Abstract
Objective
Sensorineural hearing loss leads to the progressive degeneration of spiral ganglion cells (SGC). Next to postoperative fibrous tissue growth, which should be suppressed to assure a close nerve–electrode interaction, the density of healthy SGC is one factor that influences the efficiency of cochlear implants (CI), the choice of treatment for affected patients. Rolipram, a phosphodiesterase-4 inhibitor, has proven neuroprotective and anti-inflammatory effects and might also reduce SGC degeneration and fibrosis, but it has to pass the cellular membrane to be biologically active.
Methods
Lipidic nanocapsules (LNC) can be used as biodegradable drug carriers to increase the efficacy of conventional application methods. We examined the biological effects of rolipram and LNC’s core encapsulated rolipram on SGC and dendritic cell (DC) tumor necrosis factor-α (TNF-α) production in vitro and on SGC survival in systemically-deafened guinea pigs in vivo.
Results
Our results prove that rolipram does not have a beneficial effect on cultured SGC. Incorporation of rolipram in LNC increased the survival of SGC significantly. In the DC study, rolipram significantly inhibited TNF-α in a dose-dependent manner. The rolipram-loaded LNC provided a significant cytokine inhibition as well. In vivo data do not confirm the in vitro results.
Conclusion
By transporting rolipram into the SGC cytoplasm, LNC enabled the neuroprotective effect of rolipram in vitro, but not in vivo. This might be due to dilution of test substances by perilymph or an inadequate release of rolipram based on differing in vivo and in vitro conditions. Nevertheless, based on in vitro results, proving a significantly increased neuronal survival when using LNC-rolipram compared to pure rolipram and pure LNC application, we believe that the combination of rolipram and LNC can potentially reduce neuronal degeneration and fibrosis after CI implantation. We conclude that rolipram is a promising drug that can be used in inner ear therapy and that LNC have potential as an inner ear drug-delivery system. Further experiments with modified conditions might reveal in vivo biological effects.
In industrialized nations, sensorineural hearing loss is a common disease and is usually caused by a primary loss of auditory hair cells, which are the inner ear’s sensory cells. In addition to hereditary factors, acquired causes, such as increased noise exposure or the application of ototoxic drugs, play a crucial role in the development of sensorineural hearing loss.Citation1,Citation2
An effective treatment for damaged hair cells or for the regeneration of already degenerated sensory cells of the inner ear in humans is not yet possible. Instead, the prosthetic forms of therapy are performed to replace the damaged organ’s function. Severely impaired and deaf patients are treated by implantation of electronic inner ear prostheses called cochlear implants. Deaf patients treated with cochlear implants gain a renewed sense of hearing and have a greater opportunity to communicate using audible speech.Citation3 This leads to improved social integration and a significant increase in the patient’s quality of life.
Cochlear implants use a multichannel electrode that is inserted into the scala tympani to replace the function of the missing hair cells. Through the formation and transmission of spiral ganglion cell (SGC) action potentials, an artificially-generated sound impression is achievable. However, following the primary loss of hair cells, a progressive decrease in SGC density is observed.Citation4 This is caused by missing electrical and neurotrophic stimulation, as well as other forms of stimulation.Citation5,Citation6 To achieve better speech recognition with cochlear implants, a high number of SGC must be ensured.Citation6
A large number of neurotrophic factors have a protective effect on SGC after primary hair cell loss. The systemic treatment of inner ear diseases is complicated by the presence of the blood–cochlear barrier. Adequate drug levels are only reached through the application of very high drug doses, which leads to an increased risk of side effects. Through local application of neurotrophic factors, the progressive degeneration of SGC may be decreased. Important representatives of this substance group are brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor, and fibroblast growth factor.Citation7,Citation8 Nevertheless, continuous therapy is necessary because previous studies detected a progressive reduction of SGC after the cessation of therapeutical application of BDNF.Citation9 The first attempts to produce continuous drug-delivery systems were realized using osmotic pumps. These pumps allowed continuous application periods into the inner ear for weeks. Because of delivery time limitations and the necessity of pump changes that would allow for longer application duration, this approach is not feasible for human therapeutic applications.
Another option for chronic neurotrophic treatment is gene therapy. By introducing the gene sequences of neurotrophic factors to the cellular nucleus, the transfected cells may provide a sustainable endogenous production of neuroprotective substances.Citation10,Citation11 Via viral gene transfer, these factors were already implemented in the inner ear, although an expression of the introduced sequences did not demonstrate cell-specific gene transfer.Citation12,Citation13 Furthermore, only a temporary expression of about 3 weeks was achieved, which is not desirable in terms of long-term therapy.Citation14 In addition, the use of viral vectors has considerable disadvantages, such as potential infection and the ability to trigger inflammatory or immunological processes.Citation15
In addition to the viral vectors, artificially manufactured nanoparticles offer a promising ability to transport drugs and gene sequences with the ability of cargo release over a long period of time. Today, a variety of manufactured nanoparticles, which consist of organic and inorganic parts, can be assembled. In addition to their common uses in industry, a wide range of applications in health and medicine are achievable using nanoparticles.Citation16 The advantages of nanoparticles are the impossibility of infection and the production of biodegradable scaffolds, for which a low response of the immune system is expected. Furthermore, nanoparticles can be produced inexpensively. By considering the property of the production of biological building principles, nanoparticles are ideal for the transport of medical drugs or genes. In addition to the transport function, it is possible to modify the surface of nanoparticles with specific receptors so that they will selectively bind to specific cells. The use of nanoparticles as a targeted treatment of specific cell populations results in a significant reduction of side effects.Citation16
Recent research proved that fluoresceine-5-isothiocyanate (FITC)-labeled lipidic nanocapsules (LNC), locally inserted into the scala tympani of guinea pigs, infiltrated cell populations of the inner ear without causing functional or anatomical damage. Citation17 Those data were the first to demonstrate the ability of drug-unloaded LNC to enter inner ear cells. However, LNC-mediated drug delivery was not examined in that study.
Besides the density of healthy SGC, the optimal effectiveness of cochlear implants is influenced by another important factor. In the postoperative onset of foreign body reaction, the organism encapsulates the implant through the production of fibrous tissue growth. This results in decreased nerve– electrode interaction, increased spread of neuronal excitation, reduced frequency selectivity, and increased impedances.Citation18
Rolipram, an intracellular-acting phosphodiesterase-4 inhibitor, showed neuroprotective properties in former studies.Citation19,Citation20 Furthermore, anti-inflammatory effects have also been proven in animal models.Citation21 We hypothesize that rolipram can potentially protect SGCs and reduce inflammatory reactions. If applied systemically, intravenously, or orally, rolipram has a bioavailability of 74%–77%.Citation22 However, due to the blood–brain barrier, systemically-applied drugs that are used for inner ear treatment must be administered in such high dosages to have a biological effect on their target cells in the inner ear, that side effects may occur. To avoid massive side effects, local treatment of the inner ear is favored. In order to decrease the drugs applied locally to the inner ear, a targeted drug delivery is preferable. By encapsulating rolipram into a vehicle, such as LNC, a targeted drug delivery may be realized in the future. This will decrease the amount of drugs that are needed for human therapy. In the inner ear, a targeted drug delivery will lead to a significant dosage reduction and, as a result, to a lower risk of side effects when compared to systemic treatment. Additionally, the lower dose is also economically beneficial. We hypothesize that LNC provide a feasible drug-delivery system that can be used to transport rolipram into the cytoplasm. Once in the cytoplasm, rolipram will be released and induce its biological effects.
This study examines the effect of rolipram and rolipram encapsulated in LNC on the survival rate, soma diameter, and neurite length of neonatally-harvested SGC in order to determine whether the drug’s effect is increased using LNC as a drug-delivery system.
Furthermore, the effect of rolipram and the combination of LNC and rolipram is evaluated on the tumor necrosis factor-α (TNF-α) secretion of lipopolysaccharide (LPS)- stimulated dendritic cells (DC). This experiment indirectly provides information about the release of the payload and may demonstrate the potential of LNC as a delivery system for rolipram. Additionally, the results provide further insight into the anti-inflammatory effect of rolipram. This effect is due to the key role that TNF-α plays in the proinflammatory processes, which is appreciable in reducing postoperative reactions on cochlear implants.
Based on in vitro results, we performed experiments on systemically deafened guinea pigs in order to examine LNC’s suitability for rolipram delivery in vivo.
Materials and methods
Lipidic nanocapsules
In order to formulate LNC, the laboratory “Ingénierie de la Vectorisation Particulaire”, University of Angers (Angers, France) used a novel phase inversion-based method.Citation23 The nanoparticles, which consisted of a liquid core, were surrounded by a cohesive surfactant layer at the oil-aqueous interface (). The lipophilic Labrafac® WL 1349 (Gattefossé SA, Saint-Priest, France), which is comprised of capric and caprylic acid triglycerides, was used to obtain the oily phase of the particles, whereas the aqueous phase included NaCl (Prolabo, Fontenay-sous-Bois, France) that was dissolved in Milli-Q-plus® purified water (Millipore, Billerica, MA). Hence, the LNC preparation required two tensioactive surfactants: Lipoïd® S75-3 (Lipoïd GmbH, Ludwigshafen, Germany), a soybean lecithin made of phosphatidylcholine (69%), phosphatidylethanolamine (10%), and other phospholipids, and Solutol® HS 15 (BASF, Ludwigshafen, Germany), a composition of free polyethylene glycol (PEG) 660 and PEG 660-hydroxystearate. In order to prepare LNC with a mean diameter of 50 nm, the compounds were used in the following amounts: 1.028 g Labrafac®, 0.075 g Lipoïd®, 0.846 g Solutol®, 0.089 g NaCl, and 2.962 g pure water. Magnetic stirring (200 rpm) at room temperature resulted in an oil-in-water emulsion of all compounds. A progressive heating rate of 4°C/minute obtained the inverted phase (water-in-oil) at a temperature of 85°C, with a phase inversion zone of approximately 70°C. This phase inversion zone corresponded to a microemulsion state and three temperature cycles, ranging from 60°C to 85°C, were performed to ensure a stable oil-in-water emulsion. The LNC formulation was accomplished using a fast cooling-dilution process during the phase inversion zone, with 12.5 mL cold water in a ratio of 1:2.5. The LNC that were obtained had a diameter of 50 nm. For this purpose, the dilution was stirred for 5 minutes at 2°C.
Figure 1 (A) Lipidic nanocapsule; (B) lipidic nanocapsule labeled with FITC, and (C) lipidic nanocapsule with rolipram encapsulation and FITC label.
Abbreviations: DSPE-PEG2000-amino, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(PEG)2000]; FITC, fluorescein isothiocyanate.
![Figure 1 (A) Lipidic nanocapsule; (B) lipidic nanocapsule labeled with FITC, and (C) lipidic nanocapsule with rolipram encapsulation and FITC label.Abbreviations: DSPE-PEG2000-amino, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(PEG)2000]; FITC, fluorescein isothiocyanate.](/cms/asset/abf46bef-cea1-4c7e-9a0e-bce0988d4903/dijn_a_29712_f0001_c.jpg)
To encapsulate rolipram (Sigma-Aldrich, Lyon, France), the drug was added before starting the three temperature cycles at a concentration of 1 mg/mL in Labrafac®.
The LNC surface was modified using a 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(PEG)2000] (DSPE-PEG2000-amino) post-insertion process. 1.75 mL LNC (1017 particles/mL) were incubated with a 1.25 mL aqueous micellar solution (15 mg/mL) of DSPE-PEG2000- amino (Avanti®; Polar Lipids Inc, Alabaster, AL) for 90 minutes at 60°C.Citation24 The obtained suspension was vortexed every 15 minutes and quenched in ice water for 60 seconds.
FITC-labeling of the LNC was performed with FITC () purchased from Sigma-Aldrich. 1 mL of FITC (2 mg/mL) was added to the 2.95 mL LNC suspension. By adding an appropriate amount of 0.1 M Na3PO4, the pH was increased to 9. The sample, which was placed in a water bath and protected from light, was stirred at a temperature of 37°C for 45 minutes before being cooled down in an ice bath for 3 minutes to stop the process.
In order to separate the free FITC from the labeled LNC size, exclusion chromatography on a Sephadex G-25 column (Pharmacia, Uppsala, Sweden) was executed. Fluorescence spectroscopy (FITC detection), as well as turbidity at 680 nm (LNC detection), was measured for each fraction. Integrity control of the LNC was performed by size measuring using a dynamic light scattering (DLS) procedure on the fractions that contained the particles.
DLS analysis and electrokinetic measurements indicated a hydrodynamic diameter of 52 ± 5 nm and a ζ-potential of −50 ± 10 mV. Finally, the concentration of the LNC solution was 4 × 1015 particles/mL.
For rolipram loading in the LNC, as well as for the modification by FITC (), the purification step was modified. After the grafting step, the mixture was dialyzed (MWCO 15 kDa) against pure water over night and the concentration was adjusted so as to obtain 4 × 1015 particles/mL. Hydrodynamic diameter and ζ-potential were checked and were found to be comparable with those obtained by LNC without rolipram.
In vitro experiments
Spiral ganglion cell culture
The dissociation of SGC from 3–5-day-old Sprague–Dawley rats was performed in accordance with the German law on protecting animals (§4/03). Before cell preparation, 96-multiwell culture plates (Nunc GmbH, Wiesbaden, Germany) were sequentially coated with polyornithine (Sigma, St, Louis, MO) and laminin (Invitrogen, Karlsruhe, Germany).Citation25 A total number of 1.5 × 104 cells were seeded per well in 100 μL of modified panserin-based media. Panserin (15 mL) was supplemented with 375 μL 1 M HEPES (Sigma, Steinheim, Germany), 275 μL phosphate-buffered saline (PBS; Invitrogen), 225 μL penicillin (Grünenthal, Aachen, Germany), 82.5 μL glucose (30% in PBS), 35 μL insulin (Invitrogen), and 15 μL N2-supplement (Invitrogen) to prepare the media. The following experimental groups were performed in different concentrations: LNC labeled with FITC (LNC– FITC), rolipram (rolipram; TOCRIS Bioscience, Ellisville, MO), and LNC–FITC with rolipram (LNC–FITC–rolipram). BDNF (50 ng/mL; R&D Systems, Wiesbaden, Germany) was used as a positive control.
Rolipram was used in concentrations of 38 ng (1:50 dilution, n = 20), 19 ng (1:100 dilution, n = 20), 9.5 ng (1:200 dilution, n = 20), and 6.3 ng (1:300 dilution, n = 20) per 0.1 mL medium. The stock solution of LNC contained 45 mg/mL (4.2 × 1015 LNC/mL) FITC-labeled LNC and 19 μg/mL rolipram, respectively. The used dilutions contained 0.9 mg/mL LNC (1:50 dilution), 0.45 mg/mL LNC (1:100 dilution), 0.225 mg/mL LNC (1:200 dilution), and 0.15 mg/mL LNC (1:300 dilution).
After 48 hours cultivation time at 37°C and 5% CO2, cells were fixed with 1:1 methanol/acetone. Immunochemical staining with 200-kD neurofilament antibodies (Vectastain ABC Kit ELITE Mouse IgG; Vector Laboratories, Burlingame, CA) enabled a clear identification of SGC. Untreated SGC, cultured with supplemented panserin only, served as a negative control. In order to determine the absolute number of seeded SGC, the seeding control was fixed after 4 hours. The mean of positive 200-kD-stained cells of the seeding control was set as 100%.
Vital SGC were defined as positive-stained neurons that showed a neurite outgrowth of at least three soma diameters. From five different fields of view within each well, one neuron was selected for the purpose of measuring the length of the neuron and the neurite as described previously.Citation26 Cell survival rate was determined by the number of vital neurons in correlation to the seeding control data.
SGC culture: investigation of LNC–FITC neuroprotective effect
The first SGC in vitro test revealed the neuroprotective potential of the LNC–FITC nanoparticles (), even though they were not loaded with the payload rolipram. A second test was performed to verify whether the nanoparticle itself or the fluorescein FITC had a positive effect on the SGC survival of the drug-unloaded LNC–FITC particles.
LNC–FITC nanoparticles were added to the SGC culture as described above in three different concentrations (1:100, 1:200, 1:300 dilution of stock solution of 45 mg/mL LNC). LNC Blank particles (LNC without FITC labeling) were added in an analogous procedure. Furthermore, BDNF 50 ng/mL-treated cells were used as a positive control. The cell culture medium of the seeding and the negative control remained untreated.
SGC were seeded in a mean density of 1.5 × 104 (247.3 ± 59.8 SGC in seeding control [mean ± standard deviation]; n = 6). In three independently performed experimental settings (plates), each group included three wells per setting.
Dendritic cell culture
DCs were generated from femoral BALB/c mice (Charles River, Sulzfeld, Germany) bone marrow, according to an approved protocolCitation27 and in accordance with the German law on protecting animals (§4/03). The bone marrow was flushed with 5 mL 4°C PBS out of the femur and centrifuged for 10 minutes at 290 g at 4°C. The supernatant was discarded to resuspend the cell pellet with RPMI 1640 (Biochrom, Berlin, Germany), which was supplemented with 10% fetal calf serum (FCS) and 50 μmol/L 2-mercaptoethanol (Sigma, Deisenhofen, Germany). A total amount of 2.5 × 106 cells was added to 10 mL supplemented RPMI in a petri dish (Cell+; Sarstedt, Nümbrecht, Germany). Before cultivation at 37°C and 5% CO2, 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D System, Minneapolis, MN) was added. On day 3, 10 mL of fresh medium containing 20 ng/mL GM-CSF was added. On day 6 and 8, 10 mL of the culture media was removed and centrifuged to harvest the included cells. The cell pellet was resuspended in 10 mL of fresh media, which was supplemented with 20 ng/mL GM-CSF. On day 10, a number of 5 × 104 cells were seeded in 200 μL RPMI-media per well on a 96-multiwell plate. Rolipram (Tocris Bioscience), LNC–FITC–rolipram, LNC–FITC, and LNC–Blank were added in four concentrations (10 μM, n = 12; 1 μM, n = 12; 0.1 μM, n = 12; 0.01 μM, n = 12). One hour after incubation with the test substances, DCs were stimulated with LPS to induce TNF-α production. After 24 hours, the supernatant was collected and the TNF-α concentration was measured using ELISA (R&D Systems, Minneapolis, MN).
In vivo study
Experimental animals
Female nonpigmented Dunkin Hartley guinea pigs (Charles River GmbH, Sulzfeld, Germany), weighing between 330–460 g, were used. After confirmation of physiological hearing via acoustically evoked auditory brainstem response (AABR) measurement on day 0, the animals were systemically deafened. On day 7, in order to confirm deafness, AABR measurement was repeated and animals were treated with LNC–FITC (n = 7), LNC–FITC–rolipram (n = 6), rolipram (n = 7), or PBS (n = 6; control group) via unilateral cochleostomy. Contralateral PBS was applied as a control. After 3 weeks of deafness, animals were sacrificed for tissue harvesting.
The guinea pigs’ care and all experiments were carried out in accordance with the German law on protecting animals and were permitted by the regional government (LAVES, registration number 07/1266).
Anesthesia
Guinea pigs were anesthetized using medetomidine (0.3 mg/kg, Domitor®; Pfizer, Berlin, Germany) and ketamine (40 mg/kg, Ketamin®; WDT, Garbsen, Germany) for electrophysiological measurements and surgery. If necessary, animals received an additional injection of half of the original dosage for surgery. On experimental day 21, after final AABR measurement, all animals received a second injection of the full dosage of anesthetics 15 minutes before sacrifice as described above. One hour before surgery, 0.05 mg/ kg atropine sulfate (B Braun Melsungen AG, Melsungen, Germany) and 5 mg/kg carprofen (Rimadyl®; Pfizer GmbH, Karlsruhe, Germany) were applied. Before surgery in the postauricular area and before perfusion in the region to be incised, local anesthesia was performed with prilocaine (Xylonest®; AstraZeneca GmbH, Wedel, Germany).
Acoustically evoked auditory brainstem response measurement
AABR measurements were performed on day 0 to confirm the physiological hearing threshold of the animals. Hearing thresholds of ≤80 dB SPL at 1 kHz, ≤70 dB SPL at 4 kHz, ≤50 dB SPL, and ≤60 dB SPL at the middle frequencies 8 kHz and 16 kHz, ≤70 dB SPL at 32 kHz, and ≤80 dB SPL at 40 kHz were set as thresholds for normal hearing. On day 7, AABR was measured again to verify the efficacy of the deafening procedure. A threshold shift of ≥50 dB or above 90 dB SPL was determined as deaf.Citation28 On experimental day 21, a final measurement was carried out just before sacrifice. All AABR measurements were performed with a system (Tucker Davis Technologies, Alachua, FL) that consisted of two RX6 multifunction processors, a loudspeaker driver unit (ED1), and two electrostatic speakers (EC1). The anesthetized animals were placed on a heating pad in a soundproof box where they were exposed to acoustic stimuli by electrostatic speakers (EC1) via modified ear tips. To record the neurological responses, four needle electrodes were subdermally implanted as follows: the reference electrode was attached to the nose, the auditory evoked potentials were derived via two electrodes relative to the left and right mastoid, and the ground electrode was placed on the neck to suppress interfering signals caused by heart rate or muscle tension. The recording electrodes were broadcasting the potentials of the low-impedance converter (RA4LI) to a preamplifier (RA4PA), which transferred a digitalized signal to the multifunction processor (RX6). Measurements were controlled by BioSigRP software (Tucker Davis Technologies). The dynamic range of the stimuli (10 ms sinusoidal pulses; 1 ms cosine-in and fade-out-function) was settled in 10 dB steps from 20–90 dB SPL, whereas frequencies of 1 kHz, 4 kHz, 8 kHz, 16 kHz, 32 kHz, and 40 kHz were presented. In order to suppress background noise, bandpass filters were settled from 300 Hz to 3 kHz at 3 dB. In order to suppress other artifacts, a threshold of 70 μV was chosen. For graphical representation, each stimulus was repeated 270 runs and averaged. The hearing threshold was determined by visual analysis as soon as a significant local maximum was observed in the course of the averaged wave.Citation29
Deafening procedure
All animals were systemically deafened on day 0 using a common ototoxic procedure as described by Versnel and colleagues.Citation30 After the preliminary AABR measurement, 400 mg/kg kanamycine were injected subcutaneously. In the next two hours, the jugular vein was exposed under incision of the overlaying musculature in order to inject the loop diuretic furosemide (100 mg/kg). The coadministration of the aminoglycoside kanamycine with loop diuretics results in a complete loss of sensory cells, which initiates the degeneration of SGC. Before tissue harvesting, deafness was confirmed on day 7 and 21 by AABR measurement.
Application of the test substances
On day 7 of the experiments, animals were placed in ventral alignment on a heating pad after AABR measurement was performed in order to confirm the deafness of the animals. A postauricular incision was made under aseptic conditions. The overlying muscles were removed in order to examine the Bulla tympani, which was opened in the following step. Using a microscope (Carl Zeiss AG, Oberkochen, Germany), the cochlea was visualized in the opened middle ear cavity. A small hole was drilled under the use of a lancet in the basal turn of the cochlea and the scala tympani was opened. An injection catheter was assembled using a 27 G cannula (B Braun Melsungen AG) connected with a silicon tube (inner diameter 0.3 mm; LiquidScan GmbH and Co, KG, Überlingen, Germany) and an inserted polyimide tube (inner diameter 0.12 mm; Micro Lumen Inc, Tampa, FL) at the top. In order to avoid deep penetrations of the cochlea, a 2 mm sphere of carboxylate cement (ESPE Dental AG, Seefeld, Germany) was formed on the tip. The catheter was then inserted into the scala tympani to deliver a total volume of 5 μL of the test substances over a period of 5 to 10 minutes into the inner ear using a 10 μL Hamilton syringe (Hamilton Bonaduz AG, Bonaduz, Switzerland). The left ears were treated with the test substances (LNC–FITC [n = 7], rolipram [n = 6], LNC–FITC–rolipram [n = 7], and PBS [n = 6]) in a dilution of 1:100. As a control, the right ears were treated with PBS. Subsequently, the cochlea and the bulla tympani were sealed with carboxylate cement (Durelon®; ESPE Dental AG) and the soft tissue trauma was sutured in two layers.
Histological analysis
Anesthetized animals were sacrificed after final AABR measurement on day 21 via transcardial perfusion by 200 mL PBS followed by 200 mL of paraformaldehyde (PFA; 4%; Merck, Darmstadt, Germany) infused in the left heart chamber. Cochleae were harvested and opened carefully at the apex and round window to exchange resisting perilymph for a fixative by slowly rinsing with 1 mL PFA (4%). Samples were placed in PFA for 2 hours for further fixation. Before decalcification, the cochleae were rinsed with PBS three times. Afterwards, they were placed in 20% EDTA (Sigma- Aldrich Chemie GmbH, Steinheim, Germany) for 4–5 weeks at 37°C. Decalcification media was changed every 2 or 3 days. Before paraffin embedding, dehydration with 50%–100% ethanol (BÜFA Chemikalien, Altmoorhausen, Germany) was performed. 5 μm midmodiolar sections were generated from paraffin blocks, mounted on glass slides and stained with hematoxylin and eosin.
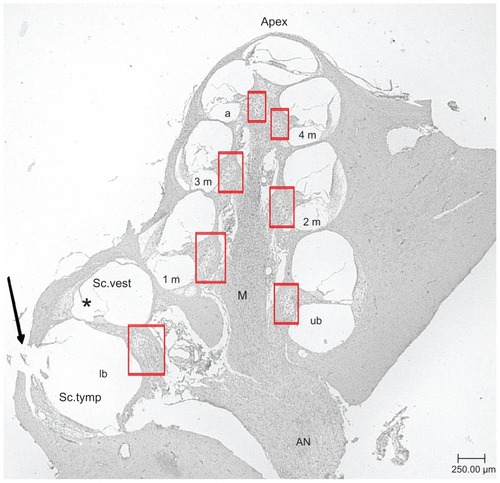
For analysis, all cochlea turns were identified with an Axiovert 25C Microscope (Carl Zeiss AG). The turns were named lower basal turn (lb), upper basal turn (ub), first middle turn (1 m), second middle turn (2 m), third middle turn (3 m), fourth middle turn (4 m), and apical turn (a) (). Due to preparation methods, the 4 m and a turn could not always be analyzed separately. Therefore, the SGC densities of these areas, if available, were added for analysis. The first midmodiolar section was selected randomly. Every fifth following section was chosen for analysis to ensure a separation of 25 μm between the sections. In total, five sections per cochlea were analyzed. Next to the perikaryal diameter, the number of surviving SGC with a visible nucleus was examined. The number of surviving SGC in correlation to the measured cross-sectional area of the Rosenthal’s canal provides the SGC density, which can be expressed as cells/10.000 μm2. Soma diameter measurement was performed by dividing the area of each Rosenthal’s canal in four equal parts. In each part, two representative SGC were chosen for measurement. In case of less than eight surviving SGC, every visible cell was measured.
Figure 2 Representative midmodiolar hematoxylin and eosin-stained section (5 μm) of a deafened guinea pig cochlea. Rosenthal’s canal areas are highlighted in red squares. Lower basal turn (lb), upper basal turn (ub), first middle turn (1 m), second middle turn (2 m), third middle turn (3 m), fourth middle turn (4 m), and apical turn (a) of the scala tympani are labeled. The arrow marks the cochleostomy where the solutions were injected into the scala tympani. The modiolus, the axis of the inner ear, and the apex, the upper part of the inner ears are marked.
Note: *scala media.
Abbreviations: AN, auditory nerve; M, modiolus; Sc. tymp, scala tympani; Sc. vest, scala vestibuli (magnification: 40-fold).
Statistical analysis
All data passed the normality test (Kolmogorov–Smirnov). Student–Newman–Keuls analysis of variance (ANOVA) was performed for in vitro data analysis. In vivo data were first analyzed using a paired t-test to compare the treated and untreated ears of one animal group. Comparisons between the treated (left) ears of all groups, as well as the comparisons with the untreated (right) ears were performed using Student–Newman–Keuls ANOVA.
Results
SGC in vitro study – neuronal survival
SGC were seeded in a mean density of 1.5 × 104 cells/well. The seeding control was evaluated after 4 hours of cultivation and contained 229.6 ± 18.1 (mean ± standard deviation [SD]; n = 12) SGC. This was set as the reference value (100 %) for the experimental groups that were fixated after 48 hours. In five independently performed experimental settings (independent plates on different days), each group included 4–8 wells. Thus, each concentration of the test substances (1:50, 1:100, 1:200, 1:300) was tested in a minimum of 20 wells. For BDNF treatment, 30 wells were used as positive control; for the untreated negative control, 12 wells were examined. depicts the mean and SD percentage of surviving SGC for each experimental group.
Figure 3 (A) Survival rates of cultured SGC treated with LNC–FITC, LNC–FITC– rolipram, rolipram, and BDNF in different concentrations. Rolipram was used in concentrations of 38 ng (1:50 dilution; n = 5; total: 20 wells), 19 ng (1:100 dilution; n = 5; total: 20 wells), 9.5 ng (1:200 dilution; n = 5; total: 20 wells), and 6.3 ng (1:300 dilution; n = 5; total: 20 wells) per 0.1 mL medium. Stock solution of LNC contained 45 mg/mL FITC (4.2 × 1015 LNC/mL). LNC–FITC–rolipram particles contained 19 μg/mL rolipram. BDNF (50 ng/mL, n = 5; total: 30 wells) served as positive control. (B) Neurite outgrowth of cultured SGC treated with LNC–FITC, LNC–FITC– rolipram, rolipram, and BDNF in different concentrations (mean ± SD). LNC–FITC 1:50 decreased the SGC–neurite length highly significantly (P < 0.001) compared to all other experimental groups (not marked in the graph) except the LNC–FITC– rolipram 1:50 group (P < 0.01; left upper line). Significant differences among treatment groups compared to the negative control are marked above the columns.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: BDNF, brain-derived neurotrophic factor; FITC, fluorescein isothiocyanate; LNC, lipidic nanocapsules; SD, standard deviation; SGC, spiral ganglion cells.
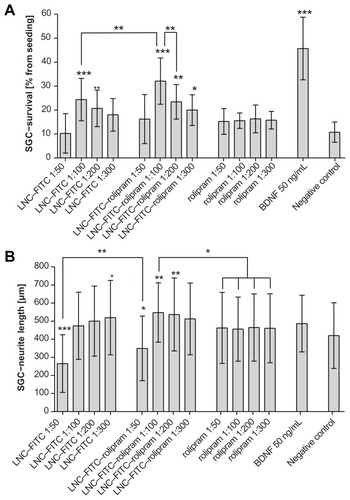
BDNF had the best results with a highly significant increased neuron survival in comparison to the negative control (45.7% ± 12.6% versus 10.7% ± 4.2%; P < 0.001), as well as compared to all other experimental groups (P < 0.001). Pure rolipram did not enhance neuronal survival. Homogenous values of 15.7% ± 4.5% survival rate in mean for each dilution of rolipram induced no significant difference when compared to the negative control, although a tendency toward an increased survival rate was visible. In combination with LNC, rolipram increased the neuronal survival in the 1:100 (32.6% ± 9.7%; P < 0.001) dilution. In comparison to the negative control, the 1:200 (23.4% ± 7.2%; P < 0.01) and 1:300 dilution (20.0% ± 6.4%; P < 0.05) proved to be neuroprotective as well. The 1:50 (16.2% ± 10.2%) dilution could not induce any benefits.
Treatment with unloaded LNC (FITC-labeled, but not containing rolipram) resulted in increased neuronal survival as well, though the effect was weaker than in the LNC–FITC–rolipram group. In this group, the 1:100 (24.4% ± 8.8%; P < 0.001) and the 1:200 (20.7% ± 7.6%; P < 0.01) dilution showed positive effects.
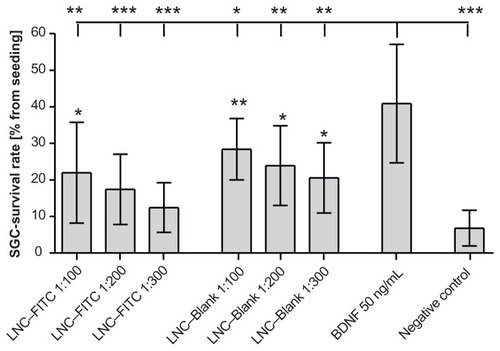
Because LNC–FITC nanoparticle treatment resulted in increased neuronal survival, an additional test was performed to evaluate which nanoparticle component – the nanoparticle itself or the fluorescein FITC – has neuroprotective properties. The results revealed neuroprotective effects of the particle itself. As shown in , LNC–Blank particles significantly increased the survival rate in comparison to the negative control.
Figure 4 Survival rates of cultured SGC treated with LNC–FITC and LNC–Blank in different concentrations (n = 9 for each group, mean ± SD). Stock solution of LNC contained 45 mg/mL FITC (4.2 × 1015 LNC/mL). BDNF (50 ng/mL, n = 9) served as positive control. The SGC survival rate of all experimental groups differed significantly from the survival rates of neurons treated with BDNF (upper horizontal line; *P < 0.05; **P < 0.01; ***P < 0.001). Statistically significant differences of LNC (with and without FITC)-treated SGC compared to the negative control are marked above the respective column.
Abbreviations: BDNF, brain-derived neurotrophic factor; FITC, fluorescein isothiocyanate; LNC, lipidic nanocapsules; SD, standard deviation; SGC, spiral ganglion cells.
SGC in vitro study: neurite lengths
Neurite lengths of the negative control were measured with a mean of 420.1 ± 181.5 μm (mean ± SD). BDNF and rolipram did not influence the neurite outgrowth, whereas the 1:50 dilutions of the LNC–FITC (265.6 ± 159.4 μm; P < 0.001) and LNC–FITC–rolipram group (349.3 ± 178.3 μm; P < 0.05) induced significantly decreased neurite lengths. In comparison to the negative control, LNC–FITC showed a positive effect on the neurite lengths in the 1:300 dilution (519.1 ± 205.4 μm; P < 0.05), LNC–FITC–rolipram in the 1:100 (547.6 ± 164.1 μm; P < 0.01), and 1:200 dilution (536.8 ± 201.1 μm; P < 0.01), whereas the other dilutions did not differ from the negative control ().
SGC in vitro study: soma diameter
The mean soma diameter of the negative control was 14.5 ± 2.6 μm (mean ± SD, n = 60). Only the cell diameter of the 1:50 LNC–FITC (stock solution: 45 mg/mL LNC)- treated SGC showed a significant decrease in soma diameter (12.8 ± 1.9 μm; P < 0.01). None of the other groups provided statistically significant differences when compared with the average cell diameter of the negative control. Values are shown in .
Table 1 Results of the measured soma diameter of SGC (mean ± SD) after 48 hours cultivation with LNC–FITC, LNC– FITC–rolipram, rolipram, or BDNF in the shown concentrations
DC in vitro study – TNF-α inhibition
Per well 5 × 104 DCs were seeded. For each concentration and experimental group, four wells were performed per plate. Three repeated settings resulted in a total of 12 wells per concentration and group.
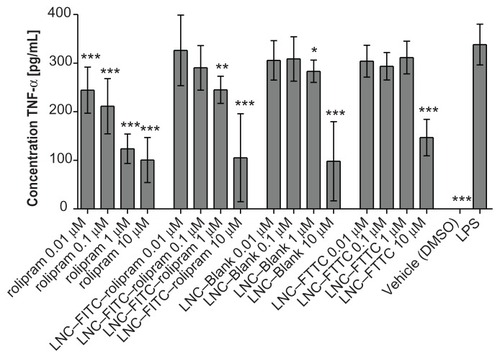
The TNF-α production of all rolipram-treated DC was significantly inhibited when compared to the positive control (338.18 ± 41.82 pg TNF-α/mL; mean ± SD). The inhibition was dose-dependent: higher rolipram concentrations induced higher TNF-α suppression (). A similar decrease was shown in the LNC–FITC–rolipram-treated group, though the values demonstrated a weaker inhibition of the TNF-α production. LNC–Blank nanoparticles caused a slight inhibition of the cytokine in the concentration of 1 μM (283.11 ± 23.01 pg/mL; P < 0.05). A strong decrease of TNF-α secretion was caused by LNC–Blank particles with the concentration 10 μM (98.11 ± 81.29 pg/mL; P < 0.001). The LNC–FITC nanoparticles showed resembling characteristics when compared to the LNC–Blank particles with a very strong inhibition in the 10 μM concentration (146.90 ± 7.64 pg/mL; P < 0.001). By microscopic examination of the DC culture, granulation and misshaping of the cells in the 10 μM nanoparticles groups (LNC–Blank, LNC– FITC, LNC–FITC–rolipram) was observed. Cell vitality tests confirmed toxic effects in these concentrations (data in Supplementary materials).
Figure 5 TNF-α-secretion of DCs treated with rolipram, LNC–FITC–rolipram, LNC–Blank, and LNC–FITC in different concentrations. DC were stimulated with LPS 1 hour after incubation with the test substances. Data show mean ± SD from three independent experiments: each consisted of n = 12 wells per concentration per group. Statistically significant differences in TNF-α secretion compared to the LPS-control group are plotted above the columns.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: BDNF, brain-derived neurotrophic factor; DC, dendritic cells; FITC, fluorescein isothiocyanate; LNC, lipidic nanocapsules; SD, standard deviation; SGC, spiral ganglion cells; TNF-α, tumor necrosis factor-α.
In vivo results
AABR measurements
To determine the hearing threshold, animals underwent AABR measurements on day 0, 7 and 21 of the study.
A preliminary AABR was performed just before the systemic deafening on day 0 to confirm the physiological hearing property of the animals. At 1 kHz, the threshold was shown at 72.90 ± 12.43 dB SPL, at 4 kHz 61.44 ± 12.55 dB SPL, at 8 kHz 36.14 ± 11.62 dB SPL, at 16 kHz 48.86 ± 11.76 dB SPL, at 32 kHz 49.02 ± 9.85 dB SPL, and at 40 kHz 63.91 ± 13.61 dB SPL (graphed in Supplementary material). The mean difference between the left and the right ear was 5.13 dB. The generated data showed normal hearing physiology of the tested animals according to previous studies.
The AABR measurements on day 7 and 21 were performed to confirm the deafening procedure. In no case of the measured frequencies was a positive AABR waveform visible until the measuring limit of 90 dB SPL. According to the literature, a threshold shift of 50 dB confirmed a successful deafening procedure.Citation28
SGC densities in vivo
No significant differences concerning the SGC densities were observed when the data from the treated left ears were compared (). The same results occurred when the SGC densities of the right ears were compared. Furthermore, the paired t-test revealed no differences between the right and left ears within the test groups. Only the SGC-densities of the left and right ears of the PBS-control group varied significantly (4.14 SGC ± 1.40/ 10.000 μm2; mean ± SD versus 5.23 SGZ ± 1.67/ 10.000 μm2; P < 0.05; ). Representative images of Rosenthal’s canal of all four treatment groups are shown in .
Figure 6 (A) Mean SGC density (cells/10.000 μm2) of deafened guinea pigs treated with LNC–FITC (n = 7), LNC–FITC–rolipram (n = 6), rolipram (n = 7), and PBS (n = 6) in 1:100 dilution (mean ± SD; *P < 0.05). (B) Mean SGC soma diameter of deafened guinea pigs treated with LNC–FITC, LNC–FITC–rolipram, rolipram, and PBS in 1:100 dilution (mean ± SD; *P < 0.05; **P < 0.01). Only LNC–FITC- and rolipram-treated animals showed a significant difference in SGC soma diameters between left (le, test substance) and right (ri, control).
Abbreviations: FITC, fluorescein isothiocyanate; LNC, lipidic nanocapsules; PBS, phosphate-buffered saline; SD, standard deviation; SGC, spiral ganglion cells.
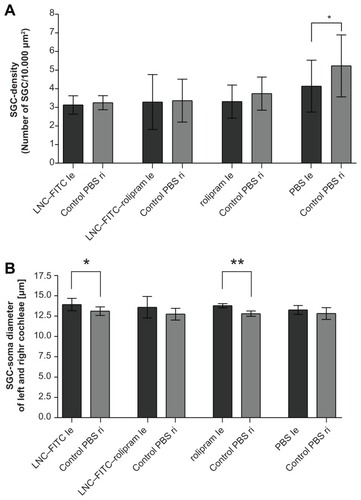
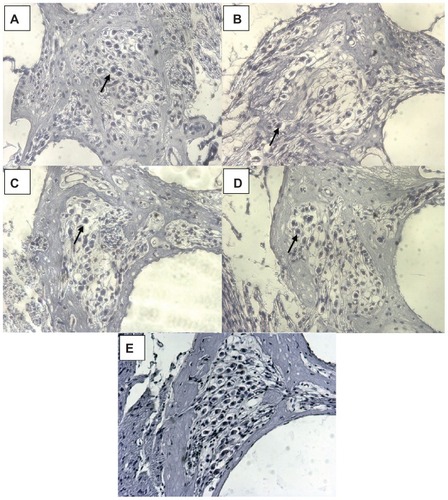
Figure 7 (A–D) Representative images of guinea pigs’ Rosenthal’s canals 21 days after deafening. (E) illustrates the SGC density in normal hearing animals as compared to the deafened ones in (A–D) (the picture is from a previous experiment registered at LAVES with the same registration number as the experiments described here: 07/1266). (A) is from an animal from the LNC–FITC group receiving the nanoparticle in a 1:100 dilution (stock solution 45 mg/ml LNC). (B) represents the Rosenthal’s canal of animals treated with LNC–FITC–rolipram (1:100 dilution of 45 mg/mL LNC and 19 μg/mL rolipram in stock solution) and (C) is from an animal that received rolipram (1:100 dilution of 19 μg/ml rolipram stock solution). An image of Rosenthal’s canal from the control group is shown in (D).
Notes: In each picture, an arrows point to one representative vital SGC. Magnification: 200-fold.
Abbreviation: SGC, spiral ganglion cells.
SGC diameter in vivo
Soma size was measured to evaluate the effect of the test substances on the cellular size. The LNC–FITC group showed a significant increase of the soma diameter between the left treated and the right control ears (14.00 ± 0.76 μm [mean ± SD] versus 13.14 ± 0.52 μm; P < 0.05). An even more evident increase of soma diameter was observed between the left and right ear of the rolipram-treated animals (13.78 ± 0.26 μm versus 12.73 ± 0.32 μm; P < 0.01). The SGC diameter in the LNC–FITC–rolipram group (left: 13.91 ± 1.33 μm versus right. 12.68 ± 0.72 μm) and the PBS control group (left: 13.27 ± 0.56 μm versus right: 12.68 ± 0.79 μm) did not differ significantly, although a difference by trend might be assumed (). There were no significant differences when the soma diameter of the left ears of all groups were compared. The same data unity was observed when comparing the right ears of all groups.
Discussion
This study demonstrates that BDNF (50 ng/mL) shows the most effective neuronal protection regarding the survival rate of cultured SGC. Former studies already confirmed a maximum survival rate of SGC with BDNF in this concentration. Citation31 Due to that property, BDNF was chosen as a positive control, although the main focus was on the examination of the neuroprotective effects of rolipram and in LNC-incorporated rolipram.
Pure administration of rolipram demonstrated no efficient SGC protection in vitro in any of the used concentrations. This might be due to the intracellular mechanism of phosphodiesterase- 4 inhibitors. Rolipram has to be translocated in the cytoplasm where phosphodiesterase 4 is located. Once in the cytoplasm, rolipram inhibits phosphodiesterase 4, which leads to increased levels of cyclic adenosine mono-phosphate (cAMP). Proteinkinase A, which depends on cAMP, phosphorylates cAMP responsive element binding protein (CREB) in the cellular nucleus.Citation32 The activated CREB triggers the transcription of BDNF,Citation33 which leads to a neuroprotective effect. To induce this effect, the application of rolipram via LNC was chosen to enhance the intracellular concentration of rolipram, which subsequently leads to neuronal protection.
Results confirm an increased SGC survival rate using the LNC–rolipram combination. The dilution of 1:100 showed the best results in vitro, therefore this dilution was used for in vivo experiments as well. Decreasing effects of the 1:200 and 1:300 dilutions are probably caused by low concentrations, whereas the 1:50 dilution may have a toxic effect on cellular metabolism. Recent studies lead to the assumption that mutual reactions between the phospholipids of the particle shell and the cell membrane may cause an imbalance in endo- and exocytotic reactions.Citation34 This consideration refers to former observations revealed in a study with polylysine nanoparticles.Citation35 Another experiment also shows good biocompatibility of the lipid nanocapsules on fibroblast cultures. Cytotoxic effects occurred only above a concentration of 2.2 mg/mL.Citation36 Our own observations revealed the effects of decreased SGC survival already in the 1:50 dilution (0.9 mg/ mL), suggesting that neuronal cells are more metabolically sensitive.
The synergistic effect of rolipram and the nanoparticle is evident; the pure administration of rolipram did not significantly affect the SGC survival.
It is important to note that we found a distinct neuroprotective effect of the unloaded LNC–FITC particles as well. Compared to the LNC–FITC–rolipram combination, the SGC survival was significantly lower, which must be caused by the payload rolipram; hence the experimental groups differ only in this characteristic. To verify whether the nanoparticle itself or the fluorescein FITC are responsible for the neuroprotective effect of the drug-unloaded LNC–FITC particles, an additional SGC culture experiment was performed with FITC-unlabeled LNC. The results prove that the particle itself is responsible for the neuroprotective effects. We hypothesize that the positive effect of the unloaded particles is caused by the particle component PEG. Inserted into the particle shell, this polymer protects the drug-delivery system against the complement system of the organism to maintain a prolonged circulation time in the body.Citation37,Citation38 Furthermore, PEG is used for the covalent binding of FITC.
Former experiments demonstrated the neuroprotective effects of PEG in dogs suffering from intervertebral disc prolapse.Citation39 Other studies with spinal cord injury models confirmed the beneficial effects of PEG; damaged cells were literally sealed by this polymer.Citation40 Thus, it is remarkable that our LNC are neuroprotective themselves and are appropriate for delivering drugs, such as rolipram, to target cells in vitro to significantly increase this protective effect.
After primary hair cell loss, a progressive decrease of neurite length and SGC density is observed.Citation41 To ensure an optimal electrical stimulation via cochlear implants, neurite preservation and, if possible, outgrowth is desired. Therefore, we measured the SGC neurite length in vitro to evaluate their outgrowth depending on the different test conditions. Rolipram did not affect the neurite outgrowth of SGC in any of the applied concentrations. Because of rolipram inhibiting phosphodiesterase 4 in the cytoplasm of cells, we hypothesize that the drug was not taken up by the neurons and therefore was not able to induce a biological effect on SGC. In contrast to this, an elongation of the neurite outgrowth was demonstrated when SGC were treated with rolipram encapsulated in LNC–FITC nanoparticles. The 1:100 and 1:200 dilutions of the LNC–FITC–rolipram particles increased the neurite outgrowth significantly. This is an indirect indication of LNC-mediated improved rolipram uptake into SGC. Next to this, cells treated with LNC–FITC 1:300 revealed a significant neurite outgrowth as well, probably due to the PEG component, as described before concerning neuronal survival. Higher concentrations (1:50 dilution) of both experimental groups induced rather short neurites, which indicates negative effects on the SGC. The rolipram-induced mechanism on neurite elongation is not clearly discovered, though a cAMP-dependent activation of protein kinase A might be involved.Citation42 Taking this, our findings support Xu et al’s report concerning the biphasic effect of the cAMP analog cpt-cAMP on neurite length. They were able to show an increased length at lower concentrations and reduced length at higher concentrations.Citation43 On the other hand, in the present study, rolipram-loaded nanoparticles significantly elongated the neurites in 1:50 dilution when compared to the unloaded LNC in 1:50 dilution. One could speculate that this divergence is based on the rolipram and that this finding supports the hypothesis that rolipram positively affects neurite outgrowth. Until recently, limited research was available on rolipram effects and mechanisms of effects on neuronal cells. Further studies are necessary to confirm or refute the hypotheses.
Results of the perikaryal diameter showed no significant differences between the experimental groups except the SGC treated with 1:50 LNC. High concentration of LNC might induce negative effects on the cell metabolism, as described before, which influences the cell diameter.
The preincubation of DC with rolipram caused a highly significant, dose dependent inhibition of TNF-α secretion in all tested concentrations (0.01 μM, 0.1 μM, 1 μM, 10 μM) after stimulation with LPS. The collected data are in accordance with the results of a former study,Citation34 which reported a dose-dependent reduction of LPS stimulated human DC with rolipram as well. The LNC–FITC–rolipram combination shows a similar dose-dependent decrease of TNF-α, though the weaker effects in comparison to the pure administration might be caused by an insufficient release of the payload in the incubation time of 24 hours. Other LNC groups showed no or rather weak TNF-α inhibition in the concentrations of 0.01 μM, 0.1 μM, and 1 μM. Upon microscopic examination, nanoparticle concentrations of 10 μM caused toxic effects on the cells. Granulated and misshaped cells, as well as crystal formations, were also visible. Thus, the low concentrations of TNF-α in these experimental groups are caused by decreased cellular vitality and not by an TNF-α inhibition, which was confirmed by a cell vitality test (CellTiter 96® AQueos One Solution Cell Proliferation Assay; Promega, Madison, WI; see Supplementary material).
By reducing the TNF-α transcription through cAMP-dependent protein kinases,Citation44 rolipram can alleviate inflammations, hence TNF-α plays a key role in proinflammatory processes. In combination with cochlear implants, benefits may be achieved by inhibiting postinsertion inflammation processes.
Our data proves that rolipram penetrates the DC cell membrane and evokes biological responses in this cell type. Conversely, rolipram does not cause a biological reaction in SGC without the support of the tested drug-delivery system LNC. Even if a small amount of pure rolipram can influence neuronal cells, the encapsulation in nanocarriers would be beneficial. As LNC encapsulation of rolipram increases the SGC survival rate and would allow a targeted drug delivery in future, this approach would be economically and clinically beneficial in terms of lower costs for drugs and decreasing side effects.
The positive results of in vitro experiments, which provided the basis for the performed in vivo studies, could not be confirmed in vivo. The comparison between the test substance-treated left ears with the right control ears (PBS-treated) revealed no differences in SGC-densities. The only significant difference was shown in the control group, where both sides were inoculated with PBS.
These results might be caused by an additional perilymphatic dilution of the test substances which leads to an ineffective concentration. In future experiments, the neuroprotective effect of a higher dosage of LNC rolipram particles might be examined, although the risk of causing toxic effects on the neuronal cells is a possibility. By administrating high dosages at the base of the cochlea, basal cells could be influenced by the high concentration before the desired dilution is achieved in the whole cochlea volume. Furthermore, a leakage of the injected substances via the ductus perilymphaticus is assumed since a contralateral transfer between treated and untreated cochleae was confirmed.Citation45
Another reason for the different results of the in vitro and in vivo study may be the usage of different species of different ages. On the one hand, neonatal rats were used to perform the in vitro culture; on the other hand, adult guinea pigs were used for in vivo studies. Thus, different trophic requirements and receptor expressions for neonatal and adult SGC were presumed, which are likely to differ in the young and adult stage.Citation9 The inhomogeneous results might be due to an inadequate trophic support in the adult stage of the in vivo experiment.
Regarding the increased SGC degeneration following the cessation of neurotrophic factors,Citation9 a preliminary protection could be assumed for the test substances used in this study. By performing single administration, the neuroprotective effect might be achieved in the first period of the study. The positive effect could be distinguished after the accelerated degeneration, as described by Gillespie and colleagues.Citation9 A later experiment could not reproduce the effect of an increased SGC degeneration after cessation of neurotrophic factors, whereas electrical stimulation for hearing threshold measurements was performed,Citation28 which themselves have neuroprotective potential.Citation46,Citation47
As mentioned before, the only significant difference in SGC density in vivo was detected between the PBS-treated ears of the control group. Considering that the SGC density of both ears should be equal because of equal treatment, one could speculate that the difference is based on an additional trauma due to PBS injection and manipulation that increases the deafness-induced SGC degeneration at the left side. The surgeon’s handedness could also be an explanation for this. But the left ears’ SGC density was the same as one of the other experimental groups. Additionally, the SGC density of PBS-treated right ears of the PBS group was higher than the density of all other PBS-treated right ears of the other experimental groups. Therefore, it is not an increased SGC degeneration in left PBS-treated ears of the control group but a decreased SGC degeneration in right PBS-treated ears of the PBS group. We do not yet have an explanation for this effect.
We were able to demonstrate significantly increased soma diameters of the treated ears in the LNC–FITC group and the rolipram group in vivo. In the LNC–FITC–rolipram group, the SD’s soma diameter was relatively wide and, even if the graph () looks like there may be a significant difference between the treated and nontreated ears of this group, no statistically significant difference was observed. Several previous studies reported an increase of soma diameter after treatment with neurotrophic factors such as BDNF in deafened animals.Citation48,Citation49 The underlying mechanisms of the soma diameter increase and the functional impact are not yet known, which complicates a final appraisal of this parameter.
Conclusion
This study demonstrates the SGC protective effects of LNC-mediated rolipram delivery in vitro in terms of concentration dependent neuronal survival and neurite outgrowth. We hypothesize that this biological effect indirectly demonstrates that the lipidic nanoparticles improve the transport of rolipram through the neuronal cell membrane into the cytoplasm of the neurons where the increase of rolipram and LNC induces significant neuronal protection. Additionally, we were able to demonstrate a strong anti-inflammatory effect of rolipram when cultivated with activated dendritic cells. The LNC rolipram combination presented an anti-inflammatory effect as well, though weaker, which might be due to the release rate of rolipram out of the LNC.
The in vivo study did not confirm the in vitro results, which might be due to either perilymphatic dilution or insufficient duration of drug application.
Even if the in vitro results that we were able to report on could not be confirmed in vivo, we still believe that rolipram is a promising drug for neuronal protection in inner ear therapy and that LNC are a feasible drug-delivery system for treatment of inner ear cells if in vivo methods are modified. Future work has to thoroughly investigate the effect of LNC on SGC and DC, and has to identify the correct LNC/rolipram ratio to cause a biological effect in vivo. Additionally, experiments may be conducted to investigate the effect of LNC/rolipram on connective tissue proliferation around an implanted device in vivo. Controlled uptake of LNC into cochlear cells should be investigated to decrease the amount of LNC that have to be applied. Nevertheless, at present, LNC are promising tools to deliver drugs to the inner ear since they are non-toxic to the inner ear tissue in vitro and in vivo if applied in sufficient dilution. They also enable the biological effect of rolipram in vitro. This opens the exciting possibility of using these nanoparticles as a drug-delivery system for inner ear structures.
Acknowledgments
This study was supported by the European Community 6th Framework Programme on Research, Technological Development and Demonstration (Nanotechnology-based Drug Delivery. Contract Number: NMP4-CT-2006-026556, Project acronym: NANOEAR). We would like to thank Dr Henning Voigt, Hannover Medical School, for his support in quality management.
Disclosure
The authors report no conflict of interest in this work.
Supplementary material
Vitality test
To determine the vitality of dendritic cells at the end of the experiment (day 10), a CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was performed. In addition, the cells were subjected to microscopic inspection to note any morphological differences. This examination allows a trend statement about a possible influence of the test substances. The results of the viability test reveal possible cellular impairments caused by the test substances. Thus, a lower production of tumor necrosis factor-α (TNF-α) depends not only on the inhibition of cytokine production but also on an increased degeneration of cells. This assay is based on nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH), which catalyzes the conversion of the yellow methyltetrazolium to a violet formazan product. NADPH/NADH are only found in living cells. Consequently, a color change by 490 nm measured by photometric analysis is an indication of cell vitality. The resulting amount of formazan is directly proportional to the number of viable cells. Supplementary Table 1 shows the measured values.
AABR measurements
Acoustically evoked auditory brainstem response measurements were performed to determine the hearing threshold of all experimental animals before and after deafening for verification of the procedure’s success. Because the used system allows stimulation with a maximum of 90 dB SPL and all animals’ hearing thresholds reached or exceeded that level 7 and 21 days after deafening, the hearing thresholds are plotted at 90 dB (Supplementary Figure 1).
Figure S1 By frequency-specific AABR measurements on day 0, a normal hearing threshold was detected in all animals (blue and pink line; mean ± SD).
Notes: The results of the measurements on day 7 and day 21 are plotted as a red line. None of the animals had residual hearing after the deafening procedure. The hearing threshold of all animals was 90 dB SPL or above on day 7 and did not recover until the final measurement on day 21.
Abbreviations: AABR, acoustically evoked auditory brainstem response; SD, standard deviation.
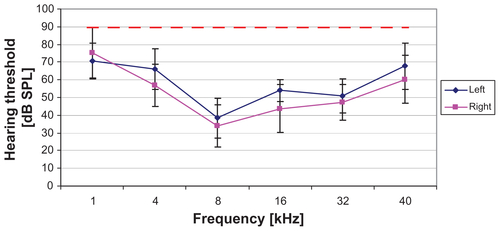
Table S1 Vitality values of dendritic cells after incubation with the test compounds and LPS stimulation; n = 3 per substance and concentration
References
- SelimogluEAminoglycoside-induced ototoxicityCurr Pharm Des200713111912617266591
- YamaneHNakaiYKonishiKFurosemide-induced alteration of drug pathway to cochleaActa Otolaryngol Suppl198844728353055805
- LenarzTCochlear implants: what can be achieved?Am J Otol199718Suppl 6S239391574
- ShibataSBBudenzCLBowlingSAPfingstBERaphaelYNerve maintenance and regeneration in the damaged cochleaHear Res20112811–2566421596129
- AltschulerRAChoYYlikoskiJPirvolaUMagalEMillerJMRescue and regrowth of sensory nerves following deafferentation by neurotrophic factorsAnn N Y Acad Sci199988430531110842602
- IncesuluANadolJBJrCorrelation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantationAnn Otol Rhinol Laryngol199810711 Pt 19069119823838
- GillespieLNShepherdRKClinical application of neurotrophic factors: the potential for primary auditory neuron protectionEur J Neurosci20052292123213316262651
- MillerJMChiDHO’KeeffeLJKruszkaPRaphaelYAltschulerRANeurotrophins can enhance spiral ganglion cell survival after inner hair cell lossInt J Dev Neurosci1997154–56316439263039
- GillespieLNClarkGMBartlettPFMarzellaPLBDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effectsJ Neurosci Res200371678579012605404
- RejaliDLeeVAAbrashkinKAHumayunNSwiderskiDLRaphaelYCochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neuronsHear Res20072281–218018717416474
- YagiMKanzakiSKawamotoKSpiral ganglion neurons are protected from degeneration by GDNF gene therapyJ Assoc Res Otolaryngol20001431532511547811
- HanJJMhatreANWareingMTransgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vectorHum Gene Ther199910111867187310446926
- SuzukiMYamasobaTSuzukawaKKagaKAdenoviral vector gene delivery via the round window membrane in guinea pigsNeuroreport200314151951195514561927
- HussemanJRaphaelYGene therapy in the inner ear using adenovirus vectorsAdv Otorhinolaryngol200966375119494571
- KhoSTPettisRMMhatreANLalwaniAKCochlear microinjection and its effects upon auditory function in the guinea pigEur Arch Otorhinolaryngol2000257946947211131371
- PautlerMBrennerSNanomedicine: promises and challenges for the future of public healthInt J Nanomedicine2010580380921042425
- ScheperVWolfMSchollMPotential novel drug carriers for inner ear treatment: hyperbranched polylysine and lipid nanocapsulesNanomedicine (Lond)20094662363519663591
- NewboldCRichardsonRHuangCQMilojevicDCowanRShepherdRAn in vitro model for investigating impedance changes with cell growth and electrical stimulation: implications for cochlear implantsJ Neural Eng20041421822715876642
- BeaumontEWhitakerCMBurkeDAHetmanMOniferSMEffects of rolipram on adult rat oligodendrocytes and functional recovery after contusive cervical spinal cord injuryNeuroscience2009163498599019635528
- WhitakerCMBeaumontEWellsMJMagnusonDSHetmanMOniferSMRolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injuryNeurosci Lett2008438220020418455876
- ZhuJMixEWinbladBThe antidepressant and antiinflammatory effects of rolipram in the central nervous systemCNS Drug Rev20017438739811830756
- KrauseWKuhneGSauerbreyNPharmacokinetics of (+)-rolipram and (−)-rolipram in healthy volunteersEur J Clin Pharmacol199038171752328751
- HeurtaultBSaulnierPPechBProustJEBenoitJPA novel phase inversion-based process for the preparation of lipid nanocarriersPharm Res200219687588012134960
- BeduneauASaulnierPAntonNPegylated nanocapsules produced by an organic solvent-free method: Evaluation of their stealth propertiesPharmaceutical Res200623921902199
- LefebvrePPMalgrangeBStaeckerHMoghadassMVan de WaterTRMoonenGNeurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitroNeuroreport1994588658688061284
- GillespieLNClarkGMBartlettPFMarzellaPLLIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitroNeuroreport200112227527911209934
- LutzMBKukutschNOgilvieALAn advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrowJ Immunol Methods19992231779210037236
- AgterbergMJVersnelHvan DijkLMde GrootJCKlisSFEnhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigsJ Assoc Res Otolaryngol200910335536719365690
- ViveroRJJosephDEAngeliSDexamethasone base conserves hearing from electrode trauma-induced hearing lossLaryngoscope2008118112028203518818553
- VersnelHAgterbergMJde GrootJCSmoorenburgGFKlisSFTime course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemideHear Res20072311–211217475424
- WefstaedtPScheperVLenarzTStoverTBrain-derived neurotrophic factor/glial cell line-derived neurotrophic factor survival effects on auditory neurons are not limited by dexamethasoneNeuroreport200516182011201416317344
- DereeJMartinsJOMelbostadHLoomisWHCoimbraRInsights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibitionClinics (Sao Paulo)200863332132818568240
- TaoXFinkbeinerSArnoldDBShaywitzAJGreenbergMECa2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanismNeuron19982047097269581763
- WolfM3g-Nanotechnology Based Targeted Drug-Delivery Using the Guinea Pig Inner Ear as a Model Target Organ [thesis]HannoverGermanyUniversity of Veterinary Medicine20099192
- SantiniMTCamettiCIndovinaPLMorelliGDonelliGPolylysine induces changes in membrane electrical properties of K562 cellsJ Biomedical Mater Res1997352165174
- ZhangYZhangWLoblerMInner ear biocompatibility of lipid nanocapsules after round window membrane applicationInt J Pharm20114041–221121921075187
- KimBYRutkaJTChanWCNanomedicineN Engl J Med2010363252434244321158659
- VonarbourgAPassiraniCDesigauxLThe encapsulation of DNA molecules within biomimetic lipid nanocapsulesBiomaterials200930183197320419329183
- LavertyPHLeskovarABreurGJA preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCIJ Neurotrauma200421121767177715684768
- KoobAOColbyJMBorgensRBBehavioral recovery from traumatic brain injury after membrane reconstruction using polyethylene glycolJ Biol Eng20082918588669
- SpoendlinHFactors inducing retrograde degeneration of the cochlear nerveAnn Otol Rhinol Laryngol Suppl198411276826431887
- AglahCGordonTPosse de ChavesEIcAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneuronsNeuropharmacology200855181718502451
- XuNEngbersJKhajaSXuLClarkJJHansenMRInfluence of cAMP and protein kinase A on neurite length from spiral ganglion neuronsHear Res20122831–2334422154930
- HeystekHCThierryACSoulardPMoulonCPhosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacityInt Immunol200315782783512807821
- StoeverTYagiMRaphaelYCochlear gene transfer: round window versus cochleostomy inoculationHear Res19991361–212413010511631
- MitchellAMillerJMFingerPAHellerJWRaphaelYAltschulerRAEffects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pigHear Res19971051–230439083802
- ChikarJAColesaDJSwiderskiDLDi PoloARaphaelYPfingstBEOver-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neuronsHear Res20082451–2243418768155
- GlueckertRBitscheMMillerJMDeafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factorJ Comp Neurol200850741602162118220258
- McGuinnessSLShepherdRKExogenous BDNF rescues rat spiral ganglion neurons in vivoOtol Neurotol20052651064107216151360