Abstract
Background
The combination of a radioisotope with a chemotherapeutic agent in a liposomal carrier (ie, Indium-111-labeled polyethylene glycol pegylated liposomal vinorelbine, [111In-VNB-liposome]) has been reported to show better therapeutic efficiency in tumor growth suppression. Nevertheless, the challenge remains as to whether this therapeutic effect is attributable to the combination of a radioisotope with chemotherapeutics. The goal of this study was to investigate the pharmacokinetics, biodistribution, and correlation of Indium-111 radioactivity and vinorelbine concentration in the 111In-VNB-liposome.
Methods
The VNB-liposome and 111In-VNB-liposome were administered to rats. Blood, liver, and spleen tissue were collected to determine the distribution profile of the 111In-VNB-liposome. A liquid chromatography tandem mass spectrometry system and gamma counter were used to analyze the concentration of vinorelbine and radioactivity of Indium-111.
Results
High uptake of the 111In-VNB-liposome in the liver and spleen demonstrated the properties of a nanosized drug delivery system. Linear regression showed a good correlation (r = 0.97) between Indium-111 radioactivity and vinorelbine concentration in the plasma of rats administered the 111In-VNB-liposome.
Conclusion
A significant positive correlation between the pharmacokinetics and biodistribution of 111Indium radioactivity and vinorelbine in blood, spleen, and liver was found following administration of the 111In-VNB-liposome. The liposome efficiently encapsulated both vinorelbine and Indium-111, and showed a similar concentration-radioactivity time profile, indicating the correlation between chemotherapy and radiotherapy could be identical in the liposomal formulation.
Introduction
Vinorelbine is a semisynthesized vinca alkaloid belonging to the Catharanthus alkaloid group.Citation1 Vinca alkaloids are potent anticancer agents that act by binding to tubulin and preventing tubulin assembly into microtubules, that can ultimately lead to mitotic inhibition and induction of apoptosis.Citation2 Vinorelbine has been approved as a treatment for various cancers, including metastatic breast cancer and nonsmall cell lung cancer.Citation3,Citation4 Vinorelbine is better tolerated than other vinca alkaloids because of a lower propensity for axonal microtubules to cause neurotoxicity.Citation5 Many studies have put effort into maintaining the vinorelbine concentration surrounding tumor cells in order to improve the anticancer activity of vinorelbine.Citation6,Citation7 Liposomal encapsulation is practical for vinorelbine to extend its circulation time and to increase its accumulation in tumor tissue.
The liposome is a drug carrier system that may prolong drug retention in the blood circulation. Liposomes consist of phospholipid bilayers and have an aqueous cavity in the inner phase, which can be a stable shelter for pharmacologic agents, including chemotherapeutic drugs used in cancer therapy, antisense oligonucleotides used in gene therapy, peptides used in the treatment of infectious diseases, antigens that stimulate an immune response, and radiopharmaceuticals used for targeting diagnosis and therapy.Citation8–Citation11 The application of a liposomal drug delivery system in cancer therapy has many advantages, such as increasing drug stability in vivo, enhancing drug bioavailability, and targeting the site of action.Citation12,Citation13
Radiolabeling technology has been utilized for drug development to evaluate the biodistribution and pharmacokinetics of investigational new drugs. In addition, radioisotopes can be used for diagnostic and therapeutic purposes.Citation10,Citation14,Citation15 Nowadays, finding the optimal strategy for cancer therapy is still a challenge.Citation16 Concurrent or sequential combination of chemotherapy and external beam radiotherapy is recognized as a standard therapeutic procedure for treating many cancers.Citation17,Citation18 The rationale for combining various therapeutic modalities is to expand the therapeutic index by synergistic drug effects and reducing the overlapping spectrum of side effects or toxicity.Citation19 Radionuclide therapy integrated into anticancer drug-loaded nanocarrier delivery systems may provide a potential therapeutic strategy for cancers.Citation20
Previous reports on the encapsulation of Indium-111 into the vinorelbine liposome (111In-VNB-liposome) and Rhenium- 188 (188Re-DXR liposome) have shown that combination therapy can be realized and provides better tumor-targeting therapeutic activity.Citation21–Citation28 There are still no reports correlating the pharmacokinetics of a radiolabeled tracer and vinorelbine concentration. In this investigation, both the pharmacokinetics and biodistribution of the vinorelbine liposome (VNB-liposome) and 111In-VNB-liposome are discussed. Results from two experiments show a correlation between vinorelbine concentration and Indium- 111 radioactivity.
Materials and methods
Materials
Methanol, ammonium formate, and formic acid were purchased from Merck (Darmstadt, Germany). Vincamine, heparin sodium, and 8-hydroxyquinoline (oxine) were obtained from Sigma-Aldrich (St Louis, MO). Aerrane (isoflurane) was purchased from Baxter (San Juan, Puerto Rico), and vinorelbine was obtained from Orient Europharma (Taipei, Taiwan). The VNB-liposome (NanoVNB) was kindly provided by the Taiwan Liposome Company (Taipei, Taiwan). Deionized water (Millipore, Bedford, MA) was used throughout the entire experiment.
Preparation of VNB-liposome
Preparation of liposomes and the VNB-liposome has been previously described.Citation21,Citation29 Briefly, PEGylated liposomes were prepared from distearoyl phosphatidylcholine, cholesterol, and PEG-DSPE (molar ratio 3:2:0.045). Small unilamellar vesicles (100 nm in diameter) were produced by a combination of the standard thin-film hydration method, the freeze-thaw method, and repeated extrusion. The extraliposomal salt was removed by a Sephadex™ G-50 column (Bio-Rad, Hercules, CA) and elution with histidine-sucrose buffer (pH 6.0). Vinorelbine, an anticancer agent was encapsulated into nanoliposomes using a polyanionic gradient. After removing the extra-liposomal salt using a Sephadex G-50 column, vinorelbine was added immediately to the solution at a concentration of 3.5 mg/10 μmol of phospholipid. The mixture of liposomes and vinorelbine was incubated in a water bath at 60°C for 30 minutes with agitation at 100 rpm. After loading, the liposomal vinorelbine was sterilized by 0.2 μm filtration and stored at 4°C–6°C before use. The mean particle size of the VNB-liposome and the concentration of vinorelbine in the VNB-liposome was 95.2 ± 4.9 nm and 2.08 mg/mL, respectively.
Preparation of 111In-VNB-liposome
The method used to label VNB-encapsulated PEGylated liposomes with 111In-oxine has been detailed in a previous report.Citation25 Briefly, 111In-oxine residue was dissolved in 20 μL of ethanol, added to 80 μL of distilled water, and then incubated with 2 mL PEGylated VNB-liposomes for 30 minutes at 37°C. About 100 μL of reaction solution were loaded onto a column (40 × 8 mm, Bio-Rad) containing Sephadex G-50 fine gel and eluted with normal saline. The labeling efficiency was determined by dividing the radioactivity of the PEGylated VNB-liposome fractions after separation by total radioactivity before separation. The particle size of 111In- VNB-liposome (after the radioactivity decay to background) was determined using an ultraviolet-visible spectrophotometer (V-530; Jasco, Tokyo, Japan). The radiochemical purity values for the 111In-VNB-liposome were all greater than 90%. The average particle size of the 111In-VNB-liposome was 102 ± 6.9 nm, which is similar to that of VNB-liposome (95.2 ± 4.9 nm).
Experimental animals
Male Sprague-Dawley rats (National Yang-Ming University Animal Center, Taipei, Taiwan), were housed on a 12-hour light and 12-hour dark cycle. Free access to food (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN) and water was allowed at all times. All animal protocols were approved by the Institutional Animal Care and Use Committee at National Yang-Ming University (Taipei, Taiwan) and the Institute of Nuclear Energy Research (Taoyuan, Taiwan). The rats weighed 250 ± 10 g. The animals were separated into two groups, one for pharmacokinetic experiments and the other for biodistribution experiments.
For the pharmacokinetics, ten rats (five rats per group) were anesthetized with 1.5% isoflurane, and each rat was given the VNB-liposome (containing vinorelbine 0.3 mg/kg) or 111In-VNB-liposome (vinorelbine 0.3 mg/kg, Indium-111 2.22 MBq per rat) through the tail vein. A 0.3 mL blood sample was collected as blank plasma prior to drug administration, and further blood samples were collected at 0.25, 1, 4, 24, 48, and 72 hours after drug administration. Following blood collection, radioactivity was measured using a Cobra II auto-gamma counter (1470 Wizard Gamma Counter; Wallac, Turku, Finland). The percentage of injected dose per mL (% ID/g) was calculated by comparison with standards representing the injected dose per animal. Plasma samples were stored at −20°C before analysis.
For the biodistribution study, 30 rats (three rats at each time point in each group) were anesthetized with 1.5% isoflurane, and each rat was given the VNB-liposome (containing vinorelbine 0.3 mg/kg) or 111In-VNB-liposome (vinorelbine 0.3 mg/kg, Indium-111 2.22 MBq per rat) through the tail vein. At 1, 4, 24, 48, and 72 hours following injection, the rats were sacrificed by CO2 asphyxiation and the liver and the spleen were collected, rinsed with normal saline, weighed, and the radioactivity was measured using the Cobra II auto-gamma counter. The %ID and the %ID/g were calculated by comparison with standards representing the injected dose per animal. The data were expressed as the mean ± standard deviation. The organs were preserved at −20°C for further treatment and vinorelbine analysis.
Sample preparation
Protein precipitation was used to extract vinorelbine from the rat plasma samples. Briefly, 240 μL of methanol with 10 ng/mL of vincamine (as the internal standard) was added to 80 μL of collected plasma, and the mixture was vortexed for 10 minutes then centrifuged at 16,000 g and 4°C for 10 minutes. The supernatant was collected and dried using a centrifugal vaporizer. The dried sample was reconstituted with 80 μL of 80% methanol, and filtered using a 0.2 μm filter (Millipore, Millex®-GV, Bedford, MA). The filtrate was analyzed using a liquid chromatography tandem mass spectrometry system. The standard samples were prepared in the same protein precipitation method by spiking stock solution (20 μL vinorelbine standard) in plasma (80 μL). The mixed sample was then added to 300 μL of methanol with 10 ng/mL vincamine and followed the previous procedure, but was reconstituted with 100 μL of 80% methanol. The final filtrate was also analyzed using a liquid chromatography tandem mass spectrometry system.
Liver and spleen samples were extracted using a solid-phase extraction cartridge (Oasis HLB, 1 mL, 10 mg). The organ samples were first homogenized (Polytron PT-MR 2100, Kinematica AG, Lucerne, Switzerland) with 50% methanol (5:1 v/w for spleen and 3:1 v/w for liver) at 20,000 rpm for 10 minutes. The supernatant was obtained by centrifugation at 10,000 rpm for 10 minutes. A 100 μL supernatant sample was mixed with 100 μL of vincamine (5 ng/mL) and 800 μL of 1% formic acid. The mixture was loaded into a solid-phase extraction cartridge, washed with 10 mM ammonium formate, 20% methanol in 10 mM ammonium formate, and eluted with 90% methanol in 10 mM ammonium formate. The collected elution was dried using a centrifugal vaporizer, reconstituted with 100 μL of 80% methanol, and filtered using a 0.2 μm filter (Millipore). The filtrate was analyzed using a liquid chromatography tandem mass spectrometry system. The standard samples were prepared by the solid-phase extraction method using a spiking stock solution (20 μL vinorelbine standard) in plasma (80 μL). The sample was mixed using the same solid-phase extraction preparation method. The final reconstituted sample was also analyzed using the liquid chromatography tandem mass spectrometry system.
Liquid chromatography-tandem mass spectrometry
The system consisted of a Waters 2690 Alliance LC with an automatic liquid chromatographic sampler and injector and a Micromass Quattro Ultima tandem quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ionization interface which was in acquired positive mode. Ultrapure argon was used as the collision gas, and high-pure nitrogen was used as cone gas. Multiple-reaction monitoring analysis was used for quantitation and the samples were quantified using peak area. The multiple-reaction monitoring transitions were m/z 779.2 to m/z 122.0 for vinorelbine and m/z 355.2 to m/z 337.2 for vincamine, which was used as the internal standard. The electrospray ionization-tandem mass spectrometry parameters were set as follows: capillary voltage, 3.0 kV; source temperature, 110°C; desolvation temperature, 350°C; cone gas flow, 100 L/hour; and desolvation gas flow, 500 L/hour. To obtain optimal responses, the cone voltage was set at 30 V, and the collision energy was adjusted to 20 eV for vinorelbine and 45 eV for the internal standard, vincamine. MassLynx 3.5 (Micromass) software was used for data processing. A Phenomenex Luna C18 column (5 μm and 50 × 4.6 mm) maintained at an ambient temperature was used to separate the vinorelbine. The mobile phase consisted of 70% methanol and 30% 10 mM ammonia acetate with 0.8% formic acid, and the flow rate was 0.2 mL/minute. The injection sample volume was 5 μL.
Method validation
Calibration curves were established using blank samples (plasma, liver, and spleen) spiked with different amounts of vinorelbine. Stock solution diluted with 50% methanol was used to form a series of concentrations from 25 to 2500 ng/mL. The concentration-response relationship for this method indicated linearity over a concentration range of 5–500 ng/mL, with a coefficient of determination (r2) of at least 0.999. The intra-assay and interassay variabilities were determined by quantitating six replicates at concentrations of 5, 10, 20, 50, 100, 200, and 500 ng/mL on the same day and consecutive days, respectively. The limit of detection was defined as a signal-to-noise ratio of 3, the lower limit of quantitation was defined as 10, and the lowest concentration of the linear regression defined the limit of quantitation. The accuracy (bias%) was calculated from the mean value of observed concentration (Cobs) and the nominal concentration (Cnom) as follows: accuracy (%) = [(Cobs − Cnom)/Cnom] × 100. The relative standard deviation (RSD) was calculated from the observed concentrations as follows: precision (%) = [standard deviation (SD)/Cobs] × 100. Accuracy and precision values within ±20% covering the actual range of experimental concentrations were considered acceptable.
Three sets of samples were prepared to evaluate the matrix effect and the recovery of the quantitative bioanalytical method:Citation30
Neat sample – samples were prepared using a vinorelbine standard solution diluted with normal saline to the target concentrations of 5, 50, and 500 ng/mL.
Postextraction fortification – samples were prepared by spiking appropriate concentrations of standard solutions of vinorelbine to the postextracted blank plasma sample, with target concentrations of 5, 50, and 500 ng/mL.
Pre-extraction fortification – samples were prepared by spiking appropriate concentrations of standard solutions of vinorelbine to the pre-extracted blank plasma sample with target concentrations of 5, 50, and 500 ng/mL. These samples were further processed by the protein precipitation methods before analysis. By comparing the peak areas of set 1 and set 2, the data allow determination of the matrix effect, which represents ion suppression or enhancement association. By comparing the peak areas of set 2 and set 3, the data enable determination of the recovery sample treatment procedure.
Pharmacokinetic parameters and statistical analysis
The pharmacokinetic parameters were calculated by WinNonlin (v 5.0.1; Pharsight Corporation, Mountain View, CA) and included half-life (t1/2), maximum concentration (Cmax), and area under the concentration-time curve (AUC). All data are presented as the mean ± standard deviation or the mean ± standard error of the mean. The unpaired t-test was used for group comparisons. Values of P <0.05 were considered significant. Coefficient of correlation (r) was used to estimate the correlation between radioactivity of Indium-111 and vinorelbine concentrations in this study.
Results
Method validation
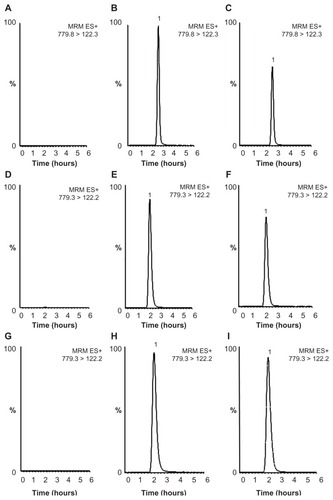
Selectivity was confirmed by a chromatogram of a blank sample and a blank sample spiked with vinorelbine. Under the given conditions, vinorelbine was eluted at a retention time of 2.7 minutes for the plasma sample and 2.2 minutes for liver and spleen samples, and there was no interference at the same retention time. Liquid chromatography tandem mass spectrometry images of vinorelbine in plasma, liver, and spleen are shown in . Calibration curves for vinorelbine were shown to have linear regression in the concentration range of 5 to 500 ng/mL for plasma, liver, and spleen, and a coefficient of determination (r2) >0.999 for all curves, demonstrating good linear regression in the concentration range tested. demonstrates that intraday and interday precisions (RSD%) were less than 13.5%, and accuracies (RE%) for intraday and interday assays were less than 16.0%. The signal-to-noise ratio of 3, defined as the limit of detection, was 0.25 ng/mL; that of 10, defined as the lower limit of quantification, was 0.83 ng/mL; and the lowest concentration of the linear regression, defined as the limit of quantification, was 5 ng/mL. The matrix effect and recovery for determination of vinorelbine are summarized in , and these results show that the detection and extraction methods for these samples were reliable and acceptable.
Table 1 Intraday and interday assay for accuracy and precision for determination of vinorelbine
Table 2 Matrix effect and recovery for the determination of vinorelbine
Figure 1 Typical liquid chromatography tandem mass spectrometry images of vinorelbine. (A) Blank plasma sample, (B) blank plasma spiked vinorelbine sample (200 ng/mL), (C) real plasma sample (156 ng/mL, t = 24 hours), (D) blank liver sample, (E) blank liver spiked vinorelbine sample (200 ng/mL), (F) real liver sample (141 ng/mL, t = 4 hours), (G) blank spleen sample, (H) blank spleen spiked vinorelbine sample (500 ng/mL), (I) real spleen sample (424 ng/mL, t = 1 hour).
Note: The maximal signal intensities for plasma, liver and spleen samples were 9.0 × e5, 1.3 × e6, and 8.5 × e5, respectively.
Pharmacokinetics of VNB-liposome
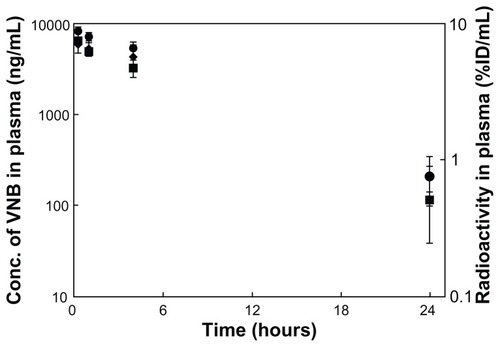
The vinorelbine concentration-time profile in the rat is shown in . It is clear that in rats administered 0.3 mg/kg of the VNB-liposome, the concentration of vinorelbine gradually decreased over 24 hours, and the concentration of vinorelbine at the 24-hour sampling point was 204 ± 64 ng/mL. According to the time-concentration profile, the pharmacokinetic parameters were estimated as: t1/2 = 4.8 ± 1.2 hours; C0 = 6.27 ± 2.68 μg/mL; AUC0–24 h = 65.5 ± 26.6 h*μg/mL. These data are listed in .
Table 3 Pharmacokinetic parameters of vinorelbine in plasma of rats administered with VNB-liposome (0.3 mg/kg VNB) and 111In-VNB-liposome (0.3 mg/kg vinorelbine, 2.22 MBq/rat)
Figure 2 Vinorelbine concentration-time curve and radioactivity-time curve in plasma. ◆ Vinorelbine concentration of the VNB-liposome (vinorelbine 0.3 mg/kg) intravenous administration group, ■ vinorelbine concentration of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, Indium-111 2.22 MBq/rat) intravenous administration group, and ▴ radioactivity of of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, Indium-111 2.22 MBq/rat) intravenous administration group.
Note: Data are expressed as the mean ± standard error of the mean (n = 5 for each group).
Abbreviation: VNB, vinorelbine.
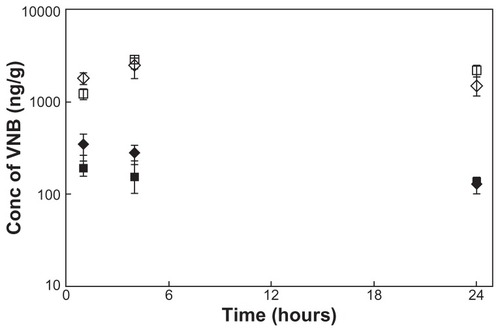
The concentration of vinorelbine in the liver and spleen versus time was also observed in this investigation, and the results are shown in . The concentration of vinorelbine in these two organs could be maintained for at least 24 hours. The results also show that the concentration of vinorelbine in the spleen was significantly higher than that in the liver (P < 0.05). From the concentration-time profile of these two organs, two parameters could be obtained: Cmax = 0.35 μg/g, AUC0–24 h = 5.26 h*μg/g and Cmax = 2.47 μg/g, AUC0–24 h = 47.0 h*μg/g for the liver and spleen, respectively.
Figure 3 VNB concentration-time curve and radioactivity-time curve in liver and spleen. ◆ Vinorelbine concentration of liver in the group of VNB-liposome (vinorelbine 0.3 mg/kg) intravenous administration; ⋄ Vinorelbine concentration of spleen in the group of VNB-liposome (vinorelbine 0.3 mg/kg) intravenous administration; ■ vinorelbine concentration of liver in the group of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, 2.22 MBq/rat) intravenous administration; □ vinorelbine concentration of spleen in the group of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, 2.22 MBq/rat) intravenous administration; • radioactivity of liver in the group of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, 2.22 MBq/rat) intravenous administration; ○ radioactivity of spleen in the group of 111In-VNB-liposome (vinorelbine 0.3 mg/kg, 2.22 MBq/rat) intravenous administration.
Note: Data are expressed as mean ± standard error of the mean (n = 3 for each time point per group).
Abbreviation: VNB, vinorelbine.
Pharmacokinetics of 111In-VNB-liposome
The vinorelbine concentration-time profile of the rats administered 111In-VNB-liposome (0.3 mg/kg vinorelbine, Indium- 111 2.22 MBq/rat) was similar to that in rats administered 0.3 mg/kg VNB-liposome (). After administration of the 111In-VNB-liposome, the concentration of vinorelbine still reached 116 ± 77 ng/mL at 24 hours. From the time-concentration profile, the pharmacokinetic parameters were obtained: t1/2 = 3.3 ± 1.3 hours; C0 = 7.15 ± 1.60 μg/mL; AUC0–24 h = 44.5 ± 31.0 h*μg/mL. These data are listed in . The radioactivity-time profile of the rats is demonstrated in . Radioactivity could be detected in rat plasma 24 hours after administration of the 111In-VNB-liposome, which was 0.7610 ± 0.3003 %ID/mL, and based on the profile, the pharmacokinetic parameters obtained were C0 = 8.88 ± 0.43 %ID/mL and AUC0–24 h = 115.46 ± 16.83 h*%ID/mL.
The concentration of vinorelbine in the liver and spleen versus time are shown in . The results were similar to that of rats administered the VNB-liposome. From the concentration-time profile of these two organs, the pharmacokinetic parameters could be calculated as Cmax = 0.19 μg/g, AUC0–24 h = 3.54 h*μg/g and Cmax = 2.86 μg/g, AUC0–24 h = 57.5 h*μg/g for liver and spleen, respectively. The radioactivity could also be detected, and based on the profile, the pharmacokinetic parameters were calculated as: Cmax = 4.8%ID/g, AUC0–24 h = 87.7 h*%ID/g and Cmax = 21.5%ID/g, AUC0–24 h = 418 h*%ID/g, respectively.
Radiolabeled tracer and vinorelbine concentration in 111In-VNB-liposome
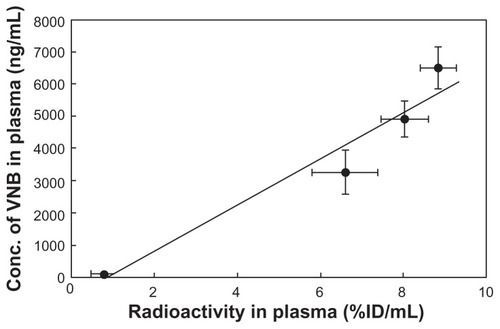
The radioactivity-time profile clearly matched the vinorelbine concentration-time profiles in 111In-VNB-liposome experiments (). This result showed that liposome encapsulated concurrently with Indium-111 and vinorelbine did not affect blood pharmacokinetic parameters of vinorelbine, which was evidenced by the similar vinorelbine concentration-time profiles between the 111In-VNB-liposome group and VNB- liposome group. From the result of vinorelbine concentration as a function of radioactivity profiles (), the linear regression between radioactivity and vinorelbine concentration in rats administered with 111In-VNB-liposome was y = 725.98 × −688.13, where r = 0.97, showing that the radioactivity and vinorelbine concentration was in good correlation.
Figure 4 Correlation of pharmacokinetics between radiolabeled tracer and vinorelbine concentration in 111In-VNB-liposome (vinorelbine 0.3 mg/kg, 2.22 MBq/rat) post intravenous administration.
Note: Data are expressed as mean ± standard error of the mean (n = 5 for each time point per group).
Abbreviation: VNB, vinorelbine.
Discussion
The characteristics of the 111In-VNB-liposome including pharmacokinetics, biodistribution, histology, and molecular imaging, have been reported.Citation21–Citation25 In addition to these pharmacological studies, 111In-VNB-liposomal combination therapy can provide better tumor-targeting therapeutic efficacy in C26 colorectal carcinoma-bearing mouse models.Citation21,Citation22,Citation24 In this study, we have further shown a correlation between radioactivity and chemotherapeutics of the 111In-VNB-liposome in the pharmacokinetics and biodistribution in the rat. The improved kinetic properties of vinorelbine attributable to the liposomal carrier were evidenced by an extended circulation time in rat blood and increased accumulation in the reticuloendothelial system (ie, liver and spleen). The concentration of vinorelbine rapidly decreased from approximately 7.5 μg/mL to 0.2 μg/mL within 4 hours of intravenous administration of vinorelbine 20 mg/kg in mice.Citation31 Different formulations, such as phosphatidylserine liposome and lipid microspheres, have been designed to improve the pharmacokinetics of vinorelbine and overcome the problem of its rapid elimination from the circulation.Citation32 Our liposomal vinorelbine formulation had improved in vivo pharmacokinetic properties, achieving the highest concentration at 24 hours (204 ± 64 ng/mL) with approximately one thirtieth of the dose administered (0.3 mg/kg, ).
Liposomal drug delivery systems have been studied extensively as a method to increase the therapeutic index of chemotherapy.Citation33,Citation34 Our previous work has demonstrated that liposomal doxorubicin has a better ability to penetrate the blood-brain barrier.Citation29 Nanoliposomes, which are double-membrane lipid vesicles with a particle size from 10 nm to 100 nm, are important carriers capable of packaging drugs in various drug delivery applications via the enhanced permeability and retention effect at leaky tumor sites. The reticuloendothelial system is the main turnover organ for liposomal drug delivery.Citation35,Citation36 Several investigations have indicated high uptake of liposome drug delivery systems in reticuloendothelial organs such as the liver and spleen.Citation24,Citation37 Our previous study has demonstrated that reticuloendothelial system-rich organs, ie, the liver and spleen, are the major sites of uptake of liposomal vinorelbine, which is evidenced by calculating the accumulation of radioactivity.Citation24 In this work, the average concentrations of vinorelbine in blood, liver, and spleen were 204 ± 64 ng/mL, 126 ± 26 ng/g, and 1499 ± 345 ng/g, respectively, at 24 hours after administration of liposomal vinorelbine 0.3 mg/kg (). The concentration of vinorelbine in the spleen was 7.3-fold higher than the concentration in blood.
A significant positive correlation between doxorubicin concentration (ng/mg) and 99mTc radiotracer (%ID/g) in tumor tissue has been reported previously.Citation38 The combined therapeutic efficacy of the 111In-VNB-liposome has been evaluated in colorectal carcinoma-bearing mice.Citation22,Citation23 Indium-111 is a radionuclide commonly used for scintigraphic imaging (t1/2 2.81 days, 172 and 247 keV photon emission), emitting 14.7 Auger electrons (mean energy 0.46 keV) on average per decay, and may also be suitable for radiotherapy.Citation39 Our current study found a good pharmacokinetic correlation between radioactivity and vinorelbine concentration in rats administered the 111In-VNB-liposome. A significant positive correlation (r = 0.97) between vinorelbine concentration (ng/mL) and the Indium-111 radiotracer (%ID/g) in plasma was observed (). Our work demonstrates that the biodistribution of radiolabeled tracer correlates well with chemotherapeutic vinorelbine, which can be used to estimate the distribution profile of vinorelbine in a liposomal formulation (111In-VNB-liposome) and for drug uptake in vivo. However, characterization of the vinorelbine versus tracer uptake relationship still needs further validation.
In conclusion, this investigation identified a valid and reliable liquid chromatography tandem mass spectrometry method to determine the concentration of vinorelbine in rat plasma, liver, and spleen after administration of the VNB-liposome and 111In-VNB-liposome in rats. We found that the nanoliposomal formulation improved the kinetic properties of vinorelbine and prolonged the circulation time in rat blood. Vinorelbine in an Indium-111-encapsulated liposome exhibits a correlated concentration/radioactivity-time profile, providing evidence that the strategy of combinatorial chemoradiotherapy is practical in vivo.
Acknowledgments
Funding for this study was provided in part by research grants (NSC99-2113-M-010- 001-MY3, and NSC99-2628- B-010-008-MY3) from the National Science Council, Taiwan, grant TCH 10001-62-007 from Taipei City Hospital, Taiwan, and 982001INER067 from the Institute of Nuclear Energy Research, Taiwan. We also thank Yun-Long Tseng from the Taiwan Liposome Company for preparation of the VNB-liposome.
Disclosure
The authors report no conflicts of interest in this work.
References
- PotierPThe synthesis of navelbine prototype of a new series of vinblastine derivativesSemin Oncol1989162 Suppl 4242540531
- JordanMAThrowerDWilsonLMechanism of inhibition of cell proliferation by vinca alkaloidsCancer Res1991518221222222009540
- WeberBLVogelCJonesSIntravenous vinorelbine as first-line and second-line therapy in advanced breast cancerJ Clin Oncol19951311272227307595730
- GridelliCDe VivoRVinorelbine in the treatment of non-small cell lung cancerCurr Med Chem20029887989111966450
- DrummondDCNobleCOGuoZImproved pharmacokinetics and efficacy of a highly stable nanoliposomal vinorelbineJ Pharmacol Exp Ther2009328132133018948499
- BawejaMSumanVJFitchTRPhase II trial of oral vinorelbine for the treatment of metastatic breast cancer in patients > or = 65 years of age: an NCCTG studyAnn Oncol200617462362916520332
- OkounevaTHillBTWilsonLJordanMAThe effects of vinflunine, vinorelbine, and vinblastine on centromere dynamicsMol Cancer Ther20032542743612748304
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- PhillipsWTGoinsBBaoARadioactive liposomesWiley Interdiscip Rev Nanomed Nanobiotechnol200911698320049780
- TingGChangCHWangHELeeTWNanotargeted radionuclides for cancer nuclear imaging and internal radiotherapyJ Biomed Biotechnol2010 953537
- TorchilinVPRecent advances with liposomes as pharmaceutical carriersNat Rev200542145160
- AllenTMBrandeisEHansenCBKaoGYZalipskySA new strategy for attachment of antibodies to sterically stabilized liposomes resulting in efficient targeting to cancer cellsBiochim Biophys Acta199512372991087632714
- DrummondDCMeyerOHongKKirpotinDBPapahadjopoulosDOptimizing liposomes for delivery of chemotherapeutic agents to solid tumorsPharmacol Rev199951469174310581328
- HamoudehMKamlehMADiabRFessiHRadionuclide delivery systems for nuclear imaging and radiotherapy of cancerAdv Drug Deliv Rev200860121329134618562040
- TingGChangCHWangHECancer nanotargeted radiopharmaceuticals for tumor imaging and therapyAnticancer Res200929104107411819846958
- DanceyJEChenHXStrategies for optimizing combinations of molecularly targeted anticancer agentsNat Rev200658649659
- ChoyHKimDWChemotherapy and irradiation interactionSemin Oncol2003304 Suppl 931012908132
- JainRKNormalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapyNat Med20017998798911533692
- HuberPEBischofMJenneJTrimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapyCancer Res20056593643365515867359
- WongJYSystemic targeted radionuclide therapy: potential new areasInt J Radiat Oncol Biol Phys200666Suppl 2S748216979445
- ChowTHLinYYHwangJJTherapeutic efficacy evaluation of 111In-labeled PEGylated liposomal vinorelbine in murine colon carcinoma with multimodalities of molecular imagingJ Nucl Med200950122073208119949027
- ChowTHLinYYHwangJJDiagnostic and therapeutic evaluation of 111In-vinorelbine-liposomes in a human colorectal carcinoma HT-29/luc-bearing animal modelNucl Med Biol200835562363418589307
- ChowTHLinYYHwangJJImprovement of biodistribution and therapeutic index via increase of polyethylene glycol on drug-carrying liposomes in an HT-29/luc xenografted mouse modelAnticancer Res20092962111212019528471
- LeeWCHwangJJTsengYLTherapeutic efficacy evaluation of 111In-VNB-liposome on human colorectal adenocarcinoma HT-29/luc mouse xenograftsNucl Instrum Meth A2006569497504
- LinYYLiJJChangCHEvaluation of pharmacokinetics of 111In-labeled VNB-PEGylated liposomes after intraperitoneal and intravenous administration in a tumor/ascites mouse modelCancer Biother Radiopharms2009244453460
- ChangYJChangCHYuCYTherapeutic efficacy and microSPECT/CT imaging of 188Re-DXR-liposome in a C26 murine colon carcinoma solid tumor modelNucl Med Biol20103719510420122674
- ChenLCChangCHYuCYPharmacokinetics, micro-SPECT/CT imaging and therapeutic efficacy of 188Re-DXR-liposome in C26 colon carcinoma ascites mice modelNucl Med Biol200835888389319026950
- ChenMHChangCHChangYJMicroSPECT/CT imaging and pharmacokinetics of 188Re-(DXR)-liposome in human colorectal adenocarcinoma-bearing miceAnticancer Res2010301657220150618
- HsiehYJChangCHHuangSPEffect of cyclosporin A on the brain regional distribution of doxorubicin in ratsInt J Pharm20083501–226527117935917
- WuYTHuangCMLinCCDetermination of melamine in rat plasma, liver, kidney, spleen, bladder and brain by liquid chromatography-tandem mass spectrometryJ Chromatogr A20091216447595760119493536
- SempleSCLeoneRWangJOptimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activityJ Pharm Sci20059451024103815793796
- WebbMSJohnstoneSMorrisTJIn vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserineEur J Pharm Biopharm200765328929917123800
- HongRLHuangCJTsengYLDirect comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: is surface coating with polyethylene glycol beneficial?Clin Cancer Res19995113645365210589782
- KobayashiSSakaiTDalrymplePDWoodSGChasseaudLFDisposition of the novel anticancer agent vinorelbine ditartrate following intravenous administration in mice, rats and dogsArzneimittelforschung19934312136713778141830
- ChoiceEMasinDBallyMBMelocheMMaddenTDLiposomal cyclosporine. Comparison of drug and lipid carrier pharmacokinetics and biodistributionTransplantation1995609100610117491673
- GabizonAIsacsonRLibsonEClinical studies of liposome-encapsulated doxorubicinActa Oncol19943377797867993646
- GabizonAShmeedaHBarenholzYPharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studiesClin Pharmacokinet200342541943612739982
- KleiterMMYuDMohammadianLAA tracer dose of technetium-99 m-labeled liposomes can estimate the effect of hyperthermia on intratumoral doxil extravasationClin Cancer Res200612226800680717121901
- KereiakesJGRaoDVAuger electron dosimetry: report of AAPM Nuclear Medicine Committee Task Group No. 6Med Phys199219613591461197