob Agents Chemother19923611251825221489196
- Lamy-FreundMTFerreiraVFNFaljonialarioASchreierSEffect of aggregation on the kinetics of autoxidation of the polyene antibiotic amphotericin-BJ Pharm Sci19938221621668383201
- HartselSCBauerEKwongEHWasanKMThe effect of serum albumin on amphotericin B aggregate structure and activityPharm Res20011891305130911683244
- JunghannsJUButtleIMullerRHSolEmuls technology: A way to overcome the drawback of parenteral administration of insoluble drugsPharm Dev Technol200712543744517963143
- AntoniadouADupontBLipid formulations of amphotericin B: where are we today?J Mycol Med2005154230238
- BekerskyIFieldingRMBuellDLawrenceILipid-based amphotericin B formulations: from animals to manPharm Sci Technol Today19992623023610366838
- JulianoRLGrantCWMBarberKRKalpMAMechanism of the selective toxicity of amphotericin-B incorporated into liposomesMol Pharmacol19873111113807887
- AraujoIBBritoCRUrbanoIASimilarity between the in vitro activity and toxicity of two different fungizone™/lipofundin™ admixturesActa Cir Bras200520Suppl 125726116186987
- SouzaLCMaranhaoRCSchreierSCampaAIn-vitro and in-vivo studies of the decrease of amphotericin-B toxicity upon association with a triglyceride-rich emulsionJ Antimicrob Chemother19933211231328226403
- CheronMPetitCBolardJGaboriauFHeat-induced reformulation of amphotericin B-deoxycholate favours drug uptake by the macrophage-like cell line J774J Antimicrob Chemother200352690491014613963
- GaboriauFCheronMLeroyLBolardJPhysico-chemical properties of the heat-induced ‘super-aggregates’ of amphotericin BBiophys Chem199766111217029866
- GaboriauFCheronMPetitCBolardJHeat-induced superaggregation of amphotericin B reduces its in vitro toxicity: a new way to improve its therapeutic indexAntimicrob Agents Chemother19974111234523519371331
- HartselSCBaasBBauerEHeat-induced superaggregation of Amphotericin B modifies its interaction with serum proteins and lipoproteins and stimulation of TNF-alphaJ Pharm Sci200190212413311169529
- PetitCYardleyVGaboriauFBolardJCroftSLActivity of a heat-induced reformulation of amphotericin B deoxycholate (Fungizone) against Leishmania donovaniAntimicrob Agents Chemother19994323903929925541
- RogersPDBarkerKSHerringVJacobMHeat-induced superaggregation of amphotericin B attenuates its ability to induce cytokine and chemokine production in the human monocytic cell line THP-1J Antimicrob Chemother200351240540812562711
- SivakOBartlettKWasanKMHeat-treated Fungizone retains amphotericin B antifungal activity without renal toxicity in rats infected with Aspergillus fumigatusPharm Res20042191564156615497680
- AraujoIBDamascenoBPde MedeirosTMSoaresLAdo EgitoESDecrease in Fungizone™ toxicity induced by the use of Lipofundin™ as a dilutent: an in vitro studyCurr Drug Deliv20052219920516305421
- BartnerEZinnesHMoeRAKuleskaJSStudies on a new solubilized preparation of amphotericin BAntibiot Annu1957–195855358
- ErnstCDupontGRinnertHLematreJEffect of Temperature- Changes on Circular-Dichroism, Absorption-Spectra and Light- Scattering of Amphotericin-B in Aqueous and Hydro-Alcoholic SolutionsComptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences Serie B197828615175178
- BaasBKindtKScottAScottJMikuleckyPHartselSCActivity and kinetics of dissociation and transfer of amphotericin B from a novel delivery formAAPS Pharm Sci199913E10
- CailletJBergesJLangletJTheoretical study of the self-association of amphotericin BBiochim Biophys Acta1995124021791958541289
- HartselSCBenzSKAyenewWBolardJNa+, K+ and Cl− Selectivity of the permeability pathways induced through sterol-containing membrane-vesicles by amphotericin-B and other polyene antibioticsEur Biophys J19942321251328050397
- BarwiczJChristianSGrudaIEffects of the aggregation state of amphotericin-B on its toxicity to miceAntimicrob Agents Chemother19923610231023151444311
- JolyVFarinottiRSaintjulienLCheronMCarbonCYeniPIn vitro renal toxicity and in vivo therapeutic efficacy in experimental murine cryptococcosis of amphotericin-B (Fungizone) associated with IntralipidAntimicrob Agents Chemother19943821771838192439
- SwensonCEPerkinsWRRobertsPIn vitro and in vivo antifungal activity of amphotericin B lipid complex: Are phospholipases important?Antimicrob Agents Chemother19984247677719559780
- LeonCTaylorRBartlettKHWasanKMEffect of heat-treatment and the role of phospholipases on Fungizone®-induced cytotoxicity within human kidney proximal tubular (HK-2) cells and Aspergillus fumigatusInt J Pharm2005298121121815950412
- van EttenEWvan VianenWRooversPFrederikPMild heating of amphotericin B-desoxycholate: Effects on ultrastructure, in vitro activity and toxicity, and therapeutic efficacy in severe candidiasis in leukopenic miceAntimicrob Agents Chemother20004461598160310817715
- BauPBolardJDupouy-CametJHeated amphotericin to treat leishmaniasisLancet Infect Dis20033418812679259
- StoodleyRWasanKABizzottoDFluorescence of Amphotericin B-deoxycholate (Fungizone) monomers and aggregates and the effect of heat-treatmentLangmuir200723178718872517637009