Abstract
Purpose
The purpose of this study was to evaluate the permeability of the blood–brain barrier after sonication by pulsed high-intensity focused ultrasound and to determine if such an approach increases the tumor:ipsilateral brain permeability ratio.
Materials and methods
F98 glioma-bearing Fischer 344 rats were injected intravenously with Evans blue with or without blood–tumor barrier disruption induced by transcranial pulsed high-intensity focused ultrasound. Sonication was applied at a frequency of 1 MHz with a 5% duty cycle and a repetition frequency of 1 Hz. The permeability of the blood–brain barrier was assessed by the extravasation of Evans blue. Contrast-enhanced magnetic resonance images were used to monitor the gadolinium deposition path associated with transcranial pulsed high-intensity focused ultrasound, and the influencing size and location was also investigated. In addition, whole brain histological analysis was performed. The results were compared by two-tailed unpaired t-test.
Results
The accumulation of Evans blue in brains and the tumor:ipsilateral brain permeability ratio of Evans blue were significantly increased after pulsed high-intensity focused ultrasound exposure. Evans blue injection followed by sonication showed an increase in the tumor:ipsilateral brain ratio of the target tumors (9.14:1) of about 2.23-fold compared with the control tumors (x4.09) on day 6 after tumor implantation. Magnetic resonance images showed that pulsed high-intensity focused ultrasound locally enhances the permeability of the blood–tumor barrier in the glioma-bearing rats.
Conclusion
This method could allow enhanced synergistic effects with respect to other brain tumor treatment regimens.
Introduction
A significant number of advances have recently been made in agents for the chemotherapeutic treatment of brain tumors, but the blood–brain barrier (BBB) often prevents cytotoxic levels being achieved. The survival of patients with malignant glioma is on average less than 2 years, even after surgical resection and extensive treatment using chemotherapy and high-dose irradiation.Citation1,Citation2 New therapeutic modalities that improve the life expectancy of these patients are critically needed. Although the blood–tumor barrier (BTB) is more permeable than the BBB, malignancies of the brain remain hard to treat with chemotherapy because the selective permeability of the BTB still blocks many agents from reaching their target.Citation3 Therefore, the efficacy of therapeutic agents will be improved and any side effects minimized if an approach is available that allows the efficient delivery of a therapeutic dose to a planned target volume without harming the brain.
Despite the fact that the integrity of the BBB is often reduced near a brain tumor, antitumor agents are rarely effective in patients with brain tumors because of the BTB.Citation4 The regions of BBB disruption that occur in tumors, if they occur at all, are generally near the tumor center; this region has been found to generally have a higher permeability than normal brain. The BBB in the tissues surrounding brain tumors and at the peripheral margin of the brain tumor are usually intact. However, malignant cells may have already invaded these areas. Therefore, in order to improve the efficacy of treatments of malignant glioma, drugs need to be delivered not only to tumor cells in the central region where the BTB has broken down but also where the migrating tumor cells are infiltrating the intact regions.
Many approaches have been tried to enhance the delivery of drugs to brain tumors but these have met with limited success. Chemotherapy accompanied by biochemical or osmotic BBB disruption may moderately augment the delivery of drugs to the brain.Citation5,Citation6 A solution of mannitol produces a hyperosmotic environment in which the BBB endothelial cells are temporarily dehydrated and therefore shrink, which opens the tight junctions and so temporarily disrupts the BBB. Our previous study has demonstrated that after BBB disruption by injection of mannitol the concentration of boron in tumors and the tumor:normal brain ratio of boron are significantly higher than that without BBB disruption.Citation7 However, these methods require intra-arterial catheterization and produce nonfocal BBB disruption within the entire tissue volume supplied by the injected artery branch.Citation8,Citation9
Pulsed high-intensity focused ultrasound (HIFU) has been shown to locally and reversibly increase the permeability of the BBB, and these changes to the BBB are affected by the applied pressure amplitude and dose of ultrasound contrast agent (UCA).Citation10–Citation12 Pulsed HIFU can produce mechanical effects that increase the permeability of the sonicated tissue in a noninvasive manner. Previous studies have shown that enhanced delivery of various chemotherapeutic agents to tumors occurs after pulsed HIFU and that this improves their antitumor effects.Citation13 The purpose of this study was to investigate the use of pulsed HIFU to enhance delivery of a relatively large amount of drugs to gliomas during tumor progression and determine if such an approach increases the tumor:ipsilateral brain ratio.
Materials and methods
F98 glioma brain tumor model
All animal experiments were performed according to the appropriate guidelines and approved by the National Yang-Ming University’s Animal Care and Use Committee. Male Fischer 344 rats (9–12 weeks, approximately 290–340 g) were anesthetized by an intraperitoneal administration of pentobarbital at a dose of 40 mg/kg of body weight. Then 1 × 105 F98 rat glioma cells in 10 μL Hanks’ balanced salt solution without Mg2+ and Ca2+ were injected into the brains of the rats. The glioma cells were stereotactically injected into one location in each hemisphere (5.0 mm posterior and 3.0 mm lateral to the bregma) of each rat’s brain at a depth of 5.0 mm from the brain surface. Next, the holes in the skull were sealed with bone wax and the wound was flushed with iodinated alcohol. The twenty-seven glioma-bearing rats were randomly divided into 3 groups. All rats in groups 1 and 2 were sonicated, group 1 was used for Evans blue (EB), and groups 2 and 3 were used for magnetic resonance (MR) imaging and histology.
Pulsed HIFU system and sonication
The pulsed HIFU was generated by a 1 MHz single-element focused transducer (A392S, Panametrics, Waltham, MA) with a diameter of 38 mm and a radius of curvature of 63.5 mm. The half-maximum of the pressure amplitude of the focal zone had a diameter and length of 3 mm and 26 mm, respectively. The whole system has been described in detail in our previous report.Citation14
The rat’s head was mounted on the stereotaxic apparatus with the nose bar positioned 3.3 mm below the interaural line. UCA (SonoVue, Bracco International, Amsterdam, The Netherlands) was injected into the femoral vein of the rats approximately 15 seconds before each sonication. The UCA contains phospholipid-coated microbubbles with a mean diameter of 2.5 μm, and at a concentration of 1 × 108 to 5 × 108 bubbles/ml. Sonication was pulsed with a burst length of 50 ms at a 5% duty cycle and a repetition frequency of 1 Hz. The duration of the sonication was 60 seconds. The pulsed HIFU was delivered to one location in the right hemisphere of the brain at the location of tumor cell implantation. For all of the animal experiments, the rats were sonicated after an injection of 300 μL/kg UCA at an acoustic power of 5.72 W.
Assessment of BBB disruption
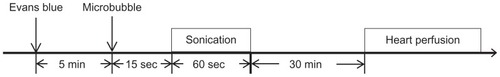
The permeability of the BBB can be quantified based on the extravasation of EB, which acts as a marker of albumin extravasation.Citation11,Citation14,Citation15 EB (Sigma, St Louis, MO) (100 mg/kg) was injected intravenously approximately 5 minutes before pulsed HIFU exposure. The animals were sacrificed approximately 30 minutes after the sonication (). The rats were perfused with saline via the left ventricle until colorless perfusion fluid appeared from the right atrium. After perfusion and brain removal, the hemispheres of the brain were dissected into tumor tissue and normal brain tissue before measuring the amount of EB extravasation. The left unsonicated brains acted as the controls. Samples were weighed and then soaked in 50% trichloroacetic acid solution. After homogenization and centrifugation, the extracted dye was diluted with ethanol (1:3), and the amount of dye present measured using a spectrophotometer (PowerWave 340, BioTek, Winooski, VT) at 620 nm. The EB present in the tissue samples was quantified using a linear regression standard curve derived from seven concentrations of the dye; the amount of dye was denoted in absorbance per gram of tissue. Results are typically expressed as means ± standard error of the mean. Any differences in EB concentration were analyzed by t-test. Statistical significance was defined as a P value ≤ 0.05.
Figure 1 Experimental time line for BTB disruption. EB was injected intravenously several minutes before sonication. Pulsed HIFU was applied 15 seconds after UCA administration in the right hemispheres. Rats were perfused 30 minutes after sonication to allow EB quantification.
Abbreviations: EB, Evans blue; HIFU, high-intensity focused ultrasound; UCA, ultrasound contrast agent.
MR imaging
Magnetic resonance imaging (MRI) of the glioma-bearing rats was performed using a 3T MRI system (TRIO 3-T MRI, Siemens AG MAGNETOM, Erlangen, Germany). A loop coil (Loop Flex Coil, small, approximately 4 cm in diameter; Siemens AG) for radio frequency reception was used. Each rat was injected intravenously with 1 mmol/kg of gadolinium (Gd-DTPA, Omniscan, GE Healthcare, Cork, Ireland) immediately after sonication. The rats were anesthetized with 1.5% isoflurane mixed with O2, and maintained on 1% isoflurane during the imaging procedure. A multislice spin echo sequence was performed to obtain 20 slices of the T1-weighted MR image covering the whole brain in order to monitor BBB disruption. The imaging plane was located across the tumor at the depth of the tumor center. In addition, tumor volumes were assessed from T2-weighted images by adding the tumor area measured from each slice and multiplying by the slice thickness (1 mm). The parameters for the MRI scans are listed in . MRI contrast enhancement was analyzed 30 minutes after the gadolinium injection. The contour maps describing the spatial distribution of the contrast enhancement were quantified in a second group of experiments. For each rat, the regions of contrast enhancement above four, eight, twelve, and 16 standard deviations of the averaged spatial normal brain regions were color-coded, allowing the distinguishable features to be easily observed.
Table 1 MRI parameters used in this study
Histological observations
After the MRI scanning, the second group of rats was prepared for histological evaluation. The rat was perfused with saline and 10% neutral buffered formalin. The brain was removed and embedded in paraffin and then serially sectioned into 6 μm slices. The slices were stained with hematoxylin-eosin (H&E) in order to confirm tumor progression. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (DeadEnd Colorimetric TUNEL system, G7130, Promega, Madison, WI) was used for the detection of DNA fragmentation and apoptotic bodies in the cells. The histological evaluation was carried out by light microscopy (Olympus BX61, Olympus, Shinjuku-Ku, Tokyo, Japan).
Results
Body weight and tumor progression
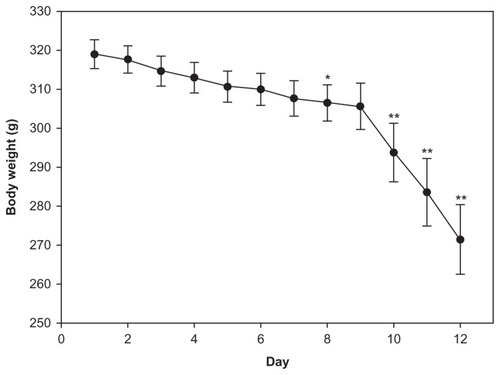
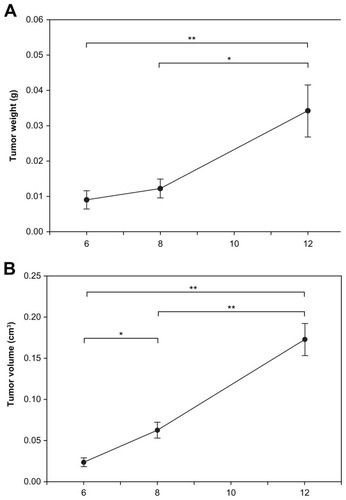
The rats’ mean body weight was greatest immediately after tumor implantation and decreased as a function of time due to growth of the tumor. Compared to the first day following tumor implantation, the mean body showed no obvious difference on day 6. However, it was significantly decreased on day 8 and had declined rapidly by day 12 (). The permeability of the BTBs was therefore assessed in rat brains on days 6, 8, and 12 after tumor cell implantation. Tumor progression was evaluated in terms of weight and volume (). The mean tumor weight and tumor volume at day 12 were significantly greater than on day 6 or day 8. The mean tumor volume of the day 8 group was significantly greater than those of the day 6 group, but no significant difference in mean tumor weight was evident between the day 6 and day 8 groups.
Assessment of EB extravasation
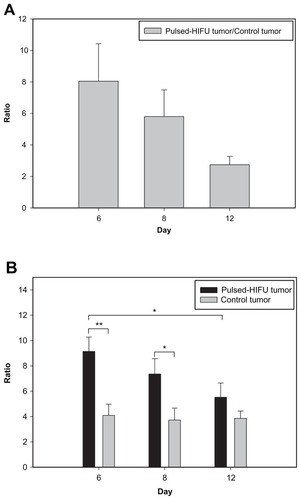
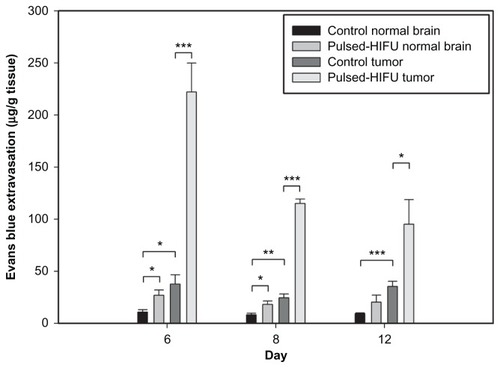
The BBB disrupted region was observed to occur in the focal zone of the ultrasound beam as assessed by EB extravasation. illustrates the degree of EB staining in the right and left hemispheres with and without sonication at the three time points after tumor implantation. Both the size and color intensity of the EB staining increased with tumor progression and that of the sonicated right hemispheres was greater than the nonsonicated left hemispheres for the three chosen days following tumor implantation. shows the mean extravasation of EB per unit mass (in micrograms per gram of tissue) for the brain tumors and the neighboring normal brain tissues with or without sonication at three time points after tumor implantation. EB extravasation was quantified in each tumor-implanted hemisphere brain; both the sonicated tumor and contralateral unsonicated control tumor were examined. Not only was the permeability of the control tumor BBB significantly greater than that of the adjacent normal brain region, but it was also found that the BTB disruption was significantly greater at the tumor site after sonication than in the control tumor for all three days. Pulsed HIFU exposure administered after EB introduction increased the EB concentration in the tumor by 805%, 580%, and 274% on days 6, 8, and 12, respectively (). There was no obvious difference in the derived tumor:ipsilateral brain ratios in the control tumor across the three days. Importantly, however, the derived tumor:ipsilateral brain ratios were greater after sonication than without sonication for all days; this change was significant on day 6 and day 8 (). The detailed ratios are listed in .
Table 2 Tumor:ipsilateral normal brain ratios
Figure 4 Distribution of BBB disruption for brain tumors as evaluated by extravasation of EB into the brain. Right brain: tumor with pulsed HIFU exposure. Left brain: control tumor without pulsed HIFU exposure. The tumor area and sonication path are consistent with the MR images in .
Abbreviations: BBB, blood–brain barrier; EB, Evans blue; HIFU, high-intensity focused ultrasound; MR, magnetic resonance.

Figure 5 Concentration of EB in the tumor and neighboring normal brain regions with and without sonication. The EB extravasation in the brain tumor with sonication was significantly higher than in brain tumor without sonication. Compared with normal tissues neighboring the control tumors, there was a significant difference for the control tumors and for the neighboring normal tissues of sonicated tumors on all days and on days 6 and 8, respectively.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviation: EB, Evans blue.
MRI analysis and histology
MRI studies were carried out in a subset of animals to noninvasively quantify tumor growth as a function of time; furthermore, the sonication pathway can be monitored using MR images in the right sonicated hemispheres (). In addition, the corresponding H&E and TUNEL-stained sections were observed for tumor progression and apoptotic evaluation (). Based on the histological observations, tumor progression was consistent with the MR images and no significant difference in apoptosis was found between the sonicated and control tumors. To better understand the extent of deposition of gadolinium, the contour maps of the spatial distribution of gadolinium for tumors with and without sonication are presented in . The contrast-enhanced regions in the right sonicated tumor were greater than in the left control tumor, especially for tumors on day 6 and day 8. During histological analysis of the sonicated and control tumors, local displacement and increased extravasation of red blood cells were seen in the sonicated tumor tissues in and around the focal region ().
Figure 7 Images of the tumors with and without pulsed HIFU exposure by (A) T1-weighted MR images, (B) hematoxylin-eosin-stained sections, and (C) TUNEL staining on days 6, 8, and 12 after implantation. The tumor progression and sonication pathway can be monitored in the MR images. H&E and TUNEL stained sections were observed for histology and apoptotic evaluation.
Abbreviations: H&E, hematoxylin-eosin; HIFU, high-intensity focused ultrasound; MR, magnetic resonance; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
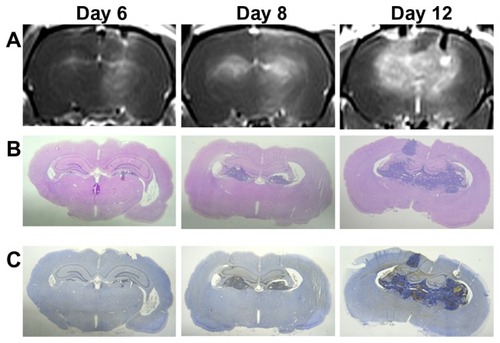
Figure 8 In vivo magnetic resonance images of rats bearing F98 gliomas in the (A) coronal view and (B) axial view. The spatial distribution of brain tumor BBB disruption with and without sonication in the right and left hemispheres, respectively, is shown. The rat brains were analyzed 30 minutes following gadolinium administration. Regions of contrast enhancement >4, >8, >12, and >16 standard deviations above the average MRI signal intensity of the normal brain tissue are shown in green, yellow, blue, and red respectively.
Abbreviations: BBB, blood–brain barrier; MRI, magnetic resonance imaging.
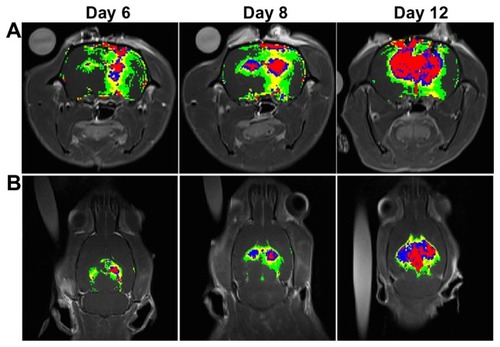
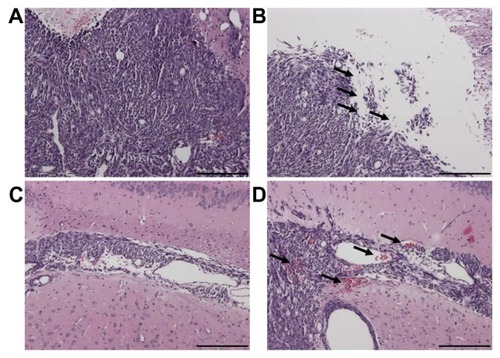
Figure 9 The structure of the contralateral brain tumor without sonication (A and C) and of the brain tumor tissue with sonication (B and D) by H&E staining (original magnification × 100 from ; scale bar = 200 μm). Local displacement and increased extravasation of red blood cells were indicated by arrows in the sonicated tumor tissues in and around the focal region.
Abbreviation: H&E, hematoxylin-eosin.
Discussion
This study demonstrates that pulsed HIFU can not only significantly increase the permeability of the BTB in brain tumors, but also significantly elevated the tumor:brain drug ratio in the focal region that was elicited by an ultrasound beam passing through the intact skull. The brain entry of drugs is impeded by the BBB, even though the permeability of this barrier may be partially increased due to the presence of a tumor. However, selective delivery of chemotherapeutic agent to brain tumor cells across the BTB remains a major obstacle to many approaches to brain tumor treatment. Our previous studies have shown that injecting an appropriate quantity of UCA effectively increases and localizes the BBB disruption that is induced by pulsed HIFU exposure.Citation11,Citation14,Citation15 In this study, a combination of pulsed HIFU and microbubbles increased the permeability of the BTB as measured by EB extravasation. The use of MRI contrast enhancement also revealed that this approach increased the level of gadolinium entering the brain tumor tissue.
The main limitation of this study was the size of the ultrasound beam focal zone produced by our device, which was not large enough to produce delivery enhancement for the whole tumor region on day 8 and day 12. This may be why the enhancement induced by pulsed HIFU decreased from day 6 to day 12 after implantation. This phenomenon suggests that the permeability of the BTB is increased only locally by pulsed HIFU. In addition, pulsed HIFU also enhanced permeability in the normal brain tissue surrounding the sonicated tumor on day 6 and day 8. Nevertheless, the enhanced uptake in the tumor tissue would be advantageous when treating larger tumors in humans using a phased array transducer for multiple focal sonications. and reveal that there were no differences in EB extravasation per gram of control tumor tissue or in the control tumor:ipsilateral brain ratio as the tumors progressed. Moreover, pulsed HIFU was able to significantly increase the permeability of the BTB and the ratio of tumor to brain, especially for the smaller tumors on day 6 and day 8; this was when the focal zone almost covered the whole tumor region.
Gadolinium deposition and the pattern of contrast enhancement were monitored by signal intensity level. shows that these are larger at high intensity level sites in the sonicated tumor on all days, especially on day 6 and day 8. This is consistent with the EB extravasation results. The sonication pathway can be observed from the brain surface to the bottom of the brain (). Additionally, the pattern of contrast enhancement while the pulsed HIFU beam is targeted over a non-homogeneous tumor tissue does not correspond to the circular pattern of the ultrasound beam on the cross section ().
One recent study has demonstrated that sonication after EB injection can lead to nearly a fivefold increase in EB extravasation in target hepatocellular carcinoma compared with contralateral controls. However, this effect is reduced while EB is administered after sonication.Citation16 If the previous hypothesis is correct, then pulsed HIFU should be able to be used for the enhancement of local drug delivery when there is a transient increase in capillary permeability. Therefore, the present study was performed using pulsed HIFU after EB injection in order to elevate extravasation of EB in the brain tumor tissue as much as possible. Furthermore, both focused and unfocused ultrasound exposure have previously been shown to produce a widening of intercellular gaps.Citation17,Citation18 Compared with the control tumors (), H&E staining showed pulsed HIFU exposure led to a widening of intercellular gaps and an increase in red blood cell extravasation (). Thus, the driving force behind the induction of BTB disruption by sonication may be increased drug extravasation due to presence of large gaps in the endothelium.Citation19
Many methods having been tested to facilitate drug delivery through the disruption of the BTB, but none have been practical when applied clinically. For instance, the BTB has been shown to be disrupted after an intracarotid artery injection of a hyperosmotic solution such as mannitol using boron neutron capture therapy. Nevertheless, due to restrictions on drug brain entry by the BTB and BBB, the selective delivery of a drug to individual brain tumor cells or the tissues surrounding brain tumors remains one of the major challenges to the boron neutron capture therapy of brain tumors, and many approaches have been reported.Citation20 Another study demonstrated that pulsed HIFU increased the uptake of antibody into surrounding tissue, but the net increase was marginal.Citation21 The current research has shown that completely noninvasive focal disruption of the BTB in the tumor and BBB in the adjacent normal brain tissue is possible through pulsed HIFU. indicates that BTB disruption induced by sonication causes a 2.23-fold increase in the tumor:ipsilateral brain ratio for EB in the target tumors compared with the control tumors. The results of this pilot study therefore suggest that the further evaluation of other treatment strategies is warranted – this may include multiple sonications to increase delivery of chemotherapeutic agents to larger brain tumors.
Acknowledgments
This study was supported by grants from the National Science Council of Taiwan (no NSC 100-2321-B-010-010 and NSC 99-2321-B-010-017), Cheng Hsin General Hospital Foundation (no 100F117CY25), Veterans General Hospitals University System of Taiwan Joint Research Program (#VGHUST100- G1-3-3 and V100E6-007), Yen Tjing Ling Medical Foundation (grant CI-100-17), Department of Health of Taiwan (DOH101-TD-PB-111-TM012 and DOH100-TD-C-111-007).
Disclosure
The authors report no conflicts of interest in this work.
References
- BremHMahaleyMSJrVickNAInterstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomasJ Neurosurg19917434414461993909
- FlorellRCMacdonaldDRIrishWDSelection bias, survival, and brachytherapy for gliomaJ Neurosurg19927621791831730945
- BlackKLNingarajNSModulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumorCancer Control200411316517315153840
- NeuweltEAImplications of the Blood-Brain Barrier and its ManipulationNew York, NYPlenum1989
- HaluskaMAnthonyMLOsmotic blood-brain barrier modification for the treatment of malignant brain tumorsClin J Oncol Nurs20048326326715208820
- KemperEMBoogerdWThuisIBeijnenJHvan TellingenOModulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours?Cancer Treat Rev200430541542315245774
- HsiehCChenYChenFEvaluation of pharmacokinetics of 4-borono-2-18F-fluoro-L-phenylalanine for boron neutron capture therapy in a glioma-bearing rat model with hyperosmolar blood–brain barrier disruptionJ Nucl Med200546111858186516269600
- AbbottNJRomeroIATransporting therapeutics across the blood-brain barrierMol Med Today1996231061138796867
- KrollRANeuweltEAOutwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other meansNeurosurgery199842510831099 discussion 1099–11009588554
- HynynenKMcDannoldNSheikovNAJoleszFAVykhodtsevaNLocal and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonicationsNeuroimage2005241122015588592
- YangFFuWChenWYehWLinWQuantitative evaluation of the use of microbubbles with transcranial focused ultrasound on blood-brain- barrier disruptionUltrason Sonochem200815463664317910929
- TreatLHMcDannoldNVykhodtsevaNZhangYTamKHynynenKTargeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasoundInt J Cancer2007121490190717437269
- FrenkelVLiKCPotential role of pulsed-high intensity focused ultrasound in gene therapyFuture Oncol20062111111916556078
- YangFYLiuSHHoFMChangCHEffect of ultrasound contrast agent dose on the duration of focused-ultrasound-induced blood-brain barrier disruptionJ Acoust Soc Am200912663344334920000948
- YangFFuWYangRLiouHKangKLinWQuantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruptionUltrasound Med Biol20073391421142717561334
- BekeredjianRKrollRFeinEUltrasound targeted microbubble destruction increases capillary permeability in hepatomasUltrasound Med Biol200733101592159817618040
- FrenkelVKimmelEIgerYUltrasound-induced intercellular space widening in fish epidermisUltrasound Med Biol200026347348010773379
- MesiwalaAHFarrellLWenzelHJHigh-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivoUltrasound Med Biol200228338940011978420
- BoucherYBaxterLTJainRKInterstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapyCancer Res19905015447844842369726
- ChenWMehtaSLuDSelective boron drug delivery to brain tumors for boron neutron capture therapyAdv Drug Deliv Rev1997262–323124710837545
- KhaibullinaAJangBSSunHPulsed high-intensity focused ultrasound enhances uptake of radiolabeled monoclonal antibody to human epidermoid tumor in nude miceJ Nucl Med200849229530218199622