?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this paper, novel liver-targeting nanoparticles (NPs), lactosyl-norcantharidin (Lac-NCTD)-associated N-trimethyl chitosan (TMC) NPs (Lac-NCTD-TMC-NPs), were prepared using ionic cross-linkage. The physical properties, particle size, and encapsulation efficiency of the nanoparticles were then investigated. The continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells (Caco-2) cell monolayer model was used to study the transport mechanism of Lac-NCTD, and the effects of factors such as time, temperature, pH level, drug concentration, enhancers, and inhibitors. This model was also used to indicate the differences among Lac-NCTD, Lac-NCTD-associated chitosan NPs (Lac-NCTD-CS-NPs), and Lac-NCTD-TMC- NPs in the absorption and transportation of membranes. Drug concentration levels were measured using high-performance liquid chromatography. Active transport and paracellular transport were suggested to be both the primary and secondary mechanisms for Lac-NCTD absorption, respectively. Lac-NCTD uptake and absorption were not controlled by pH levels, but were positively correlated to uptake time, and negatively correlated to temperature. The basolateral to apical apparent permeability coefficients (Papps) were higher than those of the apical to basolateral values. The inhibitor of P-glycoprotein and the multidrug resistance-associated protein 2 significantly enhanced the uptake amount of Lac-NCTD. Compared with Lac-NCTD, Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs significantly enhanced drug absorption. Additionally, the latter exhibited stronger action. Lac-NCTD-NPs could penetrate the plasma membrane of Caco-2 cells and translocate into the cytoplasm and even into the nucleus. Nanoparticles were uptaken into Caco-2 cells through the endocytosis pathway.
Introduction
Lactosyl-norcantharidin (Lac-NCTD) is synthesized by modifying NCTD with a lactobiose-bearing galactose group to achieve active targeting. The galactose group can combine with the asialoglycoprotein receptor situated on the hepatocyte membrane.Citation1,Citation2 A previous study by the present researchers demonstrated that, compared with NCTD, Lac-NCTD has significantly higher therapy efficiency and lower toxicity.Citation3
Chitosan (CS) is a biocompatible, biodegradable, nontoxic polymer. It has been extensively investigated as a formula carrier in delivery systems for therapeutic molecules.Citation4,Citation5 However, the application of CS has been hampered largely by its solubility only in acidic environments, with pH values below 6.0. On the other hand, N-trimethyl chitosan (TMC), a partially quaternized chitosan (CS) derivative, can be soluble in the entire pH range and encompasses all the advantages of CS. It can also form nano-complexes with anionic compounds such as drugs, proteins, and DNA through ionotropic gelation. Hence, TMC has potential applications in targeted drug-delivery applications.Citation6
To improve the therapeutic efficacy of Lac-NCTD and decrease its side effects, Lac-NCTD-loaded liver-targeting chitosan nanoparticles (Lac-NCTD-CS-NPs) were successfully developed in previous studies.Citation3 Novel liver-targeting nanoparticles Lac-NCTD-associated N-trimethyl chitosan nanoparticles (Lac-NCTD-TMC-NPs) were then investigated and discussed in the present study.
For most therapeutic agents, the oral route is still the most convenient for drug administration. Based on this consideration, the present study investigated the absorption mechanisms of Lac-NCTD, and two nanoparticles. The human Caco-2 cell line has been widely used as a potent in-vitro model to predict drug absorption in humans, to explore the mechanisms of drug absorption, and to identify substrates or inhibitors of transporters.Citation7
The present study aims to use Caco-2 cells to develop a theoretical basis for the understanding of the oral absorption of Lac-NCTD and its nanoparticles, as well as to further characterize both its uptake and transepithelial transport across Caco-2 cells. The present study could provide a theoretical basis for the formulation and development of a rational design of new agents, as well as for the selection of its administration route.
Methods and materials
Materials
Lac-NCTD and TMC were synthesized in our laboratory. CS (molecular weight [MW] of 8 kDa to 10 kDa, deacetylation degree [DD] 93.1%) was purchased from Xingcheng Biochemical Co, Ltd (Nantong, China). Sodium tripolyphosphate (TPP) was obtained from Sinopharm Group Co, Ltd (Shanghai, China).
Caco-2 cells were obtained from Fu Dan University (Shanghai, China). Cell culture reagents were purchased from Gibco (Grand Island, New York, NY). All other reagents and solvents were of analytical grade and commercially available.
High-performance liquid chromatography (HPLC) was carried out using a chromatograph consisting of two pumps (LC-10AT VP, Shimadzu, Japan) and a UV-vis detector (SPD-M10A VP, Shimadzu, Japan) set at 220 nm. The reversed-phase column used was a Hypersil ODS-C18 (5 μm in 4.6 × 250 mm, Elite, Dalian, China) set to 25°C. The mobile phase was a mixture of acetonitrile and H2O (at a ratio of 10:90, adjusted to pH 3.2 by adding phosphoric acid) and the flow rate was 0.8 mL/minute.
Preparation of Lac-NCTD-TMC-NPs
Lac-NCTD-TMC-NPs were prepared according to the procedure first reported by Calvo and colleagues,Citation8 which was based on the ionic gel of TMC with TPP anions. Briefly, 0.15 g TMC was dissolved in 50 mL distilled water containing 0.03 g Lac-NCTD. Then, 25 mL of the TPP (1.2 mg/mL) aqueous solution was drip-fed into the TMC solution under magnetic stirring (500 rpm) at 30°C, resulting in crosslinkage. Finally, the opalescent suspension was filtered through a 0.45 μm filter to remove any insoluble aggregate residue, and to obtain the Lac-NCTD-TMC-NPs suspension.
Evaluation of Lac-NCTD-TMC-NPs
The particle distribution and zeta potential of Lac-NCTD-TMC- NPs’ suspension were measured using a Zetasizer nanoparticle analyzer (model HPP-5001; Malvern Instruments, Malvern, UK), which works on the principle of laser diffraction analysis. The shape of the Lac-NCTD-TMC-NPs particles was determined through transmission electron microscopy (TEM) analysis, which was performed using an H-600 transmission electron microscope (Hitachi, Tokyo, Japan).
The drug entrapment efficiency (EE) and drug-loading (DL) amount of Lac-NCTD-TMC-NPs were determined as described previously.Citation9 Briefly, the Lac-NCTD-TMC-NPs’ suspension was ultracentrifuged (250,000 × g for 30 minutes at 10°C) in an Optima MAX Centrifuge (Beckman Instruments, Palo Alto, CA). The supernate was sampled, and the Lac-NCTD concentration in the supernate was tested using a reversed-phase HPLC method.
Cell culture
Caco-2 from a passage between 30 and 50 were used in the experiments. The cells were grown in conditions similar to those reported earlier. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4.5 g/L), which was supplemented with 15% fetal bovine serum, 1% nonessential amino acids, 1% L-glutamine, and 0.5% penicillin/streptomycin. All cell cultures were maintained in a humidified incubator at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. The culture medium was changed every two days, and the cells were passaged at a ratio of 1:3, every 3 to 5 days (at 70% to 80% confluence), using 0.25% trypsin and 0.02% EDTA at 37°C.
Uptake studies
Caco-2 cells were plated on 12-well tissue culture plates (Corning, Corning, NY) at a density of 1 × 105 cells/cm2. The cell monolayers were used on the 15th day for uptake experiments.
Hank’s balanced salt solution (HBSS; pH 7.4) was used as the incubation medium. The Caco-2 cell monolayers were washed twice with the incubation medium, and then preincubated with 1 mL of the incubation medium for 15 minutes at 37°C. The medium was then removed after the preincubation period and uptake was initiated by adding 1 mL of the preincubated drug solution (Lac-NCTD, Lac-NCTD-CS-NPs, or Lac-NCTD-TMC-NPs). At various time points, the cells were quickly washed three times with cold HBSS, and then scraped off using a cell scraper (Corning). Next, the cells were homogenated, vortexed, and ultracentrifuged. The supernate was sampled, and the accumulated amount of Lac-NCTD was assayed by HPLC. The protein content was measured according to Bradford’s method.Citation10
Transport studies
Caco-2 cells were seeded at a density of 1 × 105 cells/cm2 onto a permeable polycarbonate insert (1.1 cm2, 1 μm pore size) (Millipore, Billerica, MA) in 12-well tissue culture plates (Corning). On the 21st day, the cell monolayers were used for transepithelial transport experiments. The integrity of the monolayers grown on the permeable membrane was assessed using the transepithelial electrical resistance (TEER) of the monolayers, through a Millicell-ERS apparatus (Millipore) and phenol red transport. Caco-2 monolayers could only be used in the experiments when TEER values reached 700 Ω · cm2 and phenol red permeability was <0.5% per hour.
The culture medium of both sides was removed by aspiration, and the Caco-2 cell monolayers were washed twice with the incubation medium. After washing, the monolayers were preincubated for 15 minutes at 37°C with 0.5 and 1.5 mL of the incubation medium in the apical and basolateral sides, respectively. After the preincubation, the medium was removed immediately, and the incubation medium containing Lac-NCTD and its nanoparticles (Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs) were added to either the apical side (0.5 mL) or the basolateral side (1.5 mL). The unmodified incubation medium was then added to the opposite side (basolateral, 1.5 mL; apical, 0.5 mL). Next, 50 μL samples were taken from the receiver compartment at intervals of 5, 15, 30, 45, and 60 minutes, and replaced with a fresh buffer. Concentrations in the transepithelial transport samples were determined using the HPLC method as described above.
Subcellular localization of NPs
To detect cellular uptake using a laser scanning confocal microscope, Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs were labeled with fluorescein isothiocyanate (FITC). The FITC-labeled CS and TMC were prepared by the reaction between the isothiocyanate group of FITC and the primary amino group of CS and TMC, as described by Huang and colleagues.Citation11
The Caco-2 cells were seeded on 6-well dishes at a density of 1 × 105 cells/cm2. FITC-Lac-NCTD-TMC-NPs and FITC-Lac- NCTD-TMC-NPs were added to the cells and incubated for 15, 30, and 60 minutes. The cells were washed three times with PBS, fixed with 4% paraformaldehyde, and then stained with 10 μg/mL Hochest 33258 (Sigma-Aldrich, St Louis, MO). Micrographs were taken on a Nikon laser scanning confocal microscope (Nikon Eclipse TE2000-E; Nikon, Tokyo, Japan).
Uptake process of NPs
The Caco-2 cells were seeded on 6-well dishes at a density of 1 × 105 cells/cm2. Lac-NCTD-TMC-NPs were added to the cells and incubated for 4 hours. The cells were then washed three times with PBS, twice with distilled water, and then fixed with 4% paraformaldehyde. Micrographs were taken on an atomic force microscope (AFM; MultiMode V; Veeco Instruments, Woodbury, NY).
Data and statistical analysis
The Lac-NCTD EE and DL amounts were calculated as follows:
where T is the total Lac-NCTD in the colloid, F is the free Lac-NCTD in the supernate, and W is the weight of Lac- NCTD-TMC-NPs.
The Papp was calculated according to the equation
where dQ/dt is the slope of the cumulative amount transported during the time course of the period studied, A is the area of the inserts, and C0 is the starting concentration.
Permeability direction ratios (PDR) were calculated according to the following equation:
where AP–BL is the apical to basolateral transport and BL – AP is the basolateral to apical transport.
All data were reported as mean ± standard deviation for at least three independent samples. Data were analyzed by one-way ANOVA with the post hoc Tukey’s test applied for paired comparisons using SPSS software (version 16.0; SPSS, Chicago, IL). The criteria for statistical significance were taken as *P < 0.05, **P < 0.01, #P < 0.05, and ##P < 0.01 (–; , ).
Table 1 Uptake of Lac-NCTD on Caco-2 cells with different preparations (n = 3)
Table 2 Papp of Lac-NCTD at different concentrations in Caco-2 cells (n = 3)
Table 3 Papp of Lac-NCTD on Caco-2 cells with different preparations (n = 3)
Results
Preparation and evaluation of Lac-NCTD-TMC-NPs
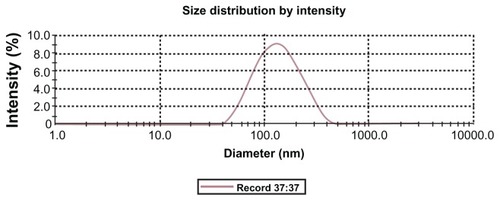
Lac-NCTD-TMC-NPs were prepared through an ionic crosslinking process and evaluated in terms of the NPs EE, DL, particle size, and polydispersity index (PDI). The particle size of Lac-NCTD-TMC-NPs was around 120 nm (PDI < 0.3) (). The zeta potential of Lac-NCTD-CS-NPs was 28.08 ± 3.95 mV, whereas that of Lac-NCTD-TMC-NPs was 37.37 ± 3.62 mV, mainly because of the high degree of TMC quaternization (65%).
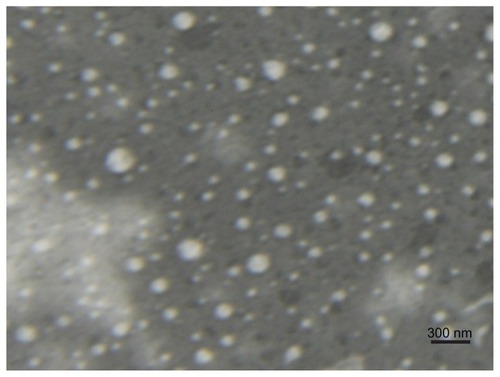
The EE and DL of Lac-NCTD-TMC-NPs were 69.29% ± 0.76% and 9.1% ± 0.07%, respectively. A TEM image of the Lac-NCTD-TMC-NPs is shown in . Under TEM, most of the NPs appeared to be uniform and round, with the majority of particles separated from one another.
Linearity, precision, and accuracy of Lac-NCTD determination
Under the conditions described above, the Lac-NCTD retention was at 5.1 minutes. The active component test was uncontaminated, and also had high specificity. In a 0.1 μg/mL to 52 μg/mL concentration range, Lac-NCTD in PBS/blank cells homogenate showed a good linear relationship. Recovery was 98.99% to 102.46% within the linear range, and relative standard deviation was below 5%, both of which fitted the requirements for analyzing the biological specimen.
Uptake of Lac-NCTD into Caco-2 monolayers
Effect of time on the uptake of Lac-NCTD
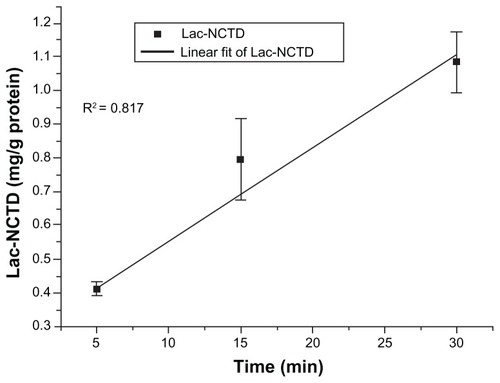
The uptake of Lac-NCTD (250 μg/mL) into Caco-2 monolayers was shown to be linear in relation to time, from 0 minutes to 30 minutes at 37°C (). To ensure the optimal absorption rate, the uptake time of the drug should be determined within its linear absorption range. Considering the nature of the drug, cell characteristics, and operational feasibility, 15 minutes was set as the uptake time for the subsequent experiments.
Effect of concentration on the uptake of Lac-NCTD
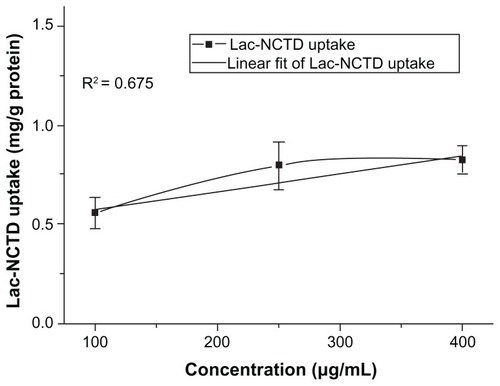
Concentration dependence or saturation was observed for the uptake of Lac-NCTD in the concentration range of 100 μg/mL to 400 μg/mL ().
Effect of pH and temperature
The uptake amount of Lac-NCTD (250 μg/mL) into Caco-2 monolayers in pH 6.0 and 7.4 were (0.82 ± 0.23) and (0.80 ± 0.15) mg/g protein, respectively. There were no significant differences between the uptake amounts in the two pH values (P > 0.05). Hence, the subsequent experiments were performed using the medium at pH 7.4.
The uptake amount of Lac-NCTD (250 μg/mL) into Caco-2 monolayers in 4°C and 37°C were (1.35 ± 0.13) and (0.80 ± 0.15) mg/g protein, respectively. The uptake of Lac- NCTD across Caco-2 cell monolayers showed temperature dependence, and was enhanced at low temperatures.
Effect of protein inhibitor
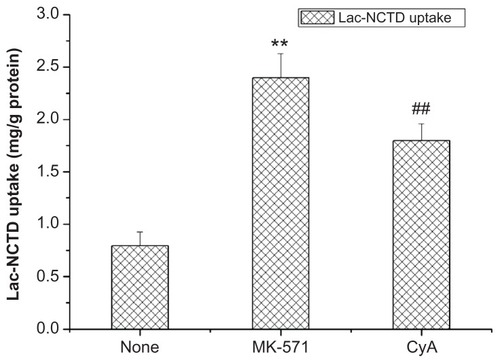
The saturation possibility of the P-glycoprotein (P-gp) and the multidrug resistance-associated protein 2 (MRP2) function was investigated in the present study. Hence, the uptake of Lac-NCTD across Caco-2 in solutions with added P-gp or MRP2 inhibitors 20 μmol/L cyclosporine (CyA) or 50 μmol/L MK-571 was investigated. Results are shown in , which indicate that CyA and MK-571 can facilitate the uptake procedure. However, the latter had an even stronger action.
Figure 5 Effect of CyA and MK-571 on Caco-2 cells uptake.
Notes: **P < 0.01 versus control group; ##P < 0.01 versus control group. Error bars represent standard error of the mean value for three determinations.
Abbreviations: CyA, cyclosporine; MK-571, Sigma-MK-571 sodium salt hydrate; Caco-2, continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells.
Uptake of Lac-NCTD-NPs into Caco-2 monolayers
The uptake of Lac-NCTD-NPs (Lac-NCTD-CS-NPs, Lac-NCTD-TMC-NPs) and Lac-NCTD solution at a dose of 250 μg/mL at 37°C was investigated. The results are shown in . Among the three formulations, the order of uptake amount was Lac-NCTD-TMC-NPs > Lac-NCTD-CS-NPs > Lac-NCTD solution.
Transport of Lac-NCTD across Caco-2 monolayers
Effect of concentration on the transport of Lac-NCTD
The influence of concentration on the transport of Lac-NCTD across the Caco-2 cell monolayers was measured (). The bidirectional transport data for Lac-NCTD showed that the secretory Papp (BL-AP) of Lac-NCTD was higher than its absorptive Papp (AP-BL) at every concentration point, indicating the existence of efflux proteins.Citation12–Citation13
Effects of protein inhibitor on bi-directional transport of Lac-NCTD
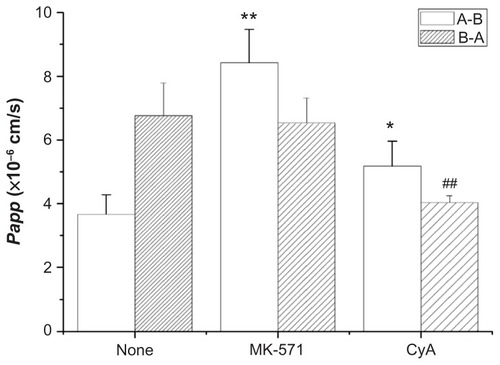
The efflux transport of Lac-NCTD (250 μg/mL) was investigated in the presence of CyA (20 μmol/L) and MK-571 (50 μmol/L). The results are shown in . When CyA was added, the Papp of the apical to basolateral direction increased, and the Papp of the basolateral to apical direction decreased. When MK-571 was added, the Papp of the apical to basolateral direction significantly increased, and the Papp of the basolateral to apical direction lightly decreased.
Figure 6 Effect of CyA, MK-571 on the transport of Lac-NCTD.
Notes: *P < 0.05 versus control group; **P < 0.01 versus control group; ##P < 0.01 versus control group. Error bars represent standard error of the mean value for three determinations.
Abbreviations: CyA, cyclosporine; MK-571, Sigma-MK-571 sodium salt hydrate; Lac-NCTD, lactosyl-norcantharitin.
Effect of endocytosis inhibitors and bypass transport enhancers
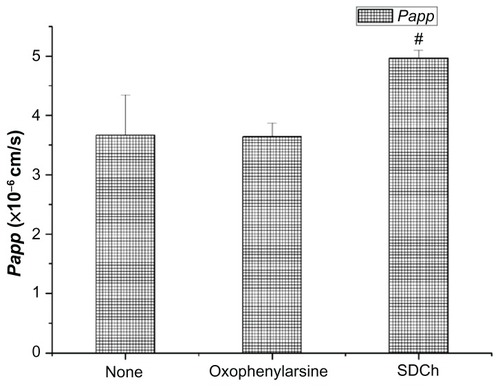
The transport of Lac-NCTD (250 μg/mL) across Caco-2 in solutions with added endocytosis inhibitor oxophenylarsine 25 mmol/L or bypass transport enhancer sodium deoxycholate (SDCh) 100 mmol/L was also investigated. As shown in , the Papp significantly increased after SDCh (P < 0.05) was added. However, no statistical difference in Papp existed between the oxophenylarsine group and the control group.
Transport of Lac-NCTD-NPs across Caco-2 mono1ayers
The bidirectional transport of Lac-NCTD-NPs (Lac-NCTD-CS-NPs, Lac-NCTD-TMC-NPs) and Lac-NCTD solution at a dose of 250 μg/mL at 37°C was also investigated. Results are shown in .
Subcellular localization of NPs
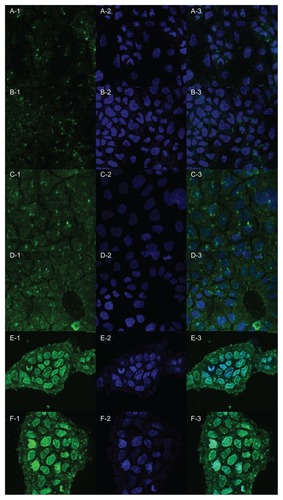
The uptake of Lac-NCTD-NPs (Lac-NCTD-CS-NPs, Lac-NCTD-TMC-NPs) by human Caco-2 cells was evaluated by laser scanning confocal microscopy (). After incubating 250 μg/mL of FITC-labeled Lac-NCTD-NPs with Caco-2 cells for 15, 30, and 60 minutes, the cells were fixed and the nuclei were stained with Hochest 33258. The fluorescence intensity profiles for both Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs were roughly comparable. After 15 minutes of incubation, the Lac-NCTD-NPs mostly gathered in the surface of the cell membrane, and a small part gathered into the cytoplasm (, green fluorescence). As the incubation time was extended, more NPs gathered in the cytoplasm, and even inside the nucleus.
Figure 8 Laser scanning confocal microscopy images showing uptake and subcellular localization of FITC-labeled Lac-NCTD-CS-NPs and FITC-labeled Lac-NCTD-TMC-NPs. Panel 1. Green fluorescence shows the location of FITC-labeled Lac-NCTD-CS- NPs or FITC-labeled Lac-NCTD-TMC-NPs. Panel 2. Blue fluorescence shows nuclear staining with Hoechst33258. Panel 3. Overlaid image of Panel 1 and Panel 2. (A, C, E): Lac-NCTD-CS-NPs groups. (B, D, F): Lac-NCTD-TMC-NPs groups. (A and B): 15 min. (C and D): 30 min. (E and F): 60 min.
Abbreviations: FITC-labeled, fluorescein isothiocyanate-labeled; Lac-NCTD-CS-NPs, lactosyl-norcantharitin (Lac-NCTD)-associated N-Trimethyl chitosan nanoparticles; Lac-NCTD-TMC-NPs, lactosyl-norcantharitin (Lac-NCTD)-associated N-Trimethyl chitosan nanoparticles.
Uptake process of NPs
The uptake of Lac-NCTD-TMC-NPs by human Caco-2 cells was observed using atomic force microscopy ().
Figure 9 Atomic force microscope micrograph of Caco-2 cells. A: Caco-2; B: Caco-2 after treatment with Lac-NCTD-TMC-NPs. (10 μm × 10 μm).
Abbreviations: Caco-2, continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells; Lac-NCTD-TMC-NPs, lactosyl-norcantharitin (Lac-NCTD)- associated N-Trimethyl chitosan nanoparticles.
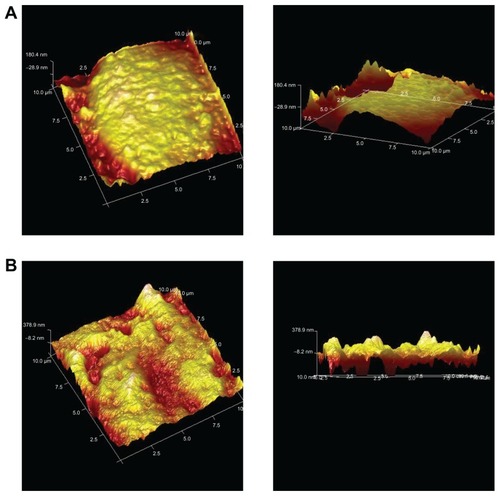
shows the surface of Caco-2 cells without any treatment. shows the cells’ surface morphology when treated with nanoparticles for 4 hours. The surface is relatively flat in , whereas multiple depressions are present in . These changes indicated the occurrence of endocytosis.
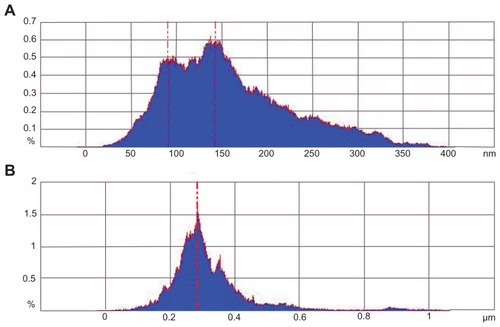
A statistical analysis of the height of the protruding particles on the cell surface () then followed. shows the Caco-2 cell surface without any treatment. The height of the protruding particles was mostly located from 100 nm to 150 nm. shows the cell surface morphology when treated with nanoparticles for 4 hours. The height of protruding particles mostly gathered at 280 nm. The height difference between 10A and B was consistent with the particle size of the nanoparticles.
Figure 10 Size distribution histogram of protrusions on Caco-2. A: Caco-2; B: Caco-2 after treatment with Lac-NCTD-TMC-NPs.
Abbreviations: Caco-2, continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells; Lac-NCTD-TMC-NPs, lactosyl-norcantharitin (Lac-NCTD)-associated N-Trimethyl chitosan nanoparticles.
Discussion
In the present study, a novel liver-targeting drug-delivery system (Lac-NCTD-TMC-NPs) with stable EE and high DL efficacy was prepared. The particle size of Lac-NCTD-TMC-NPs was 120.6 ± 1.7 nm. The majority of fenestrated liver sinusoids were, usually, smaller than 200 nm in diameter.Citation14 Additionally, several abnormalities existed between tumor blood vessels and physiologically normal vessels, including a relatively high proportion of proliferation in vivo, increased tortuosity, aberrant basement membrane formation, and enhanced permeability. Citation15–Citation17 Thus, the prepared Lac-NCTD-TMC-NPs, with a mean particle size of around 120 nm, can pass through the fenestrated sinusoids and accumulate in the tumor sites of the liver. The small particle size reduced the adverse reaction of Lac-NCTD-TMC-NPs on normal hepatic cells, by reducing the passive targeting in the liver. The TEM image suggested that these nanoparticles were probably ionically stabilized against particle agglomeration, due to the positive charges on their surface.
The intestinal transport mechanism of Lac-NCTD and its NPs was also investigated in the present study using Caco-2 cells. In recent years, the Caco-2 cell model has become a popular model of the intestinal mucosaCitation18 due to the fact that these cells have a colonic origin, and express transporters which are normally found in the intestinal mucosa. Among these transporters, some promote drug absorption.Citation19 However, others (P-gp, MRP2, and MRP1) are known to restrict drug absorption in the intestinal mucosa.Citation20
In the uptake study, concentration dependence or saturation was observed for the uptake of Lac-NCTD in the concentration range of 100 μg/mL to 400 μg/mL. This finding suggests that active transport occurred during the uptake process. The effects of pH level and temperature on the uptake of Lac-NCTD in Caco-2 monolayers were also studied. The uptake amount of Lac-NCTD in Caco-2 cells was observed to be dependent on temperature, but not on pH values. Generally, in low temperatures, the generation of adenosine triphosphate (ATP) is blocked, and so active transport is inhibited; therefore the uptake amount of Lac-NCTD in Caco-2 cells should be reduced. However, the experimental result was contrary to this finding. According to the literature,Citation21,Citation22 Caco-2 cells highly express drug efflux transporters, which are energy-dependent drug efflux pumps. The uptake amount was enhanced at a low temperature, suggesting the presence of efflux proteins.Citation22–Citation24
Previous studies have demonstrated that a class of proteins is related to drug uptake and intracellular transport.Citation25,Citation26 In addition to P-gp, MRP2 also plays a targeted transport pump role, affecting the intestinal absorption of compounds. These two types of protein are both expressed in Caco-2 cells; thus, specific inhibitors were applied in the present study to observe the interaction between Lac-NCTD and inhibitors. Results showed that P-gp and MRP2 each play an important role in the uptake and transport process of Lac-NCTD. When either CyA (20 μmol/L) or MK-571 (50 μmol/L) was added to the apical side of Caco-2 cell monolayers, the Papp of the apical to basolateral direction significantly increased by 1.41 and 2.29 times, respectively.
Both P-gp and MRP2 efflux pumps are located predominantly in the apical membranes of the epithelia (eg, small intestine, colon), making the secretion rate faster than the absorption rate.Citation27 Efflux proteins show a strong efflux effect in low drug concentrations, and become saturated in high drug concentrations. When the Lac-NCTD concentration increased from 100 μg/mL to 400 μg/mL, the ratios of Papp BL-AP to Papp AP-BL decreased from 1.91 to 1.37, respectively. Between the two types of efflux proteins, MRP2 showed a stronger action. This conclusion needs to be verified further, using a MDCK-MRP2 cell model.
shows the effects of oxophenylarsine and SDCh, which were added to the apical side of Caco-2 cell monolayers on the transport of Lac-NCTD. The paracellular transport enhancer SDCh promoted the absorption of Lac-NCTD, while the endocytosis inhibitor oxophenylarsine had no effect. This indicates that only a small proportion of Lac-NCTD was transported by the paracellular mode, which speculation is consistent with the physical and chemical properties of Lac-NCTD. Water-soluble small molecule compounds and paracellular transport are common modes of transport for these types of drugs.
The uptake and transport studies of NPs have shown that in the same concentrations, absorption of Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs was greater than that of the Lac-NCTD solution. Additionally, Lac-NCTD-TMC-NPs have a stronger action; thus, NPs can improve the cell uptake and transportation of drugs. Adsorptive endocytosis has been suggested to exist as a nonspecific interaction of the cell membrane with the NPs. Meschini and colleagues reported that higher intracellular content of a drug via endocytosis of a carrier can remarkably increase its therapeutic effect against the target cells.Citation16 On the other hand, CS and TMC can improve uptake. The mechanism is that CS and TMC can open tight junctions.Citation17 TMC’s high degree of quaternization (65%) enables high absorption-enhancing properties. Moreover, TMC-containing formulations reportedly exceed the potency of CS in mucoadhesion.Citation28 In particular, as shown in , the Papp BL-AP of NPs was greater than the Papp AP-BL, and the ratios of Papp BL-AP to Papp AP-BL were largely reduced compared with the Lac-NCTD solution.
These results suggested that after being encapsulated in CS or TMC, a small portion of Lac-NCTD was directly transported into the cells in nanoparticle form, effectively bypassing the need for efflux proteins. Therefore, for multidrug-resistant cells with a decreased drug uptake, CS-NPs or TMC-NPs can act as a transmembrane delivery vehicle, to help transport the drug molecules into the cells. Thus, CS-NPs or TMC-NPs can decrease drug efflux as well as increase cell internalization and intracellular accumulation of the drugs.Citation29
Efficient cellular internalization of nanoparticles is necessary, for both intracellular drug delivery, and efficient therapy. The results in proved that Lac-NCTD-NPs can penetrate the plasma membrane of Caco-2 cells, and, from there, translocate into the cytoplasm, and even into the nucleus. CS was regarded as a versatile polymer.Citation30 Nanoparticles made of polymer materials may pass through the nuclear pore, and then enter the nucleus during the cell division process.Citation31 Additionally, the positive charge of CS-NPs and TMC-NPs enables an electrostatic interaction with the phosphate groups of nucleic acids.Citation32 Isolated spots of green fluorescence showed higher fluorescence intensity in some cells, demonstrating the cluster of the nanoparticles. The figures obtained from atomic force microscopy () indicate that nanoparticle uptake into cells occurred through an endocytosis pathway. The combination of the nanoparticles and the cells is relatively secure.
Conclusion
Active transport and paracellular transport are suggested as both main and secondary mechanisms for the absorption of Lac-NCTD, respectively. Lac-NCTD uptake and absorption are not controlled by pH levels; however, they are positively correlated to uptake time, and negatively correlated to temperature. The Papp of the basolateral to apical direction is higher than that of the apical to basolateral direction. The P-gp and MRP2 inhibitor significantly enhances the uptake amount of Lac-NCTD. Compared with Lac-NCTD, Lac-NCTD-CS-NPs and Lac-NCTD-TMC-NPs also significantly enhance drug absorption. Lac-NCTD-NPs can penetrate the plasma membrane of Caco-2 cells and translocate into the cytoplasm, and even into the nucleus.
Acknowledgments
Technology support program of JiangSu province (BE2011670) and a project founded by the Priority Academic Program Development of JiangSu Higher Education Institutions.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiYHuangGDiakurJWiebeLITargeted delivery of macromolecular drugs: asialoglycoprotein receptor (ASGPR) expression by selected hepatoma cell lines used in antiviral drug developmentCurr Drug Deliv20085429930218855599
- MorellAGIrvineRASternliebIScheinbergIHAshwellGPhysical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivoJ Biol Chem196824311551595635941
- HuZHLiuYZhangLPreparation of a novel liver-targeting nanoparticle of norcantharidin derivative and evaluation of its antitumor activityJ Exp Nanosci201162183199
- ChewJLWolfowiczCBMaoHQLeongKWChuaKYChitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in miceVaccine20032121–222720272912798609
- WuYYangWLWangCCHuJHFuSKChitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinateInt J Pharm20052951–223524515848008
- AmidiMMastrobattistaEJiskootWHenninkWEChitosan-based delivery systems for protein therapeutics and antigensAdv Drug Delivery Rev20106215982
- YamashitaSTanakaYEndohYAnalysis of drug permeation across Caco-2 monolayer: implication for predicting in vivo drug absorptionPharm Res19971444864919144736
- CalvoPRemunan-LópezCVila-JatoJLAlonsoMJChitosan and chitosan/ethylene oxide-propylene oxide block copolymer NPs as novel carriers for proteins and vaccinesPharm Res19971410143114369358557
- YangZQXuJPanPZhangXNPreparation of an alternative freeze-dried PH-sensitive cyclosporine A nanoparticle formulation and its pharmacokinetic profile in ratsPharmazie2009641263119216227
- BradfordMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye bindingAnal Biochem197672248254942051
- HuangMMaZKhorELimLYUptake of FITC-chitosan nanoparticles by A549 cellsPharm Res20021911488149412425466
- HuMChenJZhuYDantzigAHStratfordREJrKuhfeldMTMechanism and kinetics of transcellular transport of a new beta-lactam antibiotic loracarbef across an intestinal epithelial membrane model system (Caco-2)Pharm Res19941110140514137855043
- SunHPangKSPermeability, transport, and metabolism of solutes in Caco-2 cell monolayers: a theoretical studyDrug Metab Dispos200836110212317932224
- RezaMSQuadirMAHaiderSSComparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug deliveryJ Pharm Pharm Sci20036228229112935440
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdv Drug Delivery Rev2004561116491659
- MeschiniSMolinariACalcabriniACitroGAranciaGIntracellular localization of the antitumour drug adriamycin in living cultured cells: a confocal microscopy studyJ Micros1994176Pt 3204210
- KotzéAFLuessenHLde LeeuwBJde BoerAGVerhoefJCJungingerHEComparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2)J Control Release199851135469685902
- ArturssonPPalmKLuthmanKCaco-2 monolayers in experimental and theoretical predictions of drug transportAdv Drug Deliv Rev2001461–3274311259831
- DantzigAHHoskinsJATabasLBAssociation of intestinal peptide transport with a protein related to the cadherin superfamilyScience199426451574304338153632
- KoolMde HaasMSchefferGLAnalysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell linesCancer Res19975716353735479270026
- van der SandtICBlom-RoosemalenMCde BoerAGBreimerDDSpecif icity of doxorubicin versus rhodamine-123 in assessing P-glycoprotein functionality in the LLC-PK1, LLC-PK1:MDR1 and Caco-2 cell linesEur J Pharm Sci200011320721411042226
- ArturssonPKarlssonJCorrelation between oral drug absorption in humans and drug permeability coefficients in human intestinal epithelial (Caco-2) cellsBiochem Biophys Res Commun199917538808851673839
- NakagamiTYasui-FurukoriNSaitoMTateishiTKaneoSEffect of verapamil on pharmacokinetics and pharmacodynamics of risperidone: in vivo evidence of involvement of P-glycoprotein in risperidone dispositionClin Pharmacol Ther2005781435116003291
- HaimeurAConseilGDeeleyRGColeSPThe MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulationCurr Drug Metab200451215314965249
- VarmaMVAsbokrajYDeyCSPanchagnulaRP-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancementPharm Res2003484347359
- MartinezMNAmidonGLA mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentalsJ Clin Pharmacol200242662064312043951
- CollnotEMBaldesCWempeMFMechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidityMol Pharm20074346547417367162
- FloreaBIThanouMJungingerHEBorchardGEnhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlationJ Control Release2006110235336116269199
- GottesmanMMFojoTBatesSEMultidrug resistance in cancer: role of ATP-dependent transportersNat Rev Cancer200221485811902585
- HoggardKMVarumKMIssaMImproved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomersGene Ther200411191441145215269712
- Sebolt-LeopoldJSHerreraRTargeting the mitogen-activated protein kinase cascade to treat cancerNat Rev Cancer200441293794715573115
- LaiWFLinMCNucleic acid delivery with chitosan and its derivativesJ Control Release2009134315816819100795