Abstract
Introduction
Transcription factor p53 has a powerful tumor suppressing function that is associated with many cancers. Since the molecular weight of p53 is 53 kDa, it is difficult to transport across cell membranes. Thymidine dinucleotide (pTT) is an oligonucleotide that can activate the p53 transcription factor and trigger the signal transduction cascade. However, the negative charge and high water solubility of pTT limit its transport through cellular membranes, thereby preventing it from reaching its target in the nucleus. A suitable delivery carrier for pTT is currently not available.
Objective
The purpose of this study was to employ a nanoscale liposomal carrier to resolve the delivery problem, and increase the bioavailability and efficiency of pTT.
Methodology
The approach was to employ liposomes to deliver pTT and then evaluate the particle size and zeta potential by laser light scattering (LLS), and permeation properties of pTT in vitro in a Franz diffusion assembly, and in vivo in a murine model using confocal laser scanning microscopy (CLSM).
Results
We found that dioleoylphosphatidylethanolamine (DOPE) combined with cholesterol 3 sulfate (C3S) were the best ingredients to achieve an average desired vehicle size of 133.6 ± 2.8 nm, a polydispersity index (PDI, representing the distribution of particle sizes) of 0.437, and a zeta potential of −93.3 ± 1.88. An in vitro penetration study showed that the liposomal carrier was superior to the free form of pTT at 2–24 hours. CLSM study observed that the penetration depth of pTT reached the upper epidermis and potential of penetration maintained up to 24 hours.
Conclusion
These preliminary data demonstrate that nanosized DOPE/C3S liposomes can be exploited as a potential carrier of drugs for topical use in treating skin diseases.
Introduction
The p53 tumor suppressor gene is well known and provides a major anticancer defense mechanism. Almost 80% of human cancers are associated with mutations of the p53 gene, which selectively delete or silence p53, thus promoting tumor progression and metastasis.Citation1,Citation2 Normally, there are two classes of genes: oncogenes and tumor suppressor genes. One composed of oncogenes which cause the cell to divide in an unregulated manner when a single altered copy leads to mutation, and another composed of antion-cogenes which provide a protection mechanism, also called a tumor-suppressor gene. Autoregulatory feedback mechanisms exist to achieve a balance between oncogenes and anti-oncogenes. In brief, the p53 gene acts as a regulator of anti-oncogenes or as a tumor suppressor due to its ability to maintain normal growth control and genomic stability.Citation3 Recent work showed that p53 is activated by multiple pathways, such as DNA damage, and oncogenic and oxidative stress; so different stress signals can mediate different signaling pathways.Citation4
Several skin cancers, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are also related to p53 mutations, and the site of the mutation is the basis for distinguishing skin cancers.Citation5,Citation6 Skin cancer induced by ultraviolet (UV) radiation may trigger apoptosis in a p53-dependent manner and the formation of sunburned cells (apoptotic keratinocytes). Numerous studies also indicated that triggering p53 induction inactivates the division of cancer cells.Citation7,Citation8 Thymidine dinucleotide (pTT), a fragment of an oligonucleotide, activates the p53 transcription factor and p53 protein, and triggers a cascade of DNA-repair enzymes according to in vitro and in vivo evidence which focuses on UV radiation-induced mutagenesis and carcinogenesis.Citation9 Typically, oligonucleotides have a high molecular weight, a negative charge, and high water solubility, which causes problems when transporting them through cellular membranes to generate specific reactions in nuclei.Citation10 According to the above data, the bioavailability of pTT is low when it is delivered through cellular membranes by passive diffusion.
Drug delivery systems were therefore designed to protect nucleic acids from being destroyed in environmental conditions and allow them to enter skin cells and reach the site of action. In designing formulation systems, biocompatibility is one of the most important considerations. Previous studies employed a mixed solution of 75% propylene glycol and 25% dimethyl sulfoxide (DMSO) to deliver a biologically active concentration of pTT to guinea pig skin, and despite its effectiveness, it did cause skin damage.Citation11,Citation12 Biocompatibility and safety must be considered to make such solutions feasible in clinical applications. Liposome systems are exploited as biocompatible delivery vesicles for both systemic and topical administration in clinical usage. Liposomal delivery systems can enhance the permeability of hydrophilic drugs and localize them to target tissues.Citation13 Moreover, phospholipids are nontoxic and biodegradable, and prolong the half-life of drugs to attain a sustained-release effect. Previous studies demonstrated that phospholipids exhibit their enhancing effect on the application of topical delivery.Citation14–Citation16 Phospholipids are recognized to have skin permeation enhancing ability.
The aim of this study was to improve the bioavailability of the nucleotide fragment, thymidine dinucleotide (pTT), using liposomal carriers to deliver the drug into the skin, and we focused our investigation on the skin permeation behavior. The applicability of liposomal carriers was demonstrated through extensive characterization of the particle size and zeta potential, and by transmission electron microscopic (TEM) studies. The cellular viability was evaluated using skin fibroblast and keratinocyte cell lines. Moreover, the skin delivery and penetration behavior of pTT-loaded liposomes were investigated by in vitro Franz diffusion cell and in vivo confocal laser scanning microscopy (CLSM).
Materials and methods
Materials
pTT with or without 5′ fluorescein was obtained from Protech Technology (Taipei, Taiwan). 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, with a purity of >95%) was purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol-3-sulfate (C3S) was purchased from Sigma Chemical (St Louis, MO). Other chemicals used in the study were of reagent grade.
Preparation of liposomes
Liposomes were prepared according to the thin-film hydration method. DOPE and C3S were dissolved in 5 mL of a chloroform:methanol (2:1, v/v) solution in round-bottomed flasks. Organic solvent traces were evaporated by a rotary evaporator above the transition temperature of the lipid, and solvent traces were removed under a vacuum overnight. The thin film was fully hydrated with double-distilled water containing pTT at 100 μmol/L for 20 minutes. The vesicle suspension was dispersed by a probe-type sonicator (VCX 600; Sonics and Materials, Newtown, CT) at 25 W for 10 minutes.
Vesicle size and zeta potential
The vesicle size and zeta potential of the liposomes were measured by laser light scattering (LLS) with a helium-neon laser at 630 nm (Nano ZS1 90; Malvern, Worcestershire, UK). Liposomal suspensions were directly measured. The polydispersity index (PDI) was used to measure the size distribution. All vesicle sizes and zeta potentials were measured at 25°C. Measurements were repeated three times per sample for three samples.
Morphology observation by TEM
The liposomal vesicles were examined by TEM to characterize their microstructure. A drop of liposomes was applied to a carbon film-covered copper grid to form a thin-film specimen, which was stained with 1% phosphotungstic acid. The sample was then examined and photographed with a Jeol JEM-1230 TEM (Jeol Ltd, Tokyo, Japan).
Quantification of pTT-fluorescein
The pTT-fluorescein standard was determined spectrofluorometrically (Hitachi F-2500; Hitachi, Tokyo, Japan) at an excitation wavelength of 494 nm and emission at 519 nm. The calibration curve exhibited good linearity (r = 0.97).
Cellular viability
Cellular viability was used to determine the percentage cell survival by a 3,(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazoleum bromide (MTT) assay, which produces formazan crystals in mitochondria within cells. A fibroblast cell line (Hs68) and keratinocyte (HaCat) cells were seeded on 96-well plates at a cell density of 5 × 103 cells/well until cells were confluent. Dulbecco’s modified Eagle’s medium (DMEM) was replaced by liposomal suspensions for 12 and 24 hours at 37°C in air containing 5% CO2. We then added 50 μL of the MTT solution (50 μg/mL) to each well and incubated the wells for 4 hours. In order to dissolve the water-insoluble formazan, MTT/medium was removed from each well, and 100 μL of dimethyl sulfoxide (DMSO) was added to each one. The extent of cell survival was determined by an enzyme-linked immunosorbent assay (ELISA) reader at 550 nm.
In vitro evaluation of the skin penetration ability
We measured the topical delivery of pTT-fluorescein using a modified Franz vertical diffusion assembly (Ching Fa, HsinChu, Taiwan). Normal nude mouse skin was used as the barrier membrane. The donor medium consisted of 0.5 mL of a pTT-fluorescein liposomal formulation or control solution (pTT-fluorescein in distilled water), and the receptor medium consisted of 5.5 mL of pH 7.4 citrate-phosphate buffer. The available diffusion area was 0.785 cm2. The stirring rate of the receptor was 600 rpm, and the temperature was maintained at 37°C. At appropriate intervals, 200 μL aliquots of the receptor medium were withdrawn and immediately replaced by an equal volume of fresh buffer. The concentration of pTT-fluorescein was measured by a fluorescence spectrometer (F-2500, Hitachi, Tokyo, Japan) at λexcitation of 494 nm and λemission of 519 nm. The cumulative amount of pTT-fluorescein was examined at the end of the in vitro experiments (24 hours) (n = 4).
In vivo topical delivery of the liposomal carriers
A glass cylinder with an available area of 0.785 cm2 was placed with glue on the dorsal skin of a mouse. pTT-fluorescein that was or was not encapsulated in a liposomal formulation (100 μL) was added to each cylinder for 2, 4, 6, and 24 hours. At the end of the incubation period, the formulation was removed, and the skin was wiped ten times with a cotton cloth. The amount of pTT-fluorescein retained in the skin was measured by confocal laser scanning microscopy (CLSM). All procedures were carried out in the dark to prevent the influence of ambient light.
Evaluation of the penetration behavior by CLSM
CLSM was used to scan the pTT-fluorescein signal at different skin depths. The excised nude mouse skin was positioned on the microscopic slide with the stratum corneum (SC) side facing the cover glass. The CLSM Leica TCS SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany) was used with an argon laser beam, excitation at 488 nm, and emission at 543 nm. Each skin sample was sliced in sections of 6–10 μm thick through the z-axis by CLSM. The pTT-fluorescein intensity and the permeated depth were detected by CLSM with Leica Confocal Software version 2.61 (Leica Microsystems, Wetzlar, Germany). Since CLSM was not able to be calibrated, we used arbitrary units to compare the data.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analyses were performed using unpaired Student’s t-test with WINKS 6.0 software (Texasoft, TX). A 0.05 level of probability was used as the level of significance. Analysis of variance (ANOVA) was also used.
Results and discussion
The oligonucleotide fragment pTT can activate the p53 transcription factor and trigger tumor suppressor function. However, the negative charge and high water solubility of pTT limits its transport through cellular membranes and its ability to reach its target sites. Improving the poor bio-availability of pTT is necessary to proceed with the clinical investigations. The present study demonstrates that topical delivery of pTT using liposomal carriers can successfully resolve the delivery problem. We established the properties of pTT-loaded liposomes and their permeability behavior in in vitro and in vivo models. CLSM study observed that the effect on skin reached the upper epidermis.
Characterization of pTT-loaded liposomes
The particle size, PDI, and zeta potential of the liposomes with and without pTT were measured by an LLS system, and the results are shown in . We observed no distinct, undissolved crystals in the liposomal mixtures. The morphology of DOPE liposomes with pTT was also shown under TEM observations (). DOPE liposomal vesicles were spherical. Moreover, results of the PDI were 0.39–0.44, which represent the well distribution of particle sizes. The composition with DOPE and C3S at a molar ratio 2.68 achieved the best condition. Liposome vesicles with or without pTT incorporation had particle sizes of 123 and 36 nm, respectively. Liposomes incorporating pTT were clearly larger than those without pTT (P < 0.05).
Table 1 Physicochemical characteristics of liposomes with or without encapsulated pTT- fluorescein
Figure 1 Transmission electron microscopic micrograph of liposomes. (Original magnification × 70 K).
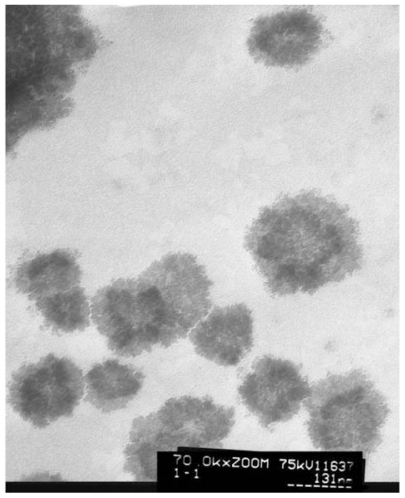
The skin penetration behavior was strongly dependent on the formulation characteristics. In our cases, diameters of the liposomal particles were controlled to <40 and 120 nm in empty and pTT-loaded liposomes, respectively. Generally, egg phosphatidylcholine (EPC) was used as phospholipid in the preparation of traditional liposome, the head group of phospholipid structure with choline N+(CH3)3 group. In this case, we chose phosphatidylethanolamine (PE) as the main phospholipid materials, with NH3+ as the head of the group. Past evidences pointed out that liposomes composed of EPC were significantly larger than those composed of PE in terms of particle size.Citation17 This phenomenon was the same in our case. The PE type of phospholipid can form smaller particle sizes when incorporated with another surfactant in a proper ratio which is mainly related to the properties of PE. In addition, PE is a phospholipid which exhibits a high tendency to form the inverted hexagonal phase, particularly at an acidic pH.Citation18 Protonation of the head group of PE, caused by a reduction of the pH in physiological conditions, neutralizes the negative charge, and the vesicles become destabilized as the PE component reverts to the hexagonal phase.Citation19 Moreover, the zeta potentials of empty liposomes and pTT-loaded liposomes were about −63.17 ± 4.11 and −93.3 ± 1.88 mV, respectively. When pTT was loaded in liposomes the negative charge significantly increased, which was mainly attributed to pTT. The liposome charge plays a particular role in skin penetration, as discussed in the following section.
Safety assessment
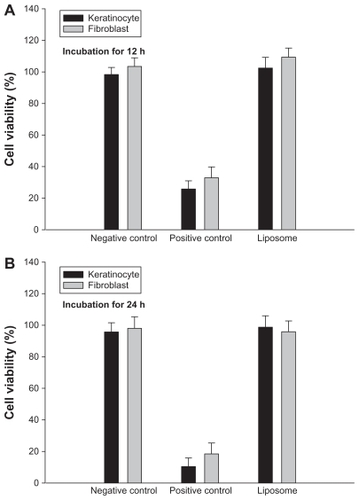
To apply liposomes as a pTT delivery carrier targeting the skin, acceptable toxicological properties should be assessed. We screened skin-associated cell lines including fibroblast and keratinocyte cells by using MTT assay. As shown in , liposomes were examined compared to a negative control (pTT dissolved in double-distilled water) and a positive control (pTT dissolved in DMSO/PG: 7:3). There was no significant difference when liposomes were compared to the negative control group in fibroblast and keratinocyte cells (P > 0.05). Moreover, empty liposomes without pTT were also tested, and it was found that the vesicles themselves had no effect on cytotoxicity in the Hs68 cell line (P > 0.05; data not shown).
Figure 2 Viability of keratinocytes and fibroblast cells following treatment with pTT in the negative/positive control and liposomal suspensions after incubation for (A) 12 hours and (B) 24 hours.
Note: Data are presented as the mean ± SD (n = 6).
Abbreviation: SD, standard deviation.
Past research attempted to utilize many approaches to transfer oligonucleotides into the skin. These approaches included topical application, iontophoresis, electroporation, sonophoresis, laser, microdermabrasion, and gene guns.Citation20–Citation22 The results were effective and appeared promising for clinical application. Although the delivery efficiency showed improvement, some methods caused skin irritation. Citation23 In the present study, we selected liposomes as the carrier for topical delivery. Liposomes were suggested to be suitable carriers for topical delivery since they are made with the same type of lipids as those present in the SC, and also the materials they are composed of are safe. In addition, the surfactants used to prepare the liposomes (most often phospholipids) are similar to those contained in the skin; liposomes easily mix with intercellular lipids of the SC. Phospholipids are not toxic; their incorporation in the SC layer changes the composition of the lipid mixture and may alter the permeability of the skin. Previous studies suggested the excellent ability of PE to facilitate drug or gene delivery into cells.Citation24,Citation25 In addition, some cases suggested that PE is easier to transfer across membranes of mammalian cells than phosphatidylcholine (PC) is.Citation26
Skin delivery ability by in vitro Franz diffusion penetration
Skin, the outermost superficial organ of the body, is an attractive target site for delivering/transferring drugs, active ingredients, and gene fragments. We evaluated the skin penetration ability of liposomal carriers to deliver pTT. The cumulative amounts (CAs) and flux of penetration were calculated from the slope after plotting the accumulated amount of pTT from each formulation as a function of time, as shown in and . The CA tendency decreased in the order of positive control > liposomes > negative control. Moreover, the CAs between the control group (consisting of a pTT aqueous solution) and liposomal carriers in nude mouse skin significantly differed (P < 0.05). In addition, significant improvements in pTT delivery in terms of the CA and flux were about 2.67- and 2.79-fold, respectively.
Table 2 In vitro permeation flux and cumulative amount of pTT across murine skin with negative control, positive control, and liposome treatment for 24 hours
Figure 3 In vitro cumulative amount-time profiles of pTT-fluorescein in various vehicles across nude mouse skin.
Note: Data are presented as the mean ± SD (n = 4).
Abbreviation: SD, standard deviation.
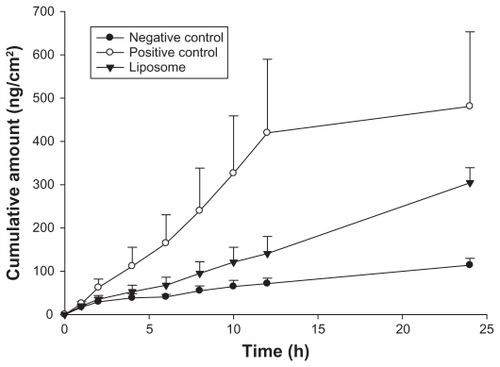
We also used a mathematical simulation to predict and more deeply understand the kinetics of the penetration mode. The model that best fitted the penetration data was selected based on the correlation coefficient (r) value in various models. Zero order was the (r) value between accumulative amounts versus time. First order was the (r) value between natural logarithm of accumulative amounts versus time. Higuchi mode was the (r) value between accumulative amounts versus square root of time. The in vitro penetration study found that pTT dissolved in the negative control solution still had low cumulative amounts which penetrated into the receptor compartment. This indicates that hydrophilic molecules may penetrate into hydrophobic skin in this case because their molecular weight was <500 kDa.Citation27 However, the cumulative amounts did not greatly increase until 24 hours compared to liposomes or the positive control group. The skin penetration model in the negative control group best fit the Higuchi order. The original data also showed a less-precise trend of the first and zero orders, for which the relative coefficients were 0.9325 and 0.9201, respectively. Accordingly, for pTT dissolved in the control solution, the penetration pattern followed Higuchi kinetics which means that the pTT concentration remaining in the receptor compartment within a period of time is limited due to the saturation capacity.
The positive control group presented high enhancement ratios in CAs and fluxes of about 4.98- and 4.21-fold compared to the negative control group; moreover, the penetration kinetics were also zero order. We divided the kinetics into two stages to discuss the kinetic modes. The initial state was from a period of 0–12 hours, in which pTT exhibited burst penetration; in the following period of 12–24 hours, it gradually achieved a steady-state. This indicates that the positive control group was saturated in the terminal period. On the other hand, in our case, enhancement ratios of CAs and flux in the first 24 hours were higher than those of the negative control group by about 2.67- and 2.79-fold. Moreover, the liposome system fit a zero order penetration model. Actually, the ideal delivery of drugs should follow zero order kinetics, wherein body circulation levels of drugs remain constant throughout the delivery period.
Skin penetration behavior by in vivo CLSM localization
We employed an in vivo CLSM technique to observe where pTT was localized in order to confirm and establish the true potential of the carrier. CLSM profiles were acquired after in vivo topical administration of the negative control, positive control, and liposomes on the back skin of nude mice for 24 hours. CLSM images suggested the accumulation, distribution, and skin penetration depth of pTT, as shown in . The skin thickness was optically scanned at 10 μm increments for nine fragments from the surface of the skin (left to right). A cumulative xyz image was merged of all optical sections. A profile of skin depth versus fluorescence intensity was directly obtained from the Leica Confocal software. Accumulation of pTT-fluorescein localization was presented by time course after the in vivo topical administration for 2, 4, 6, and 24 hours (as shown in ). According to the quantification data, all optical sections were merged, and the total intensities of pTT-fluorescein in nude mice skin (as shown in ).
Figure 4 Confocal laser-scanning microscopic (CLSM) micrographs of the pTT-fluorescein intensity after in vivo topical administration in nude mouse skin for 4 hours. Images represent negative control (pTT-fluorescein in an aqueous solution), positive control (pTT-fluorescein in DMSO/PG), and liposomes (pTT-fluorescein-loaded liposomes) sequential scans in the xyz plane, and the full thickness was divided into nine segments from the surface of the skin (left to right). Cumulative xyz images of all optical sections were merged from 0–100 μm in xyz scan. Profile of skin depth versus intensity of the negative control, positive control, and liposome by quantification from CLSM micrographs.
Figure 5 (A) Accumulation of pTT located in nude mouse skin after the in vivo topical administration for 2, 4, 6, and 24 hours (left to right images) analyzed from the negative control (pTT-fluorescein in an aqueous solution), positive control (pTT-fluorescein in DMSO/PG), and liposomes (pTT-fluorescein-loaded liposomes). (B) Quantification of fluorescence intensity from above groups.
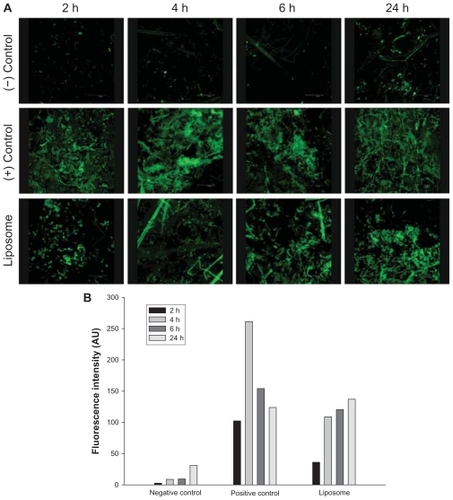

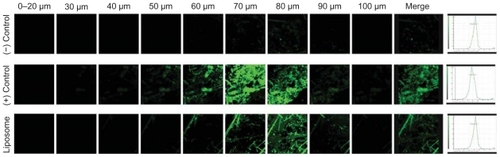
The negative control group seemed to present few penetration trends after long-term application. The positive control group with in vivo application showed a great penetration flux of pTT, particularly after being applied for 4 hours; however, as the application time increased, the pTT intensity dramatically decreased. It tended to respond as the in vitro penetration study, and we assumed that was due to a saturation effect of the positive control vehicle. DMSO and PG are well known as potential penetration enhancers by affecting the intracellular lipids or the intracellular proteins of skin structure; hence, increasing the partitioning of the drug into stratum corneum. In our case, we truly observed an enhancing effect; but it only had a short-term influence. The phenomena assumed relevant with pTT rapid release from vehicle. Past studies pointed out that DMSO is a powerful penetration enhancer with rapid effects in both hydrophilic and lipophilic compounds.Citation28 Caspers and his group indicated that DMSO remained within the tissue about 4 hours and a small fraction was detected for days, decreasing steadily with time by CLSM detection.Citation29 In this case, DMSO has significantly effects in promoting permeation, however in the limitation period.
The liposome group with in vivo application also showed significantly increased pTT intensity, and the initial release of pTT from liposomes was low followed by sustained release from the carrier. We assumed that since the liposomal carrier was a mixture, we could not separate the entrapped and free drug parts in the experiment. From our past evidence, phospholipid materials can assist hydrophilic ingredients penetrate across the skin in some circumstances.Citation30 Additionally, active pharmaceutical ingredients and their polymorphic forms, crystallinity, particle size, solubility, and pharmaceutical dosage form can influence the release and penetration behaviors.Citation31
The results suggested that liposome particle sizes and physicochemical properties play particular roles in influencing pTT penetration into hydrophobic skin. The positive results could be expected, and were contributed by the small particle size and the lipophilic property which are well known from past evidence. Moreover, Carrer and his group pointed out that the presence of a negative charge in membranes of liposomes may allow for a better efficiency of penetration.Citation32 The better penetration behavior was contributed by the lipid layer of the SC which contains a high ratio of negatively charged lipids. It is well known that the skin can act as a negatively charged membrane.Citation33 Pharmaceutical formulations that most significantly improve drug absorption are made with soft (liquid) particles rather than with solid particles. Liposomes are themselves broken at the surface of skin and penetrate skin as free molecules together with the drug. As mentioned above, it is widely accepted that the skin penetration of organic particles is strongly dependent on particle sizes.Citation34
Additionally, the pTT-loaded liposomes showed a highly negative surface charge of about –93.3 ± 1.88 mV, which would be related to the penetration behavior. Regarding the properties of the skin, the lipid layer in the SC contains a high ratio of negatively charged lipids, and it is well known that the skin can act as a negatively charged membrane.Citation35 In addition, several researchers reported that drug penetration can be influenced by modifying the surface charges of liposomes.Citation36 Negatively charged vesicles generally produce a higher flux than their positively charge counterparts, which in turn can improve drug accumulation in the superficial skin strata.Citation33
In terms of the skin barrier, negatively charged and hydrophilic oligonucleotides cannot penetrate into the skin by passive diffusion. Hence, in order to become a useful therapeutic agent, effective delivery systems are required. Nanometer-size systems have garnered attention for delivering genes, antisense oligonucleotides, and small interfering RNA in recent years.Citation10 In particular, vesicle size has a great influence on topical drug delivery systems; vesicles of <300 nm are able to deliver their contents into deeper layers of the skin to some extent.Citation37,Citation38 In the cases of liposomes with average diameters of <130 nm, there is the potential for delivery through the skin.Citation39 In fact, particle sizes of between 100 nm and 1 μm are not nanoparticles, but are correctly defined as submicron particles which are no longer regarded as “suspect” (possibly hazardous).Citation34 Hence, longer-term danger should not occur in that case.
Conclusions
Choosing a suitable vehicle is one of the key factors in developing pharmaceutics and shortening the gap to proceeding with clinical applications. Past evidence showed that topical application enhanced the DNA repair capacity and protected skin from the carcinogenesis risk of solar UV irradiation by activating the tumor suppressor gene, p53. New insights bring an alternative strategy to reduce/protect against skin cancer risks. Formulating the drug dosage and establishing the carrier activity and permeation parameters are part of the final stage of progression to clinical use. Our study contributes to knowledge of the delivery system to increase actual applications and confirm the safety and utility of the carriers.
Disclosure
The author reports no conflicts of interest in this work.
Acknowledgment
This work was supported by a grant (NSC99–2320-B-264- 001-MY2) from the National Science Council, Taipei, Taiwan.
References
- BoxNFTerzianTThe role of p53 in pigmentation, tanning and melanomaPigment Cell Melanoma Res200821552553318761658
- SigalARotterVOncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genomeCancer Res200060246788679311156366
- OrenMDamalasAGottliebTRegulation of p53: intricate loops and delicate balancesAnn N Y Acad Sci200297337438312485897
- HornHFVousdenKHCoping with stress: multiple ways to activate p53Oncogene20072691306131617322916
- BenjaminCLMelnikovaVOAnanthaswamyHNP53 protein and pathogenesis of melanoma and nonmelanoma skin cancerAdv Exp Med Biol200862426528218348463
- RodustPMStockflethEUlrichCLeverkusMEberleJUV-induced squamous cell carcinoma – a role for antiapoptotic signalling pathwaysBr J Dermatol2009161Suppl 3S107115
- AradSZattraEHebertJEpsteinEHJrGoukassianDAGilchrestBATopical thymidine dinucleotide treatment reduces development of ultraviolet-induced basal cell carcinoma in Ptch-1+/− miceAm J Pathol200817251248125518403589
- BenjaminCLUllrichSEKripkeMLAnanthaswamyHNp53 tumor suppressor gene: a critical molecular target for UV induction and prevention of skin cancerPhotochem Photobiol2008841556218173701
- GoukassianDAEllerMSYaarMGilchrestBAThymidine dinucleotide mimics the effect of solar simulated irradiation on p53 and p53-regulated proteinsJ Invest Dermatol1999112125319886259
- FattalEBarrattGNanotechnologies and controlled release systems for the delivery of antisense oligonucleotides and small interfering RNABr J Pharmacol2009157217919419366348
- AllanAEArchambaultMMessanaEGilchrestBATopically applied diacylglycerols increase pigmentation in guinea pig skinJ Invest Dermatol199510556876927594645
- GoukassianDAHelmsEvan SteegHvan OostromCBhawanJGilchrestBATopical DNA oligonucleotide therapy reduces UV-induced mutations and photocarcinogenesis in hairless miceProc Natl Acad Sci U S A2004101113933393814999099
- de LeeuwJde VijlderHCBjerringPNeumannHALiposomes in dermatology todayJ Eur Acad Dermatol Venereol200923550551619175703
- OkuNAnticancer therapy using glucuronate modified long-circulating liposomesAdv Drug Deliv Rev1999401–2637310837780
- MahjourMMauserBRashidbaigiZFawziMBEffect of egg yolk lecithins and commercial soybean lecithins on in vitro skin permeation of drugsJ Control Release199014243252
- Valjakka-KoskelaRKirjavainenMMönkkönenJUrttiAKiesvaaraJEnhancement of percutaneous absorption of naproxen by phospholipidsIn J Pharm19981752225230
- LitzingerDCHuangLPhosphatidylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applicationsBiochim Biophys Acta1992111322012271510997
- CastresanaJNievaJLRivasEAlonsoAPartial dehydration of phosphatidylethanolamine phosphate groups during hexagonal phase formation, as seen by i.r. spectroscopyBiochem J1992282Pt 24674701546961
- HwangTLLeeWRHuaSCFangJYCisplatin encapsulated in phosphatidylethanolamine liposomes enhances the in vitro cytotoxicity and in vivo intratumor drug accumulation against melanomasJ Dermatol Sci2007461112017267180
- BrandRMHannahTLNorrisJIversenPLTransdermal delivery of antisense oligonucleotides can induce changes in gene expression in vivoAntisense Nucleic Acid Drug Dev20011111611258617
- BergenJMParkIKHornerPJPunSHNonviral approaches for neuronal delivery of nucleic acidsPharm Res200825598399817932730
- PrausnitzMRMicroneedles for transdermal drug deliveryAdv Drug Deliv Rev200456558158715019747
- KikuchiYTamaiKKanedaYCutaneous gene deliveryJ Dermatol Sci2008502879817765482
- CrosassoPBrusaPDosioFAntitumoral activity of liposomes and immunoliposomes containing 5-fluorouridine prodrugsJ Pharm Sci19978678328399232525
- WongFMMacadamSAKimAOjaCRansayECBallyMBA lipid-based delivery system for antisense oligonucleotides derived from a hydrophobic complexJ Drug Target200210861562312683666
- FarhoodHSerbinaNHuangLThe role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transferBiochim Biophys Acta1995123522892957756337
- KumarRPhilipAModified transdermal technologies: breaking the barriers of drug permeation via the skinTrop J Pharma Res20076633644
- WilliamsACBarryBWPenetration enhancersAdv Drug Deliv Rev200456560361815019749
- CaspersPJWilliamsACCarterEAMonitoring the penetration enhancer dimethyl sulfoxide in human stratum corneum in vivo by confocal Raman spectroscopyPharm Res200219101577158012425479
- FangYPTsaiYHWuPCHuangYBComparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapyInt J Pharm20083561–214415218325699
- SinghviGSinghMReview: In-vitro drug release characterization modelsInt J Pharm Studies Res201127784
- CarrerDCVermehrenCBagatolliLAPig skin structure and transdermal delivery of liposomes: a two photon microscopy studyJ Control Release20081321122018761045
- SinicoCManconiMPeppiMLaiFValentiDFaddaAMLiposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle–skin interactionJ Control Release2005103112313615710506
- Marie-AlexandrineBStéphanieBYvesCNanoparticles through the skin: managing conflicting results of inorganic and organic particles in cosmetics and pharmaceuticsWiley Interdiscip Rev Nanomed Nanobiotechnol201135463478
- YooJShanmugamSSongCKSkin penetration and retention of L-ascorbic acid 2-phosphate using multilamellar vesiclesArch Pharm Res200831121652165819099237
- GilletACompèrePLecomteFLiposome surface charge influence on skin penetration behaviourInt J Pharm20114111–222323121458550
- Du PlessisJRamachandranCWeinerNMüllerDGThe influence of particle size of liposomes on the disposition of drug into the skinInt J Pharm1994103277282
- VermaDDFahrASynergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin AJ Control Release2004971556615147804
- FangYPHuangYBWuPCTsaiYHTopical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behaviorEur J Pharm Biopharm200973339139819660544