Abstract
The advent of highly active antiretroviral therapy (HAART) has significantly improved the prognosis for human immunodeficiency virus (HIV)-infected patients, however the adverse side effects associated with prolonged HAART therapy use continue. Although systemic viral load can be undetectable, the virus remains sequestered in anatomically privileged sites within the body. Nanotechnology-based delivery systems are being developed to target the virus within different tissue compartments and are being evaluated for their safety and efficacy. The current review outlines the various nanomaterials that are becoming increasingly used in biomedical applications by virtue of their robustness, safety, multimodality, and multifunctionality. Nanotechnology can revolutionize the field of HIV medicine by not only improving diagnosis, but also by improving delivery of antiretrovirals to targeted regions in the body and by significantly enhancing the efficacy of the currently available antiretroviral medications.
Introduction
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a global pandemic and is the leading infectious disease resulting in significant morbidity and mortality and consequently devastating socioeconomic effects. With the advent of multidrug, highly active antiretroviral therapy (HAART), the prognosis for HIV-infected patients has significantly improved; however, it has not eradicated HIV infection, particularly in sequestered, anatomically privileged sites, such as the brain, testes, gut, liver, kidney, and secondary lymphoid tissue. Additionally, emergence of resistant viral strains and the adverse side effects associated with prolonged use continue to slow down the application of effective antiviral therapies. Nanotechnology is an emerging multidisciplinary field that has the potential to advance the treatment and prevention of HIV/AIDS radically. The use of nanotechnology for numerous biomedical applications has become an area of intense research over the last decade.Citation1–Citation10
The potential advantages of using nanomedicine over conventional HIV therapies include the capacity to incorporate, encapsulate, or conjugate a variety of drugs to target specific cell populations and to offer tunable and site-specific drug release.Citation11–Citation20 outlines the different types of current nanotherapeutics in HIV and lists their advantages and limitations. Although HIV-1 nanotherapeutics have the potential to address key issues of traditional HIV-1 therapy, such as overcoming cellular and anatomical barriers, drug toxicity, drug resistance, suboptimal adherence, and virus sequestration, a number of obstacles remain which include safety and efficacy profiles and long-term toxicity, unwarranted immune response, and scale-up and cost considerations of large scale synthesis of these nanoparticle platforms.Citation21–Citation29
Table 1 Types of nanotherapy in HIV
Types of nanomaterials
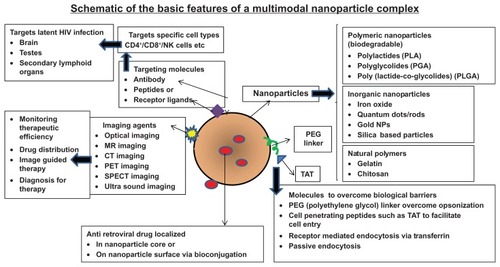
A wide range of nanomaterials have been used in biomedicine, these encompass dendrimers, liposomes, micelles, nanoemulsions, nanocapsules, nanocrystals, nanotubes, and nanoparticles.Citation30–Citation45 lists the commonly used nanomaterials in biomedical applications. The current review focuses predominantly on nanoparticles used in HIV-1 therapeutics. Nanoparticles are solid colloidal particles typically in the size range of 10–1000 nm, in which therapeutic agents either alone or in combination can be entrapped or chemically linked to the surface. Nanoparticles offer more stability to encapsulated drugs in biological fluids and also protect the encapsulated drugs against enzymatic degradation.Citation46 Due to their small size they can be taken up by cells where the uptake of larger particles is precluded. Further, nanomaterials can provide improved drug delivery, by virtue of their robustness, safety, and multimodality or multifunctionality. The multifunctional nanoparticle can simultaneously carry therapeutic agents to target molecules such as conjugated antibodies and imaging signal contrast agents (). Nanoparticles can be used for delivery of antivirals to targeted infected cells within reservoirs by virtue of targeting agents and molecules. Further, these nanoparticles can also act as imaging agents, which allows for real-time tracking within cells and within the intact organism. Advantages that nanoparticulate imaging agents have over traditional molecular imaging agents include: (a) they provide a tunable fluorescent chassis upon which targeting agents (antibodies, peptides or small molecules) can be added or changed to suit a specific need; (b) they allow for multimodality (eg, optical, magnetic resonance, and radionuclide) imaging thus permitting cross-evaluation of disease-infected sites using different imaging platforms; (c) they can be functionalized with both imaging and therapeutic abilities (d) they can be made in a large-enough size to permit multivalencyCitation42–Citation45 (ability to bind to various ligands due to different types of surface modifications, functionalization, and bioconjugation chemistries), which results in having a potential for higher affinity binding than standard agents; and (e) they can enable imaging from the single cell level to the entire, intact organism in vivo. Nanotechnology-based delivery systems also enhance and modulate the distribution of hydrophobic and hydrophilic drugs into and within different tissue compartments. This ability of these nanoscale delivery platforms means they are suitable for clinical use in HIV-1 therapeutics. In order to have clinical applicability, nanoparticles should be stable in time with a shelf life of several months, and with a long circulating time that will assure its biodistribution, allow the passive or active targeting of a specific site within the body or cell type or cell surface, respond to stimuli such as pH, temperature, etc, and to be also be usable as a contrast substance for medical imaging, ultrasonography, magnetic resonance imaging, computed tomography, and photodynamic therapy.
Table 2 Types of nanomaterials
Figure 1 Schematic of the basic features of a multimodal nanoplex capable of targeted drug delivery.
Notes: The important components of a nanoparticle used for intracellular drug delivery which include various nanomaterials (polymeric, inorganic, or natural polymers, etc), targeting molecules (antibodies, peptides, receptor ligands), cell-penetrating peptides (to promote internalization), linkers (PEG) and the incorporated drug molecules of interest (such as the antiretroviral drugs).
Abbreviation: PEG, poly(ethylene glycol); NP, natural polymers; MR, magnetic resonance; CT, computed tomography; PET, positron emission tomography; SPECT, single photon emission computed tomography; NK, natural killer; CD4, - Cluster of Differentiation Antigen 4; CD8-, Cluster of Differentiation Antigen 8.
The predominant types of nanoparticles that are typically used for biological applications are (1) inorganic, and, (2) polymeric nanoparticles. Polymeric nanoparticles such as poly(lactic-co-glycolic acid) (PLGA) or polylactide, polymethacrylic acid, polyethylene glycol (PEG), and “natural” polymers such as chitosan, gelatin, or alginate, all of which are biodegradable.Citation34,Citation35 PEGylation coating of a nanoparticle with PEG to prevent the rapid removal of nanoparticles from the bloodstream by the mononuclear phagocytic system, increases nanoparticle circulation time and theoretically improves the therapeutic capacity of the nanoparticle.Citation36 Advances in polymerization chemistries and the application of reactive, efficient and orthogonal chemical modification reactions have enabled the engineering of multifunctional polymeric nanoparticles with precise control over the architectures of the individual polymer components, to direct their assembly and subsequent transformations into nanoparticles of selective overall shapes, sizes, internal morphologies, external surface charges and functionalities. Further, incorporation of certain functionalities can modulate the responsiveness of these nanostructures to specific stimuli through remote activation.Citation34,Citation35–Citation50
Inorganic particles are much smaller, typically less than 20 nm, and circulate easily in the bloodstream and are easily cleared via renal excretion.Citation42–Citation46 Silica-based nanoparticles such as organically modified silica are known for their low toxicity and biocompatibility for targeted imaging and therapy.Citation47–Citation50 Gold nanoparticles (GNP) are an attractive drugdelivery vector due to the ease with which biomolecules such as protein or DNA can be attached to the gold surface using thiol chemistry. This process can also allow attachment of multiple targeting or functional groups to the nanoparticle surface to produce a multifunctional nanoparticle. Quantum dots (QDs) that have a colloidal core/shell such as CdSe/ZnS, CdSe/CdS/ZnS, CdTe/CdSe, and InP/ZnS are also commonly synthesized for biomedical applications.Citation51–Citation54 QDs are unique probes due to their special properties (brightness, photostability, narrow-band emission, and broadband absorption), and they have excellent biocompatibility for real-time imaging.Citation53 When QDs are functionalized with ligands, they enable the recognition of specific targets and allow tracking of dynamic functional processes with a great sensitivity for extended periods of time. Although both GNP and QDs can be easily functionalized, these nanoparticles may accumulate in tissues over time because they are not biodegradable and the long-term effects of accumulation of these nanoparticles are unknown. summarizes the pros and cons of the different types of nanomaterials used in biomedical applications.
Nanotechnology can revolutionize the field of HIV medicine since it can impact the treatment and prevention of HIV/AIDS, and also improve diagnosis. The current review will focus more on the pivotal role of nanotechnology in HIV-1 therapeutics, particularly focusing on nanotherapeutic approaches that increase the efficacy of the existing antiretrovirals in targeting sequestered viral reservoirs.
The HIV-1 lifecycle and traditional antiretroviral therapy
HIV enters the cell via endocytosis and fuses with an endosome, then the viral RNA genome is released into the cell where it undergoes reverse transcription followed by integration of the proviral DNA into the host chromosome. Subsequent to translation, immature viral particles egress the cell by assembly of the viral proteins at the cell membrane, followed by virion budding, which results in the generation of a mature virus that can infect other cells. Types of antiretroviral drugs used in treatment of HIV include: reverse transcriptase inhibitors that act by blocking the activity of the reverse transcriptase enzyme, thus preventing the construction of viral DNA; nucleoside analog reverse transcriptase inhibitors, which act by incorporation into the viral DNA leading to chain termination; nonnucleoside analog reverse transcriptase inhibitors, which block the binding potential of the reverse transcriptase enzyme; protease inhibitors, which interfere with viral assembly by blocking the protease enzyme necessary for cleaving the nascent viral proteins for final assembly into new virions; fusion inhibitors, which block the fusion of the virus with the cell membrane and subsequent entry into the host cells; integrase inhibitors, which block the integration of viral DNA into the host cell DNA; and finally, entry inhibitors, which bind to HIV-1 coreceptors on the viral membrane surface used in the entry of the virus into the host cell. Highly effective antiretroviral therapy (HAART) is a combination of three or more of the above drug classes, which is efficient in controlling infection and disease progression and minimizes the development of resistant strains by attacking the viral infection more aggressively.
Nanoformulations of antiretrovirals
Current HIV-HAART therapy exposes the entire body to multiple drugs at high doses, resulting in enormous side effects, which often limit therapy due to adherence issues leading to unsuccessful therapy. Adherence to drug regimens that constitute complexities of frequent administration and high dosages are crucial in the treatment of HIV infection. Development of targeted drug-delivery approaches for AIDS therapy will improve the safety and efficacy of anti-HIV drugs by reducing the dosage and adverse effects associated with them. The systemic distribution pattern of nanocarrier-based HIV drugs should be dictated by the properties of the nanocarrier rather than the antiretroviral drug and an ideal nanocarrier-based HIV drug should deliver higher drug concentrations and have increased residence time at target cells whereby enhanced viral load reduction can be achieved. Surface modification of nanocarriers by modulating polymer characteristics on the nanocarrier surface allows controlled release of an anti-HIV drug from nanocarriers to achieve the desired therapeutic level in target tissue.Citation11,Citation14–Citation22
Development of a biocompatible nanoformulation that can target HIV-1 in sequestered sites requires the use of functionalized nanoparticles that are engineered to deliver drugs to specific tissue or cell compartments.Citation28 Although efficient internalization within the cells and selective targeting are the major aims of such a drug carrier, ideally it should also have the characteristic of long circulation time in blood and self-regulation of drug release, and have low toxicity and minimal side effects. In general, the sizes of such nanoparticle-based delivery platforms range from 10 to 1000 nm and consist of therapeutic agents within the nanoparticle system.Citation18–Citation20 Such a nanoparticle system increases solubility and enhances stability of mostly poorly water-soluble therapeutic agents such as antiretroviral drugs which are mostly hydrophobic, and protect them from interacting with nonspecific sites, thereby reducing toxicity. lists the various antiretroviral nanoformulations used as HIV-1 therapeutics.
Table 3 Summary of antiretroviral drugs used in HIV-1 nanotherapeutics
Targeted drug delivery of antiretrovirals
Targeted delivery of antiretrovirals to HIV-1-infected T-cells and macrophages would improve the efficacy of antiviral drugs, reduce toxicity, reduce HIV-resistance frequency, and decrease viral production. However, the specificity of intracellular delivery and transport is related to many factors including the type and number of targeting ligands required for optimal cellular uptake. Various mechanisms of intracellular delivery of the antiretroviral drug include passive diffusion of free drug, nonspecific phagocytosis of a nanocarrier, pinocytosis and receptor-mediated endocytosis. Another advantage of nanocarriers includes its ability to bypass the multidrug-resistant transporters, which may efflux drugs entering freely through the plasma membrane.Citation14–Citation29
Inorganic solid lipid nanoparticles liposomes, polymeric micelles, dendrimers, cyclodextrins, and cell-based nanoformulations have been studied for delivery of drugs intended for HIV prevention or therapy.Citation43 For anti-HIV drugs to be effective, adequate distribution to specific sites in the body must be achieved, and effective drug concentrations must be maintained at those sites for the required period of time.Citation55 For effective delivery of anti HIV-1 nanotherapy, an optimal drugdelivery nanocarrier vehicle must be generated that should be of a precise geometry, whose surface (ie, zeta potential, stealth ligands), drug/biomolecule (antiretrovirals, oligonucleotides, proteins, small interfering RNA [siRNA], RNA, imaging agents) encapsulation efficiency and release, surface chemistries (targeting antibodies, PEG chains, metal chelators), and spatial distribution of ligands must be well engineered. Targeted nanocarrier delivery involves (1) the recognition of HIV-infectable target cells and tissues; (2) the ability to reach these sites; and (3) the ability to deliver multiple therapeutic agents.Citation14–Citation24
Macrophages have been used as cellular transporters for antiretroviral nanoparticles or nanoformulated antiretroviral drugs (nano-ART) distribution and that the efficacy of antiretroviral medications can be significantly improved by repackaging them into nanoparticles.Citation24,Citation29 Nowacek and Gendelman have shown that a single intravenous dose of the nano-ART can elicit high-sustained tissue and plasma drug levels of antiretroviral drugs in the reticuloendothelial system and brain.Citation26 Nano-ART can be taken up within minutes by circulating monocytes and released in tissues over a period of 2 weeks.Citation56–Citation60 Theoretically, cell-based nano-ART would travel to sites of inflammation and release drug(s) slowly with limited tissue toxicities. Such a drug-delivery system can revolutionize HIV-1 therapeutics and can particularly target virus sequestration. A recent study suggested that nano-ART has tremendous translational potential since it allows sustained delivery and targeted efficacy of the antiretroviral drugs with limited systemic toxicities, indicating that nano-ART has the potential to improve drug adherence and reduce viral resistance in HIV-1-infected subjects.Citation58
Studies by Kadiu et al show that both particle integrity and antiretroviral activity are maintained during subcellular transport of antiretroviral nanoformulations.Citation61 Recently, Shegokar and Singh have shown that nevirapine nanosuspensions that were surface modified with serum albumin, polysaccharide, and polyethylene glycol had enhanced bio-availability and targeting potential.Citation62 They also demonstrated active delivery of stavudine-loaded solid lipid nanoparticles to lymphatic tissues showing increased lymphatic drug levels and organ distribution studies demonstrating efficiency of the developed nanoparticles for prolonged residence in splenic tissues Citation62. Polymeric systems consisting of nanosuspensions of rilpivirine that were PEGylated and stabilized by other polymers showed that single-dose administration of this nanosuspension resulted in sustained release of the drug and lowered dosing frequency.Citation63–Citation65 Tat-peptide-conjugated ritonavir-loaded nanoparticles effectively controlled viral replication in HIV-1-infected brain by targeting monocyte-derived macrophages and other neural cells within the brain.Citation66 A study that evaluated pharmacokinetics of free antiretroviral drugs ritonavir, lopinavir, and efavirenz with poly(D,L-lactide-co-glycolide) nanoparticle-conjugated antiretroviral drugs (AR-NPs) showed that antiretroviral drug concentrations from AR-NPs are sustained for 28 days in vivo and anti-HIV inhibition was comparable to that of free drugs in vitro.Citation67,Citation68 Nevirapine-loaded liposomal formulations improve targeted delivery of the antiretroviral drugs to select cell compartments and alleviated systemic toxic side effects.Citation69 Carbon nanotubes show promise as anti-HIV-1 therapeutics.Citation70 All the above-mentioned studies are in various preclinical stages and significant amount of experimental and clinical validation needs to be done before nanotherapy for HIV become a reality in the clinics. Several additional nanotechnology systems for delivery of antiretroviral drugs have been outlined in recent reviews.Citation11–Citation28 We have made a comprehensive list of the antiretroviral used in HIV therapy in .
Mechanisms involved in nanoparticle based intracellular drug delivery
The mechanisms involved in nanoparticle-based intracellular drug delivery include (i) passive diffusion of free drug, (ii) nonspecific phagocytosis of the nanocarrier, (iii) nanocarrier uptake by pinocytosis, and (iv) receptor-mediated endocytosis. More than one mechanism may be involved in intracellular drug delivery. Nanoparticles may reside within lysosomal compartment following endocytosis, and premature drug release within this acidic compartment may cause drug degradation and render treatment ineffective. Therefore, cytosolic delivery and drug release within the cytosolic compartment will allow for the most effective therapy.Citation15–Citation20
Targeting HIV-1 in the brain
Most of the currently used antiretroviral drugs are effective in reducing viral load in the systemic circulation, but cannot eradicate infection from tissue reservoirs such as the brain. Nanoparticles offer great promise for neurotherapeutics.Citation25–Citation29 Nanocarriers provide a means to overcome cellular and anatomical barrier such as the blood–brain barrier (BBB) to drug delivery.Citation54 The BBB inhibits the transport of antiretroviral drugs to the brain by the presence of tight endothelial cell junctions and efflux transporters such as P-glycoprotein (P-gp), a multidrug-resistant protein. Nanocarriers bypass multidrug-resistant transporters such as P-gp that may efflux drugs entering freely through the plasma membrane. The Pluronic® (BASF - The Chemical Company, Florham Park, NJ) are block copolymers based on ethylene oxide and propylene oxide that enhance the in vivo efficacy of antiretroviralsCitation59 by influencing their distribution to sites protected by efflux mechanisms, such as the BBB, and possibly increase the brain exposure of these drugs resulting in suppression of viral replication. Pluronic P85 has shown to inhibit the interaction of P-gp with nelfinavir and saquinavir.Citation59 An elegant study using a nanosuspension of indinavir showed that macrophages loaded with this nanosuspension could bring about a measurable reduction in antiviral load in the brains of an HIV-infected rodent model.Citation56 Magnetic nanoformulations have been used for targeting active nucleotide analog reverse transcriptase inhibitors to the brain by application of an external magnetic force and thereby eliminating the brain HIV reservoirs.Citation68
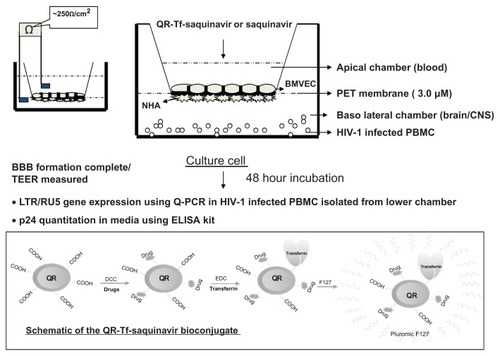
Our group has recently shown that conjugation with transferrin (Tf), allows antiretroviral drug-loaded nanoformulations to permeate across biological barriers such BBB via a receptor-mediated transport mechanism. We stably incorporated saquinavir within Tf-conjugated quantum rods (QRs), which are novel nanoparticles with unique optical properties and evaluated antiviral efficacy and the transversing ability of the QR-Tf-saquinavir nanoformulation across an in vitro model of BBB (). We have also observed that amprenavir- Tf-conjugated quantum dot (QD) (Tf-QD-amprenavir) nanoplexes cross the BBB via receptor-mediated endocytosis and release the amprenavir. The activity of amprenavir remains unaltered when conjugated to the QD or the transporter molecule, Tf.Citation71 The high photostability of QDs emitting in the near-infrared spectral region facilitates the monitoring of this transport process in real-time via high-resolution optical imaging.Citation7,Citation72–Citation75 Thus a QD/QR-based nanoplatform facilitates not only the delivery of an antiretroviral drug across the BBB via interaction with endogenous transferrin receptors on the BBB but also allows the real-time monitoring of this transendothelial migration process via optical imaging. We have demonstrated that antiretroviral drug-loaded nanoparticles can deliver effective therapeutics across the BBB and that our nanoformulation has great potential in the treatment of neuro-AIDS and other neurological disorders.Citation71,Citation75,Citation76
Figure 2 Schematic of the QR-Tf-saquinavir bioconjugate and the transversing potential of the QR-Tf-saquinavir nanoformulation across an in vitro BBB.
Copyright © 2010, Bentham Science.
Reproduced with permission from Mahajan SD, Roy I, Xu G, et al. Enhancing the delivery of anti retroviral drug “Saquinavir” across the blood brain barrier using nanoparticles. Curr HIV Res. 2010;8(5):396–404.Citation75
Abbreviations: BBB, blood–brain barrier; QR, quantum rods Tf, transferrin; PET, polyethylene terephthalate; CNS, central nervous system; PBMC, peripheral blood mononuclear cells; NHA, normal human astrocytes; Baso, basophil; HIV-1, human immunodeficiency virus-1; BMVEC, brain microvascular endothelial cells; TEER, transendothelial electric resistance; LTR, long terminal repeat; PCR, polymerase chain reaction, ELISA, enzyme-linked immunosorbent assay.
Anti-HIV-1 siRNA therapeutics
Recent advances in the field of nanotechnology offer an unprecedented opportunity to enhance the power of siRNA-mediated gene therapy by providing both an efficient delivery system as well as targeted specificity. siRNA-mediated knockdown of gene-specific messenger RNA (mRNA) levels is a great therapeutic strategy,Citation29 in which double-stranded RNAs are cleaved by the cellular nuclease Dicer into short 21–22-mer fragments referred to as siRNA, which enter a ribonuclease protein complex called the RNA-induced silencing complex. This complex mediates a specific degradation of the corresponding mRNA. Nanoparticles can form stable complexes with siRNAs, called nanoplexes, which can overcome all the impediments associated with siRNA in the free form. A nanoplex is a generic term for a nanoparticle complexed to another biological component, which could be a drug, an siRNA, a imaging agent and antibody, a peptide, etc.
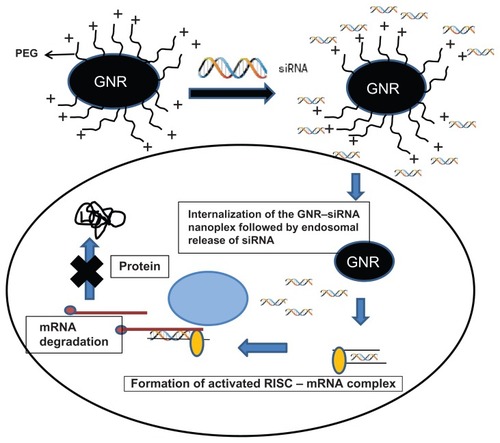
siRNA technology is a novel method to achieve complete and persistent knockdown of gene expression. Challenges to efficient siRNA delivery and activity include: (1) design of effective siRNA sequences; (2) bioconjugation with a safe and efficient delivery system such as nanoplexes; (3) formulation of a nanoplex with favorable pharmacokinetics; (4) stabilization of the siRNA in biological systems; (5) efficient delivery and entry of the siRNA nanoplex into target cells; and (6) prevention of endosomal escape of siRNA nanoplexes from the intracellular milieu. Additionally, the siRNA nanoplex should have minimal effects on nontarget genes and should avoid inadvertent stimulation of the immune system.Citation77,Citation78 Delivery is a key determinant as to whether or not RNA interference-based therapeutics will have clinical relevance and delivery encompasses extracellular transport of the nanoplex to target cells, its intracellular RNA trafficking, and processing.Citation79–Citation89 Gold nanoparticles are particularly attractive for therapeutic applications due to their biocompatibility and ease of complex formation with biomolecules. Gold nanorods (GNR) have far-reaching potential for the study of intracellular processes at the single-molecule level, using high-resolution cellular imaging, long-term observation of cell trafficking in vivo, and gene silencing.Citation73,Citation74 The hydrodynamic size of these GNR-nanoplexes under physiological condition is <100 nm, making them ideal as intracellular delivery agents. Gold nanoparticles have been used for more than a decade as a gene carrier for plasmid DNA and oligonucleotides, but recently our group was the first to report the use of GNR for the delivery of siRNA against dopamine- and cyclic-adenosine monophosphate-regulated phosphoprotein of molecular weight 32,000 (DARPP-32) in vitro.Citation90 represents a schematic of the cationically charged PEGylated GNRs electrostatically coupled with negatively charged siRNA to form stable nanoplexes. We evaluated these nanoparticles as nonviral gene carriers by investigating their specificity and efficiency in achieving DARPP-32 gene silencing in primary dopaminergic neuronal cells, which are typically difficult to transfect. These nanoplexes were also found to transmigrate across an in vitro model of the BBB without compromising the integrity of the barrier, while retaining their gene-silencing efficiency. These results have enormous implication in the treatment of drug addiction in HIV-1-infected drug-abusing patients. These observations have further underscored the tremendous benefits that nanotechnology can offer towards the safe and efficient delivery of siRNA-based therapeutics in the brain and other organs.
Figure 3 Schematic of cationically charged PEGylated GNRs electrostatically coupled with negatively charged siRNA to form stable nanoplexes.
Notes: The GNR–siRNA nanoplex can bring about the specific knockdown of a gene of interest. The siRNAs are 21–23 bp double-stranded RNAs that can initiate the enzymatic breakdown of specific mRNAs in a cell through an RNA-induced silencing complex.
Abbreviations: GNRs, gold nanorods; siRNA, small, interfering RNA.
To enhance the cellular uptake of siRNA molecules, cell-penetrating peptides (CPP)-mediated siRNA delivery employing a disulfide bond formation between peptide transduction domain (PTD)/CPP and siRNA is typically utilized. Some of the most well characterized CPPs are TAT peptide, penetratin, transportan, polyarginine and MPG. Recently, studiesCitation91,Citation92 evaluated the in vivo efficacy of structurally flexible, cationic PAMAM dendrimers as a siRNA-delivery system in a humanized mouse model for HIV-1 infection. They have also developed novel dual inhibitory anti-glycoprotein-120 aptamer–siRNA chimera with potent anti-HIV activities and have further constructed a chimerical RNA nanoparticle that contains a HIV gp120-binding aptamer escorted by the packaging RNA (pRNA) of bacteriophage phi29 DNA-packaging motor. These pRNA– aptamer chimeras specifically bind to and are internalized into cells expressing HIV gp120 and inhibit HIV function by blocking viral infectivity. This nanoplex represents a potential HIV-1 inhibitor, and provides a cell-type-specific siRNA-delivery vehicle, showing promise for systemic anti-HIV therapy.Citation87 Our group demonstrated an almost 90% viral suppression using a nanoparticle (QR)-conjugated well validated siRNA (si510) that targets the poly-A/TAR (transactivator of the HIV-1 LTR) site and suppresses viral replication in the THP-1 monocytic cells.Citation55 Dendrimers have also been successfully used to deliver and transfect siRNA to various HIV-infected human cell types resulting in gene silencing without causing cytotoxicity.Citation88,Citation89
We have observed a decrease in endothelial permeability, as reflected by reduction of transendothelial resistance across an in vitro BBB on treatment with QR-conjugated matrix metalloproteinase-9 (MMP-9)-siRNA due to downregulating the expression of MMP-9 gene in brain microvascular endothelial cells that constitute the BBB.Citation90 Thus siRNA therapeutics for HIV infection have been demonstrated in many studies, but limitations of this strategy include successful strategies to deliver siRNA to the desired target cells such as T cells, macrophages, dendritic cells, and tissues. Despite these limitations, siRNA therapeutics possesses great potential in HIV therapy.
Nanotechnology-based immunotherapy for HIV
Attempts at creating an effective HIV vaccine have been unsuccessful because immune cells that are needed for vaccine effectiveness are destroyed by the virus.Citation60 Nanoparticles can improve delivery of antigens to enhance the immune response and can encapsulate antigens in their core from which antigen-presenting cells (APCs) can process and present antigens to CD4+ and CD8+ T cells or facilitate B cells to generate humoral responses.Citation92–Citation95 Various polymeric and lipid-based nanoparticles have been used to deliver DNA-, protein-, or peptide-based antigens in vivo, which elicit strong cellular, humoral, and mucosal immune responses. However, no effective vaccine strategy has emerged in spite of promising preliminary results.Citation14–Citation18
Nanoparticles as adjuvants in HIV vaccine
The most commonly used adjuvants in vaccines are aluminum-based compounds such as aluminum hydroxide. Aluminum hydroxide adjuvants stimulate the production of specific types of immune cells called APCs. These APCs pick up the antigen and present it to T cells. Various adjuvants are considered for use with candidate AIDS vaccines. Nanomaterials can act as adjuvants in the development of the AIDS vaccine due to their unique physicochemical properties.Citation27 Surface-engineered GNRs provide a multimodal nanoplatform for vaccine neoadjuvant-delivery systems in the treatment of AIDS.Citation14,Citation24,Citation93 Nanoparticles can also be designed to provoke an immune response by either direct immunostimulation of APCs or delivering antigens to specific cellular compartments.Citation94 To eliminate an immune response to the nanoparticle itself, the particle size is important since microparticles are rapidly cleared by the reticuloendothelial system, while nanoparticles have prolonged circulation time and are efficient drug, enzyme, and protein carriers by any route of administration.
Polymeric biodegradable nanoparticles such as poly(γ-glutamic acid) can be used as a stable protein-based antigen carrier and can be utilized for antigen delivery and other immunostimulatory activities. Wang et alCitation79 have shown that γ-polyglycolides (γ-PGA) nanoparticles have a capacity comparable to that of complete Freund’s adjuvant (CFA) in inducing p24-specific serum antibody by predominantly activating p24-specific interferon-γ-producing T cells. Thus, γ-PGA nanoparticles encapsulating various antigens may have great potential as novel and efficient protein-based vaccines against HIV-1 infection.Citation11,Citation27,Citation95 Several liposome-based systems using a variety of HIV-1 antigenic peptides such as gp160, gp41, gp120, p24, tat, gag, env, and rev have been evaluated for HIV vaccine delivery, however none of them have been able to elicit a broad neutralizing antibody response.Citation32–Citation42 Although several nanoparticle-based immunotherapeutic clinical trials for treatment have been performed, none of them have provided consistent clinical improvements for patients.
Nanoparticles improve HIV-1 diagnostics
The availability of sensitive and specific detection methods to identify HIV-1 in clinical specimens is highly desirable. GNPs have been used for improving detection sensitivity of HIV-1 p24 antigen assay. A GNP-based assay can detect HIV-1 p24 antigen at levels as low as 0.1 pg/mL and these assays offer 100–150-fold enhancement in the detection limit and detected HIV-1 infection 3 days earlier than the traditional colorimetric enzyme-linked immunosorbent assay (ELISA).Citation96,Citation97 Nanolithography was used to generate nanoscale patterns of antibodies against the HIV-1 p24 antigen on a gold surface. HIV-1 p24 antigen in plasma obtained directly from HIV-1-infected patients was hybridized to the antibody array in situ, and the bound protein was hybridized to a gold antibody-functionalized nanoparticle probe for signal enhancement. The nanoarray is a three-component sandwich assay that measures less than 0.025 pg/mL of HIV-1 p24 levels suggesting that this nanoarray-based assay can exceed the limit of detection of a conventional ELISA by more than a 1000-fold. Such assay systems can have a significant impact on HIV diagnostics.Citation97
Prevention of sexual transmission of HIV using nanoparticle-based microbicides
Nanoparticles that also provide a delivery strategy for targeted or controlled delivery to the vagina using topical microbicides, which can result in HIV prevention, are urgently needed to curb the rate of new infections.Citation98,Citation99 Dendrimers, PLGA nanoparticles, and cholesterol-conjugated siRNA have been evaluated for delivery of microbicides against HIV and although in vivo studies in mice suggest proximal and distal site topical delivery of the microbicides, issues of deep penetration into the epithelial barrier and issues with degradation in the mucus physiological environment remain to be resolved.Citation13,Citation30,Citation31,Citation36–Citation41,Citation100–Citation109
HIV-1 nanotherapy: an emerging technology, its promise, and limitations
Nanotechnology can impact HIV therapy at several levels. (1) Nanoparticles by themselves have therapeutic effects since they can penetrate and neutralize the virus, by structural interference with viral assembly and thereby inhibit viral replication. (2) Nanotechnology allows improved delivery platforms for systemic delivery of antiretroviral drugs, by allowing controlled release of antiretroviral drugs in circulation, thereby enhancing their half-lives and effectiveness, all of which can have a major implication in improving adherence to drugs in HIV infected patients. (3) Gene immunotherapy can be significantly improved using various nanomaterials. Nanotechnology-based vaccines have the ability to target specific immune cells, eliciting a controlled and sustained HIV-1 specific antibody and cellular immune response. Nonviral delivery of siRNA has tremendous translational potential and although delivery of siRNA to HIV-specific cells has been demonstrated, additional research on the safety and efficacy of siRNA nanotherapeutics has to be done before these therapies are used in the treatment of HIV/AIDS.
Our group has been working in the area of nanomedicine, particularly in the context of HIV-1 therapy, for a couple of years and has used polymeric, nonpolymeric, and lately biodegradable materials for targeted drug delivery of antiretrovirals, siRNA therapeutics, and certain aspects of HIV-1 immunotherapy. All these materials represent the diversity of nanomedicine applications and although HIV-1 nanotherapy has enormous promise, several challenges remain to be addressed.
There has been tremendous development over the last decade with respect to the types of nanomaterials available for biomedical applications; an enormous range of shapes, sizes, surface modifications, and functional modality options have been developed, but the main issue of their interaction in biological systems remain to be resolved. Although, some degree of success is observed in vitro studies, many studies have not moved beyond the preclinical stage due to challenges that include unwarranted interactions with plasma proteins in systemic circulation, inability to overcome biological barriers, nonspecific distribution in the body, and aggregation and accumulation within specific organs such as kidney resulting in toxicity. Thus toxicity of nanomaterials, stability in physiological conditions, and a lack of adequate reliable and affordable HIV/AIDS animal models for in vivo studies during these nanoparticles are some of the key obstacles in HIV-1 nanotherapeutics.
In spite of these limitations, nanotechnology holds great potential for impact in the field of HIV treatment and prevention. Multidisciplinary research across disciplines of biology, medicine, chemistry, pharmaceutical sciences, and bioengineering will revolutionize the field of nanomedicine in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
- BruchezMJrMoronneMGinPWeissSAlivisatosAPSemiconductor nanocrystals as fluorescent biological labelsScience19982815385201320169748157
- ChanWCNieSQuantum dot bioconjugates for ultrasensitive non-isotopic detectionScience19982815385201620189748158
- PardridgeWMGene targeting in vivo with pegylated immunoliposomesMethods Enzymol200337350752814714424
- ShiNBoadoRJPardridgeWMReceptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomesPharm Res2001a1881091109511587478
- ShiNZhangYZhuCBoadoRJPardridgeWMBrain-specific expression of an exogenous gene after i.v. administrationProc Natl Acad Sci U S A23200198221275412759
- VinogradovSThe second annual symposium on nanomedicine and drug delivery: exploring recent developments and assessing major advancesAugust 19–20, 2004Polytechnic University, Brooklyn, NY, USAExpert Opin Drug Deliv20041118118416296729
- YongKTQianJRoyIQuantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cellsNano Lett20077376176517288490
- LeeDEKooHSunICRyuJHKimKKwonICMultifunctional nanoparticles for multimodal imaging and theragnosisChem Soc Rev20124172656267222189429
- LammersTAimeSHenninkWEStormGKiesslingFTheranostic nanomedicineAcc Chem Res201144101029103821545096
- KimBYRutkaJTChanWCNanomedicineN Engl J Med2010363252434244321158659
- ParboosingRMaguireGEMGovenderPKrugerHGNanotechnology and the treatment of HIV infectionViruses2012448852022590683
- ArmsteadALLiBNanomedicine as an emerging approach against intracellular pathogensInt J Nanomedicine201163281329322228996
- LaraHHGarza-TrevinoENIxtepan-TurrentLSinghDKSilver nanoparticles are broad-spectrum bactericidal and virucidal compoundsJ Nanobiotechnology201193021812950
- das NevesJAmijiMSarmentoBMucoadhesive nanosystems for vaginal microbicide development: friend or foe?Wiley Interdiscip Rev Nanomed Nanobiotechnol20113438939921506290
- KimJMHanSHImmunotherapeutic restoration in HIV-infected individualsImmunotherapy20113224726721322762
- MallipeddiRRohanLCProgress in antiretroviral drug delivery using nanotechnologyInt J Nanomedicine2010553354720957115
- KimPSReadSWNanotechnology and HIV: potential applications for treatment and preventionWiley Interdiscip Rev Nanomed Nanobiotechnol20102669370220860050
- ChawlaPChawlaVMaheshwariRSarafSASarafSKFullerenes: from carbon to nanomedicineMini Rev Med Chem201010866267720236059
- MamoTMosemanEAKolishettiNEmerging nanotechnology approaches for HIV/AIDS treatment and preventionNanomedicine (Lond)20105226928520148638
- Seale-GoldsmithMMLearyJFNanobiosystemsWiley Interdiscip Rev Nanomed Nanobiotechnol20091555356720049817
- HauckTSGiriSGaoYChanWCNanotechnology diagnostics for infectious diseases prevalent in developing countriesAdv Drug Deliv Rev2010624–543844819931580
- WongHLChattopadhyayNWuXYBendayanRNanotechnology applications for improved delivery of antiretroviral drugs to the brainAdv Drug Deliv Rev2010624–550351719914319
- das NevesJAmijiMMBahiaMFSarmentoBNanotechnology-based systems for the treatment and prevention of HIV/AIDSAdv Drug Deliv Rev2010624–545847719914314
- GuptaUJainNKNon-polymeric nano-carriers in HIV/AIDS drug delivery and targetingAdv Drug Deliv Rev2010624–547849019913579
- BoisselierEAstrucDGold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicityChem Soc Rev20093861759178219587967
- NowacekAGendelmanHENanoART, neuroAIDS and CNS drug deliveryNanomedicine (Lond)20094555757419572821
- BerryCCIntracellular delivery of nanoparticles via the HIV-1 tat peptideNanomedicine (Lond)20083335736518510430
- PeekLJMiddaughCRBerklandCNanotechnology in vaccine deliveryAdv Drug Deliv Rev200860891592818325628
- VyasTKShahLAmijiMMNanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sitesExpert Opin Drug Deliv20063561362816948557
- GuoPRNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapyJ Nanosci Nanotechnol20055121964198216430131
- Pedziwiatr-WerbickaEFerencMZaborskiMGabaraBKlajnertBBryszewskaMCharacterization of complexes formed by polypropylene imine dendrimers and anti-HIV oligonucleotidesColloids Surf B Biointerfaces201183236036621190815
- LeeCCMacKayJAFréchetJMJSzokaFCDesigning dendrimers for biological applicationsNat Biotechnol2005231517152616333296
- ChraiSSMurariRAhmadILiposomes: A reviewPharmaceut Tech2002262834
- KwonGSForrestMLAmphiphilic block copolymer micelles for nanoscale drug deliveryDrug Develop Res2006671522
- CroySKwonGPolymeric micelles for drug deliveryCurr Pharm Des2006124669468417168771
- ZhangJLiSLiXPolymeric nano-assemblies as emerging delivery carriers for therapeutic applications: A review of recent patentsRecent Pat Nanotechnol2009322523119958285
- Bekkara-AounallahFGrefROthmanMNovel PEGylated nanoassemblies nade of self-assembled squalenoyl nucleoside analoguesAdv Funct Mater20081837153725
- CouvreurPStellaBReddyLHSqualenoyl nanomedicines as potential therapeuticsNano Lett200662544254817090088
- MasonTGWilkingJMelesonKChangCGravesSNanoemulsions: Formation, structure, and physical propertiesJ Phys Condens Mat200618R635R666
- AZoNanoNanocapsules and dendrimers – Properties and future applications Available from: http://www.azonano.com/article.aspx?ArticleID=1649Accessed December 2, 2011
- TorchilinVPNanoparticulates as Drug CarriersLondon, UKImperial College Press2006xxix724.516
- BawaRNanopharmaceuticals: nanopharmaceuticalsEur J Nanomed201033440
- FuAGuWBoussertBSemiconductor quantum rods as single molecule fluorescent biological labelsNano Lett2006717918217212460
- LiongMLuJKovochichMXiaTMultifunctional inorganic nanoparticles for imaging, targeting, and drug deliveryACS Nano20082588989619206485
- LeeJELeeNKimHUniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug deliveryJ Am Chem Soc2010132255255720017538
- LawWCMahajanSDKopwitthayaAGene silencing of human neuronal cells for drug addiction therapy using anisotropic nanocrystalsTheranostics20122769570422896771
- KreuterJNanoparticulate systems for brain delivery of drugsAdv Drug Deliv Rev200147658111251246
- ElsabahyMWooleyKLDesign of polymeric nanoparticles for biomedical delivery applicationsChem Soc Rev20124172545256122334259
- LiuLLawWCYongKTMultimodal imaging probes based on Gd-DOTA conjugated quantum dot nanomicellesAnalyst201113691881188621373688
- DingHYongKTRoyIGold nanorods coated with multilayer poly-electrolyte as contrast agents for multimodal imagingJ Phys Chem C200711112552
- DorfsDKrahneRFalquiAMannaLGianniniCZanchetDAndrewsDLScholasGDWiederrechtGPComprehensive Nanoscience and TechnologyAmsterdam, The NetherlandsAcademic Press2011219
- CaiWShinDWChenKPeptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjectsNano Lett2006666916608262
- HuRYongKTRoyIFunctionalized near-infrared quantum dots for in vivo tumor vasculature imagingNanotechnology2010211414510520234074
- AlamMIBegSSamadAStrategy for effective brain drug deliveryEur J Pharm Sci201040538540320497904
- MallipeddiRRohanLCNanoparticle-based vaginal drug delivery systems for HIV preventionExpert Opin Drug Deliv201071374820017659
- DouHGrotepasCBMcMillanJMMacrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDSJ Immunol2009183166166919535632
- NowacekASMillerRLMcMillanJNanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug deliveryNanomedicine (Lond)20094890391719958227
- DashPKGendelmanHERoyULong-acting NanoART elicits potent antiretroviral and neuroprotective responses in HIV-1 infected humanized miceAIDS7202012 [Epub ahead of print.]
- ShaikNPanGElmquistWFInteractions of pluronic block copolymers on P-gp efflux activity: experience with HIV-1 protease inhibitorsJ Pharm Sci200897125421543318393290
- NevesJDAmijiMMBahiaBFSarmentoBNanotechnology-based systems for the treatment and prevention of HIV/AIDSAdv Drug Deliv Rev20106245847719914314
- KadiuINowacekAMcMillanJGendelmanHEMacrophage endocytic trafficking of antiretroviral nanoparticlesNanomedicine (Lond)20116697599421417829
- ShegokarRSinghKKSurface modified nevirapine nanosuspensions for viral reservoir targeting: In vitro and in vivo evaluationInt J Pharm2011421234135221986114
- BalkundiSNowacekASRoyUMartinez-SkinnerAMcMillanJGendelmanHEMethods development for blood borne macrophage carriage of nanoformulated antiretroviral drugsJ Vis Exp201046 pii246021178968
- GuptaUJainNKNon-polymeric nano-carriers in HIV/AIDS drug delivery and targetingAdv Drug Deliv Rev2010624–547849019913579
- MamoTMosemanEAKolishettiNSalvador-MoralesCEmerging nanotechnology approaches for HIV/AIDS treatment and preventionNanomedicine (Lond)20105226928520148638
- BorgmannKRaoKSLabhasetwarVGhorpadeAEfficacy of Tat-conjugated ritonavir-loaded nanoparticles in reducing HIV-1 replication in monocyte-derived macrophages and cytocompatibility with macrophages and human neuronsAIDS Res Hum Retroviruses201127885386221175357
- DestacheCJBelgumTGoedeMShibataABelshanMAAntiretroviral release from poly (DL-lactide-co-glycolide) nanoparticles in miceJ Antimicrob Chemother201065102183218720729545
- SaiyedZMGandhiNHNairMPMagnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrierInt J Nanomedicine72010515716620463931
- RamanaLNSethuramanSRangaUKrishnanUMDevelopment of a liposomal nanodelivery system for nevirapineJ Biomed Sci2010175720624325
- ChengYLiDJiBShiXGaoHStructure-based design of carbon nanotubes as HIV-1 protease inhibitors:atomistic and coarse-grained simulationsJ Mol Graph Model201029217117720580296
- MahajanSDLawWCAalinkeelRNanoparticle-mediated targeted delivery of antiretrovirals to the brainMethods Enzymol20125094364
- MichaletXPinaudFFBentolilaLAQuantum dots for live cells, in vivo imaging, and diagnosticsScience200530753854415681376
- PrasadPNIntroduction in BiophotonicsNew York, NYWiley2003
- PrasadPNNanophotonicsNew York, NYWiley2004
- MahajanSDRoyIXuGEnhancing the delivery of anti retroviral drug “Saquinavir” across the blood brain barrier using nanoparticlesCurr HIV Res20108539640420426757
- MahajanSDReynoldsJLAalinkeelREnhancing the transversing efficiency and efficacy of the anti retroviral drug “saquinavir” using nanotechnology: Implications for nanotherapeutics in neuro-AIDS. HIV Persistance during TherapyFourth International WorkshopDecember 2009Global Antiviral Journal2009524546
- XuLLiuYChenZSurface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatmentNano Lett2282012 [Epub ahead of print.]
- KalkanidisMPieterszGAXiangSDMethods for nano-particle based vaccine formulation and evaluation of their immunogenicityMethods2006401202916997710
- WangXUtoTAkagiTAkashiMBabaMPoly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccineJ Med Virol2008801111918041033
- TangSHewlettINanoparticle-based immunoassays for sensitive and early detection of HIV-1 capsid (p24) antigenJ Infect Dis2010201Suppl 1S59S6420225948
- LeeKBKimE-YMirkinCAWolinskySMThe use of nanoarrays for highly sensitive and selective detection of human immunodeficiency virus type 1 in plasmaNano Lett200441018691872
- de FougerollesAVornlocherHPMaraganoreJLiebermanJInterfering with disease: a progress report on siRNA-based therapeuticsNat Rev Drug Discov20076644345317541417
- AignerADelivery systems for the direct application of siRNAs to induce RNA interference (RNAi) in vivoJ Biomed Biotechnol200647165917057369
- HiguchiYKawakamiSHashidaMStrategies for in vivo delivery of siRNAs: recent progressBio Drugs2010243195205
- RavinaMPaolicelliPSeijoBSanchezAKnocking down gene expression with dendritic vectorsMini Rev Med Chem2010101738620380642
- BuxtonDBNanomedicine for the management of lung and blood diseasesNanomedicine (Lond)20094333133919331540
- de MartimpreyHVauthierCMalvyCCouvreurPPolymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNAEur J Pharm Biopharm200971349050418977435
- de FougerollesARDelivery vehicles for small interfering RNA in vivoHum Gene Ther200819212513218257677
- ToubNMalvyCFattalECouvreurPInnovative nanotechnologies for the delivery of oligonucleotides and siRNABiomed Pharmacother200660960762016952435
- BonoiuACMahajanSDDingHNanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neuronsProc Natl Acad Sci U S A2009106145546555019307583
- ZhouJNeffCPLiuXSystemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized miceMol Ther201119122228223821952167
- ZhouJShuYGuoPSmithDDRossiJJDual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 inhibitionMethods201154228429421256218
- MahajanSDAalinkeelRReynoldsJLNanotherapeutics using an HIV-1 poly A and transactivator of the HIV-1 LTR-(TAR-) specific siRNAPatholog Res Int2011201171913921660279
- JiménezJLClementeMIWeberNDCarbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virusBio Drugs2010245331343
- GonzaloTClementeMIChoncoLGene therapy in HIV-infected cells to decrease viral impact by using an alternative delivery methodChem Med Chem20105692192920414916
- BonoiuAMahajanSDYeLMMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrierBrain Res2009128214215519477169
- KimSSSubramanyaSPeerDShimaokaMShankarPAntibody-mediated delivery of siRNAs for anti-HIV therapyMethods Mol Biol201172133935321431696
- LetvinNLProgress toward an HIV vaccineAnnu Rev Med20055621322315660510
- MallipeddiRRohanLCProgress in antiretroviral drug delivery using nanotechnologyInt J Nanomedicine2010553354720957115
- KuoYCLeeCLMethylmethacrylate-sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood-brain barrierColloids Surf B Biointerfaces201290758222024400
- das NevesJMichielsJAriënKKPolymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirinePharm Res20122961468148422072053
- KuoYCChungCYSolid lipid nanoparticles comprising internal Compritol 888 ATO, tripalmitin and cacao butter for encapsulating and releasing stavudine, delavirdine and saquinavirColloids Surf B Biointerfaces201188268269021865017
- KuoYCYuHWPolyethyleneimine/poly-(γ-glutamic acid)/poly (lactide-co-glycolide) nanoparticles for loading and releasing antiretroviral drugColloids Surf B Biointerfaces201188115816421764569
- YangLChenLZengRSynthesis, nanosizing and in vitro drug release of a novel anti-HIV polymeric prodrug: chitosan-O-isopropyl- 5′-O-d4T monophosphate conjugateBioorg Med Chem201018111712319959368
- DestacheCJBelgumTChristensenKShibataASharmaADashACombination antiretroviral drugs in PLGA nanoparticle for HIV-1BMC Infect Dis2009919820003214
- BaertLvan’t KloosterGDriesWDevelopment of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatmentEur J Pharm Biopharm200972350250819328850
- WanLZhangXPooyanSOptimizing size and copy number for PEG-FMLF (n-formyl-methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophagesBioconjug Chem2008191283818092743
- KaurCDNaharMJainNKLymphatic targeting of zidovudine using surfaceengineered liposomesJ Drug Target2008161079880519005941
- DouHMoreheadJDestacheCJLaboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophagesVirology2007358114815816997345