?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this paper, two novel liver-targeting nanoparticles, norcantharidin-loaded chitosan nanoparticles (NCTD-CS-NPs) and norcantharidin-associated galactosylated chitosan nanoparticles (NCTD-GC-NPs), were prepared using ionic cross-linkage. The physical properties, particle size, encapsulation efficiency, and drug release characteristics of the nanoparticles were investigated in vitro. To investigate the intestinal absorption mechanisms of the two preparations, a series of experiments was carried out, including in situ circulation method, in vitro everted gut sacs, and Ussing chamber perfusion technique. The absorption rate constants (Ka) of NCTD at different segments were found to be duodenum > jejunum > ileum > colon. The concentration had no distinctive effect on absorption kinetics, suggesting that drug absorption is not dose-dependent. The transport of NCTD was found to be inhibited by P-glycoprotein (P-gp) inhibitor, indicating that NCTD might be the substrate of P-gp. The order of the absorption enhancer effects were as follows: low molecular weight chitosan (CS-8kDa) > high molecular weight chitosan (CS-30kDa) > Poloxamer > sodium dodecyl sulfate (SDS) > sodium deoxycholate (SDCh). The results indicate that the chitosan nanoparticles can improve intestinal absorption of NCTD.
Keywords:
Introduction
Norcantharidin (NCTD), a demethylation derivative of cantharidin obtained from the dried body of the Chinese blister beetle (mylabris), is used as a traditional medicine in China.Citation1 Clinical studies have shown that NCTD is effective against the primary carcinoma of liver as an inhibitor of protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A).Citation2 Recent studies have also indicated that NCTD administered orally or intravenously has potential applications in cancer chemotherapy.Citation3–Citation7 However, the clinical use of NCTD is limited because of its significant side effects, including irritation of the urinary organs.Citation8 Moreover, NCTD usually has to be administered in high doses to strengthen its tumor inhibition effects due to its low intestinal permeability. The administration of high doses causes more intense irritation at the injection site.
Chitosan (CS), as a nontoxic and biodegradable polycationic polymer with low immunogenicity, has been investigated extensively as a formula carrier and delivery system for therapeutic molecules, such as gene, protein molecule, vaccine, and ammonium glycyrrhizinate.Citation9,Citation10 Among the various mentions of chitosan derivatives in literature,Citation11,Citation12 one can differentiate specific reactions involving the free −NH2 group at the C-2 position. Lactosaminated N-succinylchitosan was found to be a good drug carrier in the treatment of liver diseases,Citation13,Citation14 because the lactose base can bind with asialoglycoprotein receptors in the liver surface to achieve active liver targeting. To improve the therapeutic efficacy of NCTD and decrease its side effects, two novel norcantharidin-loaded liver targeting chitosan nanoparticles (NCTD-NPs), including norcantharidin-loaded chitosan nanoparticles (NCTD-CS-NPs) and norcantharidin-associated galactosylated chitosan nanoparticles (NCTD-GC-NPs), were successfully developed in our previous studies.Citation15,Citation16 By virtue of their unique uptake mechanisms and liver-specific targeting properties, NCTD nanoparticles have received much attention as a suitable alternative drug carrier system.
Although the physical properties and numerous oral dosage forms of NCTD have been studied, the understanding of NCTD absorption is very limited. Drug absorption is a key process governing in vivo bioavailability of drugs. It is affected by the physicochemical properties of the drug, dosage form, biological state of the gastrointestinal (GI) tract, and coadministered food components.Citation17 Various physicochemical parameters, such as molecular size and shape, lipophilicity, hydrogen bonding capacity, and molecular surface properties, appear to play crucial roles in the absorption of drugs that cross the intestinal barrier through passive diffusion, either through the transcellular or paracellular pathway.Citation18–Citation21 In recent years, several studies have been devoted to the assessment of relationships between such parameters and drug absorption in various model systems.Citation22–Citation25
In our present study, the absorption mechanisms of two nanoparticles of NCTD, NCTD-CS-NPs and NCTD-GC- NPs, were investigated in comparison with that of NCTD. The absorption characteristics of NCTD-NPs in the intestinal barrier were studied using in vivo transport experiments, in vitro everted sac experiments, and Ussing chamber perfusion technique.
Materials and methods
Materials
Norcantharidin (lot number 20100515) with a purity of 99.21% was purchased from Surui Medicine Chemical Co, Ltd (Suzhou, Jiangsu, China). Chitosan with a deacetylation of 90.9% and a molecular weight (Mw) from 8 to 30 kDa was supplied by Xingcheng Biochemical Co, Ltd (Nantong, China). Galactosylated chitosan (GC) was synthesized as previously described by Chung et al.Citation26 Sodium tripolyphosphate (TPP) was obtained from the National Drug Group (Shanghai, China) and Pluronic F68 was purchased from Fluka Chemika (Buchs, Switzerland). Other chemicals and solvents were of analytical grade. Deionized twice-distilled water was used throughout the study.
Male Sprague–Dawley (SD) rats weighing from 220 to 270 g were obtained from the Laboratory Animals Center of the Medical College, Soochow University (Suzhou, China). All animal procedures were conducted in accordance with the guidelines for the care and use of animals of the National Institutes of Health.
Samples preparation
NCTD-NPs were prepared according to a procedureCitation15,Citation16 based on the ionic gelation of CS/GC with TPP anions, which was slightly modified. Briefly, 200 mg CS/GC was dissolved in 100 mL acetic acid aqueous solution containing 0.4 mg/mL NTCD. TPP aqueous solution was then added to the chitosan solution drop by drop under magnetic stirring, resulting in cross-linkage. After reaction for 10 minutes, 125 mg Pluronic F68 was dissolved in the solution as a stabilizer and 2% acetic acid aqueous solution concentration containing chitosan:TPP (50:20 V/V) was stirred at a constant rate of 500 rpm for 2 hours.
In the method above, NCTD gelata was prepared using equivalent distilled water instead of TPP.
The perfusion solution was a Krebs-Ringer buffer solution, which contained 7.8 g NaCl, 0.35 g KCl, 1.37 g NaHCO3, 0.02 g MgCl2, 0.22 g NaH2PO4, and glucose in 1.48 g/1000 mL purified water.
Particle size analysis and morphological characteristics
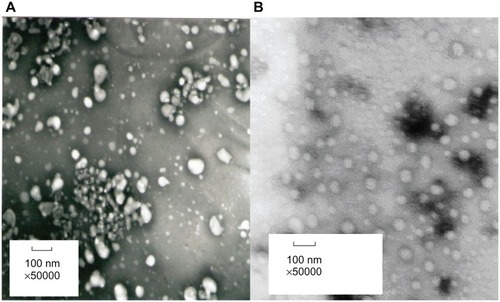
Particle size analysis of the NPs was performed by dynamic light-scattering measurement using the Zetasizer Nanoparticle Analyser (Malvern Instruments Ltd, Malvern, UK) with a range from 0.6 to 600 nm. Morphological characteristics of the nanoparticles were studied through transmission electron microscopy (TEM; JEM-1230, JEOL, Japan). Colloid samples of the NPs were condensed using a Vivaflow 50 Ultrafilter (30,000 Da polysulfone resin filter membrane; Sartorius AG, Göttingen, Germany). A drop of nanoparticle suspension was placed onto a carbon film coated on a copper grid for TEM. Negative staining was performed using phosphotungstic acid (0.05%, w/w). Observation was performed at 80 kV.Citation27
Evaluation of drug entrapment efficiency and loading capacity
The concentration of NCTD in the nanoparticles was determined by a reversed-phase HPLC method. The HPLC system was composed of two pumps (LC-10AT VP, Shimadzu, Japan) and a UV-vis detector (SPD-M10A VP, Shimadzu) set at 210 nm. The chromatographic column was a Luna C18 (4.6 × 250 mm; Hypersil ODS2, Elite, Dalian, China) maintained at 30°C. The mobile phase was a mixture of acetonitrile: H2O = 10:90 (adjusted to pH 3.1 by adding phosphoric acid), and the flow rate was 0.8 mL/min.
An appropriate volume of NPs colloid was filtered through a 0.45 μm filter to remove nonsoluble aggregate residues following the method in the previous report.Citation28,Citation29 The filtered colloid was then ultracentrifuged at 40,000 rpm for 45 minutes using the Optima MAX Centrifuge (Berkman Co, Ltd, USA). The supernatant was sampled and the NCTD concentration in the supernatant was determined by HPLC.
Encapsulation efficiency (EE%) and drug loading efficiency (DL%) were calculated using the following equations:
and
where T is the total NCTD in the colloid, F is the free NCTD in the supernatant, and W is the weight of NPs.
In vitro release studies
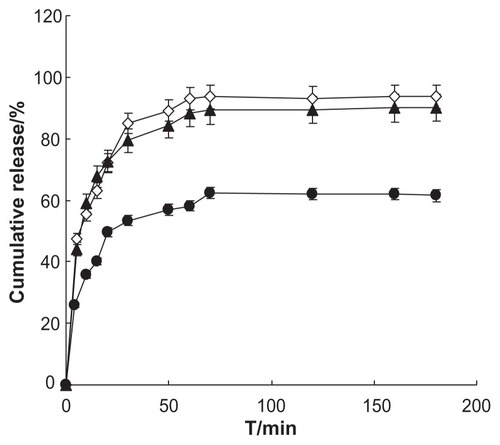
Drug release from NCTD- NPs in vitro was measured using a dialysis method and compared with that of the NCTD solution. Briefly, 6 mL NCTD-NPs was placed into a dialysis tube (8000–14,000 MWCO) and sealed at both ends with clips. The dialysis tube loaded with nanoparticles was then placed into a beaker containing 30 mL deionized twice-distilled water and incubated with stirring for 3 hours at 37°C ± 0.5°C. At various time points, 0.5 ml aliquots were withdrawn from the beaker and replaced with an equal volume of media. The NCTD concentrations were then measured by HPLC. The cumulative percentage of NCTD released was calculated as the amount of NCTD released from the solution or nanoparticles relative to the total amount of NCTD present in the formulation.
In vivo transport studies
An in vivo recirculation perfusion technique was used and performed as reported previously.Citation30–Citation32 This method was used to determine absorption characteristics at four different segments: Citation33 duodenum, jejunum, ileum, and colon. Three different concentrations were studied: 140 μg/mL, 160 μg/mL, and 180 μg/mL. The perfusate was prepared by dispersing NCTD/NCTD-NPs with different drug concentrations in the Krebs–Ringer BH solution (100 mL) containing phenol red (20 μg/mL) for the purpose of correcting the perfusion volume. Next, 0.5 mL of the sample solution was used to detect the phenol red concentration by UV detection at 559 nm. Another 0.5 mL was digested by 88% methanoic acid (0.33 mL) and then centrifuged for 10 minutes at 4000 rpm. Next, 100 μL of the supernatant was separated and placed into another centrifuge tube, into which 200 μL methanol was added. After vortexing for 3 minutes, the extraction was centrifuged for 3 minutes at 10,000 rpm. Afterward, 20 μL of the supernatant was injected onto the HPLC for determination of NCTD using the same HPLC method described previously in “Evaluation of drug entrapment efficiency and loading capacity.” The absorption percentage (PA), absorption rate constant (Ka) and absorption half-life (t 1/2a) for the NCTD solution/NCTD-NPs were calculated.
In vitro transport studies
Everted sacs of the rats were prepared using the method described previously,Citation34 which was also used to determine the absorption characteristics at different segments and with different concentrations. All samples were treated using the method described in “In vivo transport studies.”
P-gp dependency of NCTD
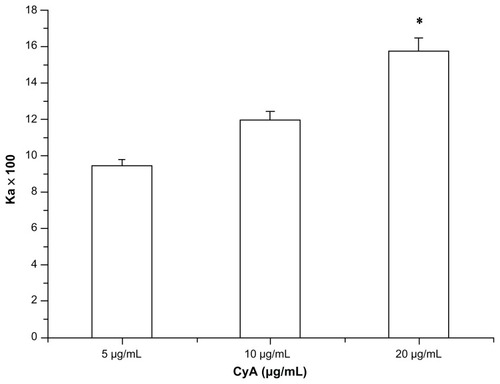
To examine the saturation potential of the P-gp function, the transport of NCTD and NCTD-NPs at the duodenum was investigated using the everted sacs method. The P-gp inhibitor, Cyclosporin A(CyA), a well-known P-gp substrate, was used as the positive control group at concentrations of 5, 10, and 20 μg/mL.
Effects of absorption enhancers
The effects of improving absorption of NCTD and NCTD-NPs used as enhancers at the duodenum were investigated using the chamber method. Five enhancers, namely, low molecular weight chitosan (CS-8kDa), high molecular weight chitosan (CS-30kDa), Poloxamer, sodium dodecyl sulfate (SDS), and Sodium deoxycholate (SDCh), were used with concentrations of 0.1%, 0.5%, and 1.0%. Apparent permeability coefficients (Papp) were calculated according to the equation below:
where dQ/dt is the permeability rate, A is the diffusion area, and C0 is the initial donor solution concentration.
Statistics
Values are expressed as χ̄ ± s (mean ± SD). Data were analyzed by one-way ANOVA with the post-hoc Tukey’s test applied for paired comparisons (SPSS 16, SPSS Inc, Chicago, USA). Moreover, the criterion of statistical significance was taken as *P < 0.05, **P < 0.01, or #P < 0.05, ##P < 0.01.
Results and discussion
Physicochemical properties of NCTD-NPs
The physicochemical characteristics of NCTD-NPs, including particle size and yield, drug loading, and encapsulation efficiency, are summarized in . The two liver-targeting NCTD-loaded nanoparticles, NCTD-CS-NPs and NCTD-GC-NPs, had small particle sizes of about 100 nm and displayed an encapsulation efficiency of about 50%, leading to the final NCTD loading values of about 10%. The values of the NCTD-GC-NPs were higher than those of the NCTD-CS-NPs.
Table 1 Physicochemical characteristics of NCTD nanoparticles (n = 3)
The majority of the fenestrate of the liver sinusoid was reported to be typically less than 200 nm in diameter.Citation35 Permeability of the vascellum endothelium existing in the liver tumor was also enhanced greatly.Citation36 Thus, the novel NCTD-CS-NPs could pass through the fenestrate sinusoid and accumulate in the tumor sites of the liver. The small particle size could reduce the adverse reaction of NCTD-NPs on normal hepatic cells and reduce passive liver-targeting. The two NCTD-NPs appeared spherical in shape, as shown in .
In vitro drug release of NCTD-NPs
The data results of in vitro NCTD release characteristics are presented in . The release curve conforms to the Weibull model; the soluble equation is plotted using the data and a good fit is achieved. Td, T50 and other parameters are calculated according to the soluble equation; the results are shown in . Analysis of the data shows that Td, T50 of the two formulations are significantly different (P < 0.01) and the parameters of NCTD-NPs are significantly less than the gelata, which means that NCTD-NPs are more conducive for the release of the drugs than the gelata.Citation37 In conclusion, the in vitro release of nanoparticles was found to follow the Weibull equation (data not shown) by data fitting (r > 0.9500). The drug release of NCTD-NPs was more complete than that of NCTD gelata. It was also argued that NCTD-NPs were more conducive to drug delivery than NCTD gelata.Citation38
Table 2 Soluble equation and parameters (n = 5)
Absorption properties of NCTD-NPs in rat intestines
Absorption of NCTD-NPs in various intestinal segments of rats
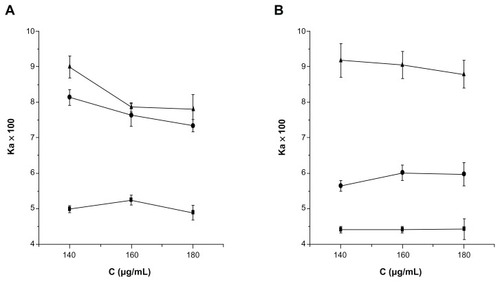
Regional differences in intestinal drug permeability have been attributed to the difference in epithelial surface area, pore radius of the paracellular pathway, mucus layer, and regional membrane fluidity.Citation39 In this NCTD-NPs study, the absorption tests were performed on four different intestinal segments to test whether the intestinal absorption of NCTD-NPs exhibited site-dependent changes. The intestinal absorption kinetics of NCTD-NPs and NCTD solution at a dose of 160 μg/mL were investigated. The results are shown in , from which it could be concluded that NCTD absorption through the duodenum and the jejunum were much higher than those through the ileum and colon. The Ka of NCTD absorption was duodenum > jejunum > ileum > colon. In addition, drug absorption is affected mainly by the physicochemical properties of drugs, drug metabolism in the gastrointestinal mucosa, lipophilicity, unionized fraction of drugs, molecular size, and drug solubility.Citation40 Changing these absorption parameters along the gastrointestinal tract may contribute to the differences in site-specific absorption of melatonin. Regional differences in the functional expression of P-gp might be one of the possible factors for site-dependent absorption. Compared to the in vitro everted sac method, Ka of the in vivo method was much higher while t1/2a was much lower. This is because during the in vitro method, the mesentery and blood vessels were cut off, which leads to less absorption. Among the three formulations, the order of Ka was NCTD-GC-NPs > NCTD-CS-NPs > NCTD solution. It has been suggested that chitosan nanoparticles could improve the intestinal absorption of NCTD because chitosan can trigger the opening of tight junctions.Citation41 In addition, there are plenty of Peyer’s patches and M cells in rat intestines, and absorption via the lymphatic tissues is an important route for drugs with low oral bioavailability.Citation42–Citation44 Nanoparticles, as compositions of intestine mucous membrane, have an important effect on the enhancement of absorption via the lymphatic route.Citation45
Table 3 Absorption parameters at various segments in rat intestines (n = 3)
Absorption of NCTD-NPs in various concentrations in rats
As shown in , the intestinal absorption percentages of NCTD at higher concentration were greater than those at lower concentrations in vivo or in vitro. However, the different concentrations did not induce a difference in the transport of NCTD (P > 0.05). It has been suggested that the concentration has no distinctive effect on absorption kinetics. A comparison of absorption kinetics among different concentrations demonstrated a non-concentration-dependent change. Results indicated that the intestinal absorption of NCTD-NPs is via passive transfer by diffusion across the intestinal membranes.Citation46
Figure 3 Intestinal absorption of NCTD-CS-NPs (×) and NCTD-GC-NPs (⋄) compared with that of NCTD solution (▴) at different concentrations in vivo (A) and in vitro (B).
Abbreviations: NCTD-CS-NPs nanoparticles, norcantharidin-loaded chitosan nanoparticles; NCTD-GC-NPs, norcantharidin-associated galactosylated chitosan nanoparticles.
P-gp dependency of NCTD
P-glycoprotein efflux pumps are located predominantly in the apical membranes of the epithelia (eg, small intestine, colon) and significantly limit oral absorption of a number of drugs.Citation47
The transport of NCTD across everted gut sacs in solutions with added 5, 10, and 20 μg/mL CyA is shown in . Results showed that the NCTD cumulative percentage in the duodenum at 120 minutes was significantly enhanced by CyA (P < 0.05). As the CyA concentration increased, the cumulative concentration of NCTD gradually increased. When the concentration of CyA was 20 μg/mL, the accumulative concentration of NCTD at 120 min was more than 200% of the control (in the absence of CyA).
Effects of absorption enhancers
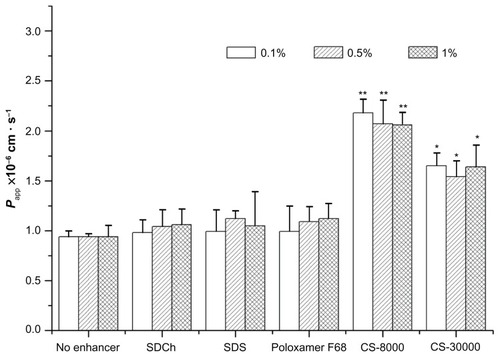
The results are shown in . Compared with the NCTD solution, absorption enhancers can enhance the absorption rate of the drug. The order of cross-mucosal permeability is CS-8-KDa > CS-30KDa > Poloxamer > SDS > SDCh. Among them, 0.1% CS-8KDa showed the most significant absorption promotion effect. With different concentrations of absorption enhancers to promote absorption between the different degrees, SDCh and Poloxamer absorption increased as the concentration was increased. SDS as an anionic surfactant promotes the role of low concentration; upon reaching a certain concentration, its effect decreased. The effect of CS-8KDa on absorption decreased as the concentration was increased. Thus it is proven that drugs containing low molecular weight chitosan as a carrier can help to promote intestinal absorption. The reason may be as follows: on the one hand, chitosan has a biological adhesive function, which can extend drug residence time in the intestines; on the other hand, because of its positively charged nature, it can connect with a variety of negatively charged protein bindings in the intestinal mucosa effectively, thereby inhibiting the physiological functions of certain proteins.Citation48
Figure 5 Effects of absorption enhancers on the apparent permeability coefficients (Papp) for different concentrations.
Notes: *P < 0.05 versus no enhancer group; **P < 0.01 versus no enhancer group.
Abbreviations: CS, chitosan; Papp, permeability coefficients; SDCh, sodium deoxycholate; SDS, sodium dodecyl sulfate.
Conclusion
Three intestinal absorption models in vivo and in vitro were used. They are simple, easy, and effective for drug absorption studies. The intestinal absorption mechanisms of NCTD solution and NCTD-NPs were investigated. Results of the study showed that NCTD-NPs significantly enhance drug absorption compared with the NCTD solution. Intestinal absorption kinetics provides methods for a more profound study of the process in vivo and in the development of new oral drugs.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program No 2007CB935800), National Key Program of New Drug Innovation (No 2009zx09310-001), Technology Support Program of Jiangsu Province (BE2011670) and a project founded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangGSMedical uses of mylabris in ancient China and recent studiesJ Ethnopharmacol19892621471622689797
- WangGSHydrolyze of norcantharidin and subtracting methyla alleviate stimulation of renal systemChin Pharm Bull19831871820
- YiSNWassJVincentPIlandHInhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitroLeuk Res199115108838861921448
- EfferthTLiPCKonkimallaVSKainaBFrom traditional Chinese medicine to rational cancer therapyTrends Mol Med200713835336117644431
- ShanHBCaiYCLiuYZengWNCytotoxicity of cantharidin analogues targeting protein phosphatase 2AAnticancer Drugs200617890591116940800
- EfferthTRauhRKahlSMolecular modes of action of cantharidin in tumor cellsBiochem Pharmacol200569581181815710358
- KokSHHongCYKuoMYComparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytesOral Oncol2003391192612457717
- LiuXHBlazsekIComissoMEffects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cellsEur J Cancer199531A69539637646929
- ChewaJLWolfowiczCBMaoHQLeongKWChuaKYChitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in miceVaccine20032121–222720272912798609
- WuYYangWLWangCCHuJHFuSKChitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinateInt J Pharm20052951–223524515848008
- LixinWHaibingHXingTRuiyingSDaweiCA less irritant norcantharidin lipid microspheres: Formulation and drug distributionInt J Pharm20063231–216116716828998
- BalaIHariharanSKumarMNPLGA nanoparticles in drug delivery: the state of the artCri Rev Ther Drug Carrier Sys2004215387422
- KatoYOnishiHMachidaYLactosaminated and intact N-succinylchitosans as drug carriers in liver metastasisInt J Pharm20012261–29310611532574
- KatoYOnishiHMachidaYBiological characteristic of lactosaminated N-succinyl-chitosan as a liver-specific drug carrier in miceJ Control Release200170329530711182200
- ZhangWLiuYChenXYTwo modelling data analytical methods applied to optimise the preparation of norcantharidin chitosan nanoparticlesJ Exp Nanosci201053271284
- WangQZhangLHuWNorcantharidin-associated galactosylated chitosan nanoparticles for dual hepatocyte-targeted deliveryNanomedicine20106237138119699319
- WagnerDSpahnLHHanafyAKoggelALangguthPIntestinal drug efflux: formulation and food effectsAdv Drug Deliv Rev200150Suppl 1S133111576693
- KarlsMSRushBDWilkinsonKFVidmarTJBurtonPSRuwartMJDesolvation energy: a major determinant of absorption, but not clearance, of peptides in ratsPharm Res1991812147714811808609
- ConradiRAHilgersARHoNFMaggioraLLThe influence of peptide structure on transport across Caco-2 cell. II. Peptide bond modification which results in improved permeabilityPharm Res1992934354391614980
- StewartBHChanOHLuRHComparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humansPharm Res19951256936997479555
- PalmKLuthmanKUngellALStrandlundGArturssonPCorrelation of drug absorption with molecular surface propertiesJ Pharm Sci199685132398926580
- BarringtonRWilliamsonGBennettRNDavisBDBrodbeltJSKroonPAAbsorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell ModelJ Funct Foods200911748720046888
- HillgrenKMKatoABorchardtRTIn vitro systems for studying intestinal drug absorptionMed Res Rev1995152831097537838
- RubasWCromwellMEMShahrokhZFlux measurements across Caco-2 monolayers may predict transport in human large intestinal tissueJ Pharm Sci19968521651698683442
- UngellALIn vitro absorption studies and their relevance to absorption from the GI tractDrug Dev Ind Pharm199723879892
- ChungTWYangJAkaikeTPreparation of alginate/galactosylated chitosan scaffold for hepatocyte attachmentBiomaterials200223142827283412069321
- ZhangXNTangLHYanXYZhangQAlbendazole polybutycyanocrylate nanoparticles-Preparation, pharmaceutical properties and tissue distribution in ratsLett Drug Des Discov20063275279
- BanerjeeTMitraSSinghAKSharmaRKMaitraAPreparation, characterization and biodistribution of ultrafine chitosan nanoparticlesInt J Pharm20022431–29310512176298
- LuEFranzblauSOnyukselHPopescuCPreparation of amino-glycoside- loaded chitosan nanoparticles using dextran sulphate as a counterionJ Microencapsul200926434635418726818
- RubinsteinAPathakYVKleinsternJRechesABenitaSIn vitro release and intestinal absorption of physostigmine salicylate from submicron emulsionsJ Pharm Sci19918076436471941560
- NornooAOZhengHLopesLBJohnson-RestrepoBKannanKReedROral microemulsions of paclitaxel: in situ and pharmacokinetic studiesEur J Pharm Biopharm200971231031718793723
- LingWRuiLCHuaJXIn situ intestinal absorption behaviors of tanshinone IIA from its inclusion complex with hydroxypropyl- β-cyclodextrinBiol Pharm Bull2007301918192217917262
- MichelCAprahamianMDefontaineLCouvreurPDamgéCThe effect of site of administration in the gastrointestinal tract on the absorption of insulin from nanocapsules in diabetic ratsJ Pharm Pharmacol1991431151676051
- SasakiITanakaKFujitaTMurakamiMYamamotoAMuranishiSIntestinal absorption of azetirelin, a new thyrotropin-releasing hormone (TRH) analogue 11: in situ and in vitro absorption characteristics of azetirelin from the rat intestineBiol Pharm Bull19951879769797581253
- LiangHFYangTFHuangCTChenMCSungHWPreparation of nanoparticles composed of poly(γ-glutamic acid)-poly(lactide) block copolymers and evaluation of their uptake by HepG2 cellsJ Control Release2005105321322515916830
- WuJLiuLYenRPolycationic liposome-mediated extracellular superoxide dismutase gene delivery prevents acute liver injury in miceHepatology2003381240241
- YangHYZhangMGTargeting Drug Delivery SystemJournal of China-Japan Friendship Hospital2001155292294
- LiQZUsing Weibull function to seek dissolution parametersChinese Journal of Hospital Pharmacy19911113031
- MasaokaYTanakaYKataokaMSakumaSYamashitaSSite of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tractEur J Pharm Sci2006293–424025016876987
- De BoerAGDrug Absorption Enhancement: Concepts, Possibilities, Limitations and TrendsChur, SwitzerlandHarwood Academic Publishers GmbH1994367389
- Cano-CebriánMJZornozaTGraneroLPolacheAIntestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: a target for drug deliveryCurr Drug Deliv20052192216305404
- HaussDJFogalSEFicorilliJVLipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitorJ Pharm Sci19988721641699519148
- HolmRMüllertzAPedersenGPKristensenHGComparison of the lymphatic transport of halofantrine administered in disperse systems containing three different unsaturated fatty acidsPharm Res20011891299130411683243
- O’DriscollCMLipid-based formulations for intestinal lymphatic deliveryEur J Pharm Sci200215540541512036717
- KhooSMShacklefordDMPorterCJEdwardsGACharmanWNIntestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogsPharm Res20032091460146514567642
- WanHJTangXYangDLAbsorption kinetics of ofloxacin in rats in situChinese Journal of Hospital Pharmacy2005257597599
- JiHYLiZZSunJRAbsorption mechanisms of protein and peptide in oral administrationShandong Pharm Ind2003223132
- CollnotEMBaldesCWempeMFMechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidityMol Pharm20074346547417367162