?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this work, racemic hybrid polypeptides poly(ethylene glycol) (PEG)-b-poly(racemic-leucine) (PRL) copolymers with different leucine residues have been synthesized and characterized. Using docetaxel as a model molecule, the high drug-loaded spherical micelles based on PEG-PRL were prepared successfully using dialysis, with a tunable particle size from 170 nm to 250 nm obtained by changing the length of the hydrophobic blocks. Facilitated drug-loading behavior (higher drug-loading ability and easier drug-loading process) of PEG-PRL compared with their corresponding levo forms (PEG-b-poly[levo leucine]) was observed and clarified for the first time. With this facilitation, the highest drug-loading content and efficiency of PEG-PRL micelles can achieve 11.2% ± 0.4% and 67.2% ± 2.4%, respectively. All drug-loaded PEG-PRL micelles exhibit a similar release behavior with a sustained release up to 72 hours. The PEG-PRL was shown to be nontoxic against MCF-7 and human umbilical vein endothelial cells up to a concentration of 100 μg/mL, displaying a good biocompatibility. Also, the docetaxel-loaded PEG-PRL micelles were more toxic than the free drug against MCF-7 human breast cancer cells – both dose and time dependent. Therefore, these high docetaxel-loaded micelles based on racemic hybrid polypeptides appear to be a novel promising nanomedicine for anticancer therapy.
Introduction
Polymeric micelles (PMs) based on amphiphilic block copolymers are one of the most promising nanodrug-delivery systems and have received much attention in recent years as anticancer drug carriers, due to their technical ease, high biocompatibility, and highly efficient drug delivery.Citation1 Taking advantage of the classical core-shell structure and nanoscopic dimensions, PMs can protect fragile molecules in their hydrophobic core from being destroyed by environmental conditions and allow them to remain unrecognized by the reticuloendothelial system during blood circulation. This facilitates their extravasations at leaky capillaries, known as the “enhanced permeability and retention effect,” leading to a passive accumulation in tumor tissues and, consequently, increased drug delivery efficiency.Citation2
The synthetic flexibility and favorable physicochemical characteristics of the block copolymers also provide additional advantages for the production of efficient micelles. Although many types of amphiphilic block copolymers can form micelles, extensive studies have shown that the hybrid polypeptides of poly(ethylene glycol) (PEG)-b-poly(amino acids) copolymers are possibly the most suitable components for designing PMs,Citation3,Citation4 due to their excellent biocompatibility and various functional groups, such as carboxyl and amino, which can be easily tailored to allow drug incorporation or the production of versatile copolymers.
Generally speaking, published studiesCitation5–Citation9 have mainly focused on poly(levo-amino acid) copolymers and their use in micelle construction because certain lengths of levo polypeptide sequences can adopt ordered conformations, such as α-helices or β-strands, which are believed to offer the benefit of an additional means of directing and stabilizing the formation of nanostructures.Citation10 Following developments in this f ield, the racemic polypeptide sequences (poly[racemic-amino acid]) in amphiphilic copolymers have also been found to possess many unique characteristics. For example, Hanson et alCitation11 reported that the use of racemic polypeptide sequences could prevent α-helix formation and increase chain flexibility, and the spherical micelles based on poly(Nε-2-[2-(2-methoxyethoxy)ethoxy] acetyl-L-lysine)100-b-poly(racemic-leucine)10 copolymer showed excellent stability at elevated temperatures and under a variety of solution conditions. Choi et alCitation12 reported the different gel-forming properties of the PEG-b-poly(racemic alanine) copolymer compared with its corresponding levo polypeptides. The unique role of poly(racemic-amino acids) segments in the formation of stable nanosized double emulsions was also found to involve increased solubility in the oil phase as well as an additional stabilizing effect by hydrogen bond formation compared with its levo forms.Citation13
However, few investigations have focused on the self-assembly behavior and the potential application as drug carriers of these racemic segment-based hybrid polypeptides, such as PEG-poly(racemic amino acid) copolymers. Also, very few investigations have compared the self-assembly behavior and drug-loading process of racemic polypeptide-based polymers compared with their levo forms. Systematic and comprehensive investigations of racemic polypeptide segments in amphiphilic polymers are still needed to identify their role in nanostructure formation as well as to explore the potential applications of this nanostructure as a useful drug-delivery vehicle.
Therefore, in this study, PEG-poly(racemic leucine) copolymers with different amounts of leucine residues, as representatives of PEG-poly(racemic amino acid) hybrid polypeptides, were synthesized and characterized. Their self-assembly behavior and the potential application as anticancer drug carriers were studied using docetaxel (DTX) as a model drug, because DTX is a potent hydrophobic anticancer drug and its current commercially available formulations (containing large amounts of Tween 80) have serious deficiencies in terms of their physical instability and the toxicity of the vehicle.Citation14 Although Abraxane® (the Food and Drug Administration-approved albumin nanoparticle-bound PTX) partially resolved the toxic issues of Tween 80, it still tends to precipitate 24 hours after being reconstituted with saline.Citation15 Moreover, the conformation-facilitated drug-loading behavior (a higher drug-loading ability and easier drug-loading process) of racemic hybrid polypeptide-based copolymers compared with the corresponding levo forms (PEG-poly[levo leucine]) was observed for the first time, and the possible mechanisms of action were examined and discussed. These results should provide the basis for the further application of the PEG-poly(racemic amino acid) hybrid polypeptides as effective carriers for anticancer drugs.
Materials and methods
Materials
Alpha-methoxyl-ω-amino poly(ethylene glycol) hydrochloride (mPEG-NH2·HCl, Mn: 5392, polydispersity index: 1.04, JenKem Technology Ltd, Co, Beijing, People’s Republic of China) was dried in nitrogen for 24 hours before use; racemic leucine (rac-Leu, Sichuan Tongsheng Amino Acid Ltd, Co, Sichuan, People’s Republic of China) and triphosgene (99%; GL Biochem, Shanghai, People’s Republic of China) were recrystallized and dried in a vacuum for 24 hours before use; and DTX (Shanghai Sanwei Pharma Ltd, Co, Shanghai, People’s Republic of China) was used as received.
All organic solvents used in the synthesis experiments were purchased from National Medicine Chemical Reagent Ltd Co (Shanghai, People’s Republic of China) and processed using the following method: ethyl acetate and dichloromethane (DCM) were dried by refluxing over CaH2 and distilled prior to use, tetrahydrofuran (THF) and petroleum ether (boiling range 30°C–60°C) were dried and distilled from sodium immediately before use, and N,N′-dimethyl formamide (DMF) was used without further purification.
Dimethyl sulfoxide (DMSO), Dulbecco’s modified Eagle’s medium, RPMI 1640 medium, fetal bovine serum, penicillin-streptomycin, trypsin, Dulbecco’s phosphate buffered saline, and 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Gibco Company (New York, NY).
The water used was double distilled. Acetonitrile and methanol were of chromatographic grade, other solvents were of analytical grade, and all other chemicals were of commercially available reagent grade.
Synthesis of racemic-N-carboxy-leucine anhydride
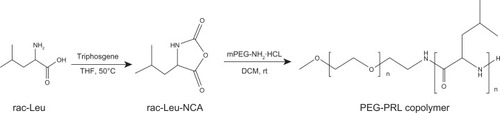
The racemic-N-carboxy-leucine anhydride (rac-Leu-NCA) was prepared according to the procedure published in the literatureCitation16 with some modification. Briefly, rac-Leu (10.0 g, 76.2 mmol) was suspended in 50 mL dry THF and heated to 55°C. After 30 minutes, a solution of triphosgene (8.0 g, 27.0 mmol) in 100 mL anhydrous THF was added dropwise under stirring, maintaining reaction until the mixture turned transparent (about 1.5 hours). Then, the reaction system was purged with nitrogen to eliminate chlorine hydride gas generated by the reaction for 30 minutes before totally evaporating the solvent under reduced pressure. The obtained crude crystals of rac-Leu-NCA were recrystallized three times from a mixture of ethyl acetate and petroleum ether at −20°C as white crystalline needles before use.
Synthesis of methoxyl poly(ethylene glycol)-poly(racemic-leucine) diblock copolymer
mPEG-poly(racemic-leucine) (PRL) diblock copolymer was synthesized via the ring-opening polymerization (ROP) of rac-Leu-NCA initiated by mPEG-NH2·HCl in anhydrous DCM with different feed ratios. The reaction system was stirred in a nitrogen atmosphere at room temperature for 72 hours. After washing twice with deionized water, the resulting mixture was evaporated under reduced pressure until a dry film was obtained, and DMF was then added to totally dissolve it. The solution was then transferred to a dialysis tube (cellulose ester, MWCO: 3500) and dialyzed with deionized water for 24 hours. The medium was replaced several times to completely remove the DMF. Finally, the fluffy white powder was collected by freeze-drying the suspension within the dialysis tube.
Polymer characterization
1H-nuclear magnetic resonance (NMR) spectra of all samples were measured on a Bruker DMX 300 spectrometer (Billerica, MA). Chemical shifts (δ) were given in ppm using tetramethylsilane as the internal standard. Fourier transform infrared spectroscopy spectra were recorded on a Bruker Tensor 27 spectrometer, and samples were prepared using KBr disks (Scharlau Chemie, Barcelona, Spain). Gel permeation chromatography (GPC) assay was performed on a Waters 1515 GPC instrument (Waters Corp, Milford, MA) equipped with three Styragel columns (Waters Corp; 105, 104, and 103 Å) in tandem and a 2414 differential refractive index detector. DMF (containing 0.1 M LiBr) was selected as the eluent at a flow rate of 1.0 mL/minute at 35°C, and the sample concentrations were approximately 2 mg/mL. The molecular weights were calibrated using polystyrene standards.
Optical rotations were determined on a PerkinElmer 241 MC polarimeter (Waltham, MA) equipped with a 1 dm quartz cell at the sodium D-line wavelength (25°C, in absolute THF). The specific optical rotation
was calculated from the following formula:Citation17
where r is the angle of rotation, l the number of decimeters of solution, and c the sample concentration (g/mL).
Determination of the critical aggregation concentration
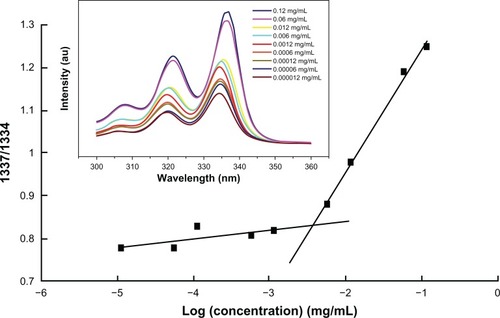
The critical aggregation concentration (CAC) of the amphiphilic PEG-PRL diblock copolymer was determined by the classical “pyrene fluorescence probe” method published elsewhere.Citation18 Specifically, 100 μL of pyrene solutions (1 × 10−5 M in acetone) was transferred to a series of 10 mL volumetric flasks, then acetone was completely volatilized under a gentle stream of nitrogen. A series of aqueous copolymer solutions were added to the acetone-free pyrene flasks and made up to the mark with deionized water, yielding final pyrene and copolymer concentrations of 1 × 10−7 M and 1 × 10−5 to 0.1 mg/mL, respectively. The resulting solutions were sonicated in an ultrasonic bath at 180 W for 30 minutes and equilibrated overnight at room temperature. The fluorescence excitation spectra of pyrene from 300 to 350 nm of pyrene were recorded on an F-7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) by fixing the emission wavelength at 390 nm and setting the slit widths for emission and excitation at 2.5 and 5.0 nm, respectively. Spectra were accumulated with a scan speed of 240 nm/minute. The fluorescence intensity ratio at 337 and 334 nm (l337/l334) of pyrene versus the logarithm of the polymer concentration was plotted from the excitation spectra, and the CAC was obtained from the inflection point of this plot. All samples were measured in parallel.
Micelle preparation
DTX-loaded micelles were prepared by dialysis method. Briefly, DTX (2 mg) and copolymer (10 mg) were completely dissolved in 2 mL DMF, and then aliquots of phosphate-buffered saline (PBS; 10 mM, pH5.5) were added dropwise to the solution using a syringe under continuous stirring. The obtained dispersion was stirred for several hours, followed by dialyzing with 500 mL PBS (10 mM, pH5.5) for 24 hours, replacing the outer medium three times (2, 6, and 12 hours) and using a cellulose ester dialysis membrane with a molecular cut-off of 14,000. Finally, the mixture was filtered through a 0.45 μm filter to remove precipitate, and the DTX-loaded micelles were obtained.
High-performance liquid chromatography analysis
DTX concentrations were determined by high-performance liquid chromatography (HPLC) (Hitachi L-2130 pump, Hitachi L-2400 UV-Vis detector) at a wavelength of 230 nm, using a Diamonsil C18 column (5 μm, 250 × 4.6 mm). A mobile phase of acetonitrile and water (70/30, v/v) was selected for the separation, and the flow rate was set at 1 mL/minute. The peak area response versus the DTX concentration was linear over the range 0.5–30 μg/mL (r2 = 0.9999).
Determination of drug-loading ability
The drug-loading ability of the DTX-loaded micelles was determined by HPLC. Briefly, 100 μL DTX-loaded micellar solution and 1 mL of THF were transferred to a 10 mL volumetric flask. The flask was placed in an ultrasonic bath and sonicated at 180 W for 10 minutes, then diluted with methanol and mixed thoroughly. The resulting solution was analyzed using the validated HPLC method described previously. The drug-loading content (DLC) and drug-loading efficiency (DLE) were calculated using the following equations:
The “weight of DTX in micelles” was calculated by the product of the DTX concentration measured by HPLC and the micellar volume. The “weight of drug-loaded micelles” was the total weight of the polymer and drug fed into the micelle preparation.
Particle size measurements
The particle size and distribution of micelles were measured by dynamic laser light scattering (DLS) with NICOMPTM 380 Submicron Particle Sizer (Particle Sizing Systems, Santa Barbara, CA) at room temperature. A laser beam at a wavelength of 632.8 nm was used, and the scattering angle was set to 90° when measurements were conducted.
Morphological analysis
The morphology of micelles was observed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM).
Samples for TEM observations were prepared by placing one drop of sample solution (0.5 mg/mL for copolymer) on to a copper grid coated with carbon. Excess solution was wiped away with the aid of filter paper, and the grid was allowed to dry for a further 10 minutes. Then, the samples were examined using a Hitachi H-600 TEM operated at an accelerating voltage of 100 kV.
Samples for SEM observations were prepared by dusting the lyophilized micellar powder on to double-adhesive conductive tape, which had been applied previously to a copper stub. After coating with a 30 nm thin layer of gold/palladium, the samples were observed using a Shimadzu SSX-500 field emission SEM (Tokyo, Japan) operating at an acceleration voltage of 10 kV.
Conformation analysis
Circular dichroism was used to analyze the conformation of the polypeptide blocks using a JASCO J-810 spectrometer (Tokyo, Japan). Hybrid polypeptides (0.01 mg/mL in deionized water) were introduced into quartz cells with a 1 cm optical path length and measured at room temperature.
In vitro release studies
The in vitro release behavior of DTX from various preparations was investigated by dialysis diffusion method.Citation19 Specifically, a drug-loaded micellar solution or free drug solution (Taxotere®; Sanofi-Aventis, Paris, France) containing about 100 μg DTX was transferred to a preswelled dialysis tube (cellulose ester, MWCO: 14,000). The tube was then incubated in 10 mL PBS (10 mM, pH7.4) containing 0.1% w/v Tween 80 to achieve sink conditions, and placed in a horizontal water bath shaker maintained at 37°C ± 0.5°C with a shaking speed of 100 rpm. At predetermined intervals, the dialysis tube was removed and placed in a new container filled with 10 mL fresh medium. The collected incubation media were analyzed by HPLC. All data were obtained in triplicate using parallel experiments and were expressed as mean values.
In vitro cytotoxicity studies
Cell culture
Human umbilical vein endothelial cells (HUVECs) and MCF-7 human breast cancer cells were generously provided by the Cell Center of the Chinese Medical University. HUVECs were cultured in RPMI 1640 medium, and MCF-7 cells were cultured in Dulbecco’s modified Eagle’s medium, both supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin (100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C in a humidified atmosphere with 5% CO2. The cells were harvested with 0.25% trypsin and 0.5 mM EDTA and rinsed. The resulting cell suspensions were used in the following experiments.
Cell viability test
The cytotoxicity of PEG-PRL copolymer, DTX, and DTX-loaded micelles was evaluated in MCF-7 and/or HUVECs using the MTT assay.Citation20 Briefly, cells were seeded in 96-well plates at a density of 104 cells per well. Following a 24-hour incubation to allow cell attachment, the growth medium was removed and replaced with 100 μL fresh medium containing different amounts of PEG-PRL, free DTX, or DTX-loaded micelles (n = 5). The micelles were sterilized with ultraviolet irradiation for 1 day prior to use. After predetermined periods of incubation, 20 μL MTT (5 mg/mL in PBS) was added to each well and incubated for another 4 hours. The culture medium was withdrawn, and formazan crystals were dissolved in 100 μL DMSO. The optical density (OD) of the resulting solution was then read at 490 nm using an ETY96 ELISA Reader (Toyo-Sokuki Inc, Tokyo, Japan). Cell viability was calculated from the following equation:Citation21
where ODtreated is the OD value of the cells treated with the samples, ODcontrol is the OD value of the cells treated with PBS alone, and ODblank is the OD value of the blank solution. IC50, the drug concentration at which 50% inhibition of cell growth occurred, was extrapolated from the dose response curves and reported in terms of DTX equivalents.
Statistical analysis
All data were presented as mean ± standard deviation and analyzed using Student’s t-test. Statistical significance was determined at a value of P < 0.05 except for special declaration.
Results and discussion
Synthesis and characterization of methoxyl polyethylene glycol-poly(racemic-leucine) copolymer
The ROP of amino acid N-carboxy-anhydrides (NCA) is still the most commonly used technique to synthesize polypeptides, because of its simplicity and ease of product purification,Citation22 although some limitations of this anionic polymerization have been reported, such as the low reaction efficiency and broad molecular weight distribution of the resulting product due to incidental side reactions.Citation23 To improve the quality of this traditional reaction, several considerations have been taken into account in this experiment, in addition to the special care needed for purifying the solvents and reagents used. Firstly, all synthesis operations were carried out using a Schlenk line. The hydrochloride of amino-terminated PEG (mPEG-NH2·HCl) was then selected as the macroinitiator because the hydrochloride molecule can effectively prevent side reactions by providing a proton and avoiding chain growth via the “activated monomer” mechanism.Citation24 Finally, the racemic-Leucine-NCA (rac-Leu-NCA) was synthesized directly from racemic leucine to simplify operations and improve the quality of NCA instead of using the existing method,Citation11,Citation13 which involves synthesizing the dextro-leucine-NCA and levo-leucine-NCA monomer in separate steps and mixing them equivalently as racemic forms.
Based on the modified reaction procedure (), three PEG-PRL copolymers with different molecular weights were obtained by varying the NCA/initiator molar feed ratios. The chemical structures of the products were confirmed by examination of the 1H-NMR and infrared spectra. It is illustrated in that the peaks at 1816 cm−1 and 1754 cm−1 are attributed to the C═O stretching vibration in the anhydride. Their disappearance, together with the emergence of an amide I and amide II bond at 1660 cm−1 and 1547 cm−1 in the PEG-PRL Fourier transform infrared spectroscopy spectra, proved the successful synthesis of the polypeptides. In addition, the marked peak at 1109 cm−1 is attributed to the C-O-C stretching vibration in the PEG block. The position, number, and splitting behavior of the NMR peaks coincide well with the structure of NCA with a smooth baseline, as shown in , confirming the purity and uniformity of the synthesized NCA monomer. In the case of the PEG-PRL NMR spectrum, the chemical shifts at 3.64 ppm and 0.91 ppm are assigned to protons of -CH2- in the PEG units and protons of (CH3)2- in the leucine residues, respectively. These two peaks were also used to calculate the molecular weight of the polymers because they appeared with good reproducibility and without any signs of interference.
Figure 1 The representative Fourier transform infrared spectroscopy spectra of: rac-Leu-NCA (A) and PEG-PRL copolymer (B).
Abbreviations: NCA, N-carboxy-anhydrides; PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
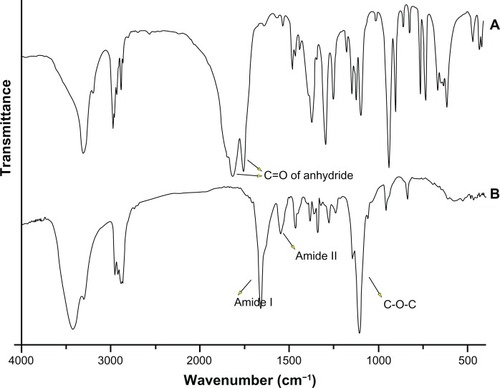
Figure 2 The representative 1H-NMR spectra of rac-Leu-NCA (A) and PEG-PRL copolymer (B) in CDCl3.
Abbreviations: NCA, N-carboxy-anhydrides; NMR, nuclear magnetic resonance; rac-Leu, racemic-leucine; PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
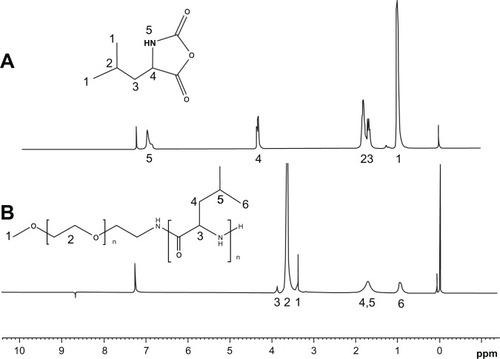
As listed in , the molecular weights of the PEG-PRL polymers calculated by 1H-NMR were in proportion to the feed ratios of the monomer/initiator with an initiator efficiency not less than 0.6. Therefore, the constitution of the polymer could be tuned via the feed ratios and conveniently based on this synthesis method with proper initiator efficiency.
Table 1 Feed compositions and some characteristic properties of hybrid polypeptide copolymers
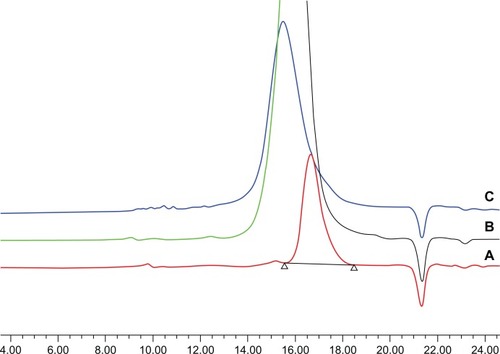
Because the GPC experiments were performed using a traditional refractive index detector and polystyrene samples as calibration standards, they were not expected to provide useful quantitative molecular weight information, partly because of the significant difference in the molecular structure between the samples and the standards.Citation25 The GPC results, however, are useful for verifying the absence of homopolymer impurities and provide an index to evaluate the molecular weight distribution of the products. As shown in , GPC traces of PEG-PRL copolymers showed a uni-modal molecular weight distribution without homopolymers. The polydispersity index of the three polymer products was always below 1.1, estimated from the GPC and listed in , which illustrates the low molecular weight distribution of the resulting polymers.
Figure 3 The gel permeation chromatography traces of PEG-PRL1 (A), PEG-PRL2 (B), and PEG-PRL3 (C) copolymers.
Abbreviations: PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
The optical activities of the PEG-PRL copolymers were analyzed by polarimetry to estimate the racemic extent of the polypeptide segments. The specific optical rotations
of the three products were all very small and negligible, as listed in , confirming the successful preparation of the racemic polypeptide block in the copolymers.
Self-assembly ability of PEG-PRL copolymers
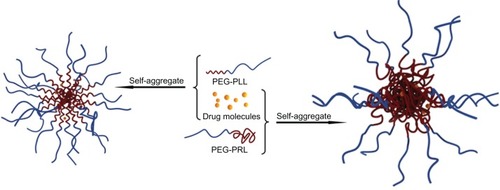
As amphiphilic polymers, the PEG-PRL products have the ability to self-assemble in selective solvents, which is the basis of their application as carriers for the delivery of hydrophobic drugs.
The CAC was determined to evaluate this ability using the “pyrene fluorescence probe” method. As demonstrated in (inset), the maximum excitation wavelength of pyrene increased from 334 nm to 337 nm with an increase in the PEG-PRL concentration. Therefore, the fluorescence intensity ratio at 337 nm and 334 nm (l337/l334) of pyrene versus the logarithm of the polymer concentration was plotted from the excitation spectra (). The inflection point in this plot represented the formation of micelles, and the corresponding concentration was recorded as the CAC of the polymer. The CAC values of the three PEG-PRL polymers, calculated and listed in , ranged from 1.1 to 6.5 mg/L, depending on the length of the hydrophobic polypeptide segments. It appeared that the greater the length of the hydrophobic block, the lower the CAC value. This indicates a stronger self-assembly and may confer greater thermodynamic stability on the resulting micelles.Citation26 The extremely low CAC values of all the synthesized PEG-PRL copolymers compared with the low molecular weight surfactants also offer the possibility of retaining micellar stability under diluted conditions, such as diluted by blood plasma after intravenous injection, which is a very favorable characteristic for the development of effective drug-delivery systems.
Preparation of DTX-loaded micelles
As one of the most commonly used methods of micellar preparation, the dialysis method was selected to prepare DTX-loaded micelles in the current study. PBS with a pH of 5.5 was selected as the dispersion medium for the micellar formulations to increase DTX stability.Citation27 Using this method, DTX-loaded micelles based on PEG hybrid polypeptides were prepared successfully. Some characteristics of these micelles – namely, the particle size and size distribution determined by DLS, DLC, and DLE measured by HPLC – are summarized in .
Table 2 Characteristics of various docetaxel-loaded micelles
The DLS results showed that the micelles based on PEG hybrid polypeptides in aqueous solution were all nanosized particles with a narrow dispersion. For PEG-PRL micelles, the intensity-averaged diameters increased from about 170 to 250 nm, depending on the length of the polypeptide segments. Regarding the well-known core-shell structure of the micelles, this phenomenon could be explained by the volume expansion of the micellar hydrophobic core, which resulted from the longer hydrophobic chains constituted and more hydrophobic drug molecules encapsulated. As well as the increase in particle size, the DLC and DLE also increased with the molecular weight growth of the copolymer.
The representative DLS graphs and corresponding TEM photographs of blank and DTX-loaded micelles prepared based on PEG-PRL3 copolymer are shown in and . A classical core-shell structure with a spherical appearance was observed in the TEM photographs.
Figure 5 The representative dynamic laser light scattering graphs of blank (A) and docetaxel-loaded (B) PEG-PRL3 micelles.
Abbreviations: PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
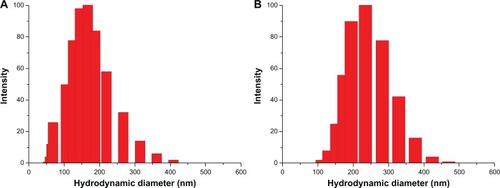
Figure 6 The representative transmission electron microscopy photographs of blank (A) and docetaxel-loaded (B) PEG-PRL3 micelles.
Abbreviations: PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
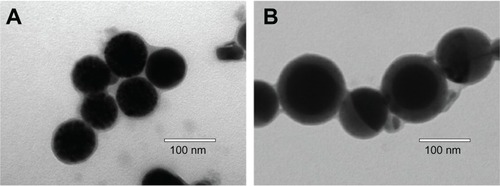
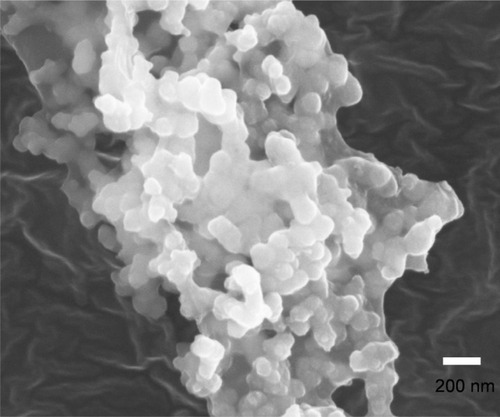
Furthermore, the diameter of the micelles increased almost two-fold after loading with DTX, from about 70 to 150 nm. This obvious expansion probably resulted from the incorporation of drug molecules, revealing the malleable characteristic of the micelle core, the dimensions of which can vary during the drug-loading process. Under all circumstances, DLS measurement showed a larger diameter than the TEM determination, probably because of dehydration of micelles and shrinkage of the PEG shell induced by water evaporation under the high vacuum before TEM observation.Citation28 The SEM photographs of the DTX-loaded PEG-PRL micelles further proved the spherical shape and showed a similar diameter to that of TEM, as shown in .
Figure 7 The representative scanning electron microscopy photograph of lyophilized docetaxel-loaded PEG-PRL3 micelles.
Abbreviations: PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
The highest drug-loading content and efficiency was found for the micelles based on the PEG-PRL3 copolymer, which possesses 38 leucine residues, with a value of 11.2% ± 0.4% and 67.2% ± 2.4%, respectively. Both values were much higher than those of drug-loaded micelles, based on the previously published data on the corresponding levo polypeptides. The poly(levo-leucine)-PEG5000-poly(levoleucine) triblock copolymers with a total of 120 leucine residues synthesized by Hua et alCitation29 were used to prepare PTX-loaded spherical micelles. The obtained DLC and DLE for PTX, which also belongs to the taxoid family and has a similar molecular structure to DTX, were only 5.5% and 11.7%, respectively. With the length of the hydrophobic chains being three times greater than a PEG-PRL3 copolymer, the levo polypeptides reached only half the drug-loading capacity of the racemic polypeptides. This apparent difference in drug loading between the two polypeptides hinted that the conformation of the hydrophobic peptide block may play an important role in the drug-loading process. Because it is well known that poly(levo amino acids) chains may adopt a special conformation, such as α-helices or β-strands,Citation12 this contrasts with the random coil conformation of racemic chains.
To further verify this conformation-determined drug-loading behavior, the PEG5000-poly(levo-leucine) diblock copolymer (PEG-PLL) with 44 leucine residues (calculated by NMR) was synthesized using the ROP of levo-leucine-NCA similar to the synthesis of PEG-PRL. The CAC of the polymer, measured by the aforementioned fluorescence method, was 0.8 mg/L (). This was not significantly different from the CAC value of the PEG-PRL3 polymer (1.1 mg/mL) and demonstrated the strong self-assembly ability of the levo polypeptides.
The TEM photographs of blank and DTX-loaded PEG-PLL micelles prepared by the dialysis method are shown in . It is clear that the morphology and particle size of the blank PEG-PLL micelles () are all similar to those of the PEG-PRL3 blank micelles (), indicating that the levo polypeptides also have the ability to self-aggregate into spherical micelles in aqueous solution. Unlike the significant volume expansion (nearly two-fold, ) after loading with DTX for PEG-PRL3 micelles, the diameter of PEG-PLL micelles exhibited no apparent change after DTX loading (), which confirmed the relatively rigid structure of the micellar core based on levo polypeptides compared with their racemic counterparts. As shown in , the DLC and DLE of DTX-loaded PEG-PLL micelles measured by HPLC were around 0.3% and 1.3%, respectively. These values were far lower than those of all the PEG-PRL micelles, which further confirmed the significant discrepancy in drug-loading ability between the racemic and levo polypeptides.
Figure 8 The transmission electron microscopy photographs of blank (A) and docetaxel-loaded (B) PEG-PLL micelles.
Abbreviations: PEG, poly(ethylene glycol); PLL, poly(levo-leucine).

To obtain a better understanding of this phenomenon, circular dichroism was used to study the secondary structure of the different polypeptides. In the circular dichroism spectrum presented in , the PEG-PLL sample showed two negative bands at 208 and 222 nm, which can be assigned to π–π*and n–π* transitions due to Cotton effects and correspond to an α-helical conformation of the peptide chain,Citation30 whereas in all the PEG-PRL samples, no secondary structure conformation was detected.
Figure 9 The CD spectrum of various copolymers in aqueous solution (0.01 mg/mL).
Abbreviations: CD, circular dichroism; PEG, poly(ethylene glycol); PLL, poly(levo-leucine); PRL, poly(racemic-leucine).
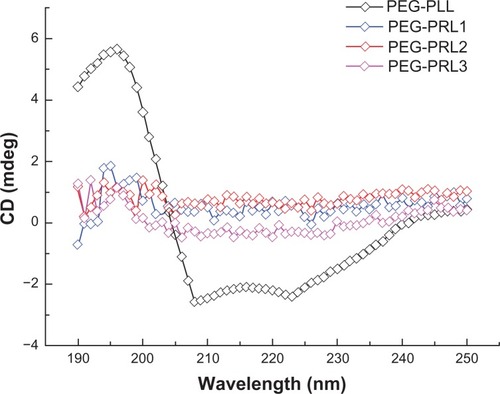
Based on this observation, the different drug-loading ability of the PEG-PLL and PEG-PRL micelles could be explained. On the one hand, as presented in , the PLL block in the PEG-PLL copolymer preferentially adopted a stable α-helical conformation and was packed to form a rigid and compact hydrophobic core of spherical micelles, leaving a limited and restricted space for loading drug molecules. However, in the case of PEG-PRL, the PRL block had no specific conformation and was coiled to form a relatively malleable and loose hydrophobic core, which could encapsulate many more drug molecules compared with PEG-PLL. On the other hand, compared with the α-helix conformation of PLL, the random coil of the PRL block has more freely exposed amide groups that are capable of facilitating H-bonding interactions between hydrophobic chains or hydrophobic chains and drug molecules in the assemblies,Citation11 which would also cause additional entrapment of DTX molecules and provide extra micelle stability simply through a physical interaction. Therefore, it is proposed that both the hydrophobic interactions and the secondary structures of the polypeptide domains control the drug-loading behavior of the PEG-poly(leucine) hybrid polypeptides, and the racemic polypeptides are capable of a much higher drug-loading capacity than the levo forms.
In addition, during the process of micellar preparation, a significant difference in solubility between PEG-PLL and PEG-PRL copolymers was observed. The PEG-PRL copolymer could dissolve easily in many common organic solvents, including acetone, chloroform, DCM, THF, DMF, and DMSO, whereas the PEG-PLL copolymer could only dissolve in strongly H-bonding solvents such as trifluoroa-cetic acid. The better solubility of PEG-PRL compared with its levo form can be ascribed to the conformation difference, because the random coil conformation of the PRL block offers much more flexibility compared with the rigid α-helix conformation of PLL segments, as also reported elsewhere.Citation11 This conformation, resulting in better solubility of PEG-PRL, also facilitated the DTX-loading behavior by providing an easier drug-loading process. In fact, the better solubility of the PEG-PRL copolymer is more favorable in the process of drug development by providing a more flexible formula and preparation technique, according to the characteristics of the drug chosen for loading and avoiding the application of toxic solvents such as trifluoroacetic acid.
In summary, the racemic hybrid polypeptides (PEG-PRL) facilitated the doxetaxol-loading behavior by providing a much higher drug-loading ability and easier drug-loading process compared with the corresponding levo forms (PEG-PLL).
In vitro release studies
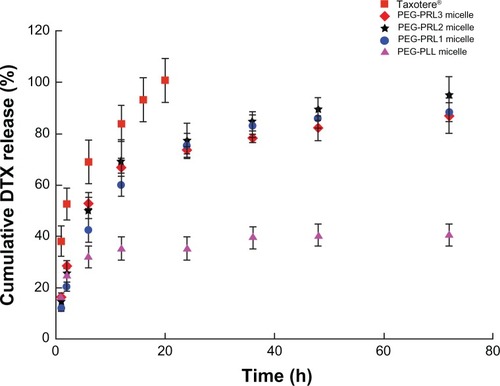
The in vitro release behavior of DTX-loaded PEG-PRL and PEG-PLL micelles was investigated by dialysis diffusion method.Citation19
As shown in , a similar typical biphasic release pattern, composed of a burst release in the initial stage followed by a sustained release up to 72 hours, was observed under all the PEG-PRL conditions, indicating that the release behavior of DTX from racemic polypeptide-based micelles depends little on the length of the hydrophobic block. An obvious difference between the release curve of PEG-PRL micelles and Taxotere was also observed with a more rapid release of DTX from Taxotere, demonstrating the sustained release properties of the PEG-PRL micelles. Furthermore, it was found that the release rate of PEG-PLL micelles was much slower than others, which released less than 40% of the loaded drug during the entire experimental period. This difference in release behavior between the micelles based on PEG-PLL and PEG-PRL copolymer further proved the different state of the hydrophobic core presented in . This is because the drug molecules can be released from the loose core formed by the racemic polypeptides more easily than from the compact core formed by levo polypeptides.
Cell cytotoxicity studies
Because the highest drug-loading content and efficiency were found in the PEG-PRL3 micelles, these micelles and the PEG-PRL3 copolymer were selected for further studies.
As a potential drug-loading carrier, the material used must be proved to be nontoxic to humans (ie, it should be highly biocompatible). Therefore, the in vitro cytotoxicity of the PEG-PRL3 copolymer was studied with MCF-7 and HUVECs to evaluate the biocompatibility of the racemic polypeptides using MTT assay.
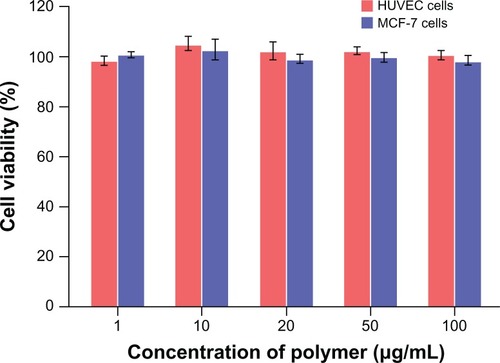
As shown in , the cell viability in the presence of various concentrations of PEG-PRL3 copolymer was in the range of 99.3% ± 1.7% to 102.5% ± 4.1% for MCF-7 cells and 99.0% ± 1.8% to 103.7% ± 2.7% for HUVECs. This result proved that PEG-PRL3 copolymer exhibited almost no cytotoxicity against these two kinds of cells up to the concentration of 100 μg/mL, demonstrating the excellent biocompatibility of PEG-PRL copolymer.
Figure 11 In vitro cytotoxicity of different concentrations of PEG-PRL3 copolymer against HUVECs and MCF-7 cells by MTT assay (n = 5).
Abbreviations: DTX, docetaxel; HUVECs, human umbilical vein endothelial cells; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
The in vitro cytotoxicity of the DTX-loaded PEG-PRL3 micelles was also studied by MTT using MCF-7 cells to evaluate the antitumor effect of the drug-loaded micelles against tumor cells. In this study, a DMSO solution of DTX was used as the free drug stock solution instead of commercially available DTX preparations such as Taxotere, because of the known cytotoxity of Tween 80, which is used as a surfactant at a high concentration in these preparations.Citation31,Citation32
The MCF-7 cell viability after 72 hours of incubation with various concentrations of free DTX and DTX-loaded micelles is shown in . In general, the viability of cells incubated with the two preparations decreased in a dose-dependent fashion. Furthermore, a much lower cell viability (P < 0.01) of micelles compared with free drug was observed at all concentrations, and the IC50 of the free DTX and DTX-loaded micelles was 0.036 (Emax: 77.7%) and 0.009 μM (Emax: 84.8%), respectively. This result suggests that the DTX-loaded micelles are more cytotoxic than the free drug against cancer cells, which is probably because the additional drug internalized in the cells resulted from endocytosis and enhanced intracellular drug accumulation by the nanoparticle uptake reported elsewhere.Citation33,Citation34
Figure 12 In vitro cytotoxicity of different concentrations of free drug and docetaxel-loaded PEG-PRL3 micelles against MCF-7 cells by MTT assay (n = 5).
Note: *P < 0.01.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).

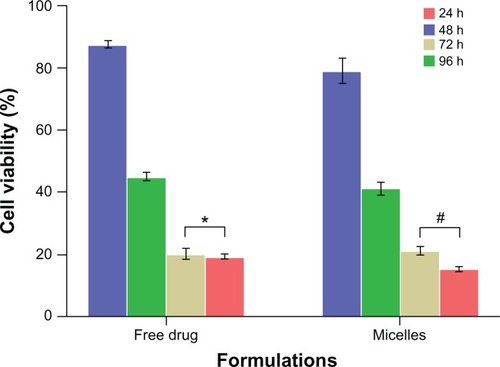
The MCF-7 cell viability after incubation with the free DTX and DTX-loaded micelles at the same concentration (10 μM calculated by DTX) after 24 hours, 48 hours, 72 hours, and 96 hours is shown in . With prolongation of the incubation time, a gradual decrease in cell viability was observed in the DTX-loaded micelles. However, the cell viability of free DTX at 72 hours and 96 hours was not significantly different (P > 0.05), with a value of 18.1% ± 1.7% and 19.8% ± 0.7%, respectively, in contrast to the significantly different cell viability (P < 0.01) of DTX-loaded micelles at 72 hours and 96 hours with a value of 21.2% ± 1.3% and 15.4% ± 0.6%, respectively. This suggests that the incubation time of 72 hours may be critical to allow the free DTX to kill MCF-7 cells, although this time threshold must be much longer for DTX-loaded micelles. This phenomenon can be attributed to the sustained release of DTX from the micelles, as already proved by the in vitro release studies.
Figure 13 In vitro cytotoxicity of free drug and docetaxel-loaded PEG-PRL3 micelles against MCF-7 cells with different incubation time by MTT assay (n = 5).
Notes: *P > 0.05; #P < 0.01.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PEG, poly(ethylene glycol); PRL, poly(racemic-leucine).
Conclusion
In summary, racemic hybrid polypeptides, PEG-PRL diblock copolymers, with different lengths of hydrophobic segments were synthesized. Based on the micellization of these amphiphilic copolymers in aqueous solution, the antitumor drug DTX could be entrapped successfully with high drug-loading capacity. In contrast to the corresponding levo polypeptide PEG-PLL, the random coil conformation of racemic polypeptides PEG-PRL facilitated drug-loading due to the looser hydrophobic micelle core, more H-bonding interactions, and much more chain flexibility. This allowed encapsulation of more drug molecules in an easier way. According to the cytotoxicity assay, the hybrid racemic poly-peptide PEG-PRL used as a drug carrier was nontoxic against MCF-7 and HUVECs up to a concentration of 100 μg/mL. Also, the anticancer efficacy of the DTX-loaded micelles was much higher than that of the free drug against MCF-7 cancer cells. These results unequivocally demonstrated that these high drug-loading PMs based on hybrid racemic polypeptides may be used as a more effective drug vehicle than their levo forms for the delivery of lipophilic antitumor drugs.
Acknowledgements
This project was financially supported by the Liaoning Provincial Science and Technology Department (2009ZX09301-012). Dr David B Jack is gratefully thanked for correcting the English in the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- KedarUPhutanePShidhayeSKadamVAdvances in polymeric micelles for drug delivery and tumor targetingNanomedicine20106671472920542144
- LavasanifarASamuelJKwonGSPoly(ethylene oxide)-block-poly (L-amino acid) micelles for drug deliveryAdv Drug Deliv Rev200254216919011897144
- LecommandouxSKlokH-ASchlaadHSelf-Assembly of Linear Polypeptide-Based Block Copolymers Block Copolymers In NanoscienceWeinheim, GermanyWiley-VCH Verlag GmbH and Co KGaA;2008117150
- BaeYKataokaKIntelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymersAdv Drug Deliv Rev2009611076878419422866
- YangDVanSJiangXYuLNovel free paclitaxel-loaded poly (L-gamma-glutamylglutamine)-paclitaxel nanoparticlesInt J Nanomedicine20116859121289985
- SunYTangYChuMSongSXinYA convenient and adjustable surface-modified complex containing poly-L-glutamic acid conjugates as a vector for gene deliveryInt J Nanomedicine20083224925618686784
- DengCChenXYuHSunJLuTJingXA biodegradable triblock copolymer poly(ethylene glycol)-b-poly(l-lactide)-b-poly(l-lysine): synthesis, self-assembly, and RGD peptide modificationPolymer2007481139149
- ShiraishiKKawanoKMinowaTMaitaniYYokoyamaMPreparation and in vivo imaging of PEG-poly(L-lysine)-based polymeric micelle MRI contrast agentsJ Control Release20091361142019331861
- HuangWWangWWangPGlycyrrhetinic acid-modified poly(ethylene glycol)-b-poly(gamma-benzyl l-glutamate) micelles for liver targeting therapyActa Biomater20106103927393520438873
- CarlsenALecommandouxSSelf-assembly of polypeptide-based block copolymer amphiphilesCurr Opin Colloid Interface Sci2009145329339
- HansonJALiZDemingTJNonionic block copolypeptide micelles containing a hydrophobic racemic-leucine coreMacromolecules201043156268626921617764
- ChoiYYJooMKSohnYSJeongBSignificance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymersSoft Matter200841223832387
- HansonJAChangCBGravesSMLiZMasonTGDemingTJNanoscale double emulsions stabilized by single-component block copolypeptidesNature20084557209858818769436
- ZhaoMSuMLinXEvaluation of docetaxel-loaded intravenous lipid emulsion: pharmacokinetics, tissue distribution, antitumor activity, safety and toxicityPharm Res20102781687170220552255
- LuoJXiaoKLiYWell-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatmentBioconjug Chem20102171216122420536174
- DalyWHPochDThe preparation of N-carboxyanhydrides of [alpha]-amino acids using bis(trichloromethyl)carbonateTetrahedron Letters1988294658595862
- SinhaVRAnithaRGhoshSNandaAKumriaRComplexation of celecoxib with β-cyclodextrin: characterization of the interaction in solution and in solid stateJ Pharm Sci200594367668715668949
- JeongYIKim doHChungCWDoxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymerInt J Nanomedicine201161415142721796244
- NieSHsiaoWLPanWYangZThermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studiesInt J Nanomedicine2011615116621499415
- RichardsonRRJrMillerJAReichertWMPolyimides as biomaterials: preliminary biocompatibility testingBiomaterials19931486276358399958
- WangYHongCZhouCXuDQuHBChengYScreening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L. seedsEvid Based Complement Alternat Med182009 [Epub ahead of print.]
- Segura-SanchezFMontembaultVFontaineLMartinez-BarbosaMEBouchemalKPonchelGSynthesis and characterization of functionalized poly(gamma-benzyl-L-glutamate) derivates and corresponding nanoparticles preparation and characterizationInt J Pharm20103871–224425220005933
- DemingTJPolypeptide materials: new synthetic methods and applicationsAdv Mater199794299311
- DimitrovISchlaadHSynthesis of nearly monodisperse polystyrene-polypeptide block copolymers via polymerisation of N-carboxyanhy-dridesChem Commun20032329442945
- FloudasGPapadopoulosPKlokHAVandermeulenGWMRodriguez-HernandezJHierarchical self-assembly of poly(γ-benzyl-L-glutamate)-poly(ethylene glycol)-poly(γ-benzyl-L-glutamate) rod-coil-rod triblock copolymersMacromolecules2003361036733683
- GaucherGDufresneMHSantVPKangNMaysingerDLerouxJCBlock copolymer micelles: preparation, characterization and application in drug deliveryJ Control Release20051091–316918816289422
- GaoKSunJLiuKLiuXHeZPreparation and characterization of a submicron lipid emulsion of docetaxel: submicron lipid emulsion of docetaxelDrug Dev Ind Pharm200834111227123718720137
- HuYZhangLCaoYGeHJiangXYangCDegradation behavior of poly(epsilon-caprolactone)-b-poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles in aqueous solutionBiomacromolecules2004551756176215360284
- HuaS-HLiY-YLiuYSelf-assembled micelles based on PEG-polypeptide hybrid copolymers for drug deliveryMacromol Rapid Commun2010311818621590841
- NormaJGApplications of circular dichroism in protein and peptide analysisTrAC1999184236244
- EsmaeiliFDinarvandRGhahremaniMHDocetaxel-albumin conjugates: preparation, in vitro evaluation and biodistribution studiesJ Pharm Sci20099882718273018972321
- ArechabalaBCoiffardCRivallandPCoiffardLJMRoeck-HoltzhauerYDComparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH releaseJ Appl Toxicol199919316316510362266
- WongHLBendayanRRauthAMXueHYBabakhanianKWuXYA mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nano-particle systemJ Pharmacol Exp Ther200631731372138116547167
- WongHLRauthAMBendayanRA new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cellsPharm Res20062371574158516786442