?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Intravesical application of an anti-inflammatory drug is an efficient strategy for acute cystitis therapy. Quercetin (QU) is a potent anti-inflammatory agent; however, its poor water solubility restricts its clinical application. In an attempt to improve water solubility of QU, biodegradable monomethoxy poly(ethylene glycol)-poly(ɛ-caprolactone) (MPEG-PCL) micelles were used to encapsulate QU by self-assembly methods, creating QU/MPEG-PCL micelles. These QU/MPEG-PCL micelles with DL of 7% had a mean particle size of <34 nm, and could release QU for an extended period in vitro. The in vivo study indicated that intravesical application of MPEG-PCL micelles did not induce any toxicity to the bladder, and could efficiently deliver cargo to the bladder. Moreover, the therapeutic efficiency of intravesical administration of QU/MPEG-PCL micelles on acute cystitis was evaluated in vivo. Results indicated that QU/MPEG-PCL micelle treatment efficiently reduced the edema and inflammatory cell infiltration of the bladder in an Escherichia coli-induced acute cystitis model. These data suggested that MPEG-PCL micelle was a candidate intravesical drug carrier, and QU/MPEG-PCL micelles may have potential application in acute cystitis therapy.
Keywords:
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections, and Escherichia coli bacteria are the main cause of UTI.Citation1–Citation4 Bacterial infection of the bladder always leads to acute cystitis. Patients with acute cystitis account for 95% of all visits to physicians for UTIs. Commonly, patients with acute cystitis have symptoms of dysuria and increased frequency and urgency of urination, which greatly affect their quality of life.Citation5 In the past decades, great efforts have been made to develop efficient therapeutics to treat acute cystitis. Despite many patients with acute cystitis having benefited from these efforts,Citation6 acute cystitis therapy still poses great challenges.
Currently, acute cystitis is commonly treated by systemic application of antibiotics and anti-inflammation agents. However, only a small amount of systemically administered drugs can reach the bladder. Intravesical administration means directly instilling the drug solution into the bladder through a urethral catheter, ensuring maximum delivery of active ingredients to the bladder.Citation7–Citation10 In addition, intravesical drug administration has other potential benefits such as avoiding the first-pass metabolism. Compared with systemic drug administration, intravesical drug administration, as a local drug delivery method, can increase drug utilization and reduce systemic toxicity and side effects. Thus, intravesical application of therapeutics attracts some attention for cystitis therapy.
Quercetin (QU; 3,3,4,5,7-pentahydroxyflavone) is one of the most abundant flavonoids found in plants with biological effects; its molecular structure is presented in .Citation11,Citation12 To the authors’ knowledge, QU has a wide range of biological properties, including anti-viral,Citation13 anti-inflammatory,Citation14 anti-oxidative,Citation15 and anti-tumor activities.Citation16,Citation17 In recent years, the anti-inflammatory effect of QU has been well recognized,Citation18,Citation19 suggesting that QU as an anti-inflammatory drug has promising clinical application. Recently, it was found that QU can be used to prevent interstitial cystitis.Citation20 The authors presumed that QU might have potential application in intravesical therapy of acute cystitis, and set out to prove this hypothesis in this work. However, QU is water insoluble, and developing an aqueous formulation for QU remains difficult, greatly restricting the clinical application of QU. Therefore, it is necessary to develop an aqueous QU formulation as well as study the application of QU in depth in vivo.
Figure 1 Molecular structure of (A) quercetin and (B) monomethoxy poly(ethylene glycol)-poly(ɛ-caprolactone) copolymer.
Nanotechnology provides a novel platform for delivery of lipophilic drugs.Citation21,Citation22 Encapsulation of hydrophobic drugs into nanoparticles can render the drug completely dispersible in solution. Biodegradable polymeric nanoparticles are regarded as excellent candidates for drug-delivery systems.Citation23–Citation25 Poly(ɛ-caprolactone) (PCL)/poly(ethylene glycol) (PEG) copolymers are biodegradable, amphiphilic, and easy to produce, showing promising applications in drug-delivery systems.Citation26,Citation27 Monomethoxy PEG (MPEG)-PCL is a diblock PCL/PEG copolymer; its molecular structure is shown in . The authors have previously used MPEG-PCL micelles to deliver curcumin, creating a novel curcumin formulation with potential application in colon cancer therapy.Citation28 In an attempt to develop an aqueous formulation for QU, MPEG-PCL micelles were used to encapsulate QU, creating QU/MPEG-PCL micelles. The effect of intravesical application of QU/MPEG-PCL micelles on acute cystitis was then evaluated. These results suggest that QU/MPEG-PCL micelles are a new formulation of QU, with potential applications in bacterial cystitis therapy.
Materials and methods
Materials
Escherichia coli DH5α was saved in the laboratory and was cultured at 37°C on solid Luria-Bertani (LB) medium or in liquid LB medium. QU was purchased from Sigma-Aldrich (St Louis, MO). MPEG-PCL diblock copolymer with a designed molecular weight of 4000 was synthesized according to previous reports.Citation27,Citation28
Female BALB/c mice (8–10 weeks old) were purchased from the Laboratory Animal Center of Sichuan University (Chengdu, China). All studies involving mice were approved by the Institute’s Animal Care and Use Committee.
Preparation of QU/MPEG-PCL micelles
QU/MPEG-PCL micelles were prepared by a self-assembly method. Briefly, QU (7 mg) and MPEG-PCL diblock copolymer (93 mg) were co-dissolved in 6 mL of organic solvent (dichloromethane [DCM]: methanol = 2:1), followed by evaporation under reduced pressure in a rotary evaporator at 55°C. Normal saline (NS) (5 mL) was then added into the polymer and drug mixture, allowing the self-assembly of MPEG-PCL and QU, creating core-shell structured QU/MPEG-PCL micelles with core-encapsulated QU. The QU/MPEGPCL micelle solution was filtered using a syringe filter (pore size: 220 nm) (Millex-LG, Millipore Co, Billerica, MA) to remove the impurity and bacteria. Finally, the prepared QU/MPEG-PCL micelles were lyophilized and stored at 4°C.
Characterization of QU/MPEG-PCL micelles
The particle size and zeta potential of QU/MPEG-PCL micelles were determined by dynamic light scattering (DLS) (Malvern Nano-ZS 90; Malvern Instruments, Malvern, UK). The temperature was kept at 25°C during the measuring process. All results were the mean of three test runs.
The morphology of QU/MPEG-PCL micelles was observed under a transmission electron microscope (H-6009IV; Hitachi, Tokyo, Japan): micelles were diluted with distilled water and placed on a copper grid covered with nitrocellulose. Samples were negatively stained with phosphotungstic acid and dried at room temperature.
The concentration of QU was determined by high- performance liquid chromatography (HPLC) (Waters Alliance 2695; Waters, Milford, MA). The solvent delivery system was equipped with a column heater and a plus autosampler. Detection was carried out on a Waters 2996 detector. Chromatographic separations were performed on a reversed phase C18 column (4.6 × 150 mm-5μm, Sunfire Analysis column), with the column temperature kept at 35°C. Methanol–water (70/30, v/v) was used as eluent at a flow rate of 1 mL·min−1.
Drug loading (DL) and encapsulation efficiency (EE) of QU/MPEG-PCL micelles were determined as follows: Briefly, 10 mg of lyophilized QU/MPEG-PCL micelles were dissolved in 0.1 mL DCM and diluted with methanol. The amount of QU in the solution was determined by HPLC. Finally, the DL and EE of QU/MPEG-PCL micelles were calculated according to Equationequations (1)(1) and Equation(2)
(2) :
In vitro drug release
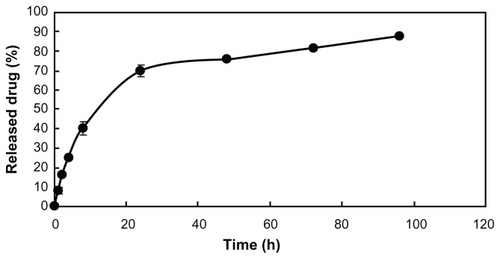
To determine the release kinetics of QU from QU/MPEG-PCL micelles, 0.5 mL of QU/MPEG-PCL micelle solution was placed in a dialysis bag (molecular weight cutoff, 3.5 kDa). Dialysis bags were incubated in 30 mL of phosphate buffer solution (pH 7.4) containing Tween® 80 (Sigma-Aldrich) (0.5% w/w) at 37°C with gentle shaking. At predetermined time points, the incubation medium was replaced with fresh incubation medium. The amount of released drug in the incubation medium was quantified by determining absorbance at 370 nm using HPLC. This study was repeated three times, and the result was expressed as mean value ± standard deviation (SD).
Analysis of the acute toxicity of MPEG-PCL micelles
Fifteen female BALB/c mice (8 weeks old) were used to evaluate the acute toxicity of MPEG-PCL micelles after intravesical application. These mice were divided into three groups (five mice per group): one group as the control did not receive any treatment, while the other two groups were treated with NS or MPEG-PCL micelles (50 mg/mL × 0.1 mL), respectively. Twenty-four hours after perfusion, all mice were killed by cervical vertebra dislocation. Bladders were immediately removed, weighted, and fixed in neutral buffer formalin (10%). The bladders were then paraffin-embedded, and sections were stained with hematoxylin and eosin (H&E). Moreover, the heart, liver, spleen, kidney, and lung of each mouse were also examined by H&E staining.
Distribution in vivo
Thirty-three female BALB/c mice (8 weeks old) were used to evaluate the distribution of MPEG-PCL micelles. These mice were divided into eleven groups (three mice per group).
Due to its specific fluorescence spectrum, coumarin-6 was used as a model drug to evaluate the capacity of MPEG-PCL micelles to deliver cargo to bladder. Coumarin-6/ MPEG-PCL micelles were prepared by a rotary evaporation method. Briefly, coumarin-6 (0.5 mg) and MPEG-PCL diblock copolymer (99.5 mg) were co-dissolved in 6 mL of DCM and evaporated to dryness under reduced pressure in a rotary evaporator. The dried films were hydrated in 5 mL of 0.9% NS.
The effect of the administration method on the bladder drug deposition of MPEG-PCL micelle-encapsulated drugs was studied in vivo. Coumarin-6/MPEG-PCL micelles (0.1 mL) were intravenously or intravesically administered into 8-week-old female BALB/c mice. At different time points (0.5, 1, 2, 4, and 6 hours) after administration, mice were killed by cervical vertebra dislocation, and their bladders were immediately harvested and examined by the curmarin-6-associated green fluorescence under a fluorescence light box (Lightools Research, Encinitas, CA). Moreover, to study the effect of administration method on the distribution of MPEG-PCL micelle-encapsulated drugs in vivo, the heart, liver, spleen, lung, and kidney of the mice were also harvested 2 hours after treatment and examined by the curmarin-6-associated green fluorescence under live image analysis instrument (Lightools Research). These experiments were repeated three times, and representative images were obtained.
Establishment of acute cystitis model
The acute cystitis model was established by intravesical application of bacterial strain E. coli DH5α into 8-week-old female BALB/c mice by transurethral catheterization as previously described.Citation29 E. coli DH5α was cultured at 37°C in 5 mL LB for 18 hours, then centrifuged for 1 minute at 10,000 rpm, washed with phosphate-buffered saline (PBS) three times and diluted to 2 × 108 CFU/mL. 50 μL of this suspension was used to infect each mouse with inoculums of ~1 × 107 CFU.
Treatment of acute cystitis by QU/MPEG-PCL micelles
Twenty 8-week-old female BALB/c mice were infected with E. coli DH5α (~1 × 107 CFU per mouse). Twenty-four hours later, the mice were divided into four groups. One group as the control did not receive any treatment, and the other groups were intravesically treated with NS (0.9%), MPEG-PCL micelle saline solution (700 μg/mL × 100 uL) and QU/ MPEG-PCL micelle saline solution (QU: 50 μg/mL × 100 uL), respectively. After 24 hours of treatment, the mice were killed by cervical vertebra dislocation. The bladder of each mouse was harvested and fixed with 10% buffered formaldehyde. The bladders were then embedded in paraffin, and at least four cross sections (5 μm thick) were taken from each bladder, followed by H&E staining.
Statistical analysis
Data were expressed as the mean ± SD. Statistical analysis was performed with one-way analysis of variance using SPSS Statistics (version 19.0.1; IBM Corporation, Somers, NY) software. P values less than 0.05 were considered to be statistically significant.
Results
Preparation and characterization of Qu/MPEG-PCL micelles
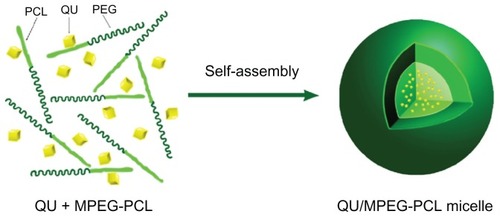
In an attempt to develop an aqueous formulation for QU, biodegradable MPEG-PCL micelles were used to encapsulate QU. QU/MPEG-PCL micelles were prepared through a self- assembly method, as schematically presented in . First, MPEG-PCL copolymer and QU were co-dissolved in a DCM and methanol mixture. Then, the organic solvent was evaporated under reduced pressure in a rotary evaporator, forming an MPEG-PCL and QU mixture. Finally, NS was added to the mixture, allowing the self-assembly of MPEG-PCL and QU in water. In the structure of MPEG-PCL, PEG is the hydrophilic segment, and PCL is the hydrophobic segment; thus, MPEG-PCL micelles always have a core-shell structure, with a PCL core and a PEG shell. The self-assembly of MPEG-PCL and QU created core-shell QU/MPEG-PCL micelles with core-encapsulated QU.
Figure 2 Preparation of QU-loaded MPEG-PCL nanoparticles. MPEG-PCL and QU were co-dissolved in organic solvent, followed by evaporation to dryness under reduced pressure in a rotary evaporator, creating QU and MPEG-PCL mixture (QU + MPEG-PCL).
Notes: The QU and MPEG-PCL mixture was then hydrated in 0.9% normal saline, allowing QU and MPEG-PCL to self-assemble into QU/MPEG-PCL micelles. This micelle has a core-shell structure (hydrophilic PEG shell and hydrophobic PCL core) with core-encapsulated QU.
Abbreviations: MPEG, monomethoxy poly(ethylene glycol); PCL, poly(ɛ-caprolactone); QU, quercetin.
The QU/MPEG-PCL micelles were characterized in detail. The QU/MPEG-PCL micelles had DL and EE of 6.9% and 98.1%, respectively. In , the particle size distribution spectrum of freshly prepared QU/MPEG-PCL micelles are presented. It was indicated that QU/MPEG-PCL micelles had a very narrow particle size distribution (polydispersity index [PDI] = 0.12), with a mean particle size of 34.1 ± 1.2 nm (determined by DLS). The zeta potential spectrum of QU/ MPEG-PCL micelles is presented in ; QU/MPEG-PCL micelles had a zeta potential of −0.269 mV. Moreover, the morphology of QU/MPEG-PCL micelles was studied by transmission electron microscopy (TEM), and the result is shown in . According to the TEM image, QU/MPEG-PCL micelles were spherical with a mean diameter of ~23 nm. TEM determined the size of dry particles, while the DLS determined the hydrodynamic diameter of particles in water. Because amphiphilic block polymeric micelles always have a loose structure in water, the particle size determined by DLS was always slightly larger than that determined by TEM.
Figure 3 Characterization of QU/MPEG-PCL micelles: (A) size distribution spectrum of QU/MPEG-PCL micelles; (B) zeta potential spectrum of MPEG-PCL micelles; (C) transmission electron microscopy image of QU/MPEG-PCL micelles; (D) the encapsulation of QU in MPEG-PCL/QU nanoparticles renders QU completely dispersible in aqueous media (a, water (pH = 7.0); b, QU in water (pH = 7.0, 2 mg/mL); c, QU/MPEG-PCL micelles in water (pH = 7.0, 2 mg/mL).
Abbreviations: MPEG, monomethoxy poly(ethylene glycol); PCL, poly(ɛ-caprolactone); QU, quercetin.
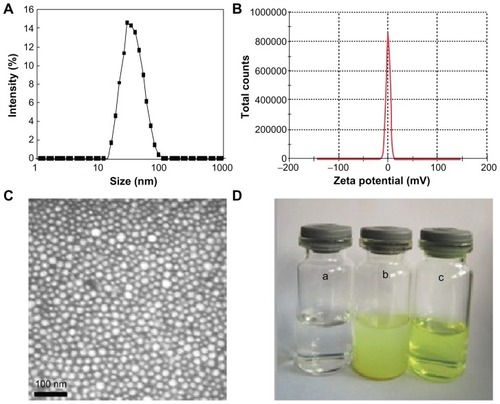
One of the major purposes of the encapsulation of QU in MPEG-PCL micelles was to make QU completely dispersible in aqueous media. The appearance of QU/MPEG-PCL micelle aqueous solution is shown in . QU cannot be dissolved in pure water, as confirmed by the observation of a turbid yellow slurry. In contrast, QU/MPEG-PCL micelle solution with an equivalent quantity of QU was transparent, indicating full dispersibility of QU in water.
The release profile of QU/MPEG-PCL micelles in vitro was studied using a dialysis method. As shown in , QU was released from QU/MPEG-PCL micelles over an extended period.
Analysis of toxicity of MPEG-PCL micelles
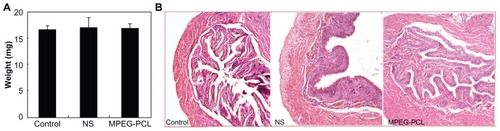
In this work, the authors attempted to treat acute cystitis by intravesical application of MPEG-PCL micelle-encapsulated QU. Thus, the toxicity of intravesically applied MPEG-PCL micelles was evaluated before treatment experiments. As shown in , intravesical application of MPEG-PCL micelles (50 mg/mL × 0.1 mL) did not significantly induce bladder weight change (control: 16.604 ± 0.739 mg; NS: 17.032 ± 1.846 mg; MPEG-PCL: 16.874 ± 0.780 mg), which implied that the intravesical application of MPEG-PCL micelles did not significantly induce bladder edema. Moreover, pathological section of bladder in each treatment group is shown in . We found that intravesical application of MPEG-PCL micelles did not induce any pathological changes to the bladder. Meanwhile, intravesical application of MPEG-PCL micelles did not cause any toxicity to the heart, liver, spleen, lung, or kidney (data not shown). These results suggest that MPEG-PCL micelles may be used as drug carriers for intravesical drug application.
Figure 5 Acute toxicity of intravenously administered MPEG-PCL micelles (50 mg/mL × 0.1 mL) to bladder. (A) The weight of bladders in different treatment groups (P > 0.05); (B) hematoxylin and eosin assay of bladders in different treatment groups.
Note: Little difference was observed among these groups, which indicated that intravesical application of MPEG-PCL had little toxicity to bladder.
Abbreviations: MPEG, monomethoxy poly(ethylene glycol); NS, normal saline; PCL, poly(ɛ-caprolactone).
Distribution in vivo
The tissue distribution of MPEG-PCL micelle-encapsulated drug after intravenous or intravesical application was studied. shows that green fluorescence appeared in the heart, lung, liver, and kidney 2 hours after intravenous injection, implying drug deposition in these tissues. However, very little green fluorescence appeared in the heart, lung, liver, kidney, and spleen after intravesical administration of courmarin-6/MPEG-PCL, suggesting that little drug can reach these tissues after intravesical administration. Thus, for bladder diseases therapy, intravesical application of therapeutics may cause lower systemic toxicity compared with intravenous application.
Figure 6 Tissue distribution of MPEG-PCL micelle-delivered cargo after intravenous (I.v.) or intravesical (I.ves.) application. The drug-associated green fluorescence indicated the deposition of drugs. (A) The fluorescence image of organs from mice 2 hours after intravenous or intravesical application of courmarin-6/MPEG-PCL micelles. (B) Fluorescence images of bladders from mice with intravenous or intravesical application of courmarin-6/MPEG-PCL micelles at different time points.
Abbreviations: MPEG, monomethoxy poly(ethylene glycol); PCL, poly(ɛ-caprolactone).
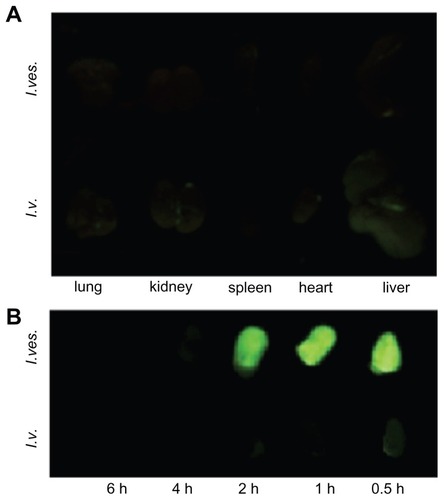
The efficiency of MPEG-PCL micelles delivering cargo to bladder was studied after intravenous or intravesical application (see ). With intravesical application of courmarin-6/MPEG-PCL micelles, the bladders emanated strong courmarin-6-associated fluorescence, implying the high drug deposition in the bladder. With intravenous application of courmarin-6/MPEG-PCL micelles, the bladders only had slight green fluorescence, suggesting the low efficiency of delivering cargo to bladder. Meanwhile, the residence time of green fluorescence in the bladder with intravesical administration was obviously longer than that with intravenous application. Thus, for bladder diseases therapy, intravesical application of therapeutics may induce higher efficiency of delivering cargos to bladder compared with intravenous application.
Treatment of acute cystitis
The anti-inflammatory effect of MPEG-PCL micelle-encapsulated QU in the acute cystitis model was assessed. As shown in , the weight of bladders in the QU/MPEG-PCL micelle treatment group was 20.50 ± 1.44 mg, while the weight of bladders in the NS and empty MPEG-PCL micelle treatment group were 43.26 ± 1.42 mg and 41.42 ± 1.68 mg, respectively. Compared with NS treatment, QU/MPEG-PCL micelle treatment significantly reduced the weight of bladders with severe inflammation (P < 0.05), but empty MPEG-PCL micelle treatment did not reduce the weight of bladders with severe inflammation (P > 0.05). This indicated that QU-loaded MPEG-PCL micelles effectively inhibited development of acute cystitis compared with NS, while empty MPEG-PCL micelles did not show any anti-inflammatory activity on acute cystitis.
Figure 7 Therapeutic effects of intravesical application of QU/MPEG-PCL micelles on acute cystitis. (A) The weight of bladders in each treatment group. The weight of the bladder in the QU/MPEG-PCL group was less than that in the MPEG-PCL or NS group (P < 0.05). (B) Representative images of the bladders in each treatment group. The bladder in the QU/MPEG-PCL group was smaller than that in the NS group, while the bladder in the MPEG-PCL group was as large as that in the NS group. (C) Hematoxylin and eosin assay of the bladders from mice with Control, NS, MPEG-PCL, or Qu/MPEG-PCL treatment.
Notes: Severe edema and lots of inflammatory cell infiltration were observed in the bladders from both the empty MPEG-PCL micelle group and the NS group, indicating that MPEG-PCL had no effect on the treatment of cystitis. However, in the QU/MPEG-PCL treatment group, no obvious edema and inflammatory cell infiltration of the bladder was detected, suggesting that QU/MPEG-PCL micelles have promising application in acute cystitis therapy.
Abbreviations: MPEG, monomethoxy poly(ethylene glycol); NS, normal saline; PCL, poly(ɛ-caprolactone); QU, quercetin.
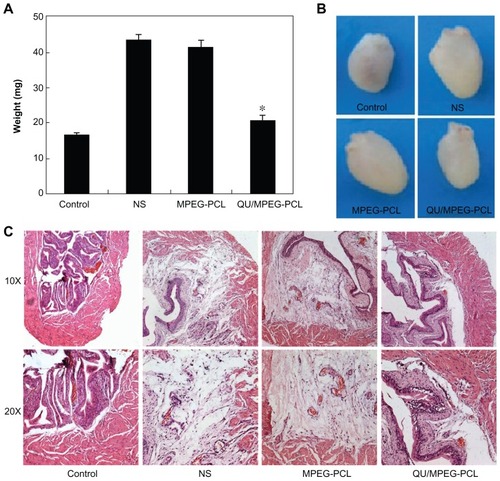
In , the appearance of the bladders in each treatment group is presented. The bladder in the QU/MPEG-PCL group was obviously smaller than that in the NS group, while the bladder in the empty MPEG-PCL micelle group was as large as that in the NS group. This indicated that the edema of the bladder efficiently lessened with QU/MPEG-PCL treatment.
In , the pathological section of bladders in each treatment group is provided. that the figure shows that the bladders in both the empty MPEG-PCL micelle group and the NS group had severe edema and lots of inflammatory cell infiltration. However, in the QU/MPEG-PCL treatment group, the bladder did not have obvious edema and inflammatory cell infiltration. These results suggest that the QU/MPEG-PCL micelles have promising application in acute cystitis therapy.
Discussion
In this work, QU/MPEG-PCL micelles of uniform size (size, 34.1 nm; PDI, 0.12) and high encapsulation rate (>98%) successfully solved the problem of poor water solubility of QU (). These QU/MPEG-PCL micelles could slowly release QU in vitro (). Moreover, QU/MPEG-PCL micelles were used to treat acute cystitis, and it was found that intravesical application of MPEG-PCL micelles did not induce any toxicity to the bladder, and intravesical administration of QU/MPEG-PCL micelles efficiently reduced the inflammation of the bladder with E. coli-induced acute cystitis.
Patients with acute cystitis always have symptoms of dysuria and increased frequency and urgency of urination, which seriously affect their quality of life. The incidence of acute cystitis is high, and the course of acute cystitis is urgent. If acute cystitis cannot be treated in a timely manner, it will be transformed into chronic cystitis. It can also be transformed into cystitis glandularis, and finally into bladder cancer. It can also induce nephritis. Therefore, timely treatment of acute cystitis is necessary. Acute cystitis therapy has received special attention in the past decades.Citation30 The urinary bladder is an ideal organ for regional therapy. The urethra provides easy access of the therapeutic agents to the bladder. Moreover, this current work has proved that intravesical drug application can more efficiently deliver drugs to the bladder, with minimal side effects compared with intravenous application. Therefore, intravesical application of drugs may be an acceptable strategy for acute cystitis therapy. However, this method may have a weakness: intravesical instillation of drugs into the bladder is inconvenient.
Nanotechnology has a wide application in medicine, with the goal of resolving some medical challenges. One of the most important applications of nanotechnology in medicine is using nanoparticles to deliver hydrophobic drugs to create aqueous formulations for these water soluble-poor drugs. QU has great prospects in the treatment of inflammation,Citation31 but it is limited in its medical application due to poor water solubility. To address this challenge, the use of liposomes to encapsulate QU has been described.Citation32 Recently, MPEG-PCL micelles were used to load curcumin and honokiol,Citation28,Citation33 successfully solving their water solubility. In this paper, MPEG-PCL micelles were used to encapsulate QU with the goal of improving the water solubility of QU and creating a novel formulation for QU. Previously, MPEG-PCL micelles had been used to deliver drugs followed by intravenous, subcutaneous, intraperitoneal, or intratumoral application. In this work, MPEG-PCL micelles were used to deliver drugs to the bladder by intravesical application. The results suggested that intravesical application of MPEG-PCL micelle-encapsulated hydrophobic drugs can efficiently deliver cargo to the bladder.
Application of anti-inflammation agents is a commonly used protocol for acute cystitis therapy. QU is a well recognized anti-inflammation agent.Citation31 In this paper, QU was used to treat acute cystitis. Results indicated that QU/MPEG-PCL micelle treatment can efficiently reduce the edema and inflammatory cell infiltration of the bladder in an E. coli-induced acute cystitis model. These data proved the hypothesis that QU had potential application in acute cystitis therapy. To the authors’ knowledge, this work may be the first attempt to reveal the potential application of QU in acute cystitis therapy.
Overall, nanotechnology provides a novel method for improving the water solubility of QU. Intravesical application of MPEG-PCL micelle-encapsulated QU has potential application in acute cystitis therapy.
Conclusion
In this paper, the nanoassemblies of QU and MPEG-PCL were studied, creating a novel QU/MPEG-PCL micelle, which is a new and interesting formulation of QU. MPEG-PCL micelles may be a safe and efficient intravesical drug-delivery system for hydrophobic drugs. Intravesical administration of MPEG-PCL micelle-encapsulated QU can efficiently reduce the edema and inflammatory cell infiltration of bladders with acute cystitis, showing great potential in acute cystitis therapy.
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (20110181120087), National Key Basic Research Program (973 Program) of China (2011CB910703), National Natural Science Foundation (NSFC30801294) and Huoyingdong Youth Fund from Ministry of Education (No 122030).
Disclosure
The authors report no conflicts of interest in this work.
References
- FoxmanBThe epidemiology of urinary tract infectionNat Rev Urol2010765366021139641
- O’NeillLABacteria fight back against Toll-like receptorsNat Med20081437037218391934
- DanielsIRZamanSRIncreasing prevalence of antimicrobial resistance among uropathogensJAMA1999282432532610432024
- StammWEHootonTMManagement of urinary tract infections in adultsN Engl J Med1993329132813348413414
- FerrySBurmanLGHolmSEClinical and bacteriological effects of therapy of urinary tract infection in primary health care: relation to in vitro sensitivity testingScand J Infect Dis1988205355443065927
- SeptimusEJKuperKMClinical challenges in addressing resistance to antimicrobial drugs in the twenty-first centuryClin Pharmacol Ther20098633633919571803
- EvansRJIntravesical therapy for overactive bladderCurr Urol Rep2005642943316238916
- BuyseGWaldeckKVerpoortenCBjörkHCasaerPAnderssonKEIntravesical oxybutynin for neurogenic bladder dysfunction: less systemic side effects due to reduced first pass metabolismJ Urol19981608928969720583
- WalkerMCMastersJRParrisCNHepburnPJEnglishPJIntravesical chemotherapy: in vitro studies on the relationship between dose and cytotoxicityUrol Res19861431371403092427
- LeeHCimaMJAn intravesical device for the sustained delivery of lidocaine to the bladderJ Control Release201114913313920971144
- MantheyJABiological properties of flavonoids pertaining to inflammationMicrocirculation200072934
- MoskaugJØCarlsenHMyhrstadMBlomhoffRMolecular imaging of the biological effects of quercetin and quercetin-rich foodsMech Ageing Dev200412531532415063108
- OhnishiEBannaiHQuercetin potentiates TNF-induced antiviral activityAntiviral Res19932243273318279819
- BootsAWWilmsLCSwennenELKleinjansJCBastAHaenenGRIn vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteersNutrition2008247–870371018549926
- MieanKHMohamedSFlavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plantsJ Agric Food Chem2001493106311211410016
- WeiYQZhaoXKariyaYFukataHTeshigawaraKUchidaAInduction of apoptosis by quercetin: involvement of heat shock proteinCancer Res199454495249578069862
- ChengSGaoNZhangZQuercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of BaxClin Cancer Res2010165679569121138867
- García-MediavillaVCrespoIColladoPSThe anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cellsEur J Pharmacol20075572–322122917184768
- HämäläinenMNieminenRVuorelaPHeinonenMMoilanenEAnti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-B activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-B activation along with their inhibitory effect on iNOS expression and NO production in activated macrophagesMediators Inflamm200720074567318274639
- KatskeFShoskesDASenderMPoliakinRGaglianoKRajferJTreatment of interstitial cystitis with a quercetin supplementTech Urol200171444611272677
- ZhangLChanJMGuFXself-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platformACS Nano200821696170219206374
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol200721275176018654426
- ShutavaTGBalkundiSSVangalaPLayer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenolsACS Nano200931877188519534472
- ShiehYAYangSJWeiMFShiehMJAptamer-based tumor-targeted drug delivery for photodynamic therapyACS Nano201041433144220166743
- WangTHeNPreparation, characterization and applications of low-molecular- weight alginate-oligochitosan nanocapsulesNanoscale2010223023920644799
- ZhouSDengXYangHBiodegradable poly (ɛ-caprolactone)- poly (ethylene glycol) block copolymers: characterization and their use as drug carriers for a controlled delivery systemBiomaterials2003243563357012809785
- GouMWeiXMenKPCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent deliveryCurr Drug Targets2011121131115021443476
- GouMMenKShiHCurcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivoNanoscale201131558156721283869
- MulveyMALopez-BoadoYSWilsonCLInduction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coliScience1998282149414979822381
- Flores-CarrerasOMartínez-EspinozaCJGonzález-RuizMIExperience in the treatment of interstitial cystitis: review of 17 casesGinecol Obstet Mex201179312513021966793
- OvermanAChuangCCMcIntoshMQuercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned mediaInt J Obes (Lond)2011351165117221224828
- YuanZPChenLJFanLYLiposomal quercetin efficiently suppresses growth of solid tumors in murine modelsClin Cancer Res200612103193319916707620
- GouMZhengXMenKSelf-assembled hydrophobic honokiol loaded MPEG-PCL diblock copolymer micellesPharm Res20092692164217319568695