?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to develop a sustained drug-release model for water-soluble drugs using silica nanoparticles.
Methods
Hollow-type mesoporous silica nanoparticles (HMSNs) were prepared using Na2CO3 solution as the dissolution medium for the first time. The water-soluble compound, silybin meglumine, was used as the model drug. The Wagner–Nelson method was used to calculate the in vivo absorption fraction.
Results
The results of transmission electron microscopy and nitrogen adsorption revealed that the empty HMSNs had uniformly distributed particles of size 50–100 nm, a spherical appearance, a large specific surface area (385.89 ± 1.12 m2/g), and ultralow mean pore size (2.74 nm). The highly porous structure allowed a large drug-loading rate (58.91% ± 0.39%). In 0.08 M Na2CO3 solution, silybin meglumine-loaded HMSNs could achieve highly efficacious and long-term sustained release for 72 hours in vitro. The results of in vitro–in vivo correlation revealed that HMSNs in 0.08 M Na2CO3 solution had a correlation coefficient R2 value of 0.9931, while those of artificial gastric juice and artificial intestinal juice were only 0.9287 and 0.7689, respectively.
Conclusion
The findings of in vitro–in vivo correlation indicate that HMSNs together with Na2CO3 solution could achieve an excellent linear relationship between in vitro dissolution and in vivo absorption for 72 hours, leading to a promising model for sustained release of water-soluble drugs.
Introduction
Over the past few decades, mesoporous silica nanoparticles have played an important role in the development of drug delivery systems. They have made a significant impact on medical technology by minimizing the size of drug delivery devices from the macro to the nano scale and significantly enhancing the performance of existing lifesaving drugs.Citation1,Citation2 Mesoporous silica nanoparticles have attracted considerable attention due to their ability to enhance solubility of poorly water-soluble drugs, as well as to achieve sustained drug release, leading to improved bioavailability.Citation3,Citation4 Advantages of mesoporous silica nanoparticles, such as large surface area, high pore volume, tunable pore size, and well defined pore structure, as well as mechanical and chemical stability and protection of the integrity of the molecular structure of the drug,Citation5–Citation7 make them ideal for controlled drug delivery systems. Most recently, mesoporous silica nanoparticles were developed for intracellular controlled drug/gene deliveryCitation8,Citation9 and targeted drug delivery to cancer cells.Citation10,Citation11 Furthermore, the safety of silica materials used as drug delivery systems has been well defined in several reports,Citation12,Citation13 which makes them desirable as perfect delivery systems. Chervenkov et alCitation12 found that oral (2 g/kg bodyweight) administration of silica dioxide xerogels did not result in significant changes in the biochemical parameters characterizing liver function and gastric mucosal damage.
So far, many attempts have been made to develop sustained- release preparations to improve the release behavior of orally delivered water-soluble drugs, such as sustained-release matrix tablets,Citation14,Citation15 nanostructured liquid crystalline matrix,Citation16 surfactant-polymer nanoparticles,Citation17 double-walled microspheres,Citation18 and mesoporous silica nanoparticles.Citation3,Citation5 However, the in vitro sustained-release duration of these formulations lasted no longer than 24 hours. Therefore, it is desirable to develop a drug-release model that can achieve in vitro sustained release for a long period of time. In the present study, hollow-type mesoporous silica nanoparticles (HMSNs) were prepared and used as an in vitro sustained drug-release model for water-soluble drugs. Due to the highly porous structure of HMSNs, drugs can be loaded within the pores or canals, achieving in vitro sustained release for 72 hours. HMSNs have most of the advantages of mesoporous silica nanoparticles, but unlike mesoporous silica nanoparticles published earlier on, HMSNs possess a spherical shape with inner pores and channels and longer-lasting drug release in vitro. The possible reason is that, unlike the regularly and orderly distributed canals in mesoporous silica nanoparticles, the HMSNs prepared in this study possessed irregular and disordered canals which may result in prolonged drug release because it is harder for the drug to escape from disordered canals than from ordered ones.
Silybin meglumine, a commercially used compound of silybin and meglumine, was used as the model drug in this work. It has been widely used for the treatment of acute hepatitis, chronic hepatitis, initial-stage hepatocirrhosis, and liver damage due to intoxication because of the excellent hepatoprotective effect of silybin. Silybin is a naturally occurring polyphenolic flavonoid extracted from the seed of the milk thistle (Silybum marianum),Citation19,Citation20 and has been widely used to treat certain liver disorders, such as chronic active hepatitis, hepatic cirrhosis, as well as alcohol-induced and various types of drug-induced and toxin-induced liver damage.Citation21,Citation22 It is known that silybin is a poorly water-soluble drug; however, once silybin is combined with meglumine, its ionization ability increases and silybin meglumine is endowed with high water solubility. The present commercial formulation of silybin meglumine is in the form of a tablet and patients have to take the medicine three times a day in order to maintain an effective plasma concentration. Obviously, it is inconvenient for most patients to take medicine so frequently. Therefore, it is important to develop a universally applicable technology for processing silybin meglumine in order to achieve high-efficacy, a long-term drug-release pattern, and reduce the frequency of drug administration. Furthermore, such a delivery system must be safe after oral or intravenous administration.
Until now, most investigations of silica nanoparticles have focused on porous silica nanoparticles and mesoporous silica nanoparticles, including preparation methods, drug-loading capacity, and in vitro release performance.Citation23–Citation25 Few attempts have been made to evaluate their in vivo absorption characteristics, and no research has been carried out to study in vitro–in vivo correlations (IVIVCs). Further, the present scientific literature shows that no report has been published about HMSNs for sustained drug delivery. In a report by Li et al,Citation26 the in vitro controlled release of loaded drugs from porous hollow silica nanoparticles could last for up to 30 days, but no in vivo investigation was carried out. Based on common knowledge, loaded drugs cannot survive up to 30 days after release in vivo. Previous studies have confirmed that the in vitro accumulated release of drug-loaded HMSNs in traditional dissolution media was less than 20%, but the drug could be eliminated within 72 hours following single-dose administration in Beagle dogs (data not shown). This inconsistency between in vitro dissolution and in vivo absorption has prompted researchers to seek out improved dissolution methods or better dissolution media in order to obtain better IVIVCs.
The in vitro dissolution medium is an important factor in constructing a good in vitro drug-release model. So far, many media, such as phosphate buffer solution,Citation27 HCl solution (pH 1–2),Citation4 and water/ethanol mixture (70:30, v/v),Citation26 have been used to dissolve formulations prepared on the basis of porous silica nanoparticles, but none of these studies has evaluated IVIVCs. A possible reason is that there still remain many difficulties in developing appropriate dissolution test methods due to fast dissolution of submicron drug substances and in separating undissolved particles from dissolution medium.Citation28 What is more important is to find a proper dissolution medium that can achieve a good IVIVC.
In an effort to address the problems mentioned above, our research group attempted to develop a high-efficacy, long-acting in vitro drug-release model based on HMSNs for the delivery of water-soluble drugs. Water-soluble silybin meglumine was directly loaded into HMSNs in this study. The in vitro release profiles of silybin meglumine-loaded HMSNs were studied for the first time using Na2CO3 solutions with different concentrations (0.01 M, 0.06 M, 0.08 M, and 0.1 M). The treatment was repeated for other dissolution media such as traditional artificial gastric juice (pH 1.2) and artificial intestinal juice (pH 6.8). Beagle dogs were used as an in vivo model to study pharmacokinetic characteristics after oral administration, and the IVIVC was then established. The use of HMSNs together with a Na2CO3 solution at a certain concentration is expected to be developed into an effective approach to obtain an in vitro drug-release model with a sustained-release pattern lasting for 72 hours, as well as a good IVIVC.
Methods and materials
Materials
Tetraethyl orthosilicate, absolute ethyl alcohol, ammonia water, cyclohexane, n-hexanol, and Na2CO3 were purchased from Chemical Reagent Co, Ltd, of the China National Pharmaceutical Group (Shanghai, China). NP-10 was obtained from Shanghai Jiafang Trade Co, Ltd (Shanghai, China). The animal experiments were performed strictly according to the experimental protocols approved by the university ethics committee for the use of experimental animals, and also conformed to the Guide for Care and Use of Laboratory Animals.
Preparation of empty HMSNs
A 6 mL quantity of nonylphenol 10 (NP-10) was added to 50 mL of cyclohexane, and shaken together. After adding 2.2 mL of n-hexanol and 1.8 mL of 25.6% ammonia water, the resulting mixture was again agitated for one hour at room temperature. Tetraethyl orthosilicate 4.2 mL was slowly added to the mixture and agitation was conducted for another 24 hours at room temperature. A 60 mL quantity of absolute ethyl alcohol was then added prior to ultrasound treatment for one hour (Wuxi Ultrasonic Devices Factory, Jiangsu, China). Centrifugal separation was performed at 15,000 per second for 15 minutes and the precipitate was washed three times with double-distilled water. After addition of water, freezing, and drying of the substance in succession, a powder of solid silica nanoparticles was obtained.
After addition of 1 g of the silica nanoparticles to 1000 mL of 0.6 mol/L Na2CO3 solution, ultrasound was administered for 5 minutes at 65°C and 200 W. The mixture was then centrifugally separated at 15,000 per second for 15 minutes and the precipitate was washed three times with double-distilled water. The resulting precipitate was then added to 10 mL of double-distilled water, followed by freezing and drying the substance in succession, and empty HMSNs were thus obtained. Fourier transform infrared analysis was carried out to examine the residual surfactant (NP-10). No absorption band characterizing NP-10 was observed, indicating that the surfactant had been completely washed away.
Preparation of silybin meglumine-loaded HMSNs
In this study, silybin meglumine was directly loaded into the HMSNs. Two grams of silybin meglumine was dissolved in 20 mL of double-distilled water, in which 1 g of empty HMSNs were soaked for 24 hours and then centrifugally separated at 15,000 per second for 15 minutes. The precipitate was washed three times with double-distilled water. It was then added to 10 mL of double-distilled water, then frozen and dried in succession. Drug-loaded nanoparticles were obtained.
Physicochemical characterization of HMSNs
The physicochemical features of HMSNs were characterized using transmission electron microscopy and the nitrogen adsorption method. The morphology and internal porous structure of the samples were observed using a transmission electron microscope (JEM-2100, JEOL, Tokyo, Japan). The specific surface area, pore volume, and pore diameter of the samples were measured by the nitrogen adsorption method using a high-speed automated surface area and pore size analyzer (Nova 2000, Quantachrome Instruments, Boynton Beach, FL) according to the Brunauer-Emmett-Teller and Barrett-Joyner-Halenda procedures.
The drug-loading rates of both silybin meglumine-loaded HMSNs and silybin meglumine-loaded solid silica nanoparticles were also determined. A 10 mg quantity of silybin meglumine-loaded HMSNs and the same amount of silybin meglumine-loaded solid silica nanoparticles were added to a certain amount of Na2CO3 solution and then water-bathed at a certain temperature for 30 minutes. The treatment was repeated for the same amount of HMSNs without a loading drug which resulted in a blank control solution.
The drug-loading rate was determined via ultraviolet spectroscopy (UV-2401PC, Shimadzu, Tokyo, Japan) at a wavelength of 288 nm. The blank control solution was used as reference. The drug-loading rate was calculated according to the following formula:
In vitro sustained-release studies
The in vitro release profiles of silybin meglumine from the sample were determined by a dialysis tubing method using dissolution testing apparatus (Tianjin University Radio Factory, Tianjin, China) according to the paddle method recorded by the Chinese Pharmacopoeia (2005). Artificial gastric juice (pH 1.2), artificial intestinal juice (pH 6.8), Na2CO3 solutions with different concentrations (0.01 M pH 11.1, 0.06 M pH 11.5, 0.08 M pH 11.6, 0.1 M pH 11.7), and 0.01 M NaOH (pH 12) were used as dissolution media in this study. The paddles were rotated at 100 rpm for 72 hours. The samples were accurately weighed and added to the dialysis bags, which were then fixed on the paddles. The samples were then put into 900 mL of artificial gastric juice (pH 1.2) or intestinal juice (pH 6.8) as the dissolution medium with the temperature adjusted to 37°C ± 0.1°C.
Test fluids were withdrawn at predetermined time intervals from each vessel and then filtered through a 0.45 μm membrane filter. The same volume of test fluid removed was then replaced by fresh medium. The quantity of silybin in the filtrate (20 μL) was analyzed using a high-pressure liquid chromatography (HPLC) system (model 510; Waters Corporation, Milford, MA) equipped with an ultraviolet detector (model 486; Waters Corporation). The HPLC conditions were as follows: reverse-phase column, Nova-pack C18 column (3.9 × 150 mm, 5 μm) at 37°C; mobile phase, dipotassium hydrogen phosphate (0.05 M): methanol 9.9:11 (v/v) mixture; flow rate, 1.0 mL/minute; and detection wavelength, 288 nm. All analyses were performed at 37°C and experiments were performed at least three times. The standard curve range was 1.68–31.8 μg/mL.
In vivo absorption study
Animal experiment
Healthy male Beagle dogs weighing 10 ± 2 kg were subjected to fasting overnight before the experiments, but allowed free access to water throughout the study. Silybin meglumine-loaded HMSNs were administered as an oral dose (21.2 mg/kg) to the test group of six dogs.
Serial blood samples of approximately 3 mL were collected at 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 16, 20, 24, 36, 48, 60, and 72 hours after oral administration. Plasma obtained after centrifugation (10 minutes, 4000 per second) was immediately added to 20 μL of internal standard solution (α-naphthol, 10 μg/mL in methanol) followed by gentle vortex agitation for 5 minutes. The mixture was centrifuged for 10 minutes at 3000 per second, and the organic layer was then transferred to a clean vial and evaporated under nitrogen at 30°C. Finally, the residue was dissolved in 100 μL of the mobile phase and transferred to the HPLC system for analysis.
HPLC analysis
Plasma silybin was determined by HPLC. A Nova-pack C18 column (150 mm × 3.9 mm, 5 μm) with a column temperature of 37.5°C and a mobile phase consisting of methanol and KH2PO4 (0.05 mol/L) with the ratio of 9.9:11 at pH 3.8 were used. The eluent was monitored at 288 nm with a flow rate of 1 mL/minute. α-Naphthol was used as an internal standard.
Calculation of in vivo absorption percentage
There are various methods that can be used to calculate the in vivo absorption percentage, including Wagner–Nelson and Loo–Riegelman methods. The former is applicable to a one-compartment model, the later is suitable for a two-compartment model, whereas according to the result determined by Akaike’s information criterion method, which was conducted by using software BAPP2.3 (supplied by the Center of Drug Metabolism of China Pharmaceutical University, China) in our previous studies, the plasma concentration-time data for free silybin released from HMSNs were fit to a one-compartment model (data not shown). Therefore, the Wagner–Nelson method was used in the present work to calculate the in vivo absorption fraction:
In the above formula, f represents the in vivo absorption fraction, Ct is the plasma concentration at time point t, and k is the elimination rate constant.
Statistical analysis
All values are represented as the mean ± standard deviation. Statistical differences between the different groups were determined using the Student’s t-test and one-way analysis of variance, with a least significant difference post-hoc test by SPSS statistical software (SPSS version 15.0, SPSS Inc, Chicago, IL). Differences between the two groups were considered to be statistically significant at P < 0.01.
Results and discussion
Preparation and physicochemical characteristics of HMSNs
So far, a number of studies have been conducted on the preparation of porous silica spheres by the modified Stober methods, in which cationic surfactants were used as porogens.Citation29–Citation31 In the present study, a Na2CO3 solution and ultrasound were used to make pores and canals within the silica spheres. After lyophilization, the hollow-type mesoporous silica nanoparticles were then obtained. In this work, Na2CO3 solution was used innovatively to dissolve the silica partially under certain conditions. The resulting HMSNs were highly porous with a uniform small size, and had disordered holes, as well as a large surface area.
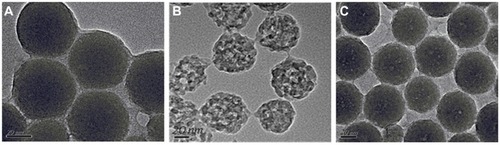
Transmission electron microscopy was used to characterize the morphology and porous structure of the HMSNs. It was observed that the empty/drug-loaded HMSNs with/ without phosphotungstic acid staining, showed images of empty/drug-loaded HMSNs (with phosphotungstic acid staining) being less desirable as compared with the other component without phosphotungstic acid staining (data not shown). Thus, the transmission electron microscopy without phosphotungstic acid staining was used in the present study. shows transmission electron microscopic images of solid silica nanoparticles and HMSNs before and after drug-loading, in which a spherical appearance and uniformly distributed particle size within the range of 50–100 nm were observed. In addition, the highly porous inner structure of the empty HMSNs () was clearly visible in comparison with the solid silica nanoparticles (). When loaded with drugs, the holes and canals on the surface of the particles disappeared, as shown in (silybin meglumine-loaded HMSNs). The most probable reason to explain this observation is that most of the canals and holes within the empty HMSNs were filled with drug.
Figure 1 Images of (A) TEM image for solid silica nanoparticles, (B) TEM image of empty HMSNs, and (C) TEM image of silybin meglumine-loaded HMSNs.
Abbreviations: HMSNs, hollow-type mesoporous silica nanoparticles; TEM, transmission electron microscopy.
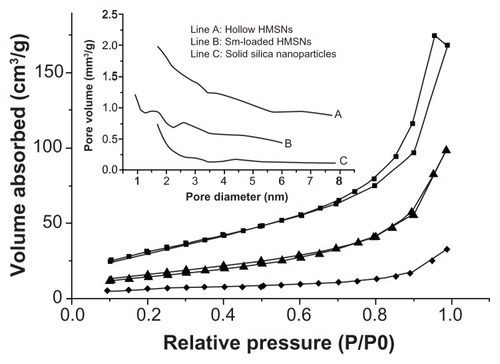
In this study, to identify the specific surface area, pore volume, and pore diameter of the samples, the nitrogen adsorption method was employed. The results are shown in , in which the nitrogen adsorption–desorption isotherms of both solid silica nanoparticles and HMSNs before and after drug-loading are demonstrated. The nitrogen adsorption–desorption isotherms for solid silica nanoparticles completely overlapped, indicating no presence of pores, while an adsorption isotherm of empty HMSNs similar to Type II Langmuir adsorption isotherms and a hysteresis loop in the range of relative pressure from 0.696 to 0.989 indicated the presence of pores or canals in HMSNs.Citation32 The average pore diameter of the empty HMSNs was 2.74 nm and the pore size was uniformly distributed within a relatively narrow range, between 1 nm and 6 nm. The empty HMSNs with an ultranarrow pore size (2.74 nm on average) prepared in this work were obviously smaller than that of the porous silica nanoparticles reported by Zhang et al.Citation3 When loaded with drug, HMSNs showed no hysteresis loop, suggesting that most pores within the HMSNs were completely filled with drug. These results are in agreement with the transmission electron microscopic image in . Additionally, nitrogen adsorption results demonstrated that the HMSNs had a much larger specific surface area (385.89 ± 1.12 m2/g) than the solid silica nanoparticles (5.84 ± 0.26 m2/g), indicating the highly porous structure of HMSNs. After adsorption of silybin meglumine, a significant decrease was observed in the surface area of HMSNs (6.42 m2/g) and the pore diameter (1.68 nm), indicating successful loading of silybin meglumine.
Figure 2 Nitrogen adsorption–desorption isotherms of solid silica nanoparticles (◆), empty HMSNs (▴), and silybin meglumine-loaded HMSNs (■). insert: line (A) represents empty HMSNs, line (B) represents silybin meglumine-loaded HMSNs, and line (C) represents solid silica nanoparticles.
Abbreviation: HMSNs, hollow-type mesoporous silica nanoparticles.
The drug-loading rate of the silybin meglumine-loaded HMSNs was 58.91% ± 0.39%, while the drug-loading rate of silybin meglumine-loaded solid silica nanoparticles was only 5.78% ± 0.12%. The results fully demonstrate the advantages of empty HMSNs over solid silica nanoparticles in drug delivery.
In vitro release studies
The in vitro release profiles of silybin meglumine from HMSNs are illustrated in , in which silybin meglumine-loaded solid silica nanoparticles were used as a comparison. In order to select a suitable dissolution medium, several kinds of solutions were tested, including artificial gastric juice (pH 1.2) and artificial intestinal juice (pH 6.8), 0.01 M NaOH solution (pH 12), as well as Na2CO3 solutions with different concentrations (0.01 M pH 11.1, 0.06 M pH 11.5, 0.08 M pH 11.6, and 0.1 M pH 11.7). For the preliminary research, artificial gastric and intestinal juices were used to dissolve samples according to the paddle method recorded by the Chinese Pharmacopoeia (2005). As shown in , the accumulated release percentages of silybin meglumin from silybin meglumine-loaded HMSNs in artificial gastric and intestinal juices were less than 20% within 72 hours. It is obvious that artificial gastric and intestinal juices were not suitable for in vitro dissolution of silybin meglumine-loaded HMSNs, and more investigations were needed in order to find a more appropriate dissolution media. Therefore, NaOH and Na2CO3 solutions were introduced and the in vitro release profiles of silybin meglumine-loaded HMSNs are presented in , which show the slowest release rate in a 0.01 M Na2CO3 solution and the fastest release rate in a 0.1 M Na2CO3 solution. It is known that silybin meglumine is an ammonium salt which is chemically stable in basic media. An accelerating dissolution rate was observed with increasing Na2CO3 concentration. Significant enhancement of dissolution was observed in 0.06 M, 0.08 M, and 0.1 M Na2CO3 solutions as compared with that in artificial gastric or intestinal juices. A similarly rapid release was also observed in 0.06 M and 0.08 M Na2CO3 solutions, with an accumulative release rate of approximately 59% and 63% at 12 hours, respectively, but a significantly faster release from 0.1 M Na2CO3 solution with an accumulative release rate of approximately 94% at 12 hours. After that silybin meglumine-loaded HMSNs in a 0.08 M Na2CO3 solution was faster than that of a 0.06 M Na2CO3 solution. For 0.08 M Na2CO3 solution, the accumulative release rate was 80.0% at 24 hours, followed by a slow increase up to 92.3% at 72 hours, while the accumulative release rate was only 62.8% at 24 hours and 77.4% at 72 hours for 0.06 M Na2CO3 solution. One of the possible mechanisms for this phenomenon is that silica exhibited weak acidityCitation33,Citation34 and Na2CO3 solution exhibited weak alkalinity, so that an acid-base chemical reaction took place between silica and Na2CO3, resulting in the water-soluble salt Na2SiO3 that existed in ion form in water. It could be speculated that the HMSN matrix disintegrated into many microparticles in Na2CO3 solution, similar to the disintegration of common tablets but at a much slower rate. Furthermore, in Na2CO3 solutions with different concentrations, there were different degrees of silica dissolution, resulting in different drug-release rates. Therefore, the in vitro release rate of loaded drugs can be controlled by using Na2CO3 solutions with different concentrations as the dissolution media. In other words, drug release from HMSNs could be affected by the pH value of the media. It is well known that silica is an acidic oxide that usually reacts with a basic oxide. As a result, silica can dissolve in alkaline media (pH > 7) with stronger alkalinity (a higher pH value), resulting in a faster reaction. Thus, with the increase in Na2CO3 concentration, the pH value of the media increased as well, leading to faster dissolution of HMSNs and quicker release of loaded drugs. However, according to the results (), a slower release profile was seen from 0.01 M NaOH solution with a pH value of 12 than from 0.06 M Na2CO3 solution. Therefore, pH value was not the only factor that in vitro degradation of HMSNs but also the concentration. The exact reason for the observation is still unknown and further investigation of the dissolution mechanism will be carried out in future works.
Figure 3 In vitro release curves of silybin from different formulations in various dissolution media. (A) In vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴); (B) in vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in Na2CO3 solutions with different concentrations [0.1 M (line A), 0.08 M (line B), 0.06 M (line C), and 0.01 M (line E)], and in 0.01 M NaOH solution (line D); (C) in vitro release curves of silybin meglumine-loaded solid silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴).
Note: Each datum point represents the mean ± standard deviation of three administrations.
![Figure 3 In vitro release curves of silybin from different formulations in various dissolution media. (A) In vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴); (B) in vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in Na2CO3 solutions with different concentrations [0.1 M (line A), 0.08 M (line B), 0.06 M (line C), and 0.01 M (line E)], and in 0.01 M NaOH solution (line D); (C) in vitro release curves of silybin meglumine-loaded solid silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴).Note: Each datum point represents the mean ± standard deviation of three administrations.](/cms/asset/511560be-a6ca-47f1-b29d-4451fc542419/dijn_a_28348_f0003_b.jpg)
shows the in vitro release profiles for silybin meglumine from silybin meglumine-loaded solid silica nanoparticles in artificial gastric and intestinal juices. The accumulated release achieved a constant level at 0.5 hours for solid silica nanoparticles in artificial intestinal juice (with an accumulated release rate of 100%) and at one hour for artificial gastric juice (with an accumulated release rate of 70%). These results confirm the advantages of HMSNs over solid silica nanoparticles in drug storage and sustained release.
In this study, Na2CO3 solutions were used as dissolution media for the first time by our research group. It is of great significance for discovering a desirable in vitro dissolution model for drug-loaded HMSNs with long-term release for 72 hours.
In vivo absorption study
The oral absorption of silybin meglumine from HMSNs was studied in fasting Beagle dogs. The fasting state was selected to exclude the effect of food lipids, which can be confused with the effect of internal lipids. Because silybin meglumine is a compound of silybin and meglumine, it was speculated that the compound could be easily degraded into silybin and meglumine in the in vivo environment. Thus, silybin blood concentration was used as an indicator of silybin meglumine absorption in vivo.
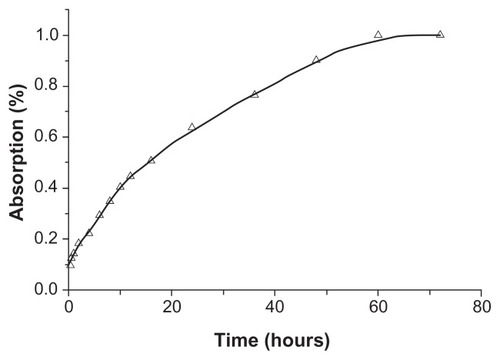
The mean plasma concentration-time data for silybin meglumine-loaded HMSNs following a single dose are presented in . Based on these data, the percentages of silybin absorbed at specific time points were calculated from the plasma concentration data by using the Wagner–Nelson method,Citation35 as shown in .
Table 1 Mean plasma concentration-time data for silybin meglumine-loaded incorporated into hollow-type silica nanoparticles following a single dose
In vitro-in vivo correlation
The correlations between in vitro and in vivo data for silybin meglumine-loaded HMSNs and silybin meglumine tablets were investigated. Three levels of IVIVC are classified according to the US Food and Drug Administration.Citation36 A level A correlation, ie, a point-to-point relationship between in vitro dissolution and in vivo absorption, was used in this present work.
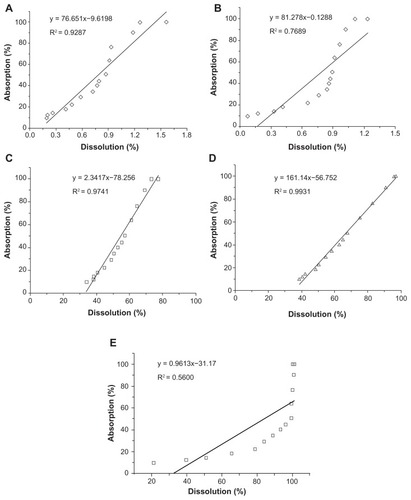
showed the correlations between the in vitro dissolved fractions and in vivo absorbed fractions for silybin meglumine-loaded HMSNs and silybin meglumine tablets in the individual beagle dogs. The fractions dissolved in artificial gastric juice (pH 1.2), artificial intestinal juice (pH 6.8), and 0.06 M, 0.08 M, and 0.1 M Na2CO3 solutions were plotted against the fractions absorbed in 72 hours in the fasting condition, in order to find out the appropriate in vitro dissolution medium leading to better IVIVC. According to the statistics, the linear relationships between the in vitro dissolved fractions and the in vivo absorbed fractions for the silybin meglumine-loaded HMSNs in artificial gastric juice (R2 = 0.9287, as shown in ) and artificial intestinal juice (R2 = 0.7689, as shown in ) were not significantly correlated. In the case of the 0.06 M Na2CO3 solution as the in vitro dissolution medium, the value of the correlation coefficient R2 was 0.9741 (). This value was lower than that for the 0.08 M Na2CO3 solution, which exhibited a correlation coefficient R2 value of 0.9931 (). Interestingly, when the concentration of Na2CO3 solution increased to 0.1 M, the correlation coefficient R2 decreased dramatically (R2 = 0.5600). This phenomenon indicated that the use of Na2CO3 solution with concentration lower than 0.08 M gave a better IVIVC with increasing Na2CO3 concentration, but when the concentration of Na2CO3 solution was beyond 0.08 M, the correlation coefficient reduced with increasing Na2CO3 concentration. The exact reasons for this will be addressed in future work. According to the results of IVIVC, the 0.08 M Na2CO3 solution was the most effective dissolution medium for silybin meglumine-loaded HMSNs. In the case of the 0.08 M Na2CO3 solution, the in vitro dissolution rate was not the same as the in vivo absorption rate in each subject (). This difference could be due to the differences between the simple in vitro environment and the complex in vivo environment for dissolution. In addition, gastric emptying and intestinal transit would regulate the absorption process,Citation24 and therefore result in the differences observed between in vitro dissolution rates and in vivo absorption rates.
Figure 5 Correlations between in vitro dissolution fractions and in vivo absorption fractions. (A) IVIVC of silybin meglumine-loaded HMSNs in artificial gastric juice; (B) IVIVC of silybin meglumine-loaded HMSNs in artificial intestinal juice; (C) IVIVC of silybin meglumine-loaded HMSNs in 0.06 M Na2CO3 solution; (D) IVIVC of silybin meglumine-loaded HMSNs in 0.08 M Na2CO3 solution; and (E) IVIVC of silybin meglumine-loaded HMSNs in 0.1 M Na2CO3 solution.
Abbreviations: HMSNs, hollow-type mesoporous silica nanoparticles; IVIVC, in vitro–in vivo correlations.
In summary, Na2CO3 solutions had been reported in this study for the first time as an in vitro dissolution medium for drug-loaded HMSNs, which exhibited excellent level A IVIVC. The drug-release model developed in this study achieved in vivo sustained release lasting for 72 hours, with advantages such as fast action, high efficacy, and long-lasting action. The results of this work have therefore had a considerable impact on the construction of sustained-release systems.
Conclusion
In this study, silybin meglumine was used as the model drug, and was loaded into HMSNs. The silybin meglumine-loaded HMSNs were successfully developed to enhance in vitro dissolution and in vivo absorption, and demonstrated an excellent IVIVC. The results of transmission electron microscopy and nitrogen adsorption showed that the empty HMSNs had a large specific surface area (385.89 m2/g) and a small pore size (average pore diameter of 2.74 nm), which led to a relatively large drug-loading capacity (58.91% ± 0.39%). The in vitro dissolution studies conducted in different media revealed that Na2CO3 solutions of particular concentrations could achieve highly effective, long-acting, sustained release lasting for 72 hours. The results of IVIVC showed that HMSNs in 0.08 M Na2CO3 solution had a correlation coefficient R2 value of 0.9931, while the correlation coefficient R2 values for the artificial gastric and intestinal juices were only 0.9287 and 0.7689, respectively. The IVIVC investigated in this work indicated that HMSNs, together with a Na2CO3 solution, could achieve an excellent linear relationship between the in vitro dissolution and in vivo absorption for up to 72 hours.
Acknowledgments
This work was supported by National Natural Science Foundation of China (30472098), Special Funds for 333 Project (BRA2010138), and industry-university-research institution cooperation (BY2009141, CY2010023, CZ2009009) in Jiangsu Province and Zhenjiang City. The authors also thank Caleb Kesse Firempong for English editing and the university ethics committee for the kind guidance in the animal experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShiJJVotrubaARFarokhzadOCLangeRNanotechnology in drug delivery and tissue engineering: from discovery to applicationsNano Lett2010103223323020726522
- MurthySKNanoparticles in modern medicine: State of the art and future challengesInt J Nanomed20072129141
- ZhangYZZhiZZJiangTYZhangJHWangZYWangSLSpherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartanJ Control Release201014525726320450945
- KapoorMPVinuAFujiiWSelf-assembly of mesoporous silicas hollow microspheres via food grade emulsifiers for delivery systemsMicropor Mesopor Mater2010128187193
- KilpeläinenMRiikonenJVlasovaMAIn vivo delivery of a peptide, ghrelin antagonist, with mesoporous silicon microparticlesJ Control Release200913716617019345247
- SlowingIIVivero-EscotoJLWuCWLinVSYMesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriersAdv Drug Deliv Rev2008601278128818514969
- SalonenJLaitinenLKaukonenAMMesoporous silicon microparticles for oral drug delivery: loading and release of five model drugsJ Control Release200510836237416169628
- Vivero-EscotoJLSlowingIITrewynBGLinVSYMesoporous slica nanoparticles for intracellular controlled drug deliverySmall201061952196720690133
- HomCLuJLiongMMesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathway in mammalian cellsSmall201161185119020461725
- MamaevaVRosenholmJMBate-EyaLTMesoporous silica nanoparticles as drug delivery system for targeted inhibition of notch signaling in cancerMol Ther2011191538154621629222
- RosenholmJSahlgrenCLindenMCancer-cell targeting and cell-specific delivery by mesoporous silica nanoparticlesJ Mater Chem20102027072713
- ChervenkovTIvanovaDGalunskaBGerovaDYankovaTToxicity of polymeric nanoparticles with respect to their application as drug carriersSimeonovaPOpopolNLusterMNanotechnology- Toxicological Issue and Environmental SafetyThe NetherlandsSpringer2007111118
- TanakaTGodinBBhavaneRIn vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administration in miceInt J Pharm201040219019720883755
- CortiGCirriMMaestrelliFMenniniNMuraPSustained-release matrix tablets of metformin hydrochloride in combination with triacetyl- β-cyclodextrinEur J Pharm Biopharm20086830330917616379
- MandalSBasuSKSaBSustained release of a water-soluble drug from alginate matrix tablets prepared by wet granulation methodAAPS Pharm Sci Tech20091013481356
- LeeKWYNguyenTHHanleyTBoydBJNanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugsInt J Pharm200936519019918790030
- ChavanpatilMDKhdairAPanyamJSurfactant-polymer nanoparticles: A novel platform for sustained and enhanced cellular delivery of water-soluble moleculesPharm Res20072480381017318416
- LeeTHWangJWangCHDouble-walled microspheres for the sustained release of a highly water soluble drug: characterization and irradiation studiesJ Control Release20028343745212387951
- FloraKHahnMRosenHBennerKMilk thistle (Silybum marianum) for the therapy of liver diseaseAm J Gastroenterol1998931391439468229
- AgarwalRKatiyarSKLundgrenDWMukhtarHInhibitory effect of silymarin, an anti-hepatotoxic flavonoid, on 12-O-tetradecanoylphorbol- 13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in Sencar miceCarcinogenesis199415109911038020140
- LigeretHBraultAVallerandDHaddadYHaddadPSAntioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injuryJ Ethnopharmacol200811550751418061382
- AbenavoliLCapassoRMilicNCapassoFMilk thistle in liver diseases: past, present, futurePhytother Res2010241423143220564545
- TengZGHanYDLiJYanFYangWSPreparation of hollow mesoporous silica spheres by a sol-gel/emulsion approachMicropor Mesopor Mater20101276772
- LiZZWenLXShaoLChenJFFabrication of porous hollow silica nanoparticles and their applications in drug release controlJ Control Release20049824525415262416
- XuWGaoQXuYWuDSunYpH-controlled drug release from mesoporous silica tablets coated with hydroxypropyl methylcellulose phthalateMater Res Bull200944606612
- LiZZXuSAWenLXControlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and releaseJ Control Release2006111818816388871
- WangFHuiHBarnesTJBarnettCPrestidgeCAOxidized mesoporous silicon microparticles for improved oral delivery of poorly soluble drugsMol Pharm2009722723619874003
- JinnoJKamadaNMiyakeMIn vitro-in vivo correlation for wet-milled tablet of poorly water-soluble cilostazolJ Control Release2008130293718582979
- WangJXiaoQZhouHJBudded, mesoporous silica hollow spheres: Hierarchical structure controlled by kinetic self-assemblyAdv Mater20061832843288
- LebedevOIvan TendelooGCollartOCoolPVansantEFStructure and microstructure of nanoscale mesoporous silica spheresSolid State Sci20046489498
- YanoKFukushimaYSynthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactantJ Mater Chem20041415791584
- WangJXWangZHChenJFYunJDirect encapsulation of water-soluble drug into silica microcapsules for sustained release applicationsMater Res Bull20084333743381
- DamyanovaSGrangePDelmonBSurface characterization of zirconia-coated alumina and silica carriersJ Catal1997168421430
- AkkariRGhorbelAEsssayemNFiguerasFSilica supported sulfated zirconia prepared by a sol-gel process: effect of solvent evacuation procedure on the structural, textural and catalytic propertiesJ Sol Gel Sci Techn200638185190
- AkborMMSultanaRUllahAAzadMAKLatifAMHasnatAIn vitro-in vivo correlation (IVIVC) of immediate release (IR) levofloxacin tabletDhaka Univ J Pharm Sci20076113119
- DressmanJBReppasCIn vitro-in vivo correlations for lipophilic, poorly water-soluble drugsEur J Pharm Sci200011S738011033429