?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Solid tumors need new blood vessels to feed and nourish them as well as to allow tumor cells to escape into the circulation and lodge in other organs, which is termed “angiogenesis.” Some tumor cells within solid tumors can overexpress integrins αvβ3 and αvβ5, which can specifically recognize the peptide motif Arg-Gly-Asp (RGD). Thus, the targeting of RGD-modified micelles to tumor vasculature is a promising strategy for tumor-targeting treatment.
Methods
RGD peptide (GSSSGRGDSPA) was coupled to poly(ethylene glycol)-modified stearic acid-grafted chitosan (PEG-CS-SA) micelles via chemical reaction in the presence of N,N′-Disuccinimidyl carbonate. The critical micelle concentration of the polymeric micelles was determined by measuring the fluorescence intensity of pyrene as a fluorescent probe. The micelle size, size distribution, and zeta potential were measured by light scattering and electrophoretic mobility. Doxorubicin (DOX) was chosen as a model anticancer drug to investigate the drug entrapment efficiency, in vitro drug-release profile, and in vitro antitumor activities of drug-loaded RGD-PEG-CS-SA micelles in cells that overexpress integrins (ανβ3 and ανβ5) and integrin-deficient cells.
Results
Using DOX as a model drug, the drug encapsulation efficiency could reach 90%, and the in vitro drug-release profiles suggested that the micelles could be used as a controlled-release carrier for the hydrophobic drug. Qualitative and quantitative analysis of cellular uptake indicated that RGD-modified micelles could significantly increase the DOX concentration in integrin-overexpressing human hepatocellular carcinoma cell line (BEL-7402), but not in human epithelial carcinoma cell line (Hela). The competitive cellular-uptake test showed that the cellular uptake of RGD-modified micelles in BEL-7402 cells was significantly inhibited in the presence of excess free RGD peptides. In vitro cytotoxicity tests demonstrated DOX-loaded RGD-modified micelles could specifically enhance the cytotoxicity against BEL-7402 compared with DOX-loaded PEG-CS-SA and doxorubicin hydrochlorate.
Conclusion
This study suggests that RGD-modified PEG-CS-SA micelles are promising drug carriers for integrin-overexpressing tumor active targeting therapy.
Introduction
It is well-known that cancer cells are inherently more vulnerable to chemotherapy than the majority of normal cells, but most anticancer drugs are nonselective and can cause injury to normal tissues. In recent years, efforts have focused on effective and safe cancer therapy by increasing tumor targeting while sparing normal cells.Citation1 It has successfully been demonstrated that polymers can form effective delivery systems for anticancer drugs via an enhanced permeation and retention (EPR) effect, termed as “passive targeting.”Citation2 Polymeric micelles as drug delivery systems (DDSs) were introduced in the early 1990s by Kataoka’s group through the development of doxorubicin-conjugated block copolymer micelles.Citation3 They have also been used for the delivery of many anticancer agents in preclinical and clinical studies.Citation4–Citation6
However, since most clinical conditions do not allow passive targeting, active targeting, where the nanoparticles are delivered to the targets by specific ligands, becomes necessary. Active-targeted delivery of anticancer drugs is a promising approach for delivery of nanoparticle systems. Long circulation times will allow effective transport of nanoparticles to the tumor site through the EPR effect, and the targeting molecule can increase endocytosis of the nanoparticles.Citation7 Polymeric micelles are the only reported DDS that conquers multiple-drug resistance through absolute targeting using various approaches such as passive targeting, folate-mediated DDSs, and pH-sensitive and thermosensitive DDSs.Citation8
Chitosan (CS), a biodegradable, biocompatible polysaccharide with low toxicity and immunity system induction,Citation9 has been widely accepted as a potential material for drug delivery carriers.Citation10,Citation11 Poly(ethylene glycol) (PEG) is a nontoxic and nonirritant hydrophilic polymer.Citation12 PEGylation is one of the most popular methods to overcome the uptake of polymeric micelles by macrophage, which may reduce the half-life of micelles in blood. As a result, PEGylation may improve the stability of the drug-loaded micelles and prolong circulation in blood.Citation13–Citation15
Previously, the authors developed PEGylated stearic acid-grafted CS (PEG-CS-SA), which is an amphiphilic block copolymer that can self-aggregate into spherical micelles in an aqueous medium. Importantly, it was found that PEG-CS-SA micelles can be rapidly internalized into tumor cells and overcome the uptake by macrophage. However, the PEG-CS-SA micelles could also be internalizated into normal cells.Citation16
By coupling specific tumor-homing ligands, such as antibodies, sugar, folate, or peptides, to the nanoparticles, an active targeting of drug to cancer cells with fewer side effects was obtained. Solid tumors need new blood vessels to feed and nourish them as well as to allow tumor cells to escape into the circulation and lodge in other organs, which is termed “angiogenesis.”Citation17 Some tumor cells within solid tumors would overexpress integrins ανβ3 and ανβ5, which can specifically recognize the peptide motif Arg-Gly-Asp (RGD).Citation18 Thus, the targeting of RGD-modified micelles to tumor vasculature is a promising strategy for tumor-targeting treatment.
Here, by coupling linear RGD to the PEG-CS-SA micelles, a novel tumor vasculature-targeting polymeric micelle was developed. These micelles were termed “RGD-PEG-CS-SA micelles.” The physicochemical properties, such as critical micelle concentration (CMC), micelle size, and zeta potential, were investigated in detail. Doxorubicin (DOX) was chosen as a model anticancer drug to investigate the drug entrapment efficiency, in vitro drug-release profile, and in vitro antitumor activities of drug-loaded RGD-PEG-CS-SA micelles in human hepatocellular carcinoma cell line (BEL- 7402) (which overexpress integrins ανβ3 and ανβ5) and human epithelial carcinoma cell line (Hela) (integrins ανβ3 and ανβ5 deficient).
Materials
CS of low molecular weight (Mw = 5.0 kDa) was prepared by enzymatic degradation of CS (95% acetylate, Mw = 450 kDa; Yuhuan Marine Biochemistry Co, Ltd, Zhejiang, China) as described in a previous work.Citation19 Stearic acid (SA) was purchased from Shanghai Chemical Reagent Co, Ltd, (Shanghai, China) and mPEG2000 with aldehyde side group, NH2-PEG-NH2, fluorescein isothiocyanate (FITC) and 3-(4, 5-dimethylthiazol- 2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Inc, (St Louis, MO). N,N′-Disuccinimidyl carbonate (DSC) was purchased from Bio Basic Inc, (Toronto, Canada). Di-tert-butyl dicarbonate ((Boc)2O) and 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDC) were purchased from Shanghai Medpep Co, Ltd, (Shanghai, China). Pyrene was purchased from Aldrich Chemical Co, (Milwaukee, WI). Doxorubicin hydrochlorate (DOX·HCl) was gifted from Zhejiang Hisun Pharmaceutical Co, Ltd, (Taizhou Zhejiang, China). Chitosanase was purchased from Dyadic International Inc, (Jupiter, Florida). Trypsin and Dulbecco’s modified Eagle’s medium were purchased from Gibco-BRL Life Technologies (Carlsbad, CA). Fetal bovine serum was purchased from Sijiqing Biology Engineering Materials Co, Ltd, (Zhejiang, China). All other chemicals used were of analytical or chromatographic grade.
Methods
Synthesis of SA-grafted CS copolymer (CS-SA) and PEGylated CS-SA (PEG-CS-SA) copolymers
CS-SA was synthesized by EDC-mediated coupling reaction of amino groups of low-molecular-weight CS and a carboxyl group of SACitation20,Citation21 with some modification. Briefly, 2.0 g CS was dissolved in 125 mL deionized (DI) water, and 0.84 g SA with 5.6 g EDC was dissolved in 100 mL anhydrous ethanol, followed by stirring in water bath at 60°C for 1 hour. The mixture was then added to the CS solution at 80°C under mechanical stirring, and the reaction was carried out for 5 hours at 80°C under mechanical stirring at 250 rpm. After the reaction, the reaction solution was dialyzed against DI water using a dialysis membrane (MWCO 3.5 kDa; Spectrum Laboratories, Laguna Hills, CA) for 48 hours to remove byproduct and this was followed by freeze drying. Then, the lyophilized powder was washed with ethanol three times to remove the unreacted SA. Finally, the purified product was lyophilized and stored at −20°C until further use.
To obtain PEG-CS-SA, 200 mg CS-SA was dissolved in 30 mL DI water. Then 55 mg mPEG2000 with aldehyde side group (PEG:CS-SA = 1:1, mol:mol) was added to the CS-SA solution. The solution was stirred overnight at room temperature and then dialyzed against DI water using a dialysis membrane (MWCO: 7 kDa; Spectrum Laboratories) for 24 hours. The final product was lyophilized.
Synthesis of RGD-modified PEGylated CS-SA (RGD-PEG-CS-SA) copolymers
Briefly, 10 mg of RGD peptide was dissolved in dried dimethyl sulfoxide (DMSO), and 3.1 μL (Boc)2O was added into the solution. The reaction was carried out for 24 hours at 40°C to protect the amine group of RGD. Then, 19.8 mg of EDC and 20.8 mg of NH2-PEG-NH2 were added, followed by stirring at 40°C for 24 hours. After that, the reaction solution was reacted with N,N′-Disuccinimidyl carbonate (DSC, NH2-PEG-NH2: DSC = 1:1, mol:mol) for 12 hours at 40°C to obtain succinimidyl t-Boc-RGD-PEG-NH2 solution.
To obtain RGD-PEG-CS-SA, CS-SA was added into the reaction solution (CS-SA: succinimidyl t-Boc-RGD-PEG-NH2 = 1:1, mol:mol) and the reaction was maintained at 40°C for 24 hours. To remove the protection of (Boc)2O to RGD peptide, 12 M hydrochloric acid was added, followed by reacting for 2 hours. Then the pH of the reaction solution was adjusted to 7.0 using 3 M sodium hydroxide. Finally, the reaction solution was dialyzed using a dialysis membrane (MWCO 14 kDa; Spectrum Laboratories) against DI water for 24 hours, followed by lyophilization.
Preparation of DOX-loaded copolymer micelles
DOX base, used for the preparation of DOX-loaded micelles, was obtained by the reaction between DOX·HCl and double mole triethylamine in DMSO for 24 hours.Citation22 DOX-loaded micelles were prepared by dialysis method.Citation23 To obtain DOX-loaded micelles, the DOX DMSO solution (DOX concentration was 1 mg/mL) was added dropwise to 5 mL PEG-CS-SA and RGD-PEG-CS-SA micelles solution (polymers concentration was 2 mg/mL). The solution was then dialyzed using a dialysis membrane (MWCO 7.0 kDa; Spectrum Laboratories). The dialyzed solution was then diluted with DI water to 10 mL (final concentration of micelles was 1 mg/mL).
Characteristics of PEG-CS-SA, RGD-PEG-CS-SA and DOX-loaded copolymer micelles (PEG-CS-SA/DOX and RGD-PEG-CS-SA/DOX)
CMC
The CMC of PEG-CS-SA and RGD-PEG-CS-SA were determined by fluorescence measurement using pyrene as a probe.Citation21,Citation24 The fluorescence emission spectra of pyrene (6 × 10−7 M) in different concentrations (varying from 1 × 10−3 to 0.25 mg/mL) of PEG-CS-SA or RGD-PEG-CS-SA solution were measured using a fluorescence spectrophotometer (F-2500, Hitachi Co, Japan), with the excitation wavelength set at 334 nm. The slits were set at 10 nm (excitation) and 2.5 nm (emission). The intensities of I1 and I3 were measured at the wavelengths corresponding to the first- and thirdhighest energy bands, which were 374 nm and 385 nm, respectively. Then the intensity ratio of I1 to I3 (I1/I3) in the pyrene emission spectra was calculated.
Determination of micelle size and zeta potential
The micelle size and zeta potential of blank micelles and DOX-loaded micelles were measured by dynamic light scattering using a Zetasizer (3000HS; Malvern Instruments Ltd, Worcestershire, UK).
Drug encapsulation efficiency and drug loading
The drug encapsulation efficiency and drug loading of DOX-loaded micelles were measured by the centrifugal-ultrafiltration method: 0.4 mL of DOX-loaded micelles was added into centrifugal-ultrafiltration tubes (MWCO 3.0 kDa, Microcon YM-10; Millipore Co, Billerica, MA), and centrifuged at 10,000 rpm for 30 minutes. The amount of DOX in ultrafiltrate (C) was determined by measuring the fluorescent intensity (excitation: 498 nm; emission: 553 nm) using a fluorescence spectrophotometer (F-2500; Hitachi Co, Tokyo, Japan). The total amount of DOX (C0) was measured after dissolving the micelles with DMSO aqueous solution (DMSO:H2O = 9:1, v/v) to dissociate the DOX-loaded micelles.
The drug encapsulation efficiency (EE, %) and drug loading (DL, %) of DOX-loaded micelles was then calculated by the following equations.
where C is the DOX concentration in ultrafiltrate; C0 is the DOX concentration in the DOX-loaded micelles solution; and Cm is the concentration of copolymer in the DOX-loaded micelles solution.
In vitro drug-release behaviors of DOX-loaded micelles
In vitro drug-release profiles from DOX-loaded micelles were performed by using pH 7.4 phosphate-buffered saline (PBS) as dissolution media. One milliliter of DOX-loaded micelles solution (DOX concentration 5 μg/mL) was put into a dialysis membrane (MWCO 3.5 kDa) and added into a plastic tube containing 10 mL of PBS solution. The plastic tube was then placed in an incubator shaker (HZ-8812S; Scientific and Educational Equipment Plant, Tai Cang, China), which was maintained at 37°C and shaken horizontally at 100 rpm. At certain time intervals, medium was withdrawn and changed with fresh medium. The DOX content was measured by fluorescence spectrophotometer (excitation: 498 nm; emission: 553 nm).
Cell culture
BEL-7402 and Hela cells were obtained from the Cell Resource Center (Chinese Academy of Medical Sciences, Beijing, China). The cells were cultured in Dulbecco’s modified Eagle’s medium at 37°C with 5% CO2 under fully humidified conditions. All the mediums were supplemented with 10% (v/v) fetal bovine serum and penicillin/streptomycin (100 U · mL−1, 100 U · mL−1). Cells were subcultured regularly using trypsin/ethylenediaminetetraacetic acid.
In vitro cytotoxicity assay
A comparison of cytotoxicity was performed on the test cells with an in vitro proliferation using the MTT method.Citation25 Briefly, BEL-7402 and Hela cells were plated in 96-well plates at a density of 1 × 104 cells/well in 200 μL of complete medium and incubated for 24 hours to allow the cells to attach. Then the cells were exposed to serial concentrations of DOX- loaded micelles at 37°C for 48 hours. At the end of incubation, 20 μL of the MTT solution was added and incubated at 37°C for another 4 hours, and then the media was replaced with 100 μL DMSO to dissolve the MTT formazan crystals. Plates were shaken for 10 minutes and the absorbance was measured at 570 nm in a microplate reader (Model 680; BioRad, Hercules, CA).
In vitro cellular uptake
BEL-7402 and Hela cells were seeded in a 24-well plate (Nalge Nunc International, Naperville, IL) and incubated for 24 hours to allow the cells to attach. A certain amount of free DOX·HCl and DOX-loaded micelles (DOX concentration 5 μg/mL) were added, and the cells were further incubated for different lengths of time (2 hours, 8 hours, and 24 hours). After washing the cells with PBS (pH 7.4) three times, the cellular uptake was observed by fluorescence microscope (DMIL; Leica Microsystems Ltd, Wetzlar, Germany).
Quantitative analysis of DOX content internalized into cells
BEL-7402 and Hela cells were seeded in a 24-well plate (Nalge Nunc International) and incubated for 24 hours to allow the cells to attach. After the cells were incubated with free DOX·HCl and DOX-loaded micelles (DOX concentration 5 μg/mL) for different lengths of time (2 hours, 4 hours, 6 hours, 10 hours, and 24 hours), the medium was removed and the cells were rinsed three times with PBS (pH 7.4). Trypsin PBS solution (50 μL) with 2.5 mg/mL concentration was then added and further incubated for 15 minutes. Then the cells were harvested by adding 950 μL DMSO solution followed by sonicate treatment in an ice water bath for 30 minutes to ensure complete lysis. After the suspension was centrifuged at 4000 rpm for 5 minutes, the drug content in the supernatant was measured by fluorescence spectrophotometer. The DOX concentration in cells was normalized with the protein content in cells, which was quantified using a BCA (bicinchoninic acid) protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China).Citation26 Briefly, the BCA working solution was preprepared by mixing the reagent A with reagent B (reagent A: B = 50:1, v/v) for further use. Then, 20 μL samples with a series of concentrations were added into 96-well plate, followed by incubated with 200 μL BCA working solution at 37°C. After 30 minutes, the assay was carried out at 570 nm by using a microplate reader (Model 680; BioRad, Hercules, CA). Typical standard curve for bovine serum albumin were used in the BCA protein assay.
To further investigate the role of RGD peptide in cellular uptake, RGD competition studies were performed. Briefly, BEL-7402 and Hela cells were pretreated with free RGD peptides for 30 minutes, and then incubated with DOX-loaded micelles. The cellular uptake percentage was determined as described above.
Statistical analysis
Data were expressed as means of the three separate experiments. The significances of the differences were determined using Student’s t-test two-tailed for each paired experiment. A P-value <0.05 was considered statistically significant in all cases.
Results and discussion
Synthesis of CS-SA, PEG-CS-SA, and RGD-PEG-CS-SA
The CS-SA was synthesized by coupling reaction between the amino groups of CS and carboxyl group of SA in the presence of water-soluble EDC, and the PEG-CS-SA was conducted by the reaction between the remaining amino groups of CS-SA and aldehyde group of PEG.
The t-Boc-RGD-PEG-NH2 was prepared by the chemical reaction between RGD (its amino terminus preprotected by (Boc)2O) and NH2-PEG-NH2 in the presence of EDC. The t-Boc-RGD-PEG-CS-SA was synthesized by conjugating the remaining amino groups of NH2-PEG-NH2 and the remaining primary amino groups in CS-SA in the presence of DSC. presents the synthesis scheme of RGD- PEG-CS-SA.
Characteristics of PEG-CS-SA, RGD-PEG-CS-SA and DOX-loaded micelles
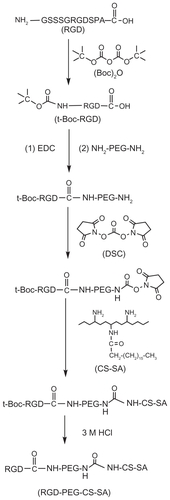
The CMC can indicate the self-aggregation ability of the amphiphilic copolymers. shows the change in the value of I1/I3 against the logarithm of PEG-CS-SA or RGDPEG- CS-SA concentration. When in the lower polymer concentration, the value of I1/I3 stayed almost constant. Once the polymer concentration reached above the CMC, the value of I1/I3 reduced sharply, indicating the formation of micelles.Citation27 There was no significant difference in the CMC values of two kinds of copolymers, and the CMC values of PEG-CS-SA and RGD-PEG-CS-SA were determined to be 25.9 ± 1.2 μg/mL and 23.5 ± 0.5 μg/mL in DI water, respectively. The CMC values were much lower than those of low-molecular-weight surfactants in water, showing that they can easily self-aggregate to form micelles in DI water.
Figure 2 Characteristics of copolymers and blank micelles. (A) Change of fluorescence intensity ratio (I374/I385) for pyrene in distilled water versus the logarithm of concentration of copolymers. The unit of concentration was μg/mL. Poly(ethylene glycol)-modified stearic acid-grafted chitosan (PEG-CS-SA) (Δ); RGD-PEG-CS-SA (□). (B) Size distribution of blank RGD-PEG-CS-SA and (C) DOX-loaded RGD-PEG-CS-SA micelles obtained by DLS.
Abbreviations: DLS, dynamic light scattering; RGD, Arg-Gly-Asp.
The particle size and surface zeta potential of blank and DOX-loaded micelles (1 mg/mL in DI water) are shown in . show the size distribution of blank RGD-PEG-CS-SA and DOX-loaded RGD-PEG-CS-SA micelles, which were obtained by dynamic light scattering determination, suggesting that the micelles had a narrow size distribution.
Table 1 Size and zeta potential of blank and DOX-loaded micelles with 1 mg/mL concentration
The size and distribution of micelles may play important roles in transport in vivo. Micelles with smaller size tended to accumulate easily in tumor sites due to the EPR effect, and gained faster internalization into cells.Citation28,Citation29 The results showed that the sizes of DOX-loaded micelles were smaller than those of blank micelles, which may benefit from hydrophobic interaction between poorly water-soluble drug and the hydrophobic cores of polymeric micelles. It was also found that the zeta potentials were reduced after the loading of drug, which may be because the change of the size and surface charge density.
The drug encapsulation efficiency and drug loading of DOX-loaded PEG-CS-SA and RGD-PEG-CS-SA micelles are shown in . The results indicate that there was no significant difference in the encapsulation efficiency of micelles after further modification by RGD peptide, suggesting that modification of RGD peptide did not affect the drug-entrapment ability of PEG-CS-SA micelles.
Table 2 Drug-loading capacity and encapsulation efficiency of PEG-CS-SA and RGD-PEG-CS-SA micelles loading DOX
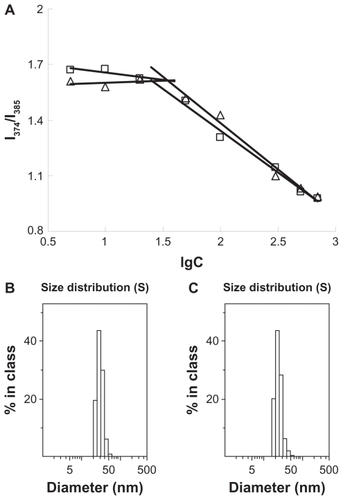
In vitro drug-release behaviors
In vitro DOX release profiles of DOX-loaded PEG-CS-SA and RGD-PEG-CS-SA micelles in pH 7.4 PBS are shown in . The in vitro release of DOX from DOX-loaded micelles could be prolonged for 9 days, and almost 35% of DOX was released during 48 hours. As shown in , the release rate of DOX from RGD-PEG-CS-SA micelles decreased significantly as compared with PEG-CS-SA/DOX micelles. These results indicate that RGD-PEG-CS-SA micelles could be employed as a drug carrier for retarding DOX release, which is helpful for decreasing drug leakage from carriers before drug-loaded carriers arrive at the target cells.
Figure 3 In vitro doxorubicin (DOX)-release profiles from poly(ethylene glycol)-modified stearic acid-grafted chitosan (PEG-CS-SA)/DOX (⋄) and RGD-PEG-CS-SA/DOX (Δ) micelles using pH 7.4 phosphate-buffered saline.
Note: Data represent the mean ± standard deviation (n = 3).
Abbreviation: RGD, Arg-Gly-Asp.
In vitro cytotoxicity assay
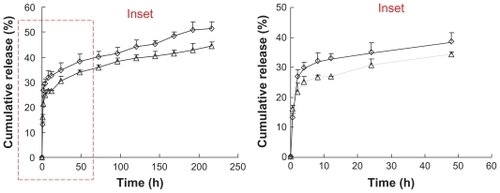
The cytotoxicity of blank and DOX-loaded micelles against BEL-7402 and Hela cells was evaluated using the MTT test. The variation of cell viability against the drug concentration of DOX·HCl solution and DOX-loaded micelles is shown in .
Figure 4 Cytotoxicity of DOX · HCl, poly(ethylene glycol)-modified stearic acid-grafted chitosan (PEG-CS-SA)/DOX and RGD-PEG-CS-SA/DOX toward (A) human hepatocellular carcinoma cell line cells and (B) human epithelial carcinoma cell line cells.
Note: Data represent the mean ± standard deviation (n = 3).
Abbreviations: DOX, doxorubicin; HCl, hydrochloric acid; RGD, Arg-Gly-Asp.
The IC50 values of PEG-CS-SA and RGD-PEG-CS-SA micelles against BEL-7402 and Hela cells were determined and these ranged from 331.2 ± 1.8 μg/mL to 490.5 ± 2.9 μg/mL, indicating that the micelles were low-toxicity carriers for cancer therapy, compared with other cationic polymers like polyethylenimine and its derivatives.Citation30
The in vitro cytotoxicities of DOX-loaded micelles were evaluated and compared with free DOX·HCl. It was found that the antitumor activities were significantly increased when DOX was loaded into micelles, and the antitumor activities against BEL-7402 cells (which overexpress integrins ανβ3 and ανβ5) were further increased after the modification of PEG- CS-SA micelles with RGD peptide (by 7.2 fold, P < 0.05). The enhanced cytotoxicities of DOX-loaded micelles, especially RGD-modified DOX-loaded micelles, were due to the faster internalization of DOX into cells mediated by the micelles and integrin-mediated endocytosis. Moreover, the modification of DOX-loaded PEG-CS-SA micelles by RGD peptide would not affect the cytotoxicity of micelles against Hela cells, which did not overexpress integrins ανβ3 and ανβ5.
In vitro cellular uptake
Using BEL-7402 and Hela cells as model tumor cells, the in vitro cellular uptakes of DOX-loaded micelles were carried out. The cellular uptake was observed by fluorescence microscope after the cells were incubated with free DOX·HCl, DOX-loaded PEG-CS-SA, and RGD-PEG-CS-SA micelles solution for different incubation times. shows the fluorescence images of DOX against BEL-7402 and Hela cells with incubation times of 2 hours, 8 hours, and 24 hours, respectively.
Figure 5 Fluorescence images of doxorubicin (DOX) after (A) human hepatocellular carcinoma cell line cells and (B) human epithelial carcinoma cell line cells were incubated with free DOX, poly(ethylene glycol)-modified stearic acid-grafted chitosan (PEG-CS-SA)/DOX and RGD-PEG-CS-SA/DOX micelles for 2 hours, 8 hours, and 24 hours, respectively.
Abbreviation: RGD, Arg-Gly-Asp.
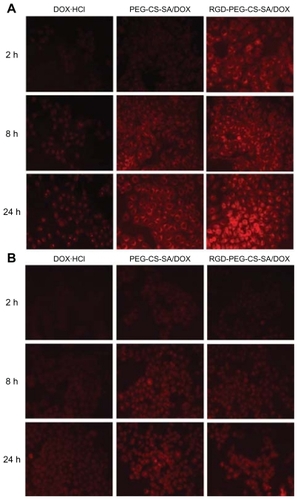
It was clear that the DOX fluorescence intensities were increased with the incubation time, which demonstrated that cellular uptakes of free DOX·HCl, DOX-loaded PEG-CS-SA, and RGD-PEG-CS-SA micelles were time dependent. At the same incubation time, the DOX fluorescence intensity in BEL-7402 and Hela cells was enhanced when DOX was loaded into micelles. Furthermore, the cellular uptakes of DOX in BEL-7402 cells were further enhanced after the modification of PEG-CS-SA micelles with RGD peptide, while not in Hela cells.
The RGD-modified micelles displayed much faster and more cellular uptake for BEL-7402 cells, which may be due to the specific binding of RGD-modified micelles to BEL- 7402 cells and internalization into cells by integrin-mediated endocytosis.
Quantitative analysis of DOX content internalized into cells
To further confirm whether RGD-modified DOX-loaded micelles could be specifically internalized into BEL-7402 cells, the DOX contents internalized into cells were quantitatively evaluated. To further investigate the role of RGD peptide in the cellular uptake of RGD-PEG-CS-SA micelles, BEL-7402 cells were pretreated with free RGD peptide for 30 minutes to saturate the overexpressing integrins and then incubated with free DOX·HCl and DOX-loaded micelles. Cellular uptake kinetics of micelles were examined for BEL-7402 and Hela cells at elapsed time points, shown in .
Figure 6 Cellular uptake percentage of free DOX, DOX-loaded micelles in (A) human hepatocellular carcinoma cell line cells and (B) human epithelial carcinoma cell line cells against incubation time.
Notes: Data represent the mean ± standard deviation (n = 3). *P > 0.05 and **P < 0.01.
Abbreviations: DOX, doxorubicin; PEG-CS-SA, poly(ethylene glycol)-modified stearic acid-grafted chitosan; RGD, Arg-Gly-Asp.
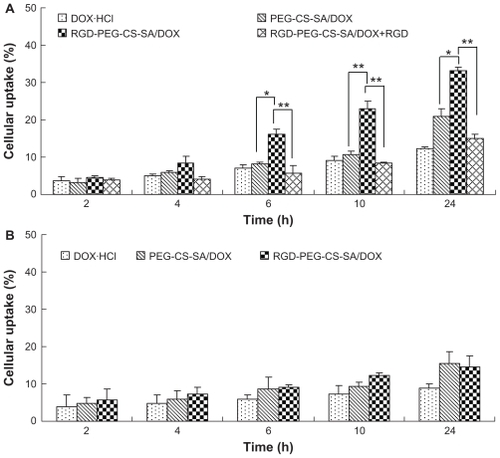
As expected, the quantitative uptakes were in accordance with the qualitative uptakes, thus confirming again that the role of integrin-mediated process in the cellular uptake of RGD-PEG-CS-SA micelles in BEL-7402 cells. Moreover, the cellular uptake of RGD-PEG-CS-SA micelles by BEL- 7402 cells was obviously inhibited after the cells were pretreated by excess free RGD peptide. It indicated that excess free RGD peptide inhibited the cell binding and uptake of the RGD-PEG-CS-SA micelles by competitive binding to integrin on the surface of BEL-7402 cells. Overall, these results show that integrin is responsible for the specific binding and uptake of RGD-modified micelles, and the cell uptake is a receptor-mediated process.
Conclusion
RGD-modified micelles were successfully synthesized and prepared by self-aggregation from copolymer in the aqueous medium with a lower CMC value of 23.5 ± 0.5 μg/mL. The cellular uptake of RGD-PEG-CS-SA micelles was significantly enhanced in the integrins overexpressed cell lines (BEL-7402), compared with the integrins deficient cell lines (Hela). Moreover, the cytotoxicity of DOX-loaded RGD-PEG-CS-SA micelles was improved sharply by 7.2-fold (P < 0.05) compared to free DOX.HCl, contributing to the increased intracellular delivery of the drug. The RGD-modified polymeric micelles that are presented may be a promising active-targeting drug carrier for tumor therapy via integrin mediation.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors are grateful for financial support of National HighTech Research and Development Program (863) of China (2007AA03Z318), the National Nature Science Foundation of China under Contract 81072583, and the Nature Science Foundation of Zhejiang province under Contract Y207785.
References
- DavisMEChenZGShinDMNanoparticle therapeutics: an emerging treatment modality for cancerNat Rev Drug Discov20087977178218758474
- DuYZWengQYuanHHuFQSynthesis and antitumor activity of stearate-g-dextran micelles for intracellular doxorubicin deliveryACS Nano20114116894690220939508
- YokoyamaMKwonGSOkanoTSakuraiYSetoTKKataokaKPreparation of micelle-forming polymer-drug conjugatesBioconjug Chem1992342953011390984
- DuncanRPolymer conjugates as anticancer nanomedicinesNat Rev Cancer2006668870116900224
- BatrakovaEVDorodnychTYKlinskiiEYAnthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activityBrit J Cancer19967410154515528932333
- NakanishiTFukushimaSOkamotoKDevelopment of the polymer micelle carrier system for doxorubicinJ Control Release2001741–329530211489509
- ByrneJDBetancourtTBrannon-PeppasLActive targeting schemes for nanoparticle systems in cancer therapeuticsAdv Drug Deliver Rev2008601516151626
- KedarUPhutanePShidhayeSKadamVAdvances in polymeric micelles for drug delivery and tumor targetingNanomedicine20106201071472920542144
- AlishahiAMirvaghefiATehraniMRChitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the nonspecific immunity system of rainbow trout (Oncorhynchus mykiss)Carbohyd Polym2011861142146
- De CamposAMSánchezAAlonsoMJChitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin AInt J Pharm20012241–215916811472825
- GazoriTKhoshayandMRAziziEYazdizadePNomaniAHaririanIEvaluation of alginate/chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterizationCarbohyd Polym2009773599606
- ZabaletaVCampaneroMAIracheJMAn HPLC with evaporative light scattering detection method for the quantification of PEGs and Gantrez in PEGylated nanoparticlesJ Pharm Biomed Anal20074451072107817587532
- GaoJQEtoYYoshiokaYEffective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administrationJ Control Release2007122110211017628160
- InadaYFurukawaMSasakiHBiomedical and biotechnological applications of PEG- and PM- modified proteinsTrends Biotechnol199513386917766222
- HongJWParkJHHuhKMChungHKwonICJeongSYPEGylated polyethylenimine for in vivo local gene delivery based on lipiodolized emulsion systemJ Control Release200499116717615342189
- HuFQMengPDaiYQPEGylated chitosan-based polymer micelle as an intracellular delivery carrier for anti-tumor targeting therapyEur J Pharm Biopharm200870374975718620050
- FolkmanJAngiogenesis in cancer, vascular, rheumatoid and other diseaseNat Med19951127317584949
- RuoslahtiERGD and other recognition sequences for integrinsAnnu Rev Cell Dev Biol199612679715
- HuFQRenGFYuanHDuYZZengSShell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxelColloids Surf Biointerfaces200650297103
- HuFQZhaoMDYuanHYouJDuYZZengSA novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studiesInt J Pharm20063151–215816616632285
- YouJHuFQDuYZYuanHPolymeric micelles with glycolipid-like structure and multiple hydrophobic domains for mediating molecular target delivery of paclitaxelBiomacromolecules2007882450245617661518
- LeeESNaKBaeYHDoxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumorJ Control Release2005103240541815763623
- LeeESNaKBaeYHPolymeric micelle for tumor pH and folate-mediated targetingJ Control Release2003911–210311312932642
- Molina-BolívarJAHierrezueloJMCarnero RuizCSelf-assembly, hydration, and structures in N-decanoyl-N-methylglucamide aqueous solutions: effect of salt addition and temperatureJ Colloid Interface Sci2007313265666417532330
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- MahmudALavasanifarAThe effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cellsColloid Surf B Biointerfaces20054528289
- ChenWChenHRHuJHYangWLWangCCSynthesis and characterization of polyion complex micelles between poly (ethylene glycol)-grafted poly (aspartic acid) and cetyltrimethyl ammonium bromideColloid Surf A: Physicochem Eng Asp20062781–36066
- MaedaHThe enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targetingAdv Enzyme Regul20014118920711384745
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000651–227128410699287
- BrownlieAUchegbuIFSchätzleinAGPEI-based vesicle-polymer hybrid gene delivery system with improved biocompatibilityInt J Pharm20042741–2415215072781