Abstract
Background
The synthesis of anisotropic silver nanoparticles is a time-consuming process and involves the use of expensive toxic chemicals and specialized laboratory equipment. The presence of toxic chemicals in the prepared anisotropic silver nanostructures hindered their medical application. The authors have developed a fast and inexpensive method for the synthesis of three-dimensional hollow flower-like silver nanostructures without the use of toxic chemicals.
Methods
In this method, silver nitrate was reduced using dextrose in presence of trisodium citrate as a capping agent. Sodium hydroxide was added to enhance reduction efficacy of dextrose and reduce time of synthesis. The effects of all four agents on the shape and size of silver nanostructures were investigated.
Results
Robust hollow flower-like silver nanostructures were successfully synthesized and ranged in size from 0.2 μm to 5.0 μm with surface area between 25–240 m2/g. Changing the concentration of silver nitrate, dextrose, sodium hydroxide, and trisodium citrate affected the size and shape of the synthesized structures, while changing temperature had no effect.
Conclusion
The proposed method is simple, safe, and allows controlled synthesis of anisotropic silver nanostructures, which may represent promising tools as effective antimicrobial agents and for in vitro diagnostics. The synthesized hollow nanostructures may be used for enhanced drug encapsulation and sustained release.
Introduction
Silver nanoparticles have unique optophysical propertiesCitation1 and have been used as catalysts and antimicrobial, antiseptic, and imaging agents.Citation2–Citation6 Applications of nanostructures depend on their size and shape.Citation6 Development of new methods for synthesis of nanostructures that allow control of size and shape are necessary to generate new nanostructures that can be employed in various applications. Hollow nanostructures were used to encapsulate and control release of drugs,Citation7,Citation8 cosmetics, and nucleic acids,Citation9 and have enhanced catalytic properties owing to their large surface area.Citation10,Citation11 Self-assembly of anisotropic silver nanostructures has been used for synthesis of biomaterials and semiconductor copolymers; however, controlling shape and size of such structures is still a challenge.Citation12–Citation14
There are several methods for fabrication of silver nanostructures with different shapes such as nanowires,Citation15 nanorods,Citation16,Citation17 and nanotubes.Citation15 However, these methods employ polymers (such as polyvinyl pyrrolidone or polyethylene glycol), toxic surfactants (such as cetyltrimethylammonium bromide, benzyldodecyldimethylammonium chloride), and reducing agents (such as sodium borohydride).Citation17–Citation19 Therefore, there is a need to develop new methods for safe and inexpensive synthesis of anisotropic silver nanostructures.
The authors have developed a fast, inexpensive, and safe method for the synthesis of three-dimensional (3D) hollow flower-like silver nanostructures which may be useful in medical applications. The synthesized hollow structures with higher pore areas may have enhanced drug encapsulation capacity and can be used for sustained release of drugs. The method employs nontoxic degradable chemicals and allows control of size and shape of the synthesized structures.
Materials and methods
Materials
Silver nitrate (AgNO3), trisodium citrate (TSC), dextrose, and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany). Hydrogen chloride and nitric acid were purchased from El-Gomhouria Co, (Cairo, Egypt). Double deionized water (DDI) was prepared using a Milli-Q™ system (Direct-Q 3, Model ZRQS0P0WW, Millipore Corporation, Billerica, MA) with a resistivity of 18 MΩcm.
Synthesis of 3D hollow silver flower-like nanostructures
The hollow flower-like structures were synthesized by the chemical reduction of AgNO3 in aqueous solution. Briefly, a round-bottom flask was cleaned thoroughly with aqua regia (3:1 hydrogen chloride:nitric acid) then rinsed with DDI water. AgNO3 (2–8 μM) was added to 3–9 mM dextrose solution and dissolved in water at room temperature. NaOH (0.01 mM) was added during stirring. The solution color changed from colorless to deep green, brown, yellow, or gray depending on the amount of AgNO3, dextrose, NaOH, and TSC (1–6 mM). Following color change, the solution was stirred for an additional 5 minutes, centrifuged, and washed three times with DDI water to remove excess dextrose.
Characterization of silver nanostructures
Scanning electron microscope
The size and morphology of the synthesized silver nanostructures were studied using scanning electron microscope (LEO SUPRA® 55; Carl Zeiss AG, Oberkochen, Germany). The samples were mounted on a silicon slide and left 2 hours to dry before imaging without sputter coating; particle size was reported as the mean diameter of randomly selected structures.
Spectrophotometry
The samples of the prepared silver nanostructures were diluted to 1/10 times in DDI water, and λ max measured using LAMBDA 950 spectrophotometer (PerkinElmer, Waltham, MA).
Nitrogen isothermal adsorption
Nitrogen isothermal adsorption was measured at 77 K on an ASAP® 2010 porosimeter (Micromeritics Instrument Corporation, Norcross, GA). The surface area was estimated by using the Brunauer–Emmett–Teller equation to measure surface area. The pore size, volume, and area distribution were calculated using the Barrett–Joyner– Halenda equation.
Atomic force microscope
The 3D morphology, surface area, and roughness of the prepared silver anisotropic nanostructures were studied using atomic force microscope (VEECO Dimension 3100 Scanning Probe Microscope, Veeco Instruments, Inc, Plainview, NY). The samples were mounted on a silicon slide and left 2 hours to dry before imaging.
Results and discussion
The size and shape of the synthesized silver nanostructures were studied using scanning electron microscopy and particle size was reported as mean diameter of randomly selected structures, as shown in and . presents micrographs of samples prepared using varying concentrations of AgNO3, dextrose, and NaOH. presents 3D hollow silver nanostructures synthesized using different concentrations of AgNO3 ranging between 2–8 μM. The particles have a 3D hollow flower-like shape, with branched edges and highly rough surface and particle size ranging between 0.2–0.5 μm with interconnected pores in the range of 50–200 nm diameters (). Pore interconnection, distribution, roughness, and branched edges increased with increasing AgNO3 concentration.
Figure 1 Scanning electron microscopy of flower-like silver nanostructures. Structures prepared by varying concentrations of different reagents. (A–C): silver nitrate 2–8 μM, dextrose 3 mM, and sodium hydroxide 0.1 mM; (D–F): silver nitrate 2 μM, dextrose 3–9 mM, and sodium hydroxide 0.1 mM; (G–I) silver nitrate 2 μM, dextrose 3 mM, and sodium hydroxide 0.1–0.5 mM.
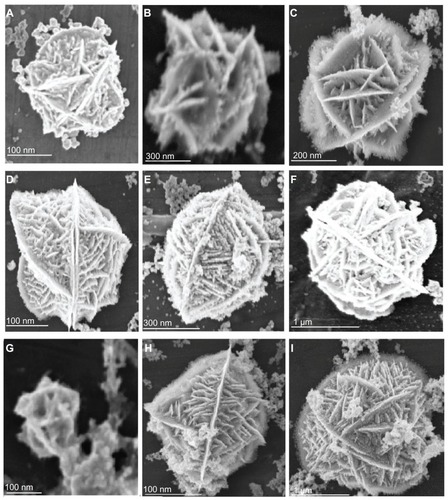
Figure 2 Scanning electron microscopy of flower-like silver nanostructures. (J–M) represent structures prepared by using silver nitrate 2 μM, dextrose 3 mM, sodium hydroxide 0.1 mM, and trisodium citrate 1–6 mM.
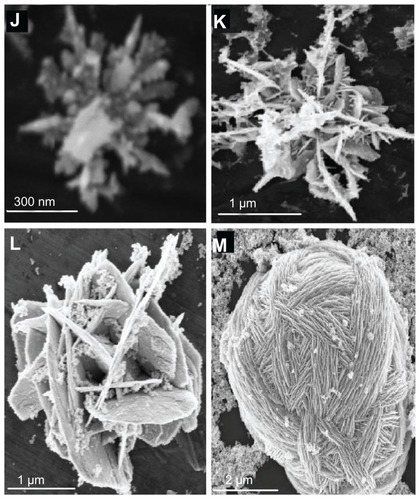
Figure 3 Particle size distributions using various concentrations of silver nitrate (A), dextrose (B), sodium hydroxide (C), or trisodium citrate (D).
Abbreviations: AgNO3, silver nitrate; NaOH, sodium hydroxide; TSC, trisodium citrate.
presents samples prepared with varying concentrations of dextrose ranging between 3–9 mM. The particles have a 3D hollow flower-like shape with more branched edges, highly rough surface, and more multilayers and interconnected channels with ordered cross layers. Particle size ranged between 0.3–1.0 μm (). Particles had distributed internal pores in the range of 20–80 nm. Layer distribution, order, and interconnection increased with increasing dextrose concentration while branched edges decreased with increasing dextrose.
presents samples prepared by varying NaOH concentration ranging between 0.1–0.5 mM. Particles have a 3D hollow flower shape with highly branched edges, highly rough surface, and elongated multilayers. Particle size ranged between 0.2–1.6 μm () with distributed pores in the range of 50–100 nm. The layers, pores, interconnection, and branched edges increased with increasing NaOH concentration.
presents samples prepared with varying concentrations of TSC ranging between 1–6 mM. Particles have a flower-like shape with size between 0.45–2.3 μm (). Increasing TSC concentration above 6 mM resulted in large multiwall hollow scaffold-like structures that ranged in size between 3.0–5.0 μm ().
Experiments were repeated several times and reproducible results were obtained indicating the ability to synthesize 3D hollow flower-like silver nanostructures with the ability to control their sizes and shapes by varying the concentrations of the four chemicals discussed above.
It should be noted that carrying out the syntheses at different temperatures, between 25°C and 70°C, alone or in combination with varying concentrations of different chemicals had no effect on particle size or shape.
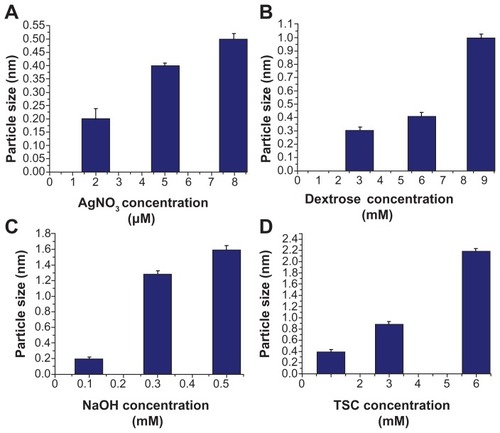
The ultraviolet-visible spectra () of samples prepared with varying concentrations of AgNO3 () showed absorption maxima between 410–450 nm. Samples prepared with varying dextrose concentration showed absorbance bands at 430 nm, 450 nm, and 450–500 nm (). shows absorption spectra of samples prepared with varying NaOH concentrations where absorption maxima were detected between 410–600 nm. shows spectra of samples prepared with varying concentrations of TSC where two absorption bands were detected at 280–300 nm and 500–525 nm. Increasing concentrations of AgNO3, dextrose, NaOH, or TSC resulted in increased size of silver nanostructures, which was reflected by the shift of absorbance bands to longer wavelength.
Figure 4 Ultraviolet absorbance spectra of flower-like silver nanostructures prepared using varying concentrations of silver nitrate (A), dextrose (B), sodium hydroxide (C), or trisodium citrate (D).
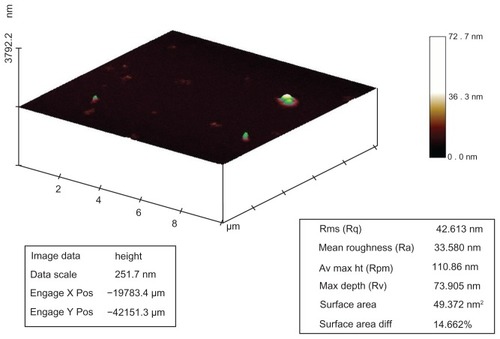
Silver nanostructures were investigated by atomic force microscopy where surface area, roughness, and surface area diffraction were 49.37 nm2, 33.5 nm, and 14.6%, respectively (). Atomic force microscopy studies confirmed the 3D structures of the silver particles.
Figure 5 Atomic force microscopy analysis of silver nanostructures. Sample prepared by reacting silver nitrate 2 μM, dextrose 3 mM, and sodium hydroxide 0.1 mM. Surface area, roughness, and surface area diffraction were 49.37 nm2, 33.5 nm, and 14.6%, respectively.
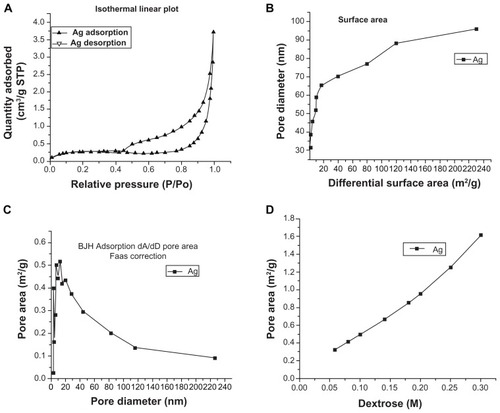
shows the surface area, pore size, pore area, and pore area relative to varying concentrations of dextrose. Porosimetry studies indicated that the surface area of 3D hollow flower-like nanostructures is high (25–240 m2/g) due to the presence of interconnected pores. The pore area increased with increased dextrose concentration.
Figure 6 Porosimetry analysis of silver nanostructures. (A) Isothermal linear plot showing adsorbed and desorbed liquid nitrogen on silver nanostructures relative to pressure; (B) surface area calculated by plotting pore diameter against differential surface area; (C) pore area calculated by Barrett–Joyner–Halenda adsorption model and flame atomic absorption spectrometry correction; (D) pore area relative to dextrose concentration.
Conclusion
The dextrose acted as a reducing agent of AgNO3 and as a preliminary capping material. TSC acted as a capping material and together with dextrose enhanced silver dispersion and directed growth of particles to highly anisotropic structures. Growth of particles depended upon concentrations of dextrose and TSC adsorbed on the surface of silver particles. NaOH augmented reduction and supported growth of nanoparticles to 3D hollow flower-like structures.
The uniformity of size and shape of the 3D hollow flower-like silver nanostructures can be controlled by manipulating AgNO3, dextrose, NaOH, and/or TSC concentrations. The smallest flower-like nanostructures (up to 200 nm) were prepared by using 2 μM AgNO3, 3 mM dextrose, and 0.01 mM NaOH. Porosimetry analysis showed 3D hollow nanostructures with high surface area ranging between 25–240 nm2/g and pore area increased with increasing dextrose concentration. Atomic force microscopy studies indicated that the prepared particles have 3D structures with surface roughness of 33.5 nm and surface area diffraction of 14.6%. Because of their unique properties and ease of preparation, the prepared structures may serve as superior vehicles for long-term release of drugs and effective antimicrobial agents and also hold the promise as effective tools for catalysis.
The use of nanoparticles as drug carriers is limited by the toxicity of used reagents, the limited control of pore size and area, and the cost of particle synthesis.Citation20,Citation21 The newly developed method allows effective control of structure porosity, employs environmental friendly reagents, and is fast and inexpensive. Therefore, the synthesized anisotropic structures could open new frontiers as efficient and safe drug carriers. As compared to spherical or nonporous nanoparticles, porous drug carriers with pore size in the range of 5–100 nm provide a larger surface area and improve drug dissolution and encapsulation efficacy.Citation22,Citation23 Noteworthy is the fact that porous nanostructures have allowed the encapsulation of large amounts of biomolecules such as peptides and nucleic acids within a relatively small carrier material mass. The new silver flower-like silver nanostructures have interconnected pores and pore size of 50–200 nm. Their enhanced porosity is predicted to allow improved drug encapsulation and loading capacity and allow their use for sustained drug release.
The antimicrobial effects of silver particles have been correlated to their large surface area which enhances their interaction with microorganisms as well as their stability in bacterial growth medium. Additionally, silver nanoparticles with different shapes have been shown to have different effects on bacteria. For spherical nanoparticles, a total silver content of 12.5 μg was needed to show bacterial inhibition, whereas rod-shaped particles needed a total of 50–100 μg of silver content to show a similar effect.Citation24 The new silver nanostructures have very large surface areas and were stable in solution up to 1 month after synthesis, and thus are predicted to have enhanced antibacterial effect. The antibacterial effect of the synthesized flower-like nanostructures will be investigated.
Silver nanoparticles exhibit enhanced absorbance and scattering properties. These properties are dependent on particle shape and size and have been utilized for clinical diagnostics. Zhou et al used triangular silver nanoparticle arrays for spectroscopic detection of p53, a marker of neck squamous cell carcinoma, in serum.Citation25 Also, Shanmukh et al developed a spectroscopic assay, based on surface enhanced Raman scattering, using silver nanorod arrays for detection and characterization of respiratory viruses.Citation26 Therefore, silver flower-like structures with their unique shape and controlled size warrant further investigation for potential utilization in clinical diagnostics.
Acknowledgments
The authors acknowledge Ms Mai Mansour (Yousef Jameel Science and Technology Research Center) for her editorial support and Mr Ramy Wasfy (Yousef Jameel Science and Technology Research Center) for his help with scanning electron microscopy. This work has been funded by a grant from Mr Yousef Jameel to Hassan Azzazy.
Disclosure
The authors report no conflicts of interest in this work.
References
- BurdaCChenXNarayananREl-SayedMAChemistry and properties of nanocrystals of different shapesChem Rev200510541025110215826010
- ChenSCarrollDLSilver nanoplates: size control in two dimensions and formation mechanismsJ Phys Chem B20041081855005506
- HoelderichWFEnvironmentally benign manufacturing of fine and intermediate chemicalsCatal Today2000621115130
- HuJOdomTWLieberCMChemistry and physics in one dimension: synthesis and properties of nanowires and nanotubesAcc Chem Res1999325435445
- PeticaAGavriliuSLunguMBurunteaNPanzaruCColloidal silver solutions with antimicrobial propertiesMater Sci Eng B20081521–32227
- El-SayedMASome interesting properties of metals confined in time and nanometer space of different shapesAcc Chem Res200134425726411308299
- ZhuYShiJShenWStimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core–shell structureAngew Chem Int Ed2005443250835087
- WeiWMaGHHuGPreparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrierJ Am Chem Soc200813047158081581018980322
- KorobkoAVJesseWvan der MaarelJREncapsulation of DNA by cationic diblock copolymer vesiclesLangmuir2005211344215620282
- ChenJWileyBMcLellanJXiongYLiZYXiaYOptical properties of Pd-Ag and Pt-Ag nanoboxes synthesized via galvanic replacement reactionsNano Lett20055102058206216218737
- ChenCWSerizawaTAkashiMPreparation of platinum colloids on polystyrene nanospheres and their catalytic properties in hydrogenationChem Mater199911513811389
- JenekheSAChenXLSelf-assembly of ordered microporous materials from rod-coil block copolymersScience199928354003723759888850
- ZhangSFabrication of novel biomaterials through molecular self-assemblyNat Biotechnol200321101171117814520402
- ZhuLPLiaoGHYangYXiaoHMWangJFFuSYSelf-assembled 3D flower-like hierarchical beta-Ni(OH)(2) hollow architectures and their in situ thermal conversion to NiONanoscale Res Lett20094655055720596326
- SunYMayersBHerricksTXiaYPolyol synthesis of uniform silver nanowires: a plausible growth mechanism and the supporting evidenceNano Lett200337955960
- SunYYinYMayersBTHerricksTXiaYUniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone)Chem Mater2002141147364745
- MurphyCJJanaNRControlling the aspect ratio of inorganic nanorods and nanowiresAdv Mater20021418082
- WileyBSunYXiaYSynthesis of silver nanostructures with controlled shapes and propertiesAcc Chem Res200740101067107617616165
- NiCHassanPAKalerEWStructural characteristics and growth of pentagonal silver nanorods prepared by a surfactant methodLangmuir20052183334333715807571
- LiongMLuJKovochichMMultifunctional inorganic nanoparticles for imaging, targeting, and drug deliveryACS Nano20082588989619206485
- LouXWArcherLAYangZHollow micro-/nanostructures: synthesis and applicationsAdv Mater2008202139874019
- HorcajadaPRámilaAPérez-ParienteJVallet-RegíMInfluence of pore size of MCM-41 matrices on drug delivery rateMicroporous Mesoporous Mater2004681–3105109
- LimnellTRiikonenJSalonenJSurface chemistry and pore size affect carrier properties of mesoporous silicon microparticlesInt J Pharm20073431–214114717600644
- PalSTakYKSongJMDoes the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coliAppl Environ Microbiol20077361712172017261510
- ZhouWMaYYangHDingYLuoXA label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinomaInt J Nanomedicine2011638138621468351
- ShanmukhSJonesLDriskellJZhaoYDluhyRTrippRARapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrateNano Lett20066112630263617090104