Abstract
Background
We have previously shown that human mesenchymal stem cells (hMSCs) can reduce toxin-induced neurodegeneration in a well characterized rodent model of Parkinson’s disease. However, the precise mechanisms, optimal cell concentration required for neuroprotection, and detailed cell tracking need to be defined. We exploited a near-infrared imaging platform to perform noninvasive tracing following transplantation of tagged hMSCs in live parkinsonian rats.
Methods
hMSCs were labeled both with a membrane intercalating dye, emitting in the near- infrared 815 nm spectrum, and the nuclear counterstain, Hoechst 33258. Effects of near-infrared dye on cell metabolism and proliferation were extensively evaluated in vitro. Tagged hMSCs were then administered to parkinsonian rats bearing a 6-hydroxydopamine-induced lesion of the nigrostriatal pathway, via two alternative routes, ie, intrastriatal or intranasal, and the cells were tracked in vivo and ex vivo using near-infrared technology.
Results
In vitro, NIR815 staining was stable in long-term hMSC cultures and did not interfere with cell metabolism or proliferation. A significant near-infrared signal was detectable in vivo, confined around the injection site for up to 14 days after intrastriatal transplantation. Conversely, following intranasal delivery, a strong near-infrared signal was immediately visible, but rapidly faded and was completely lost within 1 hour. After sacrifice, imaging data were confirmed by presence/absence of the Hoechst signal ex vivo in coronal brain sections. Semiquantitative analysis and precise localization of transplanted hMSCs were further performed ex vivo using near-infrared imaging.
Conclusion
Near-infrared technology allowed longitudinal detection of fluorescent-tagged cells in living animals giving immediate information on how different delivery routes affect cell distribution in the brain. Near-infrared imaging represents a valuable tool to evaluate multiple outcomes of transplanted cells, including their survival, localization, and migration over time within the host brain. This procedure considerably reduces the number of animal experiments needed, as well as interindividual variability, and may favor the development of efficient therapeutic strategies promptly applicable to patients.
Introduction
In the past 30 years, translation of the outstanding potential of stem cells from bench top to clinical trials in humans has represented a major objective for the scientific community. The therapeutic use of stem cells, in particular, has emerged as a promising branch of regenerative medicine for treating many aggressive and/or incurable diseases in humans, such as neurodegenerative disorders.Citation1 In particular, Parkinson’s disease has been one of the first neurodegenerative diseases considered eligible for cellular therapy.Citation2 Parkinson’s disease affects 1%–2% of individuals over 60 years and is mainly characterized by the progressive loss of a well defined population of central dopaminergic cells in the substantia nigra pars compacta. The potential efficacy of stem cells for replacement of lost neurons or as a neurotrophic support for spared neurons within the nigrostriatal compartment has been demonstrated in preclinical models of the disease. Conversely, inconclusive results have been reported in the few open-label studies conducted so far in patients. Thus, considerable progress lies ahead to reach substantial and detectable therapeutic effects.Citation3 Many regulatory pathways and environmental factors may govern stem cell behavior following transplantation in patients with Parkinson’s disease. Citation4 The development of effective cell-based therapies to treat neurodegeneration therefore requires an extensive comprehension of the progressive environmental cues and reciprocal interactions between the host and donor cells.
Human mesenchymal stem cells (hMSCs) are promising candidates for rapid clinical application of stem cell therapy in patients with Parkinson’s disease because of their simple derivation from bone marrow aspirates and ease of in vitro expansion,Citation5 absence of tumorigenic potential,Citation6 and possible autologous derivation, avoiding major ethical and immunological problems linked to other cell types. We have recently shown that in rats bearing a 6-hydroxydopamine (6-OHDA)-induced lesion of the nigrostriatal pathway, a well characterized Parkinson’s disease-like rodent model,Citation7,Citation8 intrastriatal transplantation of naïve hMSCs reduced ongoing neuronal loss in the substantia nigra pars compacta and associated behavioral alterations.Citation9 Grafted cells also positively and dose-dependently influenced endogenous neurogenesis, sustaining the commitment of neural progenitors towards neurons.Citation10
Near-infrared light imaging offers new opportunities as a sensitive and noninvasive detection technique for diagnostics. This technology holds enormous potential for a wide variety of in vivo applications and is being increasingly used in small animal research. Use of the near-infrared wavelength for imaging allows deep penetration into tissues with minimal background and high optical contrast.Citation11,Citation12 This simple technology enables live determination and imaging of biological targets without the need for exhaustive tissue sampling.Citation13 In humans, the use of noninvasive near-infrared imaging has already been proposed as a routine diagnostic tool in stroke,Citation14 and is currently employed as a valuable bedside device for in vivo targeting of cancer and other tissue abnormalities.Citation15 In the present study, we evaluated the feasibility of using near-infrared imaging to perform a longitudinal study and track in vivo the fate of hMSCs tagged with a near-infrared-excitable membrane linker in 6-OHDA parkinsonian-like rats. Outcomes of two alternative delivery routes, ie, intrastriatal or intranasal administration, were compared to clarify the progression of engraftment mechanisms by near-infrared imaging. This technology allowed rapid and efficient real- time detection of labeled cells in vivo in anesthetized animals. It also permitted easy follow-up of transplanted cells over time and within the same cohort of animals, thereby considerably reducing both the number of rats required and time-consuming analyses of serial tissue samples. Our data show that near-infrared technology is a simple and readily available technology that assures rapid semiquantitative analysis and immediate traceability of tagged hMSCs following transplantation both in vivo in anesthetized animals and ex vivo in tissue sections.
Materials and methods
Human mesenchymal stem cells
Commercial hMSCs were purchased (Cambrex, Walkersville, MD) and adherent cells were grown until confluence in the appropriate medium (MSCBM, Cambrex) according to the manufacturer’s instructions. All in vitro and in vivo experiments were conducted with cells between passages 2–6 in vitro. hMSCs were subjected to karyotypic analysis to confirm the absence of any heterochromatin aberrations, as described elsewhere.Citation9
NIR815 labeling of cultured hMSCs
Cells were labeled using the CellVue® NIR815 Midi Kit for membrane labeling (Polyscience, Warrington, PA) following the manufacturer’s instructions. This procedure allows stable incorporation of a NIR815 dye into lipid regions of the cell membrane that can be readily detected using the Odyssey® scanner and imaging system (Li-Cor® Biosciences, Lincoln, NE).
Briefly, 1 × 106 cells were washed twice with phosphate-buffered saline (Sigma-Aldrich, St Louis, MO), in the absence of Ca2+ and Mg2+, and collected. Pellets were resuspended in 50 μL of diluent C with gentle mixing, and the dye solution was added to the cell suspension and incubated for 5 minutes at room temperature. The reaction was blocked by adding fetal bovine serum (Sigma-Aldrich, 1:1 v/v), and the cells were washed to eliminate unbound dye and plated inside a T75 cm2 flask in MSCBM medium. The cells were also labeled with fluorescent dye, ie, bisbenzimide-Hoechst 33258 (1 ng/mL; Molecular Probes, Invitrogen, Carlsbad, CA) to allow their immunofluorescent detection on brain slices using conventional microscopy.
Cell survival and proliferation
Cell proliferation was tested on unlabeled hMSCs and NIR815 hMSCs over six passages in vitro. Cells were plated in a T75 cm2 flask (5 × 105 cells/flask at T0). In confluent cultures (6–7 days in vitro), cells were counted by Trypan blue exclusion and expanded in T75 cm2 flasks. Values represent the total number of NIR815-unlabeled or NIR815-labeled hMSCs counted at each passage.
Cell survival was evaluated at the same time points by a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96® AQueous Assay, Promega, Madison, WI) following the manufacturer’s instructions, starting 1 day after the near-infrared labeling procedure. Briefly, NIR815 hMSCs were plated in 96-well plate (5 × 104 cells/well) and maintained at 37°C. After 1 hour, MTS solution (20 μL/well) was added to the cells and incubation was continued at 37°C for another hour. Supernatants were then collected, centrifuged at 2000 g for 5 minutes, and optical density was read at 490 nm using a microplate reader (El × 800, Bio-Tek Instruments Inc, Winooski, VT). Each sample was performed in triplicate and at least three separate experiments were conducted for both methodologies.
Maintenance of NIR815 dye in hMSCs over time
hMSCs (P3) were labeled as described above, plated in T75 cm2 flasks (5 × 105 cells/flask) and maintained in culture. At different time points after labeling (24 hours, or 14 or 28 days in vitro) NIR815 hMSCs were collected, counted, and serial dilutions (300, 150, 80, 40, 20, 10, 5, and 2 × 103 cells) were plated on coverglasses. Cells were fixed with 4% paraformaldehyde (20 minutes at room temperature, Sigma-Aldrich) and mounted with Fluorsave (Calbiochem, La Jolla, CA). Semiquantitative evaluation of NIR815 intensity in cells was performed using the Odyssey® imager (see below). Unlabeled hMSCs (3 × 105 cells/coverglass) were used as a negative control.
Transfer of NIR815 and Hoechst 33258 dyes
hMSCs were double-labeled with NIR815 dye and Hoechst 33258, as described above (NIR815 labeling of cultured hMSCs) and plated in T75 cm2 flasks (5 × 105 cells/flask). Conditioned media were collected at different time points after labeling (24 and 72 hours) and cell debris were eliminated by a brief centrifugation step (1200 g for 10 minutes). Resulting conditioned media (CM24 and CM72) were directly added to 5 × 104 unlabeled hMSCs plated on coverglasses and incubation proceeded for a further 24 hours. Cells were then washed, fixed with 4% paraformaldehyde, and processed for immunocytochemical staining using an anti-CD90 primary antibody (BD Pharmingen, Franklin Lakes, NJ), a membrane marker expressed by hMSCs, as described elsewhere. Citation16 IRDye® 700 and AlexaFluor594 secondary antibodies (M-Medical, Milano, Italy, dilution 1:10,000) were used, respectively, for near-infrared and microscopic evaluation of dye leakage. At the end of the staining procedure, coverglasses were either placed in a 24-well plate and covered with phosphate-buffered saline (near-infrared evaluation) or mounted with Fluorsave (microscopic evaluation). Presence or absence of NIR815 was assessed using the Odyssey® imager (for near-infrared 700 and nearinfrared 800 scan intensity: 8 and 3, respectively; resolution 21 μm; focus 3 mm). Unlabeled hMSCs (CTR−) and NIR815/Hoechst+ hMSCs (CTR+; both 5 × 104 cells/coverglass) were used as negative and positive controls, respectively. Similarly, the presence of or the absence of Hoechst staining was evaluated using a fluorescent microscope (ImageM2, Zeiss, Oberkochen, Germany).
In vitro near-infrared imaging
For relative quantification of signal intensity, near-infrared images were obtained using the Odyssey® imager. Briefly, coverglasses were placed on the surface of the imager and highresolution scans were performed as follows: near-infrared 700 and near-infrared 800 scan intensity 3, resolution 21 μm, and focus 1 mm. For quantitative evaluation, a region of interest was traced around the cells, and near-infrared 700 signal intensity values were recorded using Odyssey® software.
Flow cytometry
FITC Annexin V in conjunction with propidium iodide were used to allow the identification of apoptotic, dead, and damaged cells. Briefly, NIR815 hMSCs were harvested, resuspended in 1 × binding buffer, in the presence of FITC Annexin V and propidium iodide (both from BD Pharmingen), and incubated for 15 minutes in the dark at room temperature. Cells were then washed once, acquired by a flow cytometer (FACSCanto, II Becton Dickinson, Franklin Lakes, NJ) and analyzed using Diva software within 1 hour. Unlabeled hMSCs were used as control.
Animals
Male Sprague-Dawley rats (Charles River, Calco, LC, Italy), weighing 200–250 g at the beginning of the experiment, were housed two per cage, at 20°C–22°C on a 12-hour light-dark cycle, with food and water ad libitum. Animals were left in the housing facilities for at least 1 week before the beginning of the experiments. In compliance with our national law, no specific ID for permission is required. All procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the local animal care committee.
Lesion surgery
Animals were anesthetized with sodium thiopental (50 mg/kg, Hospira, Italy), placed on a stereotaxic frame, and received an intrastriatal injection of 6-OHDA (20 μg/3 μL in saline containing 0.02% ascorbic acid; Sigma-Aldrich) into the right striatum at the following coordinates (mm) relative to bregma and the dural surface: anterior-posterior 1.0, medial-lateral 3.0, dorsal-ventral 5.0.Citation17 The animals were then randomly assigned to two subgroups for intrastriatal or intranasal infusion of NIR815 hMSCs.
Intrastriatal transplantation
Five days after 6-OHDA injection, a subgroup of animals was anesthetized (50 mg/kg thiopental, Hospira) and underwent a second surgical session () in which the rats received an injection of NIR815 hMSCs (1.8 × 105 cells/4 μL) or vehicle (phosphate-buffered saline) along two tracts in the ipsilateral striatum (anterior-posterior 1.8 mm, mediallateral 3.0 mm, dorsal-ventral 5.0 mm, and anterior-posterior 0.2 mm, medial-lateral 3.0 mm, dorsal-ventral 5.0 mm with respect to bregma and dura).Citation17
Figure 1 Schematic representation of the experimental paradigms. (A) Intrastriatal transplantation of NIR815 human mesenchymal stem cells in 6-OHDA-lesioned animals. (B) Intranasal infusion of NIR815 human mesenchymal stem cells in 6-OHDA-lesioned animals.
Note: See Materials and Methods section for detailed description.
Abbreviations: 6-OHDA, 6-hydroxydopamine; IS, intrastriatal; IN, intranasal; PBS, phosphate-buffered saline; hMSCs, human mesenchymal stem cells.
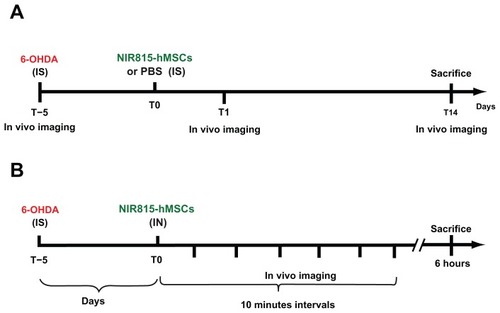
All intracerebral infusions were performed at 0.5 μL/minute, using a 10 μL Hamilton syringe with a 26-gauge needle. After injection, the needle was left in place for 10 minutes to allow complete diffusion of the medium, and wounds were clipped.
To avoid possible rejection of the xenotransplant and to mimic standard clinical procedures in humans, all animals received a daily injection of cyclosporine A 10 mg/kg that was started 2 days before the transplantation procedure. We have previously demonstrated that this treatment per se has no influence on either the 6-OHDA-induced neurodegenerative process or transplanted human cells.Citation9
One (T1) or 14 (T14) days after cell transplantation, the rats were anesthetized, placed on the surface of an Odyssey ® imager assembled with the MousePOD® Accessory (LI-COR Biosciences), and in vivo imaging was performed (see below).
Intranasal transplantation
Five days after 6-OHDA lesion, a subgroup of animals was anesthetized and received intranasal administration of NIR815 hMSCs (). Briefly, animals were placed supine on a stereotaxic frame and a cannula (32-gauge catheter system; Braintree Scientific Inc, Braintree, MA) was placed in the left nostril so that its tip reached the olfactory epithelium that lies between the nostril cavity and the olfactory bulb (15 mm deep). Hyaluronidase (Sigma-Aldrich), which has been suggested to loosen barrier function in the nasopharyngeal mucosa,Citation18 was resuspended in phosphate-buffered saline and 100 U was applied in the nostril 30 minutes prior to the application of the cells. NIR815 hMSCs (2 × 106 cells/4 μL) were administered at 0.5 μL/minute. Following injection, the cannula was left in place to avoid reflux of the cells outside of the nasal cavity. Animals were placed on the Odyssey® imager mounted with the Mouse-POD® accessory, and near-infrared images were taken, as described below, every 10 minutes over a 1-hour period and again 6 hours after intranasal administration of the cells (see experimental procedure, ).
In vivo near-infrared imaging
The snout or head of the animal was accurately shaved to reduce near-infrared background due to hair, and animals were maintained under sedation during all in vivo imaging procedures. Briefly, anesthetized animals were placed, in a supine position, on the Odyssey® Imager mounted with the MousePOD accessory so that the snout or head lay flat on the scanner surface; constant temperature of 37°C was maintained in the chamber and in vivo images were taken at 800 nm (scan intensity 7, resolution 84 μm, focus 4.0 mm). Signal intensities were displayed in pseudocolor scale using the dedicated Odyssey® software. Semiquantitative evaluation of the near-infrared signal was performed on the scanned images of the transplanted animals using the Odyssey ® software. A region of interest was drawn around the green near-infrared signal and near-infrared signal intensity values were recorded.
Sacrifice and immunohistochemistry
At the end of the experimental procedures, the animals were sacrificed, and the brains were removed immediately and conserved at −80°C. Serial coronal sections (25 μm) were cut throughout the striatum using a cryostat (Jung CM Leica, Wetzlar, Germany), picked up on poly-lysine-coated slides (Thermo Scientific, Waltham, MA), dried at room temperature, protected from light, for 1 hour and scanned (see below).
Immunohistochemical staining for tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, was carried out on every fourth section, as described elsewhere,Citation19 using a specific primary antibody (MAB318, Chemicon, Millipore, Billerica, MA, dilution 1:2,000). An IRDye®700CW goat antimouse secondary antibody (M-Medical, Milano, Italy; dilution 1:10,000) was used for detection. Slides were dried for one hour in the dark, mounted using Prolong (Invitrogen), and scanned 1 day later (see below).
Ex vivo near-infrared imaging of brain coronal sections
Dried sections were scanned immediately after sectioning and prior to any immunohistochemical staining to allow high throughput detection of the transplanted cells. Briefly, slides were placed on the Odyssey® imager and rapid low-resolution scan was performed as follows: resolution 169 μm and quality low focus 1 mm. Channel intensities were set to 3 for 800 nm to visualize tagged hMSCs and 7 for 700 nm. The latter allows detection of nonspecific background signals from the sample and permits gross localization of the transplanted cells within the cerebral tissue.
Following tyrosine hydroxylase immunohistochemistry, the slides were placed, one at a time, on the Odyssey® imager and high resolution scan was performed as follows: resolution 21 μm, quality high, and focus 1 mm. Intensity was set to 3 for both the channels (700 and 800 nm).
Three-dimensional reconstruction of transplanted striata
Images of brain sections containing transplanted NIR815 hMSCs were imported into the Neurolucida software (Microbrightfield, Williston, VT). Tracing of the brain outline, the left (intact) and right (transplanted) striata, as well as the area containing NIR815 hMSCs was performed on every fourth section (intersectional distance 100 μm), and three-dimensional brain volumes were reconstructed.
Statistical analysis
All data are expressed as the mean ± standard deviation. Data were compared by analysis of variance followed by Dunnett’s post hoc test as appropriate. Values of P < 0.05 were considered to be statistically significant.
Results
Effect of NIR815 labeling on hMSCs
Apoptosis and cell death were evaluated by flow cytometry on NIR815 hMSCs immediately and 20 hours after staining using Annexin V (that binds to phospholipid phosphatidylserine in the cell membrane in the earlier stages of apoptosis) and propidium iodide (indicative of cells that have suffered irreversible injury with damage/leakage in plasma membrane). A comparable percentage of early/late apoptotic and dead cells was observed between NIR815-labeled and unlabeled hMSCs (CTR) indicating that the dye had no effect on cell viability ().
Figure 2 Effect of NIR815 dye on human mesenchymal stem cells. (A) Flow cytometry analysis of unlabeled and NIR815 human mesenchymal stem cells, 0 and 20 hours after staining procedures. (B) Cell proliferation, cumulative number of human mesenchymal stem cells at different days in vitro following labeling with NIR815 dye. (C) Cell survival, with cell viability examined by MTS assay at different time points as indicated below the graph.
Note: Data are presented as the mean ± standard deviation from three replicates of three different experiments.
Abbreviations: OD, optical density; PI, propidium iodide; hMSCs, human mesenchymal stem cells; DIV, days in vitro.
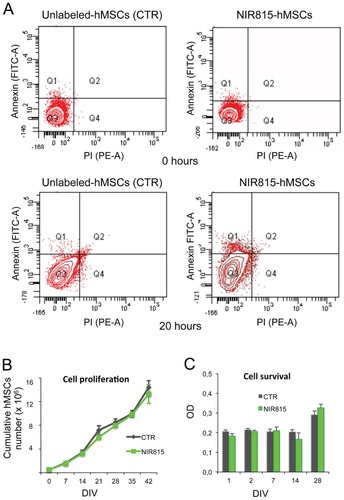
We also determined the cell proliferation and cell survival capacity of NIR815 hMSCs compared with CTR. The proliferation curve of NIR815 hMSCs was analogous to that obtained with CTR (). Both cell populations could be maintained in culture over several passages (up to seven passages, 42 days in vitro) without significant changes in duplication. Similarly, survival of NIR815 hMSCs was comparable with that observed for CTR at each time point considered ().
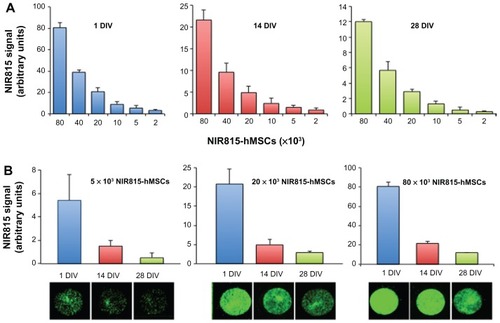
Important issues were to determine how the NIR815 signal diminished in tagged hMSCs over time as a consequence of cell division, and to evaluate the number of labeled cells that may be detected in vitro as a function of time. Serial dilutions of NIR815 hMSCs were seeded on coverglasses at one, 14, and 28 days in vitro after labeling, and near-infrared signal intensities (800 nm channel) were determined. Our data show that a quantifiable near-infrared signal was detectable at all time points considered and cell dilutions evaluated (). A significant and quantitative signal was detected and well preserved for up to 28 days in vitro, notwithstanding cell division. Relative signal intensities were also preserved between serial cell concentrations along passages ().
Figure 3 Preservation of NIR815 tag over time. Cells were labeled using the CellVue® NIR815 kit and maintained in culture. NIR815 signal intensity was determined at various time points (1, 14, and 28 days in vitro) in serial cell dilutions (80 × 103, 40 × 103, 20 × 103, 10 × 103, 5 × 103, and 2 × 103 near-infrared human mesenchymal stem cells). (A) Signal intensity detected at 1, 14, and 28 days in vitro as indicated above the graphs. Numbers below the x axis indicate the number of NIR815 human mesenchymal stem cells. (B) Signal intensity detected for 80 × 103, 20 × 103, and 5 × 103 NIR815 human mesenchymal stem cells. Numbers below the x axis indicate the days in vitro at which scan was performed. The near-infrared signal is expressed as arbitrary units.
Note: Data are presented as the mean ± standard deviation from three replicates of three different experiments.
Abbreviations: DIV, days in vitro; hMSCs, human mesenchymal stem cells.
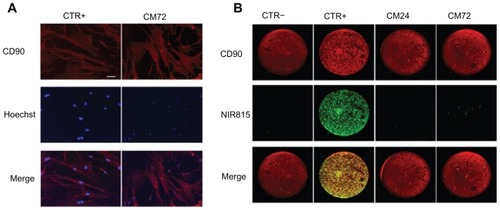
We also assessed whether some dye transfer could be detected in vitro. Our data show that while some leakage was observed for Hoechst, this phenomenon was not detected following NIR815 labeling (). CD90, a surface marker expressed on hMSCs, was used to confirm the presence and human nature of the cells. Unlabeled hMSCs incubated with supernatants from double-labeled hMSCs (CM24 and CM72) showed a weak Hoechst signal, clearly distinguishable from that observed in positive CTR (). Conversely, no NIR815 staining could be detected in cells incubated with CM72, clearly indicating that the NIR815 dye did not leak from tagged cells ().
Figure 4 Dye transfer. Cells were double-labeled with Hoechst and CellVue® NIR815, their conditioned media taken up at various time points (24 and 72 hours) and used to assess possible leakage of either dyes. (A) Detection of Hoechst staining (blue) in CD90-positive hMSCs (red). CTR+: human mesenchymal stem cells directly labeled with the NIR815 dye (positive control); CM72: CD90 human mesenchymal stem cells incubated with conditioned media obtained 72 hours after NIR815 labeling. Scale bar 10 μm. (B) Detection of NIR185 signal in CD90-positive human mesenchymal stem cells. CD90 signal is shown in red, CellVue signal is shown in green.
Abbreviations: CTR−, CD90-positive human mesenchymal stem cells in the absence of NIR815 staining; CTR+, CD90 human mesenchymal stem cells directly labeled with the NIR815 dye; CM24, CD90 human mesenchymal stem cells incubated with conditioned media obtained 24 hours after near-infrared labeling; CM72, CD90 human mesenchymal stem cells incubated with conditioned media obtained 72 hours after NIR815 labeling.
In vivo tracking of NIR815 hMSCs in parkinsonian rats
Animals received a unilateral injection of 6-OHDA in the right striatum. This toxin induces an immediate loss of dopaminergic terminals in the striatum that progresses and stabilizes over a 2-week period. Tagged hMSCs were infused 5 days after 6-OHDA injection when the toxin-induced neurodegenerative process was still ongoing.Citation7,Citation8
Intrastriatal delivery of tagged hMSCs
Near-infrared scans of the animal’s head were performed immediately before the 6-OHDA-induced lesion and 5 days later just before the transplantation procedure (see Material and Methods section and ). Near-infrared scans were then performed at different time points after intrastriatal transplantation of NIR815 hMSCs (, and B). No near-infrared background signal linked to the surgical procedures per se was observed. An intense near-infrared signal was detected at T1 and T14 after grafting; a reduction in the in vivo signal intensity was observed over time ().
Figure 5 In vivo and ex vivo detection of NIR815 human mesenchymal stem cells following intrastriatal injection. (A) Animals were anesthetized and head imaging was performed immediately before intrastriatal injection of 6-hydroxydopamine (CTR), 1 and 14 days after intrastriatal injection of NIR815 human mesenchymal stem cells. Scans were performed at 800 nm with laser intensity set at 7, a resolution of 84 μm, and focus at 4 mm. Arrows within the dotted area (shaved area) indicate the transplanted cells visualized in green at 800 nm (NIR815, white arrow) or in pseudocolors (red arrows). Pseudocolors correlate with signal intensity (arbitrary units) as indicated. Signals (green and pseudocolors) observed outside of the dotted area represent background due to the animal’s fur. (B) Signal intensity in 6-hydroxydopamine animals 1 and 14 days after transplantation. (C) At the time of sacrifice, whole brains were removed and scanned immediately; near-infrared channel 700 scan intensity 7; near-infrared channel 800 scan intensity 3; resolution 84 μm; and focus offset 4 mm. NIR815 human mesenchymal stem cells are clearly detectable (green arrow). The red signal represents nonspecific tissue autofluorescence at 700 nm. (D) Sequential ex vivo detection of NIR815 human mesenchymal stem cells following intrastriatal transplantation on coronal brain sections. (D1) Coronal brain sections were picked up on poly-lysine slides and high-throughput low-resolution scan was performed immediately (near-infrared channel 700 scan intensity 7, near-infrared channel 800 scan intensity 3, resolution 169 μm, and focus 1 mm). Localization of the transplanted NIR815 human mesenchymal stem cells is clearly visible. The red signal represents nonspecific tissue autofluorescence at 700 nm. (D2) Serial coronal sections were stained using a primary antityrosine hydroxylase antibody and revealed using a NIR700 secondary antibody. Sections were dried and high-resolution scans were performed on single slides at a time (NIR700 scan intensity 3, NIR800 scan intensity 3, resolution 21 μm, and focus offset 1 mm). The 6-hydroxydopamine-induced lesion is indicated by the absence of striatal tyrosine hydroxylase staining in the right hemisphere (dotted circle). Transplanted cells within the lesioned area are clearly visible. (D3) The same serial coronal sections used in D2 were stained using a primary anti-CD90 antibody and revealed using an AlexFluor594 secondary antibody. Sections were mounted and microscopic detection of Hoechst/CD90-positive human mesenchymal stem cells was performed as a proof-of-principle of near-infrared evaluation. Hoechst/CD90 labeled cells that colocalized with near-infrared staining as shown in the squared box in D2 demonstrate the specificity of the detected signals..
Notes: Data represent the mean ± standard deviation. *P < 0.01 versus CTR, analysis of variance; Scale bar 10 μm.
Abbreviations: NIR, near-infrared; hMSCs, human mesenchymal stem cells.
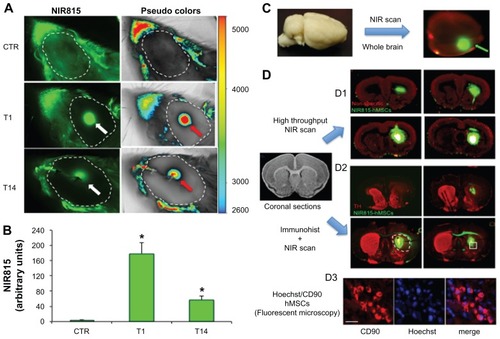
To confirm the exogenous origin of the near-infrared signal, animals were sacrificed at T14 () and whole brains were immediately scanned to detect and localize the specific labeling (, green arrow). Serial coronal brain sections containing the striatum were then obtained and directly analyzed using the near-infrared scanner. A specific intense green near-infrared signal corresponding to the tagged hMSCs was detected in situ (). High intensity scanning in the NIR700 channel allowed detection of a non-specific red signal due to the tissue and permitted regional localization of the transplanted cells within the striatum.
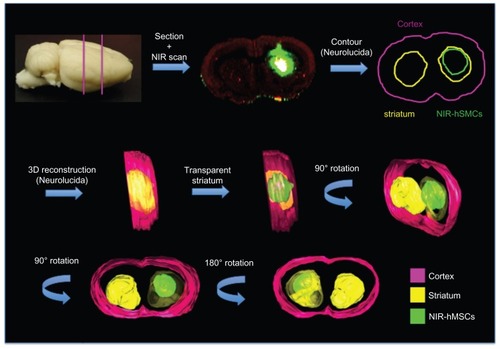
Tyrosine hydroxylase immunohistochemistry using a specific secondary antibody emitting in the NIR700 spectrum allowed simultaneous localization of the transplanted NIR815 hMSCs (, green) and tyrosine hydroxylase-positive dopaminergic terminals (, red). The lack of tyrosine hydroxylase staining in the right striatum clearly indicates the 6-OHDA-induced lesion (, dotted white circle). Fluorescent microscopy subsequently allowed detection of Hoechst-labeled/CD90-positive hMSCs perfectly matching to the near-infrared signal (). Three-dimensional reconstruction using Neurolucida further demonstrated striatal localization of the transplanted cells ().
Figure 6 Three-dimensional reconstruction. Coronal sections were scanned and images imported into the Neurolucida software; contour of whole brain, striata, and transplanted cells was performed and partial three-dimensional images of NIR815 human mesenchymal stem cells within the lesioned striatum were obtained.
Abbreviations: NIR, near-infrared; hMSCs, human mesenchymal stem cells; 3D, three-dimensional.
Intranasal delivery of tagged hMSCs
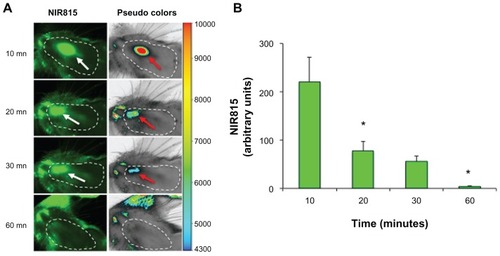
In vivo tracking of NIR815 hMSCs following intranasal injection in parkinsonian animals allowed immediate and time-dependent localization of the cells. Anesthetized animals received an intranasal administration of NIR815 hMSCs in the right nostril and were scanned every 10 minutes (see Materials and Methods section). Cells were clearly visible in the nostril close to the olfactory epithelium at the first time point (10 minutes, see and ). The signal progressively moved towards the orifice of the nostril and diminished significantly over a 30-minute period (compare ). No NIR815 hMSCs were detectable 60 minutes after intranasal injection (). Accordingly, no Hoechst signal was detected in brain coronal slices (not shown).
Figure 7 In vivo tracking of NIR815 human mesenchymal stem cells following intranasal injection. (A) Animals were anesthetized and head imaging was performed immediately after intranasal injection of NIR815 human mesenchymal stem cells. Scans were performed at 800 nm with laser intensity set at 7, resolution of 169 μm, and focus offset at 4 mm. Arrows within the dotted area (shaved area) indicate the transplanted cells visualized in green at 800 nm (white arrows) or in pseudocolors (red arrows) which clearly correlate with signal intensity (arbitrary units) as indicated. (B) Decrease of near-infrared labeling in 6-hydroxydopamine animals at various time points after intranasal injection.
Notes: Data represent the mean ± standard deviation. *P < 0.01 versus CTR, analysis of variance.
Abbreviations: NIR, near-infrared; mn, minutes.
Discussion
We have previously shown that transplantation of Hoechst-labeled hMSCs in the striatum of rats bearing a 6-OHDA lesion counteracted progressive toxin-induced nigrostriatal neurodegeneration and associated behavioral alterations,Citation9 and enhanced endogenous neurogenesis.Citation10 In the present study, we used this well characterized experimental paradigm to validate an in vivo imaging procedure to track stem cells using a near-infrared platform. To the best of our knowledge, this is the first study that addresses the use of near-infrared technology to track the fate, in living parkinsonian animals, of near-infrared-labeled cells following two different administration routes, ie, intrastriatal and intranasal.
Near-infrared fluorescence allows in vivo visualization of molecular targets both at the microscopic and whole animal macroscopic levels in relation to the chosen probe properties.Citation20,Citation21 The peculiar characteristics of near-infrared technology have been already translated into a clinical medical imaging device for image-guided surgery using exogenous contrast agents.Citation22,Citation23 The use of near-infrared light imaging has also been recently proposed to ameliorate the correct delivery of genes to target tissues and monitor therapeutic efficacy in gene therapy.Citation24 Staining cells with the NIR815 tracer is simple and can be performed rapidly using short incubation procedures that can be applied to any cell without the requirement of tedious transfection or potentially dangerous transduction protocols. Near-infrared dye represents an important advantage compared with other procedures that use retroviruses or lentiviruses and that are not directly applicable to patients. To exclude concerns regarding near-infrared cytotoxicity,Citation25 we first determined whether the NIR815 dye interfered in any way with normal stem cell metabolism and proliferation/survival. Our extensive in vitro data demonstrate that near-infrared labeling has no toxic effect on cells; the slight increase in early apoptosis observed in NIR815 hMSCs compared with unlabeled cells may be considered physiological. A clear limitation of the staining procedure might be the dilution of the near-infrared signal due to active cell proliferation. However, our results show that a close correlation between signal intensity and cell number was preserved, even after an extended period of time. The ratio for cell dilution to near-infrared intensity diminished with time, but was proportionally maintained both in vitro and in vivo. We have previously shown that hMSCs do not proliferate following intracerebral transplantation in rats with Parkinson’s disease,Citation9 thus limiting the possibility of loss-of-signal over time in vivo.
Possible leakage of Hoechst 33342 dye into host cells has been reported previously.Citation26 To minimize this problem, we used a different Hoechst compound (33258) that showed reduced passage from cell to cell in vitro. Importantly, our data clearly show that NIR815 dye was not released from labeled hMSCs even after several days in culture, and further support the use of intercalating near-infrared dyes to label cells for in vivo tracking. Our results also demonstrated that hMSCs can be efficiently tagged using intercalating NIR815 dye without effects on cell viability and proliferation.
We directly transplanted NIR815 hMSCs into the striatum of 6-OHDA-lesioned rats. In this well characterized rodent model of Parkinson’s disease,Citation7,Citation8 the toxin induces progressive retrograde neurodegeneration of the nigrostriatal pathway. In our experimental paradigm, cells are transplanted in the ipsilateral striatum 5 days after toxin injection, when neurodegenerative processes are still ongoing. The extent of progressive neurodegeneration resembles the inimical conditions that stem cell therapy would face in patients with Parkinson’s disease. In this study, we show that transplanted NIR815 hMSCs could be promptly tracked in vivo over an extended period of time in 6-OHDA lesioned rats. In vivo near-infrared imaging connected to a computer analysis system allowed longitudinal semiquantitative evaluation of cell survival in a selected cohort of transplanted animals. Therefore, near-infrared technology considerably reduced both the number of animals necessary for a given experimental setting and avoided the demanding firsthand sectioning of whole brain to perform analyses at each experimental time point. Importantly, near-infrared signal from the cells was easily detected through the scalp and skull at a depth of at least 5 mm.
Findings from in vivo near-infrared imaging after intrastriatal delivery were confirmed by ex vivo analyses of whole brain and coronal brain sections. Selected brain sections were directly scanned, operating the same near-infrared platform previously used for in vivo evaluation. Precise intracerebral localization of the transplanted cells was assessed immediately. Distribution of grafted near-infrared hMSCs could be localized ex vivo in tissue sections with high throughput by scanning many slides at once. Near-infrared imaging indicated that exogenous cells mostly remained within the site of injection and did not migrate towards other brain areas. These data were further confirmed by fluorescent microscopy showing that Hoechst-positive hMSCs were distributed in the striata of animals within the boundaries of the 6-OHDA-induced lesion. Imaging of near-infrared-labeled hMSCs could be combined with staining of selected proteins, such as tyrosine hydroxylase, using near-infrared secondary antibodies, thus allowing direct visualization of transplanted cells within the lesioned striatum, characterized by reduced tyrosine hydroxylase staining. Similar results have been obtained in a rat model of permanent middle cerebral artery occlusion.Citation27 Furthermore, the clear colocalization of the CD90 antigen, a marker of naïve hMSCs, and either Hoechst 33258 and/or NIR815, in vitro and ex vivo further confirmed the specific human nature of the transplanted cells we observed.
Intracerebral transplantation is an invasive route of administration and its use in humans remains limited for safety reasons, as well as because of the high costs linked to the procedure. Direct intranasal delivery of therapeutics from the nasal cavity into the central nervous system rapidly bypasses the blood–brain barrier, reducing systemic exposure and side effects, and provides an alternative route to invasive intracerebral methods of drug/cell administration. Citation28,Citation29 Intranasal delivery of target drugs to the central nervous system has already shown many benefits in the treatment of neurologic disorders.Citation28 However, to date, few studies have addressed the feasibility and safety of this route of cell administration.Citation18 In our study, we showed that tagged NIR815 hMSCs could be detected immediately following infusion by either the intranasal or intrastriatal route. NIR815 hMSCs injected via the intrastriatal route were detectable in vivo for up to 14 days. Conversely, no near-infrared signal was measurable 60 minutes after intranasal injection, suggesting that labeled hMSCs were rapidly expelled from the nasal cavity. We could not a priori exclude that a small percentage of cells, below the detection capacity of our near-infrared platform, had passed across the olfactory epithelium and reached the central nervous system. A subsequent thorough ex vivo analysis of brain sagittal sections, including the olfactory bulb, further confirmed the absence of labeled hMSCs (NIR815 or Hoechst), corroborating our live in vivo observations.
Our findings show that near-infrared technology allows follow-up of grafted stem cells both immediately and over time, and support the use of near-infrared imaging as a valuable method to track stem cell engraftment/homing processes after transplantation.Citation30 In the future, near-infrared technology will permit more rapid and efficient development of the correct experimental conditions necessary to obtain optimal cell transfer from nose to brain without the requirement of a large number of animals and time consuming post-experimental procedures, including brain sectioning and immunohistochemical staining.
Conclusion
Exploiting near-infrared imaging for in vivo tracking of transplanted tagged cells holds enormous potential for neurobiological research. This technology can be readily accessible in any laboratory without the requirement for expensive clinical diagnostic equipment and specialized technical abilities, compared with those required for other in vivo imaging technologies (ie, magnetic resonance). This technique allows conducting analyses from whole animals to the single cell. Importantly, near-infrared labeling avoids complicated transduction procedure using viruses and offers important opportunities to develop this noninvasive imaging procedure readily applicable to patients. Such technology will help validate novel cellular transplantation strategies for the treatment of neurodegenerative diseases, including Parkinson’s disease.
Acknowledgments
The authors thank M-Medical and LI-COR for technical assistance with the MousePOD® Accessory.
Disclosure
The authors report no conflicts of interest in this work.
References
- DassBOlanowCWKordowerJHGene transfer of trophic factors and stem cell grafting as treatments for Parkinson’s diseaseNeurology200666S8910316717256
- BrundinPBarkerRAParmarMNeural grafting in Parkinson’s disease: problems and possibilitiesProg Brain Res201018426529420887880
- WakemanDRDodiyaHBKordowerJHCell transplantation and gene therapy in Parkinson’s diseaseMt Sinai J Med20117812615821259269
- ArenasETowards stem cell replacement therapies for Parkinson’s diseaseBiochem Biophys Res Commun20103961526
- KramperaMPasiniAPizzoloGCosmiLRomagnaniSAnnunziatoFRegenerative and immunomodulatory potential of mesenchymal stem cellsCurr Opin Pharmacol2006643544116777484
- ChoumerianouDMDimitriouHPerdikogianniCMartimianakiGRiminucciMKalmantiMStudy of oncogenic transformation in ex vivo expanded mesenchymal cells, from paediatric bone marrowCell Prolif20084190992219040569
- BlandiniFLevandisGBazziniENappiGArmenteroMTTime-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old modelEur J Neurosci20072539740517284180
- BlandiniFArmenteroMTMartignoniEThe 6-hydroxydopamine model: news from the pastParkinsonism Relat Disord200814Suppl 2S12412918595767
- BlandiniFCovaLArmenteroMTTransplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the ratCell Transplant20101920321719906332
- CovaLArmenteroMTZennaroEMultiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s diseaseBrain Res20101311122719945443
- LukerGDLukerKEOptical imaging: current applications and future directionsJ Nucl Med2008491418077528
- AswathyRGYoshidaYMaekawaTKumarDSNear-infrared quantum dots for deep tissue imagingAnal Bioanal Chem20103971417143520349348
- KosakaNOgawaMChoykePLKobayashiHClinical implications of near-infrared fluorescence imaging in cancerFuture Oncol200951501151119903075
- LareauELesageFPouliotPNguyenDLe LanJSawanMMultichannel wearable system dedicated for simultaneous electroencephalography near-infrared spectroscopy real-time data acquisitionsJ Biomed Opt20111609601421950928
- HeXGaoJGambhirSSChengZNear-infrared fluorescent nanoprobes for cancer molecular imaging: status and challengesTrends Mol Med20101657458320870460
- BossolascoPCovaLCalzarossaCNeuro-glial differentiation of human bone marrow stem cells in vitroExp Neurol200519331232515869934
- PaxinosGWatsonATThe Rat Brain in Stereotaxic Coordinates4th edSan Diego, CAAcademic Press1998
- DanielyanLSchaferRvon Ameln-MayerhoferATherapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson diseaseRejuvenation Res20111431621291297
- ArmenteroMTLevandisGNappiGBazziniEBlandiniFPeripheral inflammation and neuroprotection: systemic pretreatment with complete Freund’s adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson’s diseaseNeurobiol Dis20062449250517023164
- HilderbrandSAWeisslederRNear-infrared fluorescence: application to in vivo molecular imagingCurr Opin Chem Biol201014717919879798
- HilderbrandSAShaoFSalthouseCMahmoodUWeisslederRUpconverting luminescent nanomaterials: application to in vivo bio-imagingChem Commun (Camb)20094188419019585016
- Gibbs-StraussSRosenbergMCloughBTroyanSFrangioniJFirst-in-human clinical trials of imaging devices: an example from optical imagingConf Proc IEEE Eng Med Biol Soc200912001200419964033
- GiouxSChoiHSFrangioniJVImage-guided surgery using invisible near-infrared light: fundamentals of clinical translationMol Imaging2010923725520868625
- LiuGSwierczewskaMLeeSChenXFunctional nanoparticles for molecular imaging guided gene deliveryNano Today2010552453922473061
- FerreiraLKarpJMNobreLLangerRNew opportunities: the use of nanotechnologies to manipulate and track stem cellsCell Stem Cell2008313614618682237
- IwashitaYCrangAJBlakemoreWFRedistribution of bisbenzimide Hoechst 33342 from transplanted cells to host cellsNeuroreport2000111013101610790874
- SugiyamaTKurodaSOsanaiTNear-infrared fluorescence labeling allows noninvasive tracking of bone marrow stromal cells transplanted into rat infarct brainNeurosurgery2011681036104721221028
- HansonLRFreyWHIIIntranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative diseaseBMC Neurosci20089Suppl 3S519091002
- HansonLRHafezDSvitakALIntranasal phosphoramidon increases beta-amyloid levels in wild-type and NEP/NEP2-deficient miceJ Mol Neurosci20114342442720941644
- UshikiTKizaka-KondohSAshiharaENoninvasive tracking of donor cell homing by near-infrared fluorescence imaging shortly after bone marrow transplantationPLoS One20105e1111420559437