?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
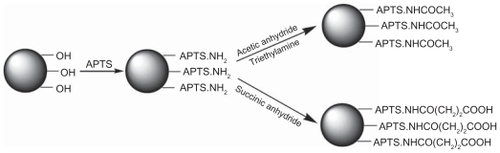
We report on aminopropyltriethoxysilane (APTS)-mediated surface modification of nanohydroxyapatite with different surface functional groups for potential biomedical applications. In this study, nanohydroxyapatite covalently linked with APTS (n-HA-APTS) was reacted with acetic anhydride or succinic anhydride to produce neutralized (n-HA-APTS. Ac) or negatively charged (n-HA-APTS.SAH) nanohydroxyapatite, respectively. Nanohydroxyapatite formed with amine, acetyl, and carboxyl groups was extensively characterized using Fourier transform infrared spectroscopy, transmission electron microscopy, 1H nuclear magnetic resonance spectroscopy, X-ray diffraction, inductively coupled plasma-atomic emission spectroscopy, and zeta potential measurements.
Results
In vitro 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay revealed that the slight toxicity of the amine-functionalized n-HA-APTS could be eliminated by post-functionalization of APTS amines to form acetyl and carboxyl groups. Blood compatibility assessment demonstrated that the negligible hemolytic activity of the pristine nanohydroxyapatite particles did not appreciably change after APTS-mediated surface functionalization.
Conclusion
APTS-mediated functionalization of nanohydroxyapatite with different surface groups may be useful for further functionalization of nanohydroxyapatite with biologically active materials, thereby providing possibilities for a broad range of biomedical applications.
Introduction
Recent advances in nanotechnology have shown that inorganic nanoparticles have attracted immense scientific and technological interest in the areas of catalysis,Citation1,Citation2 sensors,Citation3,Citation4 molecular imaging,Citation5–Citation8 tissue engineering,Citation9 and drug delivery.Citation10 This is largely due to their nanometer size, unique chemical and physical properties, and tailored surface functionality. Nanohydroxyapatite with the structural formula of Ca10(PO4)6(OH)2 is the principal inorganic ingredient of bone and teeth of the human body.Citation11 Studies from laboratory research on therapeutic applications have demonstrated that nanohydroxyapatite has a similar crystallographic structure to that of natural bone and excellent biological characteristics, such as nonimmunogenicity, noninflammatory behavior, good biocompatibility, and high osteoconductivity and osteoinductivity.Citation12,Citation13 These distinct features enable a wide range of potential applications of nanohydroxyapatite as a gene carrier,Citation14 and in bone tissue engineering,Citation15 drug delivery,Citation16 and orthopedic surgery.Citation17
As a structural material, nanohydroxyapatite possesses some inherent imperfections, such as brittleness,Citation18–Citation21 difficulties in surface functionalization,Citation22 and weakened colloidal stability in a nanocrystal form.Citation23 Consequently, much effort has been devoted to improving the dispersity and colloidal stability of nanohydroxyapatite and promoting the mechanical durability.Citation12,Citation24–Citation30 Most of these studies have involved incorporating nanohydroxyapatite into a polymer matrix to form organic/inorganic hybrid composites in the form of membranesCitation12,Citation24,Citation25 or nanofibers,Citation26,Citation27 However, these composites prepared by simply mixing nanohydroxyapatite with a polymer matrix usually lack strong molecular interaction between organic and inorganic phases which may give rise to compromised processing ability and structural damage to nanohydroxyapatite in some cases.Citation31 Hence, finding an approach to modifying or functionalizing nanohydroxyapatite without compromising its physicochemical properties is of vital importance for its biomedical applications.
Aminopropyltriethoxysilane (APTS) is a common silane agent with amino groups that can be further functionalized with general bioconjugation techniques.Citation32 In an aqueous environment, APTS can be hydrolyzed to form silanol groups, which are able to react with hydroxyl groups onto particle surfaces or planar surfaces. For instance, magnetic Fe3O4 and TiO2 nanoparticles with surface hydroxyl groups can be successfully modified with APTS.Citation33–Citation36 In a recent study, Balasundaram et alCitation37 reported the surface grafting of hydroxyapatite compacts with APTS for further conjugation with an arginine-glycine-aspartic acid (RGD) peptide sequence for bone tissue engineering. In their study, the APTS aminemodified hydroxyapatite compacts were able to be activated by N-succinimidyl-3-maleimido propionate for RGD peptide conjugation. That study clearly indicates that hydroxyapatite with a hydroxyl surface is able to be modified with APTS for further bioconjugation. It is reasonable to hypothesize that single nanohydroxyapatite particles are also able to be modified in a similar way, thereby providing many opportunities for their further biomedical applications.
In the present study, we attempted to modify nanohydroxyapatite with APTS, and then the APTS amine groups on the surface of nanohydroxyapatite were further modified with acetic anhydride and succinic anhydride to generate surface charge neutral and negatively charged nanohydroxyapatite particles, respectively. The functionalized nanohydroxyapatite derivatives were extensively characterized using Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), 1H nuclear magnetic resonance (NMR), X-ray diffraction, inductively coupled plasma-atomic emission spectroscopy (ICP-AES), and zeta potential measurement. The cytotoxicity of these functionalized nanohydroxyapatite derivatives was tested by an MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) colorimetric assay of mouse fibroblasts (L929 cells). The blood compatibility of these functionalized nanohydroxyapatite derivatives was assessed by in vitro hemolysis assay. To our knowledge, this is the first report related to the modification of nanohydroxyapatite with APTS (n-HA-APTS) and the systematic modification of n-HA-APTS to generate nanohydroxyapatite with different surface charges. We anticipate that the findings from this study will be beneficial for the development of nanohydroxyapatite-based composite materials for various biomedical applications.
Methods and materials
Materials
Nanohydroxyapatite was purchased from Aladdin Chemical Reagent Co, Ltd (Shanghai, China). APTS and MTT were obtained from J and K Chemical Co, Ltd (Shanghai, China). Acetic anhydride and succinic anhydride were from Sigma-Aldrich (St Louis, MO). L929 cells were from the Institute of Biochemistry and Cell Biology, the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle’s medium, fetal bovine serum, penicillin, and streptomycin were purchased from Hangzhou Jinuo Biomedical Technology (Hangzhou, China). All other chemicals were from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China) and used as received. Water used in all experiments was purified using a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA) with resistivity higher than 18 MΩ · cm.
Modification of nanohydroxyapatite by APTS
As shown in , nanohydroxyapatite was treated with APTS to introduce amino groups on its surface. Briefly, nanohydroxyapatite (180 mg) was dispersed into ethanol (200 mL). The mixture was sonicated for 2 hours until a homogeneous solution was obtained. Subsequently, APTS (5 mL) was added into the nanohydroxyapatite solution under vigorous magnetic stirring. In order to facilitate the silanization reaction, 1 mL of water was added into the nanohydroxyapatite suspension to promote hydrolysis of the alkoxysilane groups of APTS to form silanol groups.Citation38 The mixture was allowed to react with stirring for 12 hours at room temperature. After that, the resulting solution was washed with water two times and then with methanol five times to remove the excess reactants and byproducts, followed by centrifugation (6000 rpm, 5 minutes) and air dried to obtain n-HA-APTS.
Acetylation of surface amines of n-HA-APTS
To neutralize the positive charges of the amine-surfaced n-HA-APTS particles, the particles were reacted with acetic anhydride (). The acetylation reaction was performed according to a procedure described in our previous work.Citation39 In brief, n-HA-APTS (50 mg) was dispersed thoroughly in dimethylsulfoxide (15 mL) under magnetic stirring, followed by addition of triethylamine (1 mL) and the solution was well mixed for 30 minutes. Excess acetic anhydride (1 mL) dissolved in dimethylsulfoxide (5 mL) was then added dropwise into the n-HA-APTS/triethylamine mixture solution under vigorous magnetic stirring. The mixture was allowed to react with stirring for 24 hours. Acetylated n-HA-APTS (n-HA-APTS.Ac) particles were obtained using similar washing and centrifugation steps that were used to purify n-HA-APTS particles.
Carboxylation of surface amines of n-HA-APTS
To modify the amine groups of n-HA-APTS particles with carboxyl groups, the n-HA-APTS particles were reacted with succinic anhydride according to a procedure described in our previous report ().Citation39 Briefly, n-HA-APTS (50 mg) dispersed in dimethylsulfoxide (20 mL) was added with excess succinic anhydride (500 mg) dissolved in 5 mL of dimethylsulfoxide under vigorous magnetic stirring. The reaction was stopped after 24 hours. Thereafter, the carboxylated n-HA-APTS (n-HA-APTS.SAH) formed was purified according to the procedure used to purify n-HA-APTS.
Characterization techniques
FTIR was performed using a Nicolet Nexus 670 FTIR (Thermo Electron Corporation, Waltham, MA) spectrometer. All spectra were recorded in transmission mode ranging from 650 to 4000 cm−1 under ambient conditions. 1H NMR spectra were recorded on a Bruker AV 400 NMR spectrometer. Samples were dispersed in D2O before measurements. Zeta potential measurements were carried out using a Zetasizer Nano ZS system (Malvern, Worcestershire, UK) equipped with a standard 633 nm laser. Samples were dispersed in water at a concentration of 1 mg/mL before measurement. TEM was performed using a Jeol 2010F analytical electron microscope (Jeol, Tokyo, Japan) operating at 200 kV. A 5 μL aqueous solution (1 mg/mL) of each sample was dropped onto a carbon-coated copper grid and air-dried before measurement. The crystalline structure of nanohydroxyapatite before and after modification was analyzed by a Rigaku D/max-2550 PC X-ray diffraction system (Rigaku Co, Tokyo, Japan) using Cu Kα radiation with a wavelength of 0.154 nm at 40 kV and 200 mA. The scan was performed from 5° to 70° (2θ). The elemental composition of calcium and silicon in the APTS-modified nanohydroxyapatite sample was determined by a Leeman Prodigy ICP-AES system (Hudson, NH03051). A n-HA-APTS sample (1.15 mg) was dissolved into 5 mL of concentrated nitric acid before measurement.
Cell biology evaluation
L929 cells were cultured in 25 cm2 tissue culture flasks with 5 mL Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin in an incubator with 5% CO2 at 37°C. Cells (1 × 104 cells per well) were plated into a 96-well plate in a complete medium overnight. The medium was replaced by fresh medium containing samples with a concentration range of 0–100 μg/mL. After 24 hours of incubation, an MTT assay was used to quantify the viability of the cells according to the manufacturer’s instructions. The metabolically active cells were detected by adding MTT to each well. The plates were then read at 570 nm using a microplate reader (MK3, Thermo Electron Corporation). The mean and standard deviation for the triplicate wells for each sample were reported.
The viability of L929 cells was also evaluated by visual observation of the morphology of cells treated with the pristine nanohydroxyapatite and the APTS-modified nanohydroxyapatite samples using a Leica DM IL LED inverted phase contrast microscope with a magnification of 200× for each sample.
Hemolysis assay
Human blood stabilized with heparin was kindly provided by Shanghai First People’s Hospital (Shanghai, China). Human red blood cells were obtained by removing the serum via centrifugation and washing with phosphate-buffered solution five times according to the procedure reported in the literature.Citation40 After that, the cells were diluted 10 times with phosphate-buffered solution. Then 0.2 mL of the diluted human red blood cell suspension was transferred to a 1.5 mL Eppendorf tube containing 0.8 mL of water, phosphatebuffered solution, or phosphate-buffered solution containing dispersed nanohydroxyapatite and its derivatives at different concentrations (10, 50, 100, 250, and 500 μg/mL), respectively. The above mixtures were then incubated at 37°C for 2 hours, followed by centrifugation (10000 rpm, 2 minutes), and the absorbance of the supernatants (hemoglobin) was recorded using a Perkin Elmer Lambda 25 ultraviolet-visible spectrometer.
Statistical analysis
One-way analysis of variance statistical analysis was performed to evaluate the significance of the toxicity of the nanohydroxyapatite and its derivatives. A P value of 0.05 was considered as statistical significance level.
Results and discussion
In this study, we attempted to use APTS to silanize nanohydroxyapatite with abundant hydroxyl groups, resulting in the formation of n-HA-APTS, which possesses a large amount of primary amines on the surface. The surface amines of the n-HA-APTS particles were then acylated with acetic anhydride and succinic anhydride to form neutrally and negatively charged particles, respectively. The differently modified particles formed were extensively characterized using FTIR, 1H NMR, ICP-AES, TEM, X-ray diffraction, and zeta potential measurement.
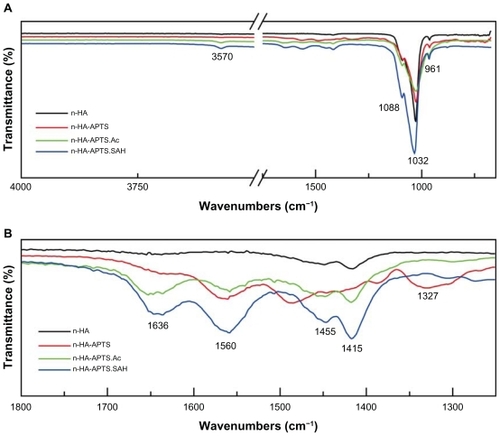
FTIR spectroscopy was carried out to confirm APTSmediated modification on the nanohydroxyapatite particle surfaces (). The typical absorption bands at 961 cm−1, 1032 cm−1, and 1088 cm−1 shown in can be assigned to the characteristic bands of PO4 3−, and the weak peak at 3570 cm−1 corresponds to the stretching vibration of hydroxyl groups on the nanohydroxyapatite surface. shows the enlarged spectra with a wavenumber range of 1250 cm−1 to 1800 cm−1. The peak located at 1327 cm−1 for n-HAAPTS is assigned to the C-N stretching vibration band of APTS, suggesting successful modification of APTS on the surface of nanohydroxyapatite particles. Compared with the spectra for nanohydroxyapatite and n-HA-APTS, a new peak at 1636 cm−1 in the spectra of both n-HA-APTS.Ac and n-HA-APTS.SAH appears, which can be assigned to the C=O stretch vibration band. The peak located at 1560 cm−1 in the spectra of n-HA-APTS, n-HA-APTS.Ac, and n-HA-APTS. SAH may be caused by moisture from the atmosphere. The peak located at 1480 cm−1 in the spectrum of n-HA-APTS can be assigned to the deformation modes of the amine groups that were strongly hydrogen-bonded with the silanol groups.Citation41 The peaks at 1455 cm−1 and 1415 cm−1 may be attributed to CO2 adsorption on the surface of nanohydroxyapatite from the atmosphere.Citation41 In general, the FTIR spectroscopy studies give relatively qualitative confirmation of APTS modification onto the nanohydroxyapatite surfaces; however, it is difficult to discern the difference between n-HA-APTS.Ac and n-HAAPTS. SAH samples.
Figure 1 Fourier transform infrared spectroscopy spectra of nanohydroxyapatite, n-HA-APTS, n-HA-APTS.Ac, and n-HA-APTS.SAH, respectively. (A) Wavenumber range of 650–4000 cm−1. (B) Wavenumber range of 1250–1800 cm−1.
Abbreviations: n-HA-APTS, nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS.Ac, neutralized nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS. SAH, negatively charged nanohydroxyapatite-aminopropyltriethoxysilane.
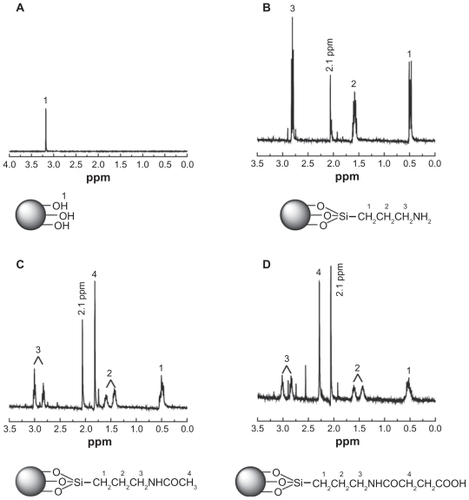
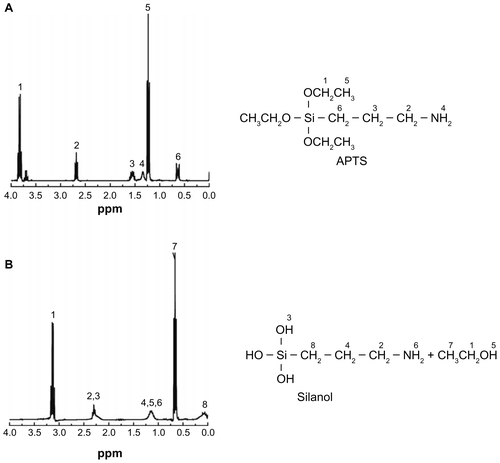
The APTS-modified nanohydroxyapatite derivatives were further characterized by 1H NMR spectroscopy (). Pristine nanohydroxyapatite only displays the –OH proton signals at 3.17 ppm, as shown in . However, after modification with APTS and APTS-mediated further functionalization, the –OH proton signals at 3.17 ppm almost disappear (). In addition, the -CH2- proton signals (peak 1 at 0.47 ppm, peak 2 at 1.60 ppm, and peak 3 at 2.82 ppm) related to APTS can be clearly observed (). These results indicate successful modification of nanohydroxyapatite with APTS. Further reaction of APTS amines to form acetyl or carboxyl groups does not significantly alter the chemical shift of the above 3 -CH2- proton signals of APTS. For the n-HA-APTS.Ac sample, the peak at 1.87 ppm (peak 4, ) is related to the -CH3 protons of the acetyl groups,Citation42 confirming successful acetylation of APTS amines. For the n-HA-APTS.SAH sample, the peak at 2.30 ppm (peak 4, ) is assigned to the -CH2- protons of the succinamic acid end groups, verifying the transformation of the APTS amines to carboxyl groups. It is worth noting that a new peak at 2.1 ppm can be clearly observed for all APTS-functionalized nanohydroxyapatite samples (), which may be associated with the silanol –OH proton signals of APTS that have not been reacted with the nanohydroxyapatite hydroxyl groups due to the much higher molar excess of the added APTS. To confirm this hypothesis further, the 1H NMR spectra of APTS dissolved in both CDCl3 and D2O were compared (). The 1H NMR spectrum of APTS in CDCl3 is different from that of APTS dissolved in D2O, because APTS can be hydrolyzed in D2O to form silanol groups. It is clear that the proton signal of -CH2- adjacent to Si shifts to the high field after hydrolysis, and a new peak at about 2.2 ppm assigned to the –OH proton signal of silanol appears. Therefore, the peak at 2.1 ppm in is likely associated with the hydrolyzed APTS silanol –OH proton signals. The much more molar excess of APTS added to the pristine nanohydroxyapatite samples may not allow complete reaction of the APTS silanol groups with the hydroxyl groups of nanohydroxyapatite.
Figure 2 1H Nuclear magnetic resonance spectra of nanohydroxyapatite (A), nanohydroxyapatite-aminopropyltriethoxysilane (B), neutralized nanohydroxyapatiteaminopropyltriethoxysilane (C), and negatively charged nanohydroxyapatite-aminopropyltriethoxysilane (D), respectively.
In order to quantify the amount of APTS modified onto the nanohydroxyapatite particles, ICP-AES was carried out to analyze the elemental composition of the n-HA-APTS sample. The mass fractions of calcium and silicon were determined to be 382.8 μg/mg and 14.3 μg/mg, respectively. With the known mass fractions of calcium and silicon, the amount of modified APTS can be calculated according to the following equation:
where ωAPTS is the mass percentage of APTS modified onto nanohydroxyapatite particles, mn−HA and mAPTS are the masses of nanohydroxyapatite and APTS in the n-HA-APTS sample, respectively, ωsi and ωca are the mass fractions of silicon and calcium in the n-HA-APTS sample, respectively, and Msi and Mca are the atomic weights of silicon and calcium, respectively. It is worth noting that M′si and M′n−HA in the equation stands for the incoming molecular mass of APTS and nanohydroxyapatite after APTS modification reaction to form n-HA-APTS,Citation43 where the value of M′si and M′n−HA is calculated to be 134 and 953, respectively. The mass of APTS reacted with nanohydroxyapatite was estimated to be 6.96% according to EquationEquation 1(1) .
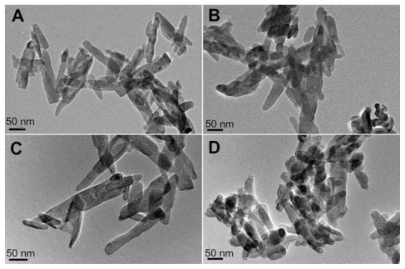
The morphology of the nanohydroxyapatite particles before and after different modifications was observed by TEM (). It is clear that the pristine nanohydroxyapatite particles have a regular rod shape with a diameter and length of 37 ± 9 nm and 118 ± 42 nm, respectively (). The modification of nanohydroxyapatite particles with APTS and further acetylation or carboxylation of the APTS amines () seem not to alter the morphology of the particles significantly when compared with pristine nanohydroxyapatite particles. No aggregation was observed for all samples dispersed in aqueous solution, suggesting that APTS is uniformly coated onto each nanohydroxyapatite particle even after further APTS amine functionalization to form acetyl or carboxyl groups. The somewhat aggregated particles shown in the TEM images of all samples are presumably due to the TEM sample preparation method. The air-drying of the aqueous suspension of the samples before TEM measurement may lead to a partial aggregation or interconnection of particles, in agreement with previous literature.Citation44
Figure 3 Transmission electron microscopic images of nanohydroxyapatite (A), nanohydroxyapatite-aminopropyltriethoxysilane (B), neutralized nanohydroxyapatite-aminopropyltriethoxysilane (C), and negatively charged nanohydroxyapatite-aminopropyltriethoxysilane (D), respectively.
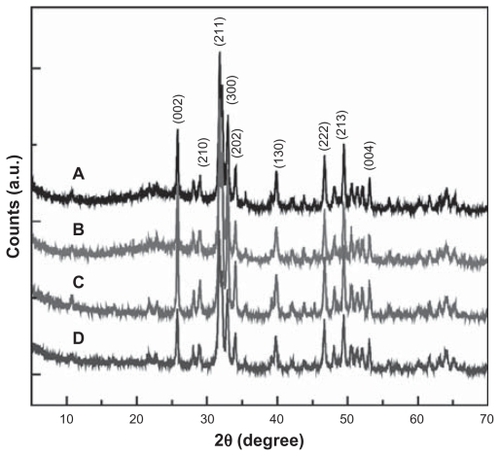
The crystalline structure of the pristine nanohydroxyapatite particles and the different APTS-modified nanohydroxyapatite particles were investigated by X-ray diffraction (). The diffraction peaks of the pristine nanohydroxyapatite particles were indexed in curve A (Joint Committee for Powder Diffraction Standards standard X-ray diffraction data for pure hydroxyapatite: 09-0432). It is clear that after grafting with APTS and further acetylation or carboxylation of APTS amines, the X-ray diffraction patterns remain similar to that of the pristine nanohydroxyapatite particles, suggesting that APTS modification reactions did not have any appreciable effect on the original crystal structure of the nanohydroxyapatite particles.
Figure 4 X-ray diffraction patterns of nanohydroxyapatite (A), nanohydroxyapatite-aminopropyltriethoxysilane (B), neutralized nanohydroxyapatite-aminopropyltriethoxysilane (C), and negatively charged nanohydroxyapatite-aminopropyltriethoxysilane (D), respectively.
The surface modification of nanohydroxyapatite was finally assessed by zeta potential measurement. The surface potential of the pristine nanohydroxyapatite (−8.22 ± 0.08 mV) was reversed to be positive (7.14 ± 0.27 mV) after modification of APTS possessing primary amine groups. Further acetylation of the APTS amines neutralized the positive charges of the n-HA-APTS particles (1.31 ± 0.08 mV), whereas carboxylation of the APTS amines of n-HA-APTS was able to reverse further the positive charge of nanohydroxyapatite to be highly negative (−21.8 ± 0.49 mV). Overall, FTIR, 1H NMR, ICP-AES, TEM, X-ray diffraction, and zeta potential measurement confirmed successful surface modification of nanohydroxyapatite particles with APTS and APTS derivatives.
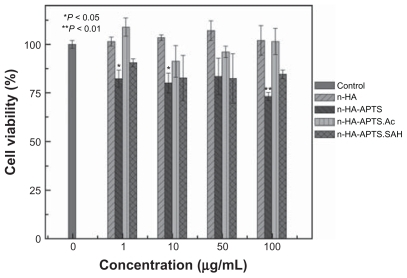
The cytotoxicity of nanohydroxyapatite and its derivatives was tested by MTT viability assay of L929 cells after treatment with nanohydroxyapatite and its derivatives. As shown in , there was no statistically significant difference in cell viability for L929 cells between the control cells treated with phosphate-buffered solution, and the cells treated with the pristine nanohydroxyapatite, the n-HA-APTS.Ac, and the n-HA-APTS.SAH particles at the concentration range of 0–100 μg/mL (P > 0.05). However, there is a statistically significant difference in cell viability between the phosphate-buffered solution control and the n-HA-APTS particles at different concentrations (P < 0.05 at 1 and 10 μg/mL, P < 0.01 at 100 μg/mL), implying that the modified APTS on the nanohydroxyapatite surface without neutralization of the amine groups inhibits cell growth to some extent. This also suggests that the APTS amine-induced cytotoxicity of nanohydroxyapatite particles can be readily reduced by acetylating or carboxylating of the APTS amine groups. These results are consistent with our previous report.Citation39 The relatively toxic APTS-modified nanohydroxyapatite particles with surface amine groups should be due to the electrostatic interaction between the positively charged particles and the negatively charged cell membranes.Citation45–Citation47 The neutralization of the surface amine groups to form acetamide or carboxyl groups can significantly reduce the electrostatic interaction between the particles and the cells, thereby improving the biocompatibility of APTS-modified nanohydroxyapatite particles.Citation39
Figure 5 MTT viability assay of L929 cells after treatment with nanohydroxyapatite particles and aminopropyltriethoxysilane-modified nanohydroxyapatite derivatives for 24 hours.
Notes: Mean and standard deviation for triplicate wells were reported. Data are expressed as the mean ± standard deviation. Statistical significance was calculated using analysis of variance. *P < 0.05; **P < 0.01.
Abbreviations: n-HA-APTS, nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS.Ac, neutralized nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS. SAH, negatively charged nanohydroxyapatite-aminopropyltriethoxysilane.
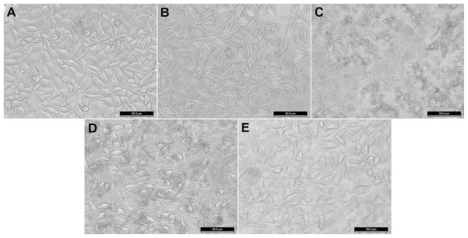
The cytotoxicity of nanohydroxyapatite and its derivatives was further confirmed by phase contrast microscopic visualization of cell morphology changes after treatment with nanohydroxyapatite with different surface functionalities for 24 hours. shows the morphology of untreated L929 cells (), L929 cells treated with pristine nanohydroxyapatite particles (), n-HA-APTS particles (), n-HA-APTS.Ac particles (), and n-HA-APTS.SAH particles () at a concentration of 100 μg/mL. It is clear that the pristine nanohydroxyapatite particles, n-HA-APTS.Ac particles with neutral surface charge, and n-HA-APTS.SAH particles with a negative surface charge do not induce appreciable cell morphology changes when compared with untreated L929 cells, confirming their excellent biocompatibility. In contrast, when L929 cells were treated with positively charged n-HA-APTS particles, a portion of cells became rounded and nonadherent, indicating that the cells had undergone apoptosis. These results are consistent with the MTT assay data.
Figure 6 Phase-contrast photomicrograph of (A) untreated L929 cells (phosphate-buffered control) and L929 cells treated with 100 μg/mL of (B) nanohydroxyapatite, (C) nanohydroxyapatite-aminopropyltriethoxysilane, (D) neutralized nanohydroxyapatite-aminopropyltriethoxysilane, and (E) negatively charged nanohydroxyapatite-aminopropyltriethoxysilane for 24 hours, respectively.
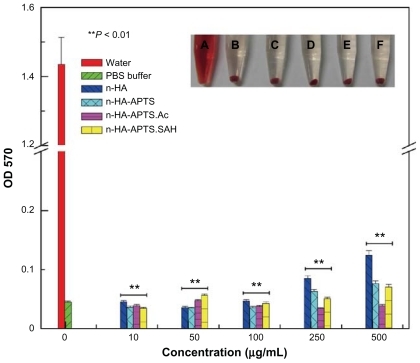
For practical biomedical applications, especially in circumstances where nanoparticles need to be intravenously injected, blood compatibility becomes a major concern for the utility of materials. Blood compatibility of nanohydroxyapatite and its derivatives was evaluated by in vitro hemolysis assay (). No obvious hemolysis phenomenon was observed after exposure of nanohydroxyapatite and its derivatives with different surface functionalities to human red blood cells, even when the sample concentration is up to 500 μg/mL (inset of ). The hemolytic effects of each sample at different concentrations were further determined by recording absorbance of the supernatant at 570 nm using ultraviolet-visible spectroscopy. A significant difference (P < 0.01) in absorbance at 570 nm associated with hemoglobin was found between the control group (human red blood cells exposed to distilled water) and the experimental groups. This indicates that, in the concentration range studied, nanohydroxyapatite and its derivatives with different functionalities do not show any appreciable hemolytic effect, implying that the surface modification of nanohydroxyapatite via APTS-mediated functionalization does not compromise the compatibility between pristine nanohydroxyapatite particles and blood.
Figure 7 Hemolytic assay of human red blood cells after treatment with distilled water, phosphate-buffered solution, and differently functionalized nanohydroxyapatite derivatives for 24 hours. The mean and standard deviation for triplicate samples were reported. The inset shows a photograph of human red blood cells exposed to (A) distilled water, (B) phosphate-buffered solution, (C) nanohydroxyapatite, (D) n-HA-APTS, (E) n-HA-APTS.Ac, and (F) n-HA-APTS.SAH for 2 hours (sample concentration 500 μg/mL), followed by centrifugation.
Note: Statistical significance was calculated using analysis of variance. **P < 0.01.
Abbreviations: n-HA-APTS, nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS.Ac, neutralized nanohydroxyapatite-aminopropyltriethoxysilane; n-HA-APTS. SAH, negatively charged nanohydroxyapatite-aminopropyltriethoxysilane.
Conclusion
In summary, a simple approach was developed to modify nanohydroxyapatite particles via an APTS-mediated chemical reaction. The silanization of nanohydroxyapatite particles with APTS creates particles with abundant primary amines on their surfaces, which can be further acetylated or carboxylated to make nanohydroxyapatite particles with a neutral surface charge or negative surface charge, respectively. We showed that pristine nanohydroxyapatite particles, neutral (n-HA-APTS.Ac), and negatively charged (n-HA-APTS. SAH) n-HA derivatives do not display cytotoxicity at a concentration up to 100 μg/mL. However, without further modification, APTS-modified nanohydroxyapatite particles are slightly cytotoxic at the concentrations studied. The hemolytic effect of pristine nanohydroxyapatite particles is not compromised after APTS-mediated surface functionalization. The significance of our study is that through APTS modification and further functionalization, the surface charge of nanohydroxyapatite particles can be regulated to be positive, neutral, and negative with different functional groups. We anticipate that APTS-functionalized nanohydroxyapatite with reactive surface functional groups may be beneficial for further conjugation with other polymers or biological ligands onto the particle surfaces for various biomedical applications.
Disclosure
The authors report no conflicts of interest relevant to this research.
Acknowledgments
This research is financially supported by the National Basic Research Program of China (973 Program, 2007CB936000, 2011CB510106, 2011CB504300), the National Natural Science Foundation of China (20974019, 81101150, 81071747), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (XS and JW), the Innovation Funds of Donghua University Doctorate Dissertation of Excellence (BC201107), and the Fundamental Research Funds for the Central Universities (MS, RG, XC, XS). XS gratefully acknowledges the Fundação para a Ciência e a Tecnologia and Santander bank for the Chair in Nanotechnology.
Supplementary material
Figure S1 Citation1H nuclear magnetic resonance spectra of aminopropyltriethoxysilane (APTS) dissolved in CDCl3 (A) and D2O (B).
References
- FangXMaHXiaoSFacile immobilization of gold nanoparticles into electrospun polyethyleneimine/polyvinyl alcohol nanofibers for catalytic applicationsJ Mater Chem20112144934501
- DanielMCAstrucDGold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnologyChem Rev200410429334614719978
- LuoXMorrinAKillardAJSmythMRApplication of nanoparticles in electrochemical sensors and biosensorsElectroanalysis200618319326
- XuJJLuoXLDuYChenHYApplication of MnO2 nanoparticles as an eliminator of ascorbate interference to amperometric glucose biosensorsElectrochem Commun2004611691173
- GaoJGuHXuBMultifunctional magnetic nanoparticles: Design, synthesis, and biomedical applicationsAcc Chem Res2009421097110719476332
- ShenMShiXDendrimer-based organic/inorganic hybrid nanoparticles in biomedical applicationsNanoscale201021596161020820690
- WangHZhengLPengCComputed tomography imaging of cancer cells using acetylated dendrimer-entrapped gold nanoparticlesBiomaterials2011322979298821277019
- YangHZhangCShiXWater-soluble superparamagnetic manganese ferrite nanoparticles for magnetic resonance imagingBiomaterials2010313667367320144480
- ItoATakizawaYHondaHTissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cellsTissue Eng20041083384015265301
- TaskerLHSparey-TaylorGJNokesLDMApplications of nanotechnology in orthopaedicsClin Orthop Relat Res200745624324917224843
- HouCHHouSMHsuehYSLinJWuHCLinFHThe in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapyBiomaterials2009303956396019446329
- ShiZHuangXCaiYTangRYangDSize effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cellsActa Biomater2009533834518753024
- HornezJCChaiFMonchauFBlanchemainNDescampsMHildebrandHFBiological and physico-chemical assessment of hydroxyapatite (HA) with different porosityBiomol Eng20072450550917900978
- ZhuSHHuangBYZhouKCHydroxyapatite nanoparticles as a novel gene carrierJ Nanopart Res20046307311
- FabbriPBondioliFMessoriMBartoliCDinucciDChielliniFPorous scaffolds of polycaprolactone reinforced with in situ generated hydroxyapatite for bone tissue engineeringJ Mater Sci Mater Med20102134335119653069
- ZhangCLiCHuangSSelf-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug deliveryBiomaterials2010313374338320122726
- PetitRThe use of hydroxyapatite in orthopaedic surgery: a ten-year reviewEur J Orthop Surg Traumatol199997174
- CookeFWCeramics in orthopedic surgeryClin Orthop Relat Res19922761351461537144
- AkaoMAokiHKatoKMechanical properties of sintered hydroxyapatite for prosthetic applicationsJ Mater Sci198116809812
- LopesMAMonteiroFJSantosJDGlass-reinforced hydroxyapatite composites: fracture toughness and hardness dependence on micro-structural characteristicsBiomaterials1999202085209010535820
- CengizBGokceYYildizNAktasZCalimliASynthesis and characterization of hydroxyapatite nanoparticlesColloids Surf A Physicochem Eng Asp20083222933
- WeiGMaPXStructure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineeringBiomaterials2004254749475715120521
- AokiHKutsunoTLiWNiwaMAn in vivo study on the reaction of hydroxyapatite-sol injected into bloodJ Mater Sci Mater Med200011677215348049
- PengJLiXGuoGPreparation and characterization of poly (vinyl alcohol)/poly(-caprolactone)-poly(ethylene glycol)-poly (-caprolactone)/nano-hydroxyapatite composite membranes for tissue engineeringJ Nanosci Nanotechnol2011112354236021449393
- DengCYangXBWengJFabrication and in vitro bioactivity of poly (ɛ-caprolactone) composites filled with silane-modified nano-apatite particlesInt J Polym Mater201160374383
- SeyedjafariESoleimaniMGhaemiNShabaniINanohydroxyapatite-coated electrospun poly (l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formationBiomacromolecules20101131183125
- KimHWLeeHHKnowlesJCElectrospinning biomedical nanocomposite fibers of hydroxyapatite/poly (lactic acid) for bone regenerationJ Biomed Mater Res A20067964364916826596
- XiaoFYeJWangYRaoPDeagglomeration of HA during the precipitation synthesisJ Mater Sci20054054395442
- ZhangRMaPXPoly (α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphologyJ Biomed Mater Res A1999444446455
- ZuoYLiYLiJNovel bio-composite of hydroxyapatite reinforced polyamide and polyethylene: Composition and propertiesMater Sci Eng A Struct Mater2007452–453512517
- ZhangYVenugopalJREl-TurkiARamakrishnaSSuBLimCTElectrospun biomimetic nanocomposite nanof ibers of hydroxyapatite/chitosan for bone tissue engineeringBiomaterials2008294314432218715637
- TangTFanHAiSHanRQiuYHemoglobin (Hb) immobilized on amino-modified magnetic nanoparticles for the catalytic removal of bisphenol AChemosphere20118325526421237483
- SongYHildebrandHSchmukiPPhotoinduced release of active proteins from TiO2 surfacesElectrochem Commun20091114291433
- PanBGaoFGuHDendrimer modified magnetite nanoparticles for protein immobilizationJ Colloid Interface Sci20052841615752777
- MaMZhangYYuWShenHYZhangHGuNPreparation and characterization of magnetite nanoparticles coated by amino silaneColloids Surf A Physicochem Eng Asp2003212219226
- SongYYHildebrandHSchmukiPOptimized monolayer grafting of 3-aminopropyltriethoxysilane onto amorphous, anatase and rutile TiO2Surf Sci2010604346353
- BalasundaramGSatoMWebsterTJUsing hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGDBiomaterials2006272798280516430957
- BauerFGläelHUlrichDHartmannESauerlandVMehnertRTrialkoxysilane grafting onto nanoparticles for the preparation of clear coat polyacrylate systems with excellent scratch performanceProgress in Organic Coatings200347147153
- ShenMWangSHShiXPolyethyleneimine-mediated functionalization of multiwalled carbon nanotubes: synthesis, characterization, and in vitro toxicity assayJ Phys Chem C200911331503156
- WuHLiuGZhuangYThe behavior after intravenous injection in mice of multiwalled carbon nanotube/Fe (3) O (4) hybrid MRI contrast agentsBiomaterials2011324867487621459436
- JoschekSNiesBKrotzRGöferichAChemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural boneBiomaterials2000211645165810905406
- GabrielsonNPDanielWAcetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactionsBiomacromolecules200672427243516903692
- PradoAGSAiroldiCImmobilization of the pesticide 2,4-dichlorophenoxyacetic acid on a silica gel surfacePest Manag Sci200056419424
- ShiXBrisenoALSanedrinRJZhouFFormation of uniform polyaniline thin shells and hollow capsules using polyelectrolyte-coated microspheres as templatesMacromolecules20033640934098
- HongSBielinskaAUMeckeAInteraction of poly (amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transportBioconjug Chem20041577478215264864
- HongSLeroueilPRElizabethKInteraction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeabilityBioconjug Chem20061772873416704211
- ShiXWangSSunHBakerJRJrImproved biocompatibility of surface functionalized dendrimer-entrapped gold nanoparticlesSoft Matter200737174